Chapter 2 Noninvasive Testing
Historical Background
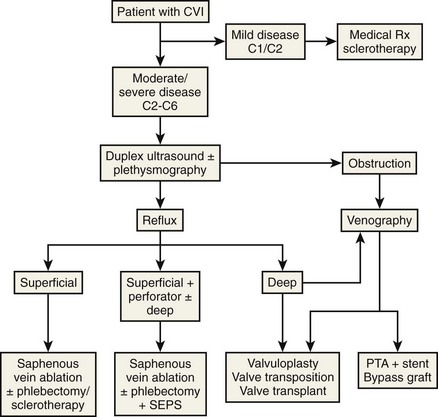
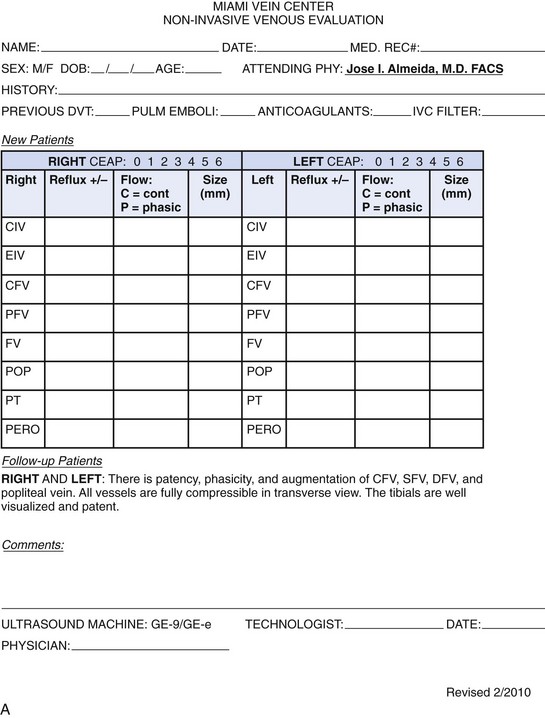
Physiologic testing is used to identify, grade, and follow venous insufficiency and to define deep venous thrombosis (DVT). Since more patients will be presenting for therapy because of improved outcomes with endovenous techniques over traditional surgery, physiologic testing will take on increasing importance. For purposes of this chapter, physiologic testing includes the various plethysmography devices and color flow duplex imaging. The goal of these studies is to provide accurate information describing the hemodynamic or anatomic characteristics of the patient with chronic venous insufficiency, precluding the need for invasive studies2 (Fig. 2-1).1
Etiology and Natural History of Disease
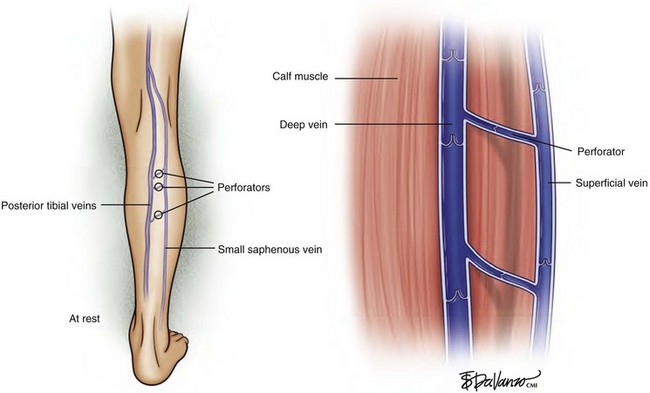
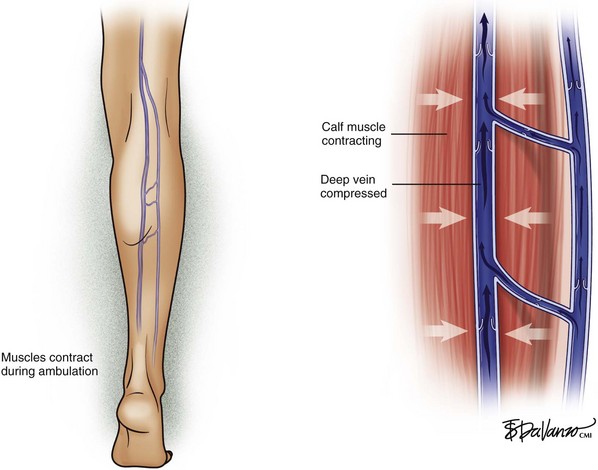
The venous system in the lower extremities is composed of three interconnected parts: the deep system, the perforating system, and the superficial system. In healthy veins, blood flows toward the right side of the heart (i.e., upward) and from the superficial system to the deep system (i.e., inward), driven by the venous muscular pump and unidirectional valves. Lower extremity muscle compartments contract during ambulation; this contraction compresses the deep veins, producing a pumping action, which propels blood upward toward the right side of the heart. Transient pressures in the deep system have been recorded as high as 5 atmospheres (atm) during strenuous lower extremity exertion. This pumping action secondary to ambulation has the effect of reducing pressure within the superficial system (Figs. 2-2 and 2-3).
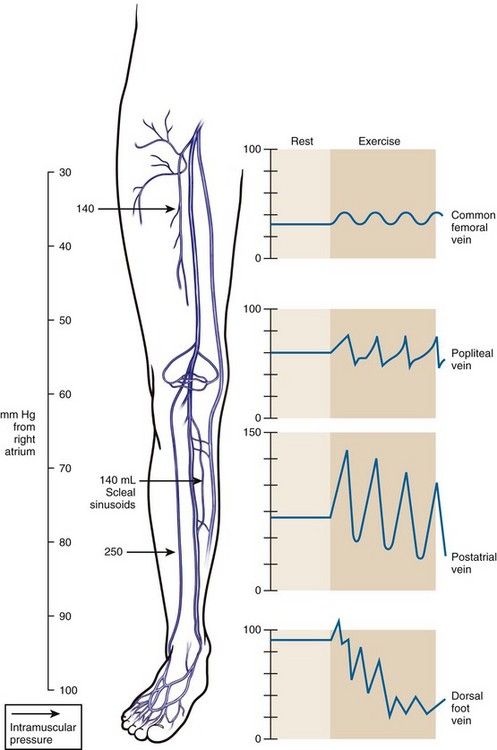
All three venous systems of the lower extremity are subjected to hydrostatic pressure. A fluid column has weight and can produce a pressure gradient. In an individual who has a height of 6 feet, the distance from the level of the right atrium to the ankle is 120 cm and produces a hydrostatic pressure of approximately 90 mm Hg (Fig. 2-4). Deep veins can withstand elevated pressure because the fascia in which they exist limits dilation. In contrast, the superficial system, surrounded by fat and elastic skin, is constructed for low pressure; therefore, elevated pressure in the superficial system can produce dilation, elongation, and valve failure. Dilation increases the diameter of the veins and elongation causes them to be more tortuous.
Instrumentation
Plethysmography
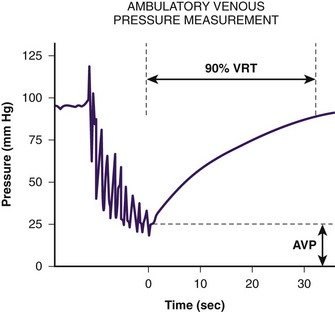
To understand lower extremity venous hemodynamics, venous pressure measurements by dorsal foot vein cannulation can be instructive. The cannula tubing is connected to a fluid column. With the subject standing erect, the fluid column will rise to the level of the right atrium. This is due to the fact that right atrial pressure is near zero and, therefore, the dorsal foot vein pressure at the cannulation site is almost entirely based on the subject’s hydrostatic blood column (the subject’s blood and the fluid in the column have nearly the same specific weight). When the subject is asked to perform repeated ankle flexion, the fluid column drops to between 50% and 60% of its resting height. This simulates walking and the reduction in superficial venous pressure secondary to the ambulatory venous pump. In subjects with venous insufficiency, the fluid column will not drop to normal levels. If a subject’s fluid column falls to normal levels during occlusion of the superficial system, the observer knows the deep system is intact and the superficial system is incompetent. If the fluid column remains elevated with exclusion of the superficial system, the observer knows the deep system is incompetent. Physiologic venous testing is based on these principles (Fig. 2-5).
Air Plethysmograph
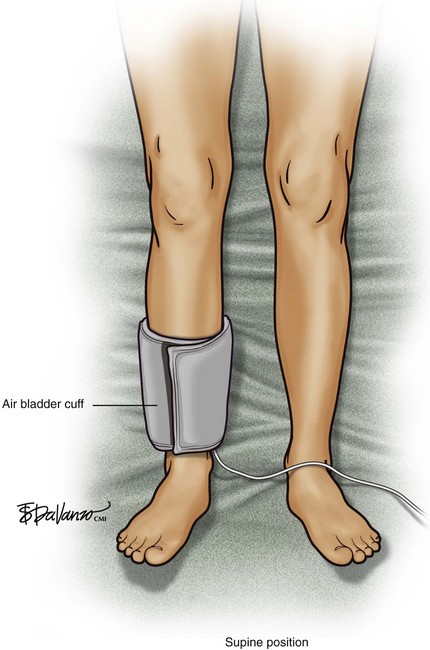
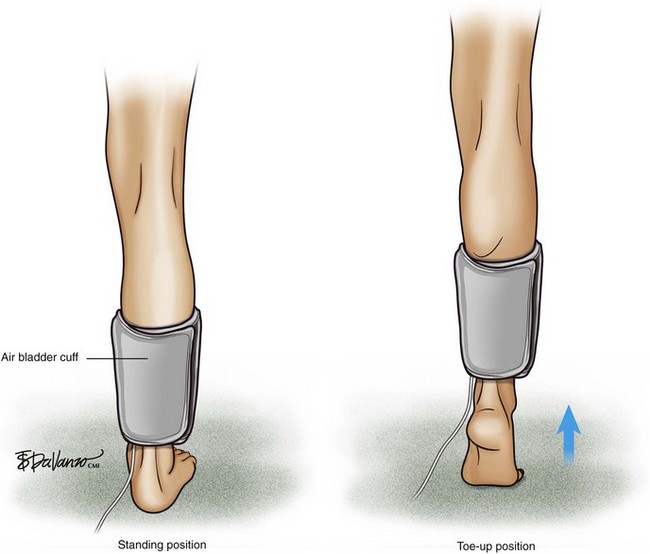
An air bladder (cuff) is connected to a console via a single rubber tube, and any change in limb volume is measured by a pressure change within the bladder. If limb volume increases, the bladder volume will decrease, but the bladder pressure will increase. An air plethysmograph (APG) is able to detect changes in venous limb volume secondary to various patient maneuvers. The APG is used in the clinical assessment of venous insufficiency and DVT. The use of the APG is based on the use of air bladders, which are devices similar to standard blood pressure cuffs. Physiologic parameters related to chronic venous disease such as chronic obstruction, valvular reflux, calf muscle pump function, and venous hypertension can be measured (Figs. 2-6 and 2-7).
Duplex Ultrasound
Deep Venous System
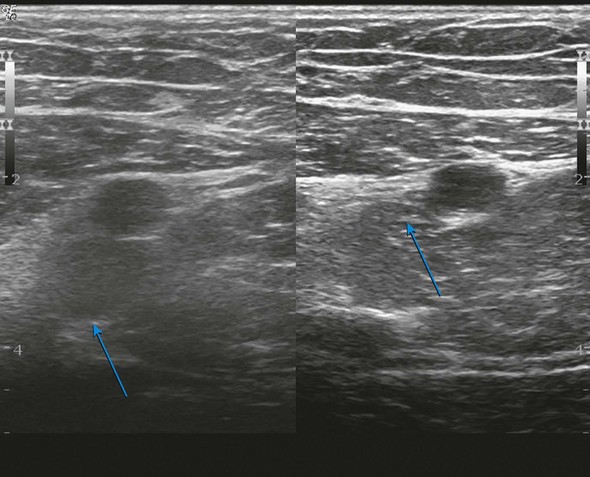
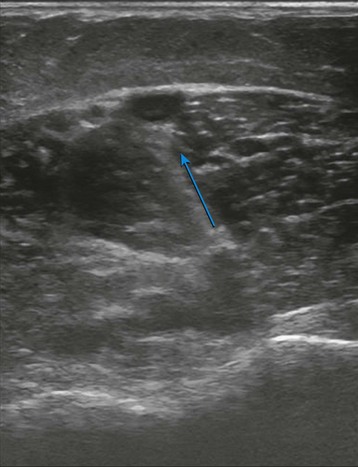
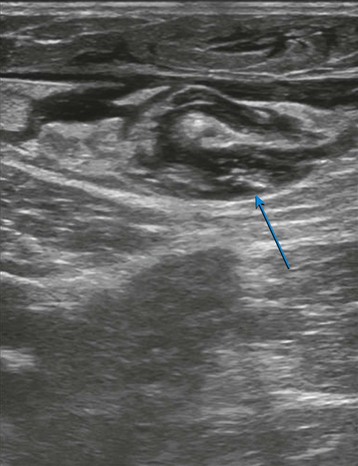
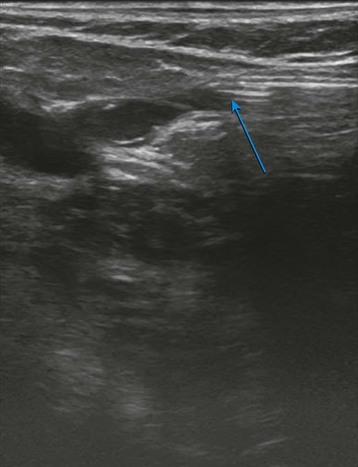
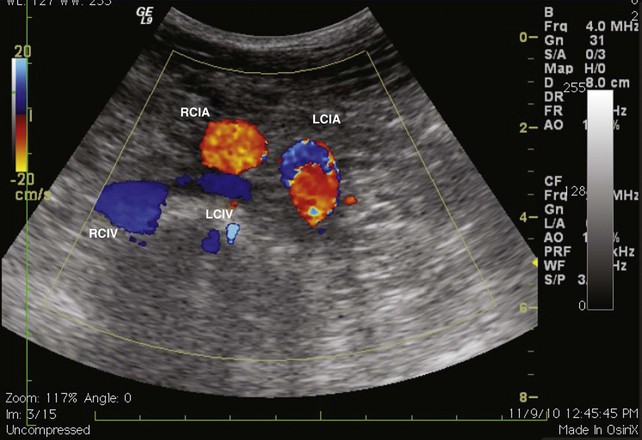
The evaluation begins at the groin using gray-scale imaging. Usually seen are the common femoral vein, common femoral artery, and great saphenous vein (GSV), forming a “Mickey Mouse image.” We have found keeping the lateral (arterial) structures on the left side of the screen for both the right and left leg to be helpful. This requires that the technologist rotate the linear array probe 180 degrees when moving from the right to the left leg. The marker on the probe should be oriented to the lateral aspect of the leg. With this orientation, Mickey’s face is the common femoral vein and is the larger and lower of the three structures. The common femoral artery forms Mickey’s right ear, and the GSV forms Mickey’s left ear. As the probe is moved distally, the GSV will disappear, and the common femoral artery will divide into the superficial femoral artery and the deep femoral artery. As the probe continues distally, the technologist should focus on keeping the superficial femoral artery and the femoral vein in clear view. The popliteal artery and the popliteal vein are difficult to visualize in the adductor canal; therefore, these structures are identified from behind by placing the probe in the popliteal crease. In the calf, the duplicated posterior tibial and peroneal veins with their associated single arteries can be viewed from a medial approach as they travel between the muscle bellies. Similarly, the gastrocnemius and soleus veins are identified; however, they are located within the muscular bellies. In general, the anterior tibial veins are not interrogated because they are rarely pathologic (Fig 2-8).
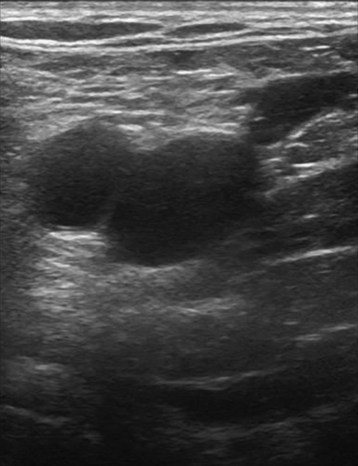
With the probe, the technologist can compress the vein in the short axis view. The ability to fully compress the vein walls—and obliterate the venous lumen momentarily—confirms vein patency and absence of thrombus formation (Fig. 2-9). If the technologist identifies thrombus, the next step is to determine its age. Acute thrombi are characterized by vein dilatation and noncompressible echolucent material, while chronic thrombi take on a speckled, hyperechoic ultrasonic appearance.
Superficial Venous System
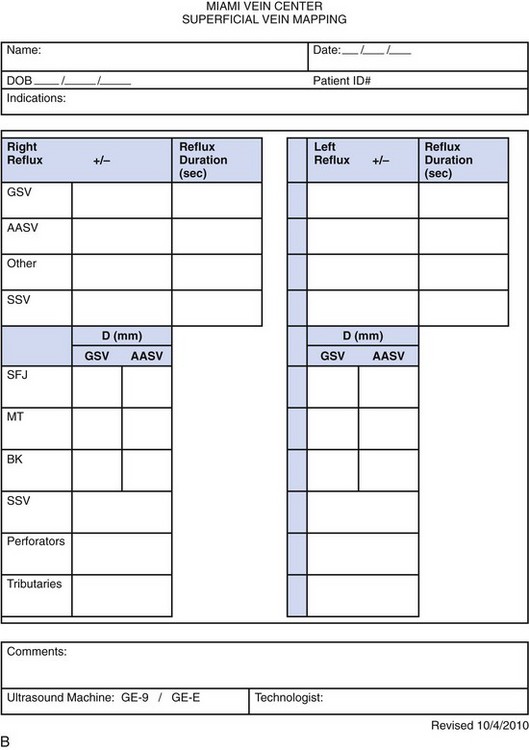
The same evaluation is repeated posteriorly for the small saphenous vein (SSV). This vein originates in the distal calf and can terminate in the upper thigh. We access this vessel with ultrasound by rotating the subject to expose the back of the legs. We identify the SSV at the distal calf and advance over the course of the SSV. Multiple levels may be assessed; however, we generally record a characteristic SSV diameter (in millimeters) and assess reflux in the most diseased location (Fig. 2-10).
Duplex ultrasound is not only diagnostic but also plays crucial roles in endovenous ablation, ultrasound-guided sclerotherapy, and monitoring the success of vein closure procedures (Figs. 2-11 through 2-17).
1 Marston WA. PPG, APG, duplex: which noninvasive tests are most appropriate for the management of patients with chronic venous insufficiency? Semin Vasc Surg. 2002;15:13-20.
2 Raines JK, Almeida JI. Role of physiologic testing in venous disorders. In: Bergan JJ, editor. The Vein Book. San Diego: Elsevier; 2007:47-55.