Chapter 58 Nonischemic Dilated Cardiomyopathy
Diagnosis and Management
Introduction and Classification
Nonischemic dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy. The hallmarks of DCM are left or often biventricular enlargement with mostly global systolic hypokinesis, although some regionally more pronounced contraction abnormality may be present.1 Several specific diseases of the heart muscle (e.g., infectious agents, chemotherapeutic agents, metabolic disorders, genetic mutations) present as clinical manifestations of DCM, which presumably represents a final common pathway of myocardial damage.1 Therefore, DCM is defined as a primary cardiomyopathy with a mixed etiologic background. Nevertheless, a considerable overlap between the different groups must be taken into account (e.g., myocarditis and DCM caused by an infectious agent). The definitions of DCM and ischemic cardiomyopathy, with the latter defined as a “dilated cardiomyopathy with impaired contractile performance not explained by the extent of the coronary artery disease or ischemic damage,” have been controversial in the past.2 The recent definitions and the classification of cardiomyopathies by the American Heart Association do not include pathologic myocardial processes and dysfunction caused directly by other cardiovascular diseases; hence the term ischemic cardiomyopathy is not supported any more.1 Therefore, although this term continues to be used, it is not directly linked to the previous definition but is meant to reflect any type of left ventricular dysfunction caused by coronary artery disease.2 In this context, one should concede that without coronary angiography, the diagnosis of significant coronary artery disease solely based on clinical findings in patients with heart failure often fails to identify this cause.3
Epidemiology and Survival
The incidence of DCM varies from 5 to 8 cases per 100,000 per year, and the prevalence is estimated to be 1 : 2500, with approximately 10,000 deaths and 46,000 hospitalizations in the United States.1 Heart failure caused by DCM represents a major health issue and is the primary indication for heart transplantation.4 Usually, DCM presents for the first time in patients between 18 and 50 years of age; however, children and older adults may also be affected.5 Furthermore, it develops almost three times more often in blacks and males than in whites and females, with apparently lower survival rates in blacks than in whites for unknown reasons.
Mortality and Causes of Death in Dilated Cardiomyopathy
The natural history of DCM is diverse. Some patients have minimal or no symptoms, whereas symptomatic patients usually experience a progressive deterioration; however, a minority improves with a reduction in cardiac size and longer survival. In some patients, clinical and functional improvements may occur years after the initial manifestation of symptoms. Recently, better survival rates have been achieved with improvement in medical (angiotensin-converting enzyme [ACE] inhibitors, β-blockers, aldosterone inhibitors) and device therapy (implantable cardioverter-defibrillator [ICD] and cardiac resynchronization therapy).6 A difference between nonfamilial and familial cases of DCM could not be demonstrated.7 Yet, the prognosis of some secondary cardiomyopathies, for example, the human immunodeficiency virus (HIV)–related form, is particularly bad. Irrespective of the underlying cause, patients with DCM are prone to ventricular arrhythmias and sudden cardiac death (SCD), with DCM representing the substrate for approximately 10% of all SCDs in adults.8 Approximately 20% of patients with DCM will die within 1 year after diagnosis; in most of them, the cause is SCD.9
Genetics and Other Causes of Dilated Cardiomyopathy
DCM presumably represents a final common or toxic pathway that is the end result of myocardial damage caused by different mechanisms.1 In about 50% of cases, patients are described as having “idiopathic” DCM because an etiologic cause or secondary reason cannot be identified.10 Several specific diseases of the heart muscle or metabolism can lead to the clinical manifestations of DCM. Evidence that many idiopathic cases still result from inherited abnormalities is increasing, since 20% to 50% of these may be familial on further evaluation.11 Genetically determined familial DCM, which refers to the presence of two or more family members with DCM, can be subdivided into at least four phenotypes12: isolated DCM, DCM with involvement of the cardiac conduction system, DCM with concomitant skeletal myopathy (with or without conduction disease), and DCM with sensorineural deafness.13 The most common mode of inheritance is autosomal dominant (56%), and in 5% to 10%, it is linked to the X chromosome.14–16 Autosomal recessive or mitochondrial forms of DCM are uncommon.17–19 Molecular analysis has revealed a great number of genes and chromosomal loci leading to DCM. The pathophysiological effects are a malfunction of force generation (because of mutations in sarcomeric protein genes) and of force transmission (due to mutations in cytoskeletal protein genes).20,21 Other causes of DCM as a secondary cardiomyopathy may be infectious disease, a tachycardiomyopathy, inflammatory cardiomyopathy, deposition diseases, medications, toxins, endocrinologic disorders, neuromuscular diseases, rheumatologic diseases, postpartum cardiomyopathy, uremia, and others.1 These causes have to be taken into account whenever a genetic cause is considered.
Pathophysiology of Arrhythmias in Dilated Cardiomyopathy
Multiple mechanisms contribute to the development of ventricular arrhythmias in patients with dilated cardiomyopathy.22 Autopsy studies have shown substantial left ventricular subendocardial scarring in 33% of patients and patchy areas of replacement fibrosis in 57%, accompanied by increased perivascular fibrous tissue and perimyocytic fibrosis in the left ventricle.23 This may be the substrate for re-entry. Other factors such as hypokalemia, hypomagnesemia, and ischemia caused by the occlusion of small intramyocardial arteries by thrombosis or emboli may serve as triggers for ventricular arrhythmias.24 An elevated sympathetic tone and increased circulating catecholamines may also favor ventricular re-entrant arrhythmias.25,26 Stretch-induced shortening of the ventricular refractory period may support the development of re-entry.27
A distinct form of ventricular arrhythmia in DCM is bundle branch re-entrant ventricular tachycardia (BBRVT). A macro–re-entry, which usually employs the right bundle branch as the antegrade limb and the left bundle branch as the retrograde limb, leads to a rapid ventricular tachycardia (VT). Apart from BBRVT, macro–re-entrant VTs involving myocardial scars at the mitral annulus are frequently observed.28,29
Ventricular arrhythmias in patients with chronic heart failure caused by DCM may be provoked by non–re-entrant mechanisms such as abnormal automaticity and triggered activity.30,31 The occurrence of triggered activity and the causative early after-depolarizations is promoted by prolonged repolarization and prolonged action potential predominately induced by the downregulation of repolarizing potassium channels.32,33 Focal ventricular arrhythmias originating from the distal Purkinje system are often nonsustained.30,31 Although frequently occurring in patients with DCM, VT is not the only cause of SCD in these patients. At end-stage heart failure, bradycardia and electromechanical dissociation are very common causes of SCD.34
Risk Stratification for Arrhythmic Death in Dilated Cardiomyopathy
Left Ventricular Function
In patients with nonischemic DCM, overall mortality is associated with left ventricular dysfunction, but only a few studies have investigated the relationship between left ventricular function and SCD directly.35 The combination of severely reduced left ventricular function (left ventricular ejection fraction [LVEF] <30%) and nonsustained VT was used to identify the highest-risk subgroup.36 Since reduced LVEF was an inclusion criterion in all ICD prophylaxis trials, it is a prominent feature of guidelines for ICD therapy and a cornerstone in daily clinical practice.37,38 Nevertheless, the majority of SCDs occur in patients with less severely reduced left ventricular function, which highlights the limited sensitivity of this parameter.39
Electrocardiography
In patients with nonischemic DCM, the presence of left bundle branch block (LBBB) has been associated with a worse outcome. The Vesnarinone Trial (VEST) and other studies confirmed a significant association between the degree of QRS duration and mortality.40–42 However, other studies were not able to demonstrate a significant association between intraventricular conduction delay and SCD.36,43,44 The Defibrillators in Nonischemic Cardiomyopathy (DEFINITE) trial was not able to show an association between QRS duration and all-cause mortality.45 The Sudden Cardiac Death–Heart Failure Trial (SCD-HeFT), which enrolled patients with ischemic cardiomyopathy and those with nonischemic cardiomyopathy, reported that ICD therapy yielded a greater mortality reduction in patients with QRS duration ≥0.12 seconds, but specific information on the relationship between QRS duration and mortality reduction in patients with nonischemic cardiomyopathy has not been presented.46 In a retrospective analysis of the Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT) database, Iuliano et al identified a prolonged QRS duration of ≥0.12 seconds as an independent predictor of total mortality and SCD in patients with heart failure.47 The role of atrial fibrillation remains controversial, too. Survival rates may be reduced by increased thromboembolic events or ventricular arrhythmias, which may be evoked by an increased dispersion of refractoriness caused by long-short cycle lengths.48
Spontaneous Ventricular Arrhythmias
The prevalence of spontaneous ventricular arrhythmias in patients with DCM is very high.49 Polymorphic premature ventricular contractions, ventricular pairs, and nonsustained VT are very common, with increasing prevalence of nonsustained VT and increasing severity of heart failure symptoms. The positive predictive value of these arrhythmias is relatively low, ranging from 20% to 50%, yet the negative predictive value has been cited as high as 95.5%.50,51 In the absence of a treatment modality with proven efficacy, specific treatment for nonsustained VT is not indicated, except in the rare circumstances of symptomatic, frequent, or very rapid episodes leading to hemodynamic instability.
Heart Rate Variability, Baroreflex Sensitivity, and Heart Rate Turbulence
Heart rate variability (HRV) and baroreflex sensitivity (BRS) provide indirect (i.e., through their effects on the sinus node) measures for the autonomic effects in the ventricle that may be important in the pathophysiology of VT and SCD. Most studies using HRV, BRS, and heart rate turbulence (HRT) as predictors of adverse arrhythmic events have been conducted in patients after the occurrence of myocardial infarction (MI). One of the few studies in DCM was conducted by Rashba et al.52 They reported a significant difference in mortality rates in a substudy of the Defibrillators in Nonischemic Cardiomyopathy (DEFINITE) trial. With decreasing standard deviation of normal R-R intervals (SDNN) as a measurement of HRV, an increase in total mortality was noted: Among 70 patients with SDNN longer than 113 ms, no deaths occurred. However, in 69 patients with SDNN between 81 and 113 ms, the mortality rates was 7%; in 72 patients with SDNN less than 81 ms, the mortality rate was 10%. Similar results were seen when the predictive value of SDNN was examined for the composite endpoint of SCD and appropriate ICD shock. On the basis of this study, the authors suggested that patients with nonischemic DCM and preserved HRV have a good prognosis and may not benefit from ICD prophylaxis and that lower levels of SDNN were associated with a progressively increased mortality risk.
HRT describes the short-term fluctuation in sinus cycle length that follows a ventricular premature beat.53,54 It has been postulated that it measures vagal responsiveness similar to BRS. It is a potentially attractive risk factor, as it can be performed with a relative small number of premature beats from 24-hour Holter electrocardiogram (ECG). In the Marburg Cardiomyopathy Study (MACAS), low HRT was a multivariate predictor of transplant-free survival, but not of arrhythmic events.55 In the same study, blunted BRS, which identified patients with a higher cardiac mortality after a recent MI in the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) study, was not a predictor of arrhythmic events.56
Microvolt T-Wave Alternans
The term microvolt T wave alternans (MTWA) refers to the presence of beat-to-beat changes in T-wave amplitude that are not detectable on the surface ECG. Bloomfield et al showed that in patients with left ventricular dysfunction (EF ≤40%), the negative predictive accuracy of MTWA was very high (≥98% at a follow-up of 2 to 3 years) regardless of the etiology of the cardiomyopathy, with significantly lower event rates in patients with normal MTWA test results.57 In contrast, a prospective substudy of the SCD-HeFT trial, which tested the predictive value of MTWA in 490 (n = 250 nonischemic heart failure) of the 2521 patients with an EF 35% or less and New York Heart Association (NYHA) class II or III heart failure randomized to ICD therapy, amiodarone, or placebo, did not show any significant differences in the composite primary end point of the first occurrence of any of the following: SCD, sustained VT or ventricular fibrillation (VF), or appropriate ICD discharge.58 In conclusion, further evidence is needed in the specific setting of nonischemic DCM.
Cardiac Magnetic Resonance Imaging
Wu et al recently observed that late gadolinium enhancement detected by cardiac magnetic resonance imaging (MRI) strongly predicts adverse cardiac outcomes, including adverse arrhythmic events in patients with nonischemic DCM, supporting earlier reports by Assomull et al.59,60 In the latter study, midwall fibrosis was detected in 35% of patients with nonischemic DCM by late gadolinium enhancement. This finding was associated with a higher rate of the predefined combined primary endpoint of all-cause death and hospitalization for a cardiovascular event and also predicted secondary outcome measures of SCD or VT.
Electrophysiological Testing
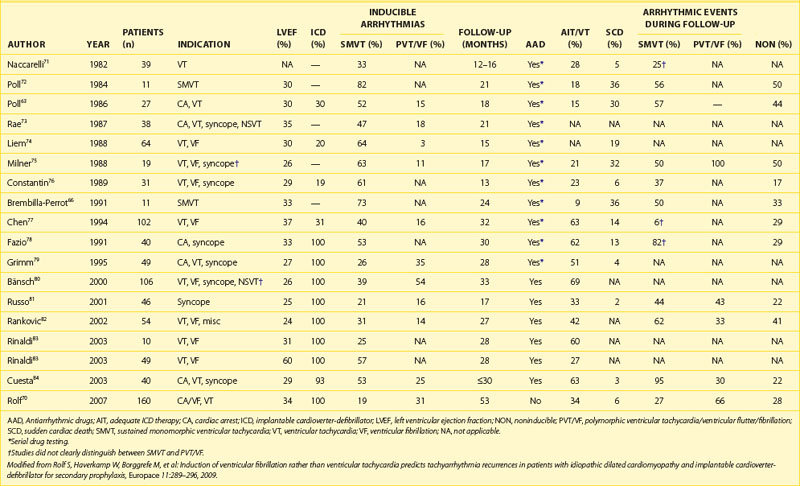
The role of electrophysiological testing (i.e., programmed ventricular stimulation) in risk stratification in patients with nonischemic cardiomyopathy and no history of sustained ventricular arrhythmias has been addressed in nine studies.61–69 The small numbers of patients in each study, the low rate of arrhythmia induction and reproducibility, and the low subsequent arrhythmia event rates have made it difficult to draw consistent conclusions. Therefore, programmed ventricular stimulation is not recommended for risk stratification in patients with nonischemic cardiomyopathy. However, Rolf et al showed that in a relatively large cohort of 160 patients with DCM who received ICDs for secondary prophylaxis, the induction of polymorphic VT or VF in contrast to the induction of monomorphic VT was associated with a high risk of subsequent fast ventricular arrhythmias during a mean follow-up of 53 months (Table 58-1). Nevertheless, a subgroup of patients with secondary prevention and sufficiently low risk that would render ICD therapy unnecessary could not be identified. A prospective study in a DCM population with primary prevention, possibly in combination with other risk factors, might be helpful in refining the indications for ICD therapy.
Management
Vasodilator Therapy
Treatment with ACE inhibitors improves ventricular function, patient well-being and reduces hospital admissions for worsening heart failure. Moreover, a reduction of total mortality and SCD by ACE inhibitor therapy has been demonstrated. Detailed information on the treatment of acute and chronic heart failure, which is beyond the scope of this chapter, is provided in current guidelines.85,86
Antiadrenergic Therapy
The pathophysiological rationale for antiadrenergic therapy in patients with DCM is to antagonize the heightened sympathetic tone and circulating catecholamines, thereby reducing the various adverse effects of these regulatory mechanisms. Several studies have shown symptomatic improvement in patients with DCM treated with β-blockers. To date, no specific studies have demonstrated the benefit of β-blockers for the prevention of SCD in DCM. However, several studies have shown that the reduction in mortality is similar in patients with ischemic heart failure or nonischemic heart failure. The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) randomized 3991 patients with an LVEF 40% or less and NYHA class II to IV to metoprolol or placebo in addition to conventional therapy. It showed a reduction in overall mortality by 34% at 21 months (30% in patients without coronary artery disease).87 Of note, a 41% reduction in SCD was observed. Similarly, the CIBIS II study showed in 1327 patients (160 patients with nonischemic DCM) in NYHA class III and IV randomized to bisoprolol, a significant 34% reduction in total mortality at 1.3 years because of a 44% reduction in SCD mortality rates.88 Taking these studies and the results of the Carvedilol Heart Failure Study Group (HFSG) trial into account, all patients with congestive heart failure should receive β-blockers and ACE inhibitors unless contraindicated.89
Antiarrhythmic Drugs
Since patients with DCM are prone to atrial and ventricular arrhythmias, antiarrhythmic drugs may be considered for treatment. However, most antiarrhythmic drugs may exhibit proarrhythmic effects and exacerbate the left ventricular dysfunction; the mortality rate may be increased when some class I or III antiarrhythmic drugs are used. Data from the Cardiac Arrhythmia Suppression Trial (CAST) on patients who have had MIs have been extrapolated to others with reduced left ventricular function and to those with structural heart disease in general.90 Therefore, despite the fact that patients with DCM frequently have symptomatic as well as asymptomatic supraventricular and ventricular arrhythmias, treating them with class Ia and Ic drugs is not advised. This also applies to D-sotalol.91–93 Proarrhythmia rarely occurs with amiodarone therapy, even in patients with depressed left ventricular function. Therefore, amiodarone is the most frequently used antiarrhythmic agent in patients with DCM. Of note, patients with nonischemic cardiomyopathy were under-represented in most studies, except for the relatively small Amiodarone Versus Implantable Cardioverter-Defibrillator: Randomized Trial in Patients with Nonischemic Dilated Cardiomyopathy and Asymptomatic Nonsustained Ventricular Tachycardia (AMIOVIRT) and SCD-HeFT (Sudden Cardiac Death-Heart Failure Trial).46,94 Since no placebo group was included in AMIOVIRT, the role of nonsustained VT for risk stratification in nonischemic DCM and the superior effectiveness of amiodarone compared with placebo remain unclear.
In the CHF-STAT (Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy), amiodarone proved to be more effective in patients with nonischemic cardiomyopathy versus those with ischemic cardiomyopathy with regard to survival without SCD or hospitalization.95 However, a reduction in overall mortality or in mortality from SCD could not be demonstrated in the entire study cohort. The outcomes of patients as a function of NYHA class were not reported.
In contrast to this, in the Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) trial, which was a randomized but open trial, all-cause mortality was reduced by 28% (P < .03), and mortality from SCD was reduced by 27% (P = .056) in the amiodarone treatment group.96 Moreover, a higher percentage of patients improved by NYHA functional class. In comparison to the CHF-STAT, the GESICA trial included fewer patients with ischemic cardiomyopathy (39% vs. 72%) but a significant number of patients with Chagas disease. Hence, the positive results of the CHF-STAT in the case of patients with nonischemic DCM might be confirmed by GESICA. Nevertheless, these two trials present conflicting data on amiodarone treatment for patients with heart failure.
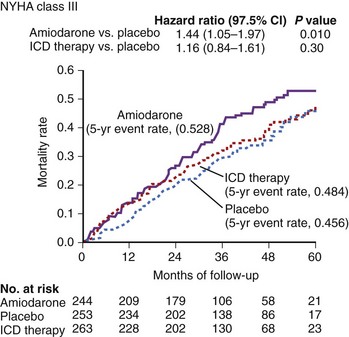
In the larger SCD-HeFT trial, which randomized patients with ischemic or nonischemic cardiomyopathy, an LVEF less than 36%, and a history of congestive heart failure to placebo, amiodarone, or an ICD, no significant difference was seen in all-cause mortality in the placebo group and the amiodarone group, but the ICD significantly reduced overall mortality. Of note, in SCD-HeFT, a relative 44% increase was seen in the risk of death in the amiodarone group compared with the placebo or ICD group among patients with NYHA III heart failure. Thus, although survival in the amiodarone and placebo arms of class II patients was identical, amiodarone was worse than placebo in class III patients (Figure 58-1).
Consistent with these findings from post hoc analyses, the Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease (ANDROMEDA) trial reported an increased early mortality related to the worsening of heart failure in patients with recent-onset heart failure or with aggravation of pre-existing heart failure.97 After randomization of 310 patients with new or worsening heart failure to the multiple-channel blocker dronedarone (25.5% with nonischemic DCM) and 317 matched patients to placebo (32.5% with nonischemic DCM), the trial was prematurely terminated after a median follow-up of 2 months because of excessive mortality in the dronedarone group (8.1% vs. 3.8% in the placebo group). Post hoc subgroup analyses revealed that patients with more severely reduced left ventricular function were at higher risk of death associated with dronedarone treatment. These data are difficult to extrapolate to patients in stable heart failure.
In conclusion, antiarrhythmic drugs are not primarily indicated to improve the prognosis of patients with nonischemic cardiomyopathy. Mostly, they may be used in addition to ICD therapy to reduce ICD shock frequency and in the treatment of symptomatic atrial fibrillation.38,98
Implantable Cardioverter-Defibrillator Therapy
The Amiodarone versus Implantable Cardioverter-Defibrillator Trial (AMIOVIRT) compared ICD therapy with amiodarone therapy in patients with nonischemic left ventricular dysfunction with a mean duration of 3 years and asymptomatic nonsustained VT.94 According to calculations, 219 patients were required in each group for an 80% power to identify a reduction in total mortality from 20% to 10%. A difference in the primary endpoint of total mortality could not be demonstrated after enrollment of 103 patients and an average follow-up of 2 years (6 deaths in the ICD group vs. 7 in the amiodarone group; P = .8). Thus, AMIOVIRT neither supported nor disproved the value of ICD therapy in this population. In contrast to AMIOVIRT, the Cardiomyopathy Trial (CAT) evaluated the significance of ICD therapy in patients with nonischemic left ventricular dysfunction after exclusion of significant coronary artery disease by angiography and recent onset of heart failure symptoms within 9 months or less before inclusion.99 The primary endpoint of CAT was all-cause mortality. However, the observed mortality rate in the non-ICD group was markedly lower than the expected mortality rate (3.7% and 30%, respectively). Over a mean follow-up of 5.5 years, 30 deaths were observed (13 deaths in the ICD group, 17 in the control group; P = .6). The trial was terminated early owing to futility. Like AMIOVIRT, CAT was underpowered, so no consistent conclusions regarding the efficacy of ICD therapy in patients with nonischemic DCM could be drawn from this trial.
The Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial evaluated the efficacy of ICD therapy in comparison with no ICD therapy in 458 patients with nonischemic left ventricular dysfunction (LVEF ≤35%) with a history of heart failure NYHA functional class I to III.45 Additionally, patients were required to have frequent premature ventricular beats (>10/hour) or nonsustained VTs. The absence of clinically significant coronary artery disease as the cause of the cardiomyopathy was confirmed by coronary angiography or by a negative stress imaging study. The mean ejection fraction (21%) was lower than that seen in CAT (24%) and SCD-HeFT (25%), and the average duration of heart failure was 2.8 years longer than that seen in CAT. During a mean follow-up of 29 months, 28 deaths occurred in the ICD group compared with 40 deaths in the control group, leading to a 35% relative reduction in mortality in the ICD group (95% confidence interval [CI] = 1.06 to 0.4; P = .08). In contrast, a significant reduction in SCD (80%; 95% CI = 0.06 to 0.71; P = .006) was observed.45
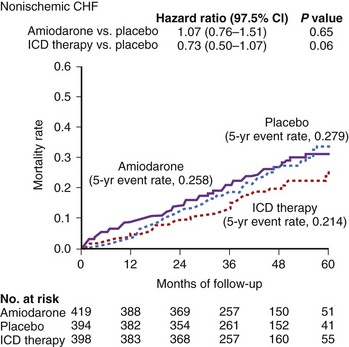
The SCD-HeFT compared the efficacies of ICD therapy, amiodarone, and placebo in 2521 patients with heart failure. The rate of heart failure medication use was higher in the SCD-HeFT than in other ICD studies. Patients with ischemic cardiomyopathy (52%) as well as those with nonischemic cardiomyopathy (48%) were enrolled. Patients randomly assigned to ICD therapy had a significantly lower risk of death compared with those assigned to amiodarone and placebo (28.9%) over 60 months with a relative risk reduction of 23% (95% CI = 0.62 to 0.96; P = .007). Moreover, the relative benefit of ICD therapy and the absolute reduction in mortality were similar in the ischemic cardiomyopathy group and the nonischemic cardiomyopathy group (21% vs. 27%, and 7.3% vs. 6.5%, respectively). However, statistical significance was not reached for the use of ICDs in patients with nonischemic DCM. In contrast to the DEFINITE trial, in which patients in NYHA functional class III derived the largest survival benefit from ICD therapy, this group of patients had no apparent reduction in the risk of death with ICD therapy (Figure 58-2).
It is not entirely clear whether a history of syncope in patients with nonischemic DCM is a prognostic indicator because these patients were excluded from the large randomized controlled ICD trials. Phang et al retrospectively compared 108 consecutive non-ICD patients with DCM and a mean LVEF of 27% presenting with syncope with 71 consecutive patients with DCM who presented with sustained ventricular arrhythmias, with regard to freedom from any ventricular arrhythmias or life-threatening arrhythmias and all-cause mortality.100 They were not able to show any significant differences between the groups in the three outcome parameters during the mean follow-up of 44 months. This supports the results of Knight et al, who found no differences in the rates of appropriate ICD shocks in 14 patients with DCM, unexplained syncope, and a negative electrophysiological study compared with 19 patients with DCM and previous cardiac arrest.101 Therefore, patients with DCM presenting with syncope are probably a high-risk group, with event rates similar to those of patients with DCM presenting with sustained arrhythmias, and should be considered for ICD therapy.
Biventricular Pacing
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial randomized 1520 patients in NYHA class II and III and a QRS duration of 0.12 seconds or greater to cardiac resynchronization therapy (CRT) plus ICD (CRT-D, 595 patients), to CRT alone (617 patients), or to optimal medical treatment (308 patients).102 In 45% of these patients, nonischemic cardiomyopathy was present. The primary endpoint was a composite of death from or hospitalization for any cause. Secondary endpoints were all-cause mortality and cardiac morbidity. The risk of the combined primary endpoint was reduced by 34% in the CRT group (P < .002) and by 40% in the CRT-D group (P < .001 for comparison with optimal medical therapy alone). CRT reduced the risk of the secondary endpoint of death from any cause by 24% (P = .059), and CRT-D reduced the risk by 36% (P = .003). The absolute risk reduction of 7% in the CRT-D group indicates that 15 patients need to be treated with a CRT-D device in order to prevent 1 death over 12 months. Of note, patients with nonischemic cardiomyopathy received a greater benefit from biventricular pacemaker–defibrillator therapy than did patients with ischemic cardiomyopathy when comparing the secondary endpoint of all-cause mortality. Most of the baseline parameters of patients in the COMPANION trial were similar to other trials of ICD in heart failure. However, the annual mortality rate in the control group in the COMPANION trial was higher than in other trials. The 12-month mortality rate in the medical therapy group in the CARE-HF trial was 12.6% versus 19% in the COMPANION trial.102,103
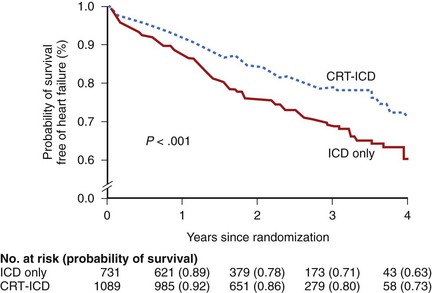
Recently, the Multicenter Automatic Defibrillator Implantation Trial–CRT (MADIT-CRT) trial randomized 1820 patients in NYHA functional class I or II (85% in class II), with an EF 30% or less and a QRS duration 0.13 seconds or longer to CRT-D or ICD therapy in a 3 : 2 fashion (1089 and 731 patients, respectively). A significant reduction in the primary endpoint death from any cause or a nonfatal heart failure event (whichever came first) was shown in patients with ischemic cardiomyopathy as well as in those with nonischemic cardiomyopathy, strengthening the earlier results of the smaller Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study, which was not significant with regard to its primary endpoint of patients’ conditions worsening because of the smaller sample size and shorter follow-up.104–106 However, a longer follow-up of the European subgroup of the REVERSE trial showed a significant improvement in the clinical status of patients in the CRT-ON group and a significantly reduced risk of first hospital stay or death and reversed left ventricular remodeling.107 The observed significant reduction of the primary endpoint in the MADIT-CRT trial was driven by a reduction of heart failure events primarily in a prespecified subgroup of patients with a QRS duration of 0.15 seconds or longer, whereas no effect on mortality was seen in this early heart failure cohort, which can be considered as a low-risk group (3% annual mortality rate) (Figure 58-3).
The Resynchronization Therapy In Narrow QRS (RETHINQ) study addressed the question whether CRT will increase peak oxygen consumption during exercise in patients with NYHA class III heart failure, an EF of 35% or less, a QRS interval of less than 0.13 seconds, and evidence of mechanical dyssynchrony on echocardiography. The trial failed to reach this primary endpoint.108
Catheter Ablation
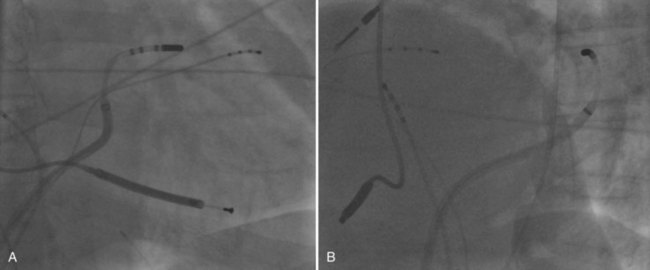
Catheter ablation of ventricular arrhythmias in patients with nonischemic DCM has been used after the authors’ initial case report with increasing frequency.109,110 The authors’ initial experience had demonstrated that monomorphic VT in nonischemic DCM may be caused by a fixed reentry circuit, similar to what occurs after myocardial infarction. Modern modalities such as electroanatomic mapping systems, irrigated tip catheters, and the integration of three-dimensional computed tomography or MRI may facilitate ablation. However, entrainment and pace mapping are still important cornerstones in the ablation procedure. Apart from the three-dimensional information, MRI with late enhancement may also provide information about the localization and extent of myocardial scars, which might be involved in the VT circuit. Integration of this additional information into modern mapping systems might add to the procedural success rate. The percutaneous epicardial approach is increasingly used also in patients with nonischemic cardiomyopathy because approximately one third of VTs in this setting can only be ablated successfully epicardially (Figure 58-4).28
Key References
Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453-1458.
Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-e62.
Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118:1497-1518.
Grimm W, Christ M, Bach J, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: Results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883-2891.
Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158.
Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816.
Massie BM, Fisher SG, Radford M, et al. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. CHF-STAT Investigators. Circulation. 1996;93:2128-2134.
Moss A, Hall W, Cannom D, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Eng J Med. 2009;361(14):1329-1338.
Rashba EJ, Estes NAM, Wang P, et al. Preserved heart rate variability identifies low-risk patients with nonischemic dilated cardiomyopathy: Results from the DEFINITE trial. Heart Rhythm. 2006;3:281-286.
Soejima K, Stevenson WG, Sapp JL, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: The importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834-1842.
Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707-1712.
Zipes DP, Borggrefe M, Buxton AE, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006;48:e247-e346.
1 Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816.
2 Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841-842.
3 Fox KF, Cowie MR, Wood DA, et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228-236.
4 Manolio TA, Baughman KL, Rodeheffer R, et al. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung, and Blood Institute workshop). Am J Cardiol. 1992;69:1458-1466.
5 Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867-1876.
6 Di Lenarda A, Pinamonti B, Mestroni L, et al. The natural history of dilated cardiomyopathy: A review of the Heart Muscle Disease Registry of Trieste [in Italian]. Ital Heart J Suppl. 2004;5:253-266.
7 Michels VV, Driscoll DJ, Miller FA, et al. Progression of familial and non-familial dilated cardiomyopathy: Long term follow up. Heart. 2003;89:757-761.
8 Neri R, Mestroni L, Salvi A, Camerini F. Arrhythmias in dilated cardiomyopathy. Postgrad Med J. 1986;62:593-597.
9 Bahler RC. Assessment of prognosis in idiopathic dilated cardiomyopathy. Chest. 2002;121:1016-1019.
10 Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077-1084.
11 Thiene G, Corrado D, Basso C. Cardiomyopathies: Is it time for a molecular classification? Eur Heart J. 2004;25:1772-1775.
12 Fatkin D, Graham RM. Molecular mechanisms of inherited cardiomyopathies. Physiol Rev. 2002;82:945-980.
13 Emery AE. The muscular dystrophies. Lancet. 2002;359:687-695.
14 Mestroni L, Rocco C, Gregori D, et al. Familial dilated cardiomyopathy: Evidence for genetic and phenotypic heterogeneity. Heart Muscle Disease Study Group. J Am Coll Cardiol. 1999;34:181-190.
15 Grünig E, Tasman JA, Kücherer H, et al. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol. 1998;31:186-194.
16 Baig MK, Goldman JH, Caforio AL, et al. Familial dilated cardiomyopathy: Cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol. 1998;31:195-201.
17 Murphy RT, Mogensen J, Shaw A, et al. Novel mutation in cardiac troponin I in recessive idiopathic dilated cardiomyopathy. Lancet. 2004;363:371-372.
18 Carmignac V, Salih MA, Quijano-Roy S, et al. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340-351.
19 Sparkes R, Patton D, Bernier F. Cardiac features of a novel autosomal recessive dilated cardiomyopathic syndrome due to defective importation of mitochondrial protein. Cardiol Young. 2007;17:215-217.
20 Taylor MR, Slavov D, Ku L, et al. Prevalence of desmin mutations in dilated cardiomyopathy. Circulation. 2007;115:1244-1251.
21 Mestroni L, Taylor MR. Lamin A/C gene and the heart: How genetics may impact clinical care. J Am Coll Cardiol. 2008;52:1261-1262.
22 Eckardt L, Haverkamp W, Johna R, et al. Arrhythmias in heart failure: Current concepts of mechanisms and therapy. J Cardiovasc Electrophysiol. 2000;11:106-117.
23 Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: Analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340-1355.
24 Schwartz CJ, Gerrity RG. Anatomical pathology of sudden unexpected cardiac death. Circulation. 1975;52:III18-III26.
25 Meredith IT, Broughton A, Jennings GL, Esler MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991;325:618-624.
26 Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85:I77-I91.
27 Franz MR, Burkhoff D, Yue DT, Sagawa K. Mechanically induced action potential changes and arrhythmia in isolated and in situ canine hearts. Cardiovasc Res. 1989;23:213-223.
28 Soejima K, Stevenson WG, Sapp JL, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: The importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834-1842.
29 Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821-2825.
30 Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034-1048.
31 Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation. 1998;98:2404-2414.
32 Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379-385.
33 Näbauer M, Kääb S. Potassium channel down-regulation in heart failure. Cardiovasc Res. 1998;37:324-334.
34 Luu M, Stevenson WG, Stevenson LW, et al. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation. 1989;80:1675-1680.
35 Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564-1575.
36 Grimm W, Christ M, Bach J, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: Results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883-2891.
37 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-e62.
38 Zipes DP, Borggrefe M, Buxton AE, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006;48:e247-e346.
39 Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death. Structure, function, and time-dependence of risk. Circulation. 1992;85:I2-I10.
40 Bode-Schnurbus L, Böcker D, Block M, et al. QRS duration: A simple marker for predicting cardiac mortality in ICD patients with heart failure. Heart. 2003;89:1157-1162.
41 Baldasseroni S, Opasich C, Gorini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: A report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398-405.
42 Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810-1816.
43 Romeo F, Pelliccia F, Cianfrocca C, et al. Predictors of sudden death in idiopathic dilated cardiomyopathy. Am J Cardiol. 1989;63:138-140.
44 Hofmann T, Meinertz T, Kasper W, et al. Mode of death in idiopathic dilated cardiomyopathy: A multivariate analysis of prognostic determinants. Am Heart J. 1988;116:1455-1463.
45 Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158.
46 Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
47 Iuliano S, Fisher SG, Karasik PE, et al. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085-1091.
48 Grönefeld GC, Mauss O, Li YG, Klingenheben T, Hohnloser SH. Association between atrial fibrillation and appropriate implantable cardioverter defibrillator therapy: Results from a prospective study. J Cardiovasc Electrophysiol. 2000;11:1208-1214.
49 Meinertz T, Hofmann T, Kasper W, et al. Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol. 1984;53:902-907.
50 Doval HC, Nul DR, Grancelli HO, et al. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA-GEMA Investigators. Circulation. 1996;94:3198-3203.
51 Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118:1497-1518.
52 Rashba EJ, Estes NAM, Wang P, et al. Preserved heart rate variability identifies low-risk patients with nonischemic dilated cardiomyopathy: Results from the DEFINITE trial. Heart Rhythm. 2006;3:281-286.
53 Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390-1396.
54 Watanabe MA, Marine JE, Sheldon R, Josephson ME. Effects of ventricular premature stimulus coupling interval on blood pressure and heart rate turbulence. Circulation. 2002;106:325-330.
55 Grimm W, Schmidt G, Maisch B, et al. Prognostic significance of heart rate turbulence following ventricular premature beats in patients with idiopathic dilated cardiomyopathy. J Cardiovasc Electrophysiol. 2003;14:819-824.
56 La Rovere MT, Bigger JT, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478-484.
57 Bloomfield DM, Bigger JT, Steinman RC, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456-463.
58 Gold MR, Ip JH, Costantini O, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: Primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118:2022-2028.
59 Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414-2421.
60 Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985.
61 Meinertz T, Treese N, Kasper W, et al. Determinants of prognosis in idiopathic dilated cardiomyopathy as determined by programmed electrical stimulation. Am J Cardiol. 1985;56:337-341.
62 Stamato N, O’Connell J, Murdock D, et al. The response of patients with complex ventricular arrhythmias secondary to dilated cardiomyopathy to programmed electrical stimulation. Am Heart J. 1986;112:505-508.
63 Poll D, Marchlinski F, Buxton A, Josephson M. Usefulness of programmed stimulation in idiopathic dilated cardiomyopathy. Am J Cardiol. 1986;58:992-997.
64 Das S, Morady F, DiCarlo L, et al. Prognostic usefulness of programmed ventricular stimulation in idiopathic dilated cardiomyopathy without symptomatic ventricular arrhythmias. Am J Cardiol. 1986;58:998-1000.
65 Gossinger H, Jung M, Wagner L, et al. Prognostic role of inducible ventricular tachycardia in patients with dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia. Int J Cardiol. 1990;29:215-220.
66 Brembilla-Perrot B, Donetti J, de la Chaise A, et al. Diagnostic value of ventricular stimulation in patients with idiopathic dilated cardiomyopathy. Am Heart J. 1991;121:1124-1131.
67 Kadish A, Schmaltz S, Calkins H, Morady F. Management of nonsustained ventricular tachycardia guided by electrophysiological testing. Pacing Clin Electrophysiol. 1993;16:1037-1050.
68 Turitto G, Ahuja R, Caref E, el-Sherif N. Risk stratification for arrhythmic events in patients with nonischemic dilated cardiomyopathy and nonsustained ventricular tachycardia: Role of programmed ventricular stimulation and the signal-averaged electrocardiogram. J Am Coll Cardiol. 1994;24:1523-1528.
69 Grimm W, Hoffmann J, Menz V, et al. Programmed ventricular stimulation for arrhythmia risk prediction in patients with idiopathic dilated cardiomyopathy and nonsustained ventricular tachycardia. J Am Coll Cardiol. 1998;32:739-745.
70 Rolf S, Haverkamp W, Borggrefe M, et al. Induction of ventricular fibrillation rather than ventricular tachycardia predicts tachyarrhythmia recurrences in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillator for secondary prophylaxis. Europace. 2009;11:289-296.
71 Naccarelli GV, Prystowsky EN, Jackman WM, et al. Role of electrophysiologic testing in managing patients who have ventricular tachycardia unrelated to coronary artery disease. Am J Cardiol. 1982;50:165-171.
72 Poll DS, Marchlinski FE, Buxton AE, et al. Sustained ventricular tachycardia in patients with idiopathic dilated cardiomyopathy: Electrophysiologic testing and lack of response to antiarrhythmic drug therapy. Circulation. 1984;70:451-456.
73 Rae AP, Spielman SR, Kutalek SP, et al. Electrophysiologic assessment of antiarrhythmic drug efficacy for ventricular tachyarrhythmias associated with dilated cardiomyopathy. Am J Cardiol. 1987;59:291-295.
74 Liem LB, Swerdlow CD. Value of electropharmacologic testing in idiopathic dilated cardiomyopathy and sustained ventricular tachyarrhythmias. Am J Cardiol. 1988;62:611-616.
75 Milner PG, DiMarco JP, Lerman BB. Electrophysiological evaluation of sustained ventricular tachyarrhythmias in idiopathic dilated cardiomyopathy. PACE. 1988;11:562-568.
76 Constantin L, Martins JB, Kienzle MG, et al. Induced sustained ventricular tachycardia in nonischemic dilated cardiomyopathy: Dependence on clinical presentation and response to antiarrhythmic agents. PACE. 1989;12:776-783.
77 Chen X, Shenasa M, Borggrefe M, et al. Role of programmed ventricular stimulation in patients with idiopathic dilated cardiomyopathy and documented sustained ventricular tachyarrhythmias: Inducibility and prognostic value in 102 patients. Eur Heart J. 1994;15:76-82.
78 Fazio G, Veltri EP, Tomaselli G, et al. Long-term follow-up of patients with nonischemic dilated cardiomyopathy and ventricular tachyarrhythmias treated with implantable cardioverter defibrillators. PACE. 1991;14:1905-1910.
79 Grimm W, Marchlinski FE. Shock occurrence and survival in 49 patients with idiopathic dilated cardiomyopathy and an implantable cardioverter-defibrillator. Eur Heart J. 1995;16:218-222.
80 Bänsch D, Böcker D, Brunn J, et al. Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol. 2000;36:566-573.
81 Russo AM, Verdino R, Schorr C, et al. Occurrence of implantable defibrillator events in patients with syncope and nonischemic dilated cardiomyopathy. Am J Cardiol. 2001;88:1444-1446. A1449
82 Rankovic V, Karha J, Passman R, et al. Predictors of appropriate implantable cardioverter-defibrillator therapy in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;89:1072-1076.
83 Rinaldi CA, Simon RDB, Baszko A, et al. Can we predict which patients with implantable cardioverter defibrillators receive appropriate shock therapy? A study of 155 patients. Int J Cardiol. 2003;88:69-75.
84 Cuesta A, Mont L, Rogel U, et al. Comparison of effectiveness of implantable cardioverter defibrillator in patients with idiopathic dilated cardiomyopathy versus those with proved coronary heart disease. Am J Cardiol. 2003;92:1227-1230.
85 Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Failure. 2008;10:933.
86 Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
87 Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Effect of metoprolol CR/XL in chronic heart failure. Lancet. 1999;353:2001-2007.
88 The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II). A randomised trial. Lancet. 1999;353:9-13.
89 Packer M, Bristow M, Cohn J, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349-1355.
90 Akhtar M, Breithardt G, Camm AJ, et al. CAST and beyond. Implications of the Cardiac Arrhythmia Suppression Trial. Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. Circulation. 1990;81:1123-1127.
91 Waldo AL, Camm AJ, deRuyter H, et al. Effect of D-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7-12.
92 The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227-233.
93 The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406-412.
94 Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707-1712.
95 Massie BM, Fisher SG, Radford M, et al. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. CHF-STAT Investigators. Circulation. 1996;93:2128-2134.
96 Doval H, Nul D, Grancelli H, et al. Randomised trial of low-dose amiodarone in severe congestive heart failure. Lancet (British edition). 1994;344:493-498.
97 Køber L, Torp-Pedersen C, McMurray JJV, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678-2687.
98 Eckardt L, Breithardt G. Is there a role for amiodarone in the era of the implantable cardioverter-defibrillator? Heart Rhythm. 2006;3:484-487.
99 Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453-1458.
100 Phang RS, Kang D, Tighiouart H, et al. High risk of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy presenting with syncope. Am J Cardiol. 2006;97:416-420.
101 Knight BP, Goyal R, Pelosi F, et al. Outcome of patients with nonischemic dilated cardiomyopathy and unexplained syncope treated with an implantable defibrillator. J Am Coll Cardiol. 1999;33:1964-1970.
102 Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
103 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
104 Moss A, Hall W, Cannom D, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329-1338.
105 Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834-1843.
106 Breithardt G. MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy): Cardiac resynchronization therapy towards early management of heart failure. Eur Heart J. 2009;30:2551-2553.
107 Daubert C, Gold MR, Abraham WT, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: Insights from the European cohort of the reverse (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837-1846.
108 Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461-2471.
109 Breithardt G, Borggrefe M, Karbenn U, et al. Successful catheter ablation of refractory incessant ventricular tachycardia in a case with dilated cardiomyopathy. Eur Heart J. 1986;7:817-819.
110 Kottkamp H, Hindricks G, Chen X, et al. Radiofrequency catheter ablation of sustained ventricular tachycardia in idiopathic dilated cardiomyopathy. Circulation. 1995;92:1159-1168.