Chapter 28 Noninvasive Respiratory Support
In this chapter, supplemental oxygen therapy, humidification systems, adjunctive respiratory therapy, and noninvasive ventilation (NIV) are discussed. NIV encompasses various methods of positive pressure ventilation, including continuous positive airway pressure (CPAP), delivered without an endotracheal tube.1
OXYGEN THERAPY
Oxygen delivery to the tissues depends on arterial oxygen content, cardiac output, and individual organ perfusion. Arterial oxygen content depends on the hemoglobin concentration and arterial oxygen saturation (Sao2; see Equation 1-13). The latter is, in turn, dependent on the oxyhemoglobin dissociation curve (see Fig. 1-14) and the arterial partial pressure of oxygen (Pao2) (see Chapter 1). Supplemental oxygen therapy is indicated to maintain the Pao2 above 8 κPa (60 mmHg) or the Sao2 above 90%. Only low concentrations of supplemental oxygen are required to treat hypoxemia due to hypoventilation. In contrast, high concentrations of oxygen may be required to treat hypoxemia due to ventilation/perfusion mismatch. Oxygen is ineffective if hypoxemia is due to pure shunt. High-concentration oxygen therapy in the setting of hypoventilation may mask the development of severe hypercarbia and respiratory acidosis (see Chapter 1).
Impaired gas exchange is almost universally present following cardiac and thoracic surgery, so oxygen is indicated for the duration of a patient’s stay in the intensive care unit (ICU). It should also be continued following discharge from the ICU if a patient is receiving opioid analgesia because that predisposes the patient to nocturnal desaturation.2 Supplemental oxygen therapy during the perioperative period has also been shown to reduce the incidence of surgical wound infections.3
Excessive oxygen therapy is not without risk. High-concentration oxygen promotes absorption atelectasis and, in patients with severe chronic obstructive pulmonary disease (COPD), it may exacerbate hypercarbia (see Chapter 27). Administration of high oxygen concentrations (FIo2 >0.6) for longer than several days may potentially cause direct pulmonary toxicity and should be avoided if possible.4 However, supplemental oxygen should not be withheld in the presence of clinically significant hypoxemia (Sao2 <85% to 90%).
Oxygen Delivery Systems
Variable-Performance Systems
Manual Resuscitators.
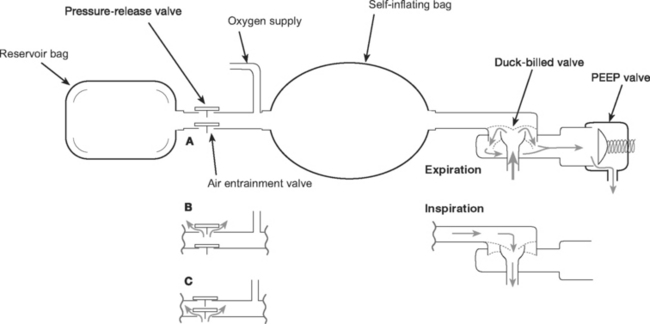
The components of manual resuscitators (such as the Laerdal and the Ambu) are illustrated in Figure 28-1. As with other variableperformance systems, FIo2 depends on the oxygen flow, tidal volume, and respiratory rate, but it can be increased by attaching an oxygen reservoir bag to the air entrainment inlet. The resuscitation bag is designed for short-term emergency use when augmentation of a patient’s respiratory effort by manual ventilation is required. Care should be taken with a patient who has respiratory distress but good respiratory drive because spontaneous breathing through a manual resuscitator increases the work of breathing, potentially worsening the respiratory distress.
HUMIDIFICATION
Humidification Devices
Heat and Moisture Exchangers
Microbiologic filters have been developed that conserve the heat and moisture of the gas expired by a patient; they use them to heat and humidify the inspired gas delivered during the next breath. Modern heat and moisture exchangers are light in weight, low in resistance, and low in volume. They provide variable degrees of humidification (approximately 20 to 30 g/m3), that decrease with duration of use and high minute ventilation. Some may be effective for several days of continuous use. Heat and moisture exchangers should not be used with noninvasive ventilation because their resistance, although low, imposes increased work of breathing.
ADJUNCTIVE RESPIRATORY THERAPIES
Physical Therapy
Lung-expansion techniques duplicate the normal sigh breath (periodic voluntary hyperinflation), increasing end-inspiratory lung volume and reversing atelectasis. The most commonly used technique is incentive spirometry. This requires proper patient instruction and encouragement and should be done several times per hour, with a target tidal volume greater than 14 ml/kg.
Bronchodilators
Bronchodilators include β2-adrenergic agents (e.g., albuterol) and anticholinergic drugs (e.g., ipratropium bromide). In addition to acting as bronchodilators, they improve mucociliary function by stimulating ciliary activity and reducing mucus production. Delivery is achieved by nebulizer or metered-dose inhaler, both of which are designed to aerosolize the drugs such that the particle size is small enough to reach the distal airways. Both routes of administration have similar efficacy in spontaneously breathing and intubated patients.5
NONINVASIVE VENTILATION
Positive-pressure ventilation increases functional residual capacity, improves oxygenation and lung mechanics, and reduces the work of breathing. In patients with auto positive end-expiratory pressure (PEEP), external PEEP (or CPAP) reduces the inspiratory threshold load (see Fig. 27-4), which reduces the work of breathing. In addition, tidal positive pressure ventilation (e.g., bilevel positive airway pressure ventilation) unloads inspiratory muscles, augments tidal volume, and reduces arterial carbon dioxide tension (Paco2). In patients with left ventricular dysfunction, cardiac performance is improved by reduction in left ventricular afterload. The rationale for NIV is to obtain these benefits without the disadvantages of endotracheal intubation, such as the need for sedation and increased risk of nosocomial pneumonia.1
Indications and Contraindications
NIV is well established for treating acute exacerbations of COPD.6–8 However, of greater relevance to the cardiothoracic ICU is the use of NIV in treating cardiogenic pulmonary edema, postoperative respiratory failure, and thoracic trauma. Patient refusal or failure to cooperate precludes the use of NIV. Other contraindications to NIV are listed in Table 28-1.
| Neurologic | Impaired level of consciousness, confusion, or inability to protect airway |
| Anatomic | Trauma or recent surgery to the upper airway, face, or esophagus |
| Upper airway obstruction | |
| Respiratory | Severe hypoxemia |
| Moderate or large quantity of sputum | |
| Weak cough | |
| Undrained pneumothorax | |
| Cardiovascular | Marked hemodynamic instability |
| Abdominal | Abdominal distension or vomiting |
Cardiogenic Pulmonary Edema
In patients with acute cardiogenic pulmonary edema, the addition of face-mask CPAP to standard medical therapy leads to a more rapid improvement in respiratory rate, heart rate, Paco2, and oxygenation than does standard medical therapy (oxygen, morphine, diuretics, and nitrates) alone.9,10 In addition, CPAP reduces the need for endotracheal intubation and may decrease mortality rates.11 Similar physiologic benefits have been demonstrated with bilevel positive airway pressure ventilation.12,13 In a head-to-head comparison of bilevel positive airway pressure and CPAP, an increased rate of myocardial infarction was reported in the former group.14 However, because a greater proportion of patients had chest pain at entry into the bilevel support group, it is possible that this outcome was not related to the mode of ventilation. Until more data are available, CPAP is the preferred mode of NIV.
Postoperative Respiratory Failure
NIV may be used to treat (rescue therapy) or prevent (prophylaxis) postoperative respiratory failure. Prophylaxis refers to the application of NIV immediately following extubation so as to prevent respiratory failure. As rescue therapy, NIV has been shown to reduce the incidence of reintubation and other complications in patients who develop respiratory distress following thoracic surgery15 and solid-organ (liver, kidney, lung) transplantation.16 It has also been shown to be beneficial as rescue therapy following major abdominal surgery.17
In patients deemed at high risk for postextubation respiratory failure, prophylactic NIV has been shown to reduce the need for reintubation,18,19 with a possible survival advantage in hypercarbic patients19; however, in both trials, postoperative patients constituted only a minority of study subjects. Neither rescue nor prophylactic NIV has been specifically studied following cardiac surgery.
CPAP is a highly efficacious treatment for obstructive sleep apnea and, although there are few trial data, prophylactic use during the postoperative period is strongly recommended in patients with this condition who were being treated with NIV preoperatively.20
Thoracic Trauma
NIV may be considered in patients who have experienced respiratory failure due to thoracic trauma (rib fractures, flail chest, pulmonary contusion). In one small, randomized study of patients with flail chest, face-mask CPAP plus patient-controlled opioid analgesia were associated with lower mortality rates than found in those undergoing invasive ventilation.21 There is the potential for any form of positive pressure ventilation to convert a simple pneumothorax into a tension pneumothorax, so pneumothoraces should be drained before instituting NIV.
Delivery systems and settings
Continuous Positive Airway Pressure Systems
Continuous Fresh-Gas-Flow Systems.
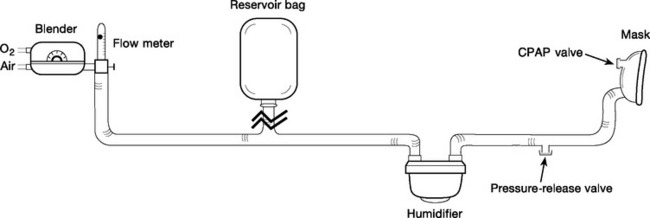
Continuous fresh-gas-flow systems consist of a humidified fixedperformance gas delivery system, an interface (tightfitting mask), and a spring-loaded valve (CPAP valve) that provides constant pressure against expiratory flow (Fig. 28-2). Gas delivery is usually provided by a high-flow system, but gas flow can be reduced and system-imposed work of breathing reduced if a weighted, high-compliance reservoir bag is incorporated into the inspiratory limb of the system.22 High fresh-gas-flow rates are generated by a pressurized gas supply, a gas turbine, or a jet Venturi mechanism. The addition of a pressure-relief valve in the inspiratory limb of the circuit provides safety against barotrauma in the event that the CPAP valve becomes obstructed.
Demand Valve Systems.
Demand valve systems are used for the application of CPAP through a mechanical ventilator; they rely on an efficient inspiratory trigger and expiratory valve mechanism. For most modern intensive care ventilators, this is an acceptable alternative to continuous-gas-flow systems, and they have the advantage of providing extra patient monitoring.23 However, in a patient with a high peak inspiratory flow and high respiratory rate, the imposed work of breathing through this type of CPAP system becomes appreciable and is undesirable.
Commonly used initial settings for treating acute cardiogenic pulmonary edema are 10 cm H2O CPAP and FIo2 1.0. With a continuous fresh-gas-flow system, the gas flow must be sufficiently high to ensure that there is flow through the CPAP valve throughout the respiratory cycle (usually 40 l/min for systems that incorporate a reservoir bag); that is, the CPAP valve should always be open. With clinical improvement, FIo2 can be progressively reduced, ensuring that Spo2 is greater than 90%. Once an FIo2 of 0.4 is reached and the patient remains clinically stable, with a respiratory rate of fewer than 30 breaths per minute, a trial without CPAP (on humidified oxygen) or a reduction in the level of CPAP to 7.5 cm H2O (and then 5 cm H2O) should be attempted.
Bilevel Positive Airway Pressure Ventilation
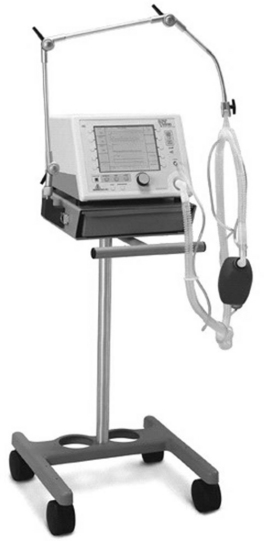
The pressure-support mode is either pressure-triggered or flow-triggered and is flow-cycled (see Chapter 29). The bilevel positive airway pressure mode is also pressure- or flow-triggered and is flow-cycled but has a facility for time triggering at a set respiratory rate (spontaneous/timed mode). Bilevel positive airway pressure has two pressure settings—the expiratory positive airway pressure (EPAP) and the inspiratory positive airway pressure (IPAP). The level of IPAP is inclusive of EPAP. (In contrast, the level of pressure support is in addition to CPAP.) Standard initial settings are an EPAP of 4 to 8 cm H2O and an IPAP of 8 to 12 cm H2O, with an FIo2 sufficient to maintain the SPo2 above 90%. These pressures can subsequently be increased according to the patient’s response and tolerance, but IPAP values over 20 cm H2O are likely to cause unacceptable leak or patient discomfort. Because bilevel positive airway pressure is primarily a spontaneous mode of ventilation, the breath rate is set to a lower rate than the patient’s expected breath rate. A rate of 8 to 12 is appropriate. Bilevel positive airway pressure (BiPAP), which is available in the Vision noninvasive ventilator (Respironics, Murrysville, PA; Fig. 28-3) is an example of a commercially available bilevel positive airway pressure NIV mode.
Patient-ventilator dysynchrony
Even with minimal interface leak, patient-ventilator dysynchrony is common with bilevel modes and may be the principal determinant of success or failure of NIV.24 Graphic monitoring of pressure-time and flow-time signals can help to detect specific patterns of dysynchrony.
Inspiratory Dysynchrony
Inspiratory dysynchrony usually occurs during the trigger phase (the signal that initiates the change to the inspiratory pressure level). It manifests as ineffective respiratory efforts, with either failure to trigger or, at the other extreme, autotriggering. Failure to trigger is likely in patients with dynamic lung hyperinflation and auto-PEEP that is much higher than the set EPAP level. Autotriggering occurs with sensitive trigger settings and either a variable leak or water in the tubing. Flow triggering reduces the inspiratory effort and the trigger delay more than pressure triggering does, but all ventilators have unavoidable, intrinsic trigger-activation delays, usually greater than 120 ms.25,26 The theoretic advantage of flow triggering may be less significant in modern ventilators that have pressure sensors closer to the patient, thus reducing trigger delay.
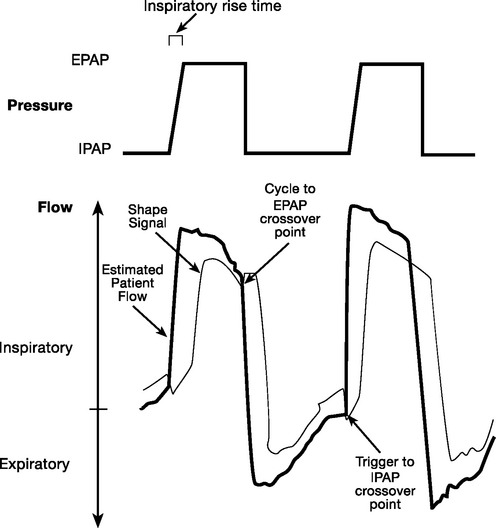
The flow waveform method of triggering (BiPAP Vision, Respironics) results in a transition from expiration to inspiration by means of volume triggering or by the shape signal method (Fig. 28-4). The flow waveform method results in fewer episodes of trigger failure but more frequent autotriggering than standard flow triggering, although this has been studied only in intubated patients.25,27
Inspiratory dysynchrony may also result from an inappropriate rate of pressure rise to the set inspiratory pressure level (rise time; see Fig. 28-4). A rapid rise time reduces inspiratory effort but increases air leak and worsens patient tolerance.28
Expiratory Dysynchrony
Expiratory dysynchrony occurs when the ventilator’s transition from inspiration to expiration occurs before or after the end of the patient’s inspiratory effort. This manifests as “fighting the ventilator” if the patient actively expires when the transition is delayed or as retriggering if termination of inspiratory flow is premature. The transition is determined by the method of cycling. With flow cycling, the ventilator cycles to expiration when the inspiratory flow falls to a certain proportion of the peak inspiratory flow or to a predetermined (low) flow rate. Flow cycling in the presence of a significant leak or long patient respiratory time constant can result in delayed transition to expiration and thus dysynchrony (see Chapter 4); time cycling may provide the most consistent patient-ventilator synchrony.29
The shape signal method described earlier has also been applied to the cycling variable, although in the setting of a large leak or long expiratory time constant, it too can result in a delayed transition to expiration.27 The Vision ventilator also provides the spontaneous expiratory threshold, time, and flow reversal methods of cycling in an effort to reduce this risk.
Interfaces
Oronasal Masks
The oronasal mask is the most commonly used interface for NIV in the ICU. When correctly secured, this style of mask should provide a firm seal around the patient’s nose and mouth. It is held in place by an adjustable head harness, and problems attributed to poor mask fit can be solved by diligent adjustment of the harness in conjunction with appropriate support of the NIV system’s tubing. Most masks are made of a clear polycarbonate shell that is surrounded by a silicone-based sealing cuff that may be filled with air. Masks are available in a range of shapes and sizes, and the selection of a correctly fitting mask is imperative so as to minimize leakage, reduce the risk of pressure necrosis, and maximize patient comfort and compliance. Pressure necrosis of the skin most commonly develops over the bridge of the nose. Use of cushioning materials and periods of rest from NIV help to reduce this complication. A large leak may cause eye irritation. Compared to the nasal mask, the oronasal mask can deliver higher ventilation pressures with fewer leaks, requires less patient cooperation, and permits mouth breathing, making it the most suitable type of mask for the acutely dyspneic patient in the ICU.
Complications
The most common complications are related to mask fit (described earlier). Complications attributable to positive pressure ventilation include gastric insufflation (with risk of aspiration of gastric contents), hypotension, and pneumothorax. A nasogastric tube should not be used unless there is a clear indication to do so because it exacerbates mask leak and may increase the risk for pressure-induced skin necrosis. Delay in the institution of invasive ventilation may also occur.
1 American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and la Société de Réanimation de la Langue Franaise. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute respiratory failure:. Am J Respir Crit Care Med. 2001;163:283-291.
2 Stone JG, Cozine KA, Wald A. Nocturnal oxygenation during patient-controlled analgesia. Anesth Analg. 1999;89:104-110.
3 Greif R, Akca O, Horn EP, et al. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342:161-167.
4 Jenkinson SG. Oxygen toxicity. New Horiz. 1993;1:504-511.
5 Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156:3-10.
6 Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817-822.
7 Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931-1935.
8 Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2004:CD 004104.
9 Bersten AD, Holt AW, Vedig AE, et al. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med. 1991;325:1825-1830.
10 Rasanen J, Heikkila J, Downs J, et al. Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. Am J Cardiol. 1985;55:296-300.
11 Peter JV, Moran JL, Phillips-Hughes J, et al. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary edema: a meta-analysis. Lancet. 2006;367:1155-1163.
12 Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: a multicenter randomized trial. Am J Respir Crit Care Med. 2003;168:1432-1437.
13 Park M, Sangean MC, Volpe Mde S, et al. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit Care Med. 2004;32:2407-2415.
14 Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25:620-628.
15 Auriant I, Jallot A, Herve P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164:1231-1235.
16 Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235-241.
17 Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293:589-595.
18 Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465-2470.
19 Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164-170.
20 Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081-1093. quiz 1117-1118
21 Gunduz M, Unlugenc H, Ozalevli M, et al. A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg Med J. 2005;22:325-329.
22 Bersten AD, Rutten AJ, Vedig AE. Optimizing fresh gas flow and circuit design for the delivery of continuous positive airway pressure. Crit Care Med. 1991;19:266-270.
23 Takeuchi M, Williams P, Hess D, et al. Continuous positive airway pressure in new-generation mechanical ventilators: a lung model study. Anesthesiology. 2002;96:162-172.
24 Nava S, Ceriana P. Patient-ventilator interaction during noninvasive positive pressure ventilation. Respir Care Clin North Am. 2005;11:281-293.
25 Nava S, Ambrosino N, Bruschi C, et al. Physiological effects of flow and pressure triggering during non-invasive mechanical ventilation in patients with chronic obstructive pulmonary disease. Thorax. 1997;52:249-254.
26 Stell IM, Paul G, Lee KC, et al. Noninvasive ventilator triggering in chronic obstructive pulmonary disease: a test lung comparison. Am J Respir Crit Care Med. 2001;164:2092-2097.
27 Prinianakis G, Kondili E, Georgopoulos D. Effects of the flow waveform method of triggering and cycling on patient-ventilator interaction during pressure support. Intens Care Med. 2003;29:1950-1959.
28 Prinianakis G, Delmastro M, Carlucci A, et al. Effect of varying the pressurisation rate during noninvasive pressure support ventilation. Eur Respir J. 2004;23:314-320.
29 Calderini E, Confalonieri M, Puccio PG, et al. Patient-ventilator asynchrony during noninvasive ventilation: the role of expiratory trigger. Intens Care Med. 1999;25:662-667.