Chapter 60 Noninvasive Breast Cancer
Lobular Carcinoma in Situ
Lobular carcinoma in situ (LCIS) was first described as a pathologic entity by Foote and Stewart in 1941.1 Microscopically, LCIS appears as a noninfiltrating process of lobular proliferation. LCIS has been reported to present with multicentric breast involvement in up to 90% of mastectomy specimens, with bilateral involvement documented in 35% to 59%.2–5 Histologically, LCIS is characterized by loose discohesive epithelial cells filling the acinar space.2–5 The degree of lobular involvement ranges from a simple filling of the ductal lumens to moderate distention to overt distention with extension into the adjacent extralobular ducts.6 As a result of this spectrum of appearances, the lines of histologic delineation can become blurred between atypical ductal hyperplasia, LCIS, and, when ductal extension is seen, DCIS. This introduces a source of complexity when comparing publications from varying institutions.3–5
Molecular markers have been suggested as a potential method of providing a technique for distinguishing LCIS in problematic cases.4 LCIS cells are frequently ER-positive cells, and rarely is c-ERBB2 overexpressed or the TP53 protein accumulated.4,7–9 The loss of E-cadherin (CDH1) gene expression is documented in more than 95% of LCIS cases.9,10 The E-cadherin gene is a calcium-dependent cellular adhesion molecule that is responsible for epithelial organization, and the absence of this adhesion molecule in LCIS may explain its discohesive morphologic characteristics.9,10
Publications on biopsy results report LCIS incidence rates to be 0.5% to 3.6%; these lesions represent less than 15% of all noninvasive lesions recorded.5,11 Because there are no clinical or mammographic indicators, LCIS without additional histologic findings is typically an incidental finding when biopsy is performed for alternative reasons.1–411 Although mammographically detected calcifications corresponding with LCIS have been reported, the calcifications are more commonly unassociated and are present in the adjacent tissue.12 In fact, DCIS or invasive disease, or both, is frequently identified in the subsequent lumpectomies performed (22% to 27% of cases) when LCIS is the sole histologic entity seen on core biopsy.13,14 Criteria identifying those patients for whom observation only is sufficient following core biopsy showing LCIS have not yet been clearly specified.
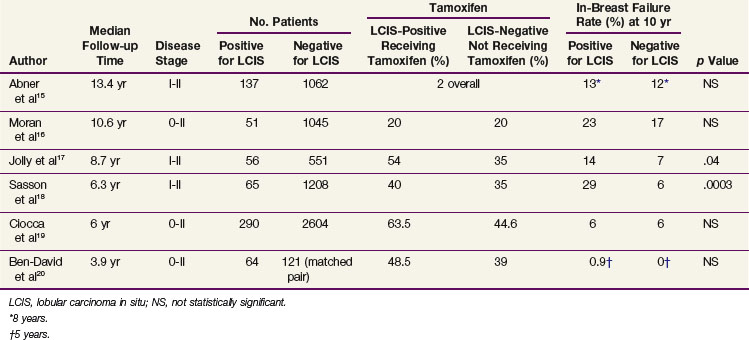
Management considerations for LCIS differ depending on whether it is associated with a diagnosis of DCIS or infiltrative disease or whether LCIS is the sole histologic entity encountered. Only 5% to 12% of early-stage breast cancers have an associated component of LCIS.15,16 Several institutional experiences have evaluated the potential impact of the presence of LCIS on breast-conserving treatment outcomes.15–20 The number of patients included in these experiences are relatively small, and there are differences in these cohorts, including pathologic assessment, length of follow-up, and use of tamoxifen (Table 60-1). However, the majority of data suggest that no statistically significant difference exists when comparing rates of in-breast disease control, distant disease-free survival, and overall survival between patients with or without a component of LCIS.15–20 The presently accepted treatment approach is to manage the breast according to the dominant histologic findings present (DCIS or invasive disease) and disregard the presence of LCIS. Additional surgery is not pursued to obtain LCIS clear margins. The need for the addition of tamoxifen and/or a more aggressive treatment approach in this group of high-risk patients is uncertain, and further study is necessary.
If LCIS is the sole histologic diagnosis, treatment recommendations range from observation to mastectomy. When first described as a pathologic entity, the significance of LCIS was unknown and mastectomy was consistently pursued.1 Knowledge of frequent contralateral involvement extended treatment recommendations to random contralateral biopsy and bilateral mastectomy.1,2 However, observational studies after lumpectomy only have led to a better understanding of the natural history of this disease, and a more conservative approach is now commonly practiced.5,11 Although studies of E-cadherin (CDH1) and loss of heterozygosity (LOH) suggest that LCIS may be a precursor to invasive disease, LCIS has historically been considered a marker for an increased risk of developing invasive disease (9 to 12 times that of the normal population); this risk requires long-term follow-up exceeding 20 years.3,12
A range of ipsilateral and contralateral breast failure rates are reported. This variation is due in part to differences in the length of follow-up, the definitions used for histologic classification (atypical lobular hyperplasia vs. LCIS), and the extent of excision used in these observational studies.21,22 In a recent 12-year follow-up publication on a 182-patient cohort treated with lumpectomy only for LCIS, the National Surgical Adjuvant Breast and Bowel Project (NSABP) reported a 14.4% in-breast tumor recurrence (IBTR) rate and a 7.8% contralateral breast tumor recurrence (CBTR) rate.22 Of the IBTRs, nine (34.6%, or 5% of the total cohort) were invasive malignant tumors and 17 (65.4%, or 9% of the total cohort) were noninvasive malignant tumors. Although the frequency of CBTRs was less than that of IBTRs, the frequency of invasive CBTRs (5.6% of the total cohort) was similar to that of invasive IBTRs (5% of the total cohort). All of the IBTRs were documented to be at the site of the index lesion except for one, characterized as pure LCIS, that was found at a remote site. This report continues to support the indolent nature of LCIS and a conservative management approach.
The impact of subsequent development of invasive disease on mortality risk has been estimated in earlier publications to be 5% to 7%.11 However, with contemporary use of close mammographic and clinical follow-up leading to early detection of subclinical abnormalities, one would expect this mortality risk to be reduced. This reduction is reflected in the lower mortality risk (1%) reported by the NSABP.22
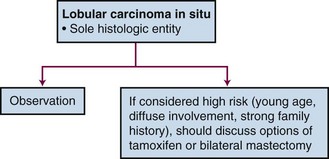
Management options vary depending on individual risk assessment (Fig. 60-1). Close observation with regular physical examinations and mammographic surveillance is the accepted management approach.5,11,21,22 At present, there is no role for radiotherapy in the management of LCIS. Knowing that this is a bilateral breast disease process leaves a unilateral treatment approach inadequate (i.e., ipsilateral mastectomy). Bilateral prophylactic mastectomy is thought to be excessive in all patients except those believed to be at higher risk (i.e., young patients, diffuse process, a significant family history of disease) for whom it could be considered. A less radical approach to be considered is the use of tamoxifen. Indeed, a cohort of patients with LCIS were entered into the NSABP P-01 trial, which compared tamoxifen with placebo for breast cancer prevention. The 5-year risk of subsequent disease development was reduced with the use of tamoxifen by 56%.23
Paget’s Disease
The clinical presentation of crusting, bleeding, and ulceration of the nipple was first described in 1856, but it was not until 1874 that the association with an underlying breast carcinoma was recognized by Sir James Paget.24,25 Paget’s disease of the nipple is characterized histologically by the presence of unique, clearly identifiable Paget’s cells that are described as large, round to oval cells that contain hyperchromatic nuclei and prominent nucleoli. Mitoses are frequently seen. The Paget’s cells occur singly or in clusters and are scattered throughout the epidermis.26–28
Reported in 0.5% to 5% of breast cancer patients, Paget’s disease is an infrequent diagnosis.26,29,30 It is most commonly unilateral, but reports of bilateral and male Paget’s disease can be found.31–33 Clinically, women describe itching and burning of the nipple and areola and crusting is often described. There is a slow progression toward an eczematoid appearance, which can extend to the skin. If neglected, bleeding, pain, and ulceration can occur.30 The differential diagnosis includes superficial spreading melanoma, pagetoid squamous cell carcinoma in situ, and clear cells of Toker.27,28 A palpable mass is detected in about 50% of patients at diagnosis. If there is a palpable mass, more than 90% will be invasive carcinoma. On the other hand, if no palpable mass is detected, 66% to 86% will have an underlying DCIS. These adjacent, underlying malignant tumors are usually located centrally; however, they have also been located elsewhere in the breast.26,30
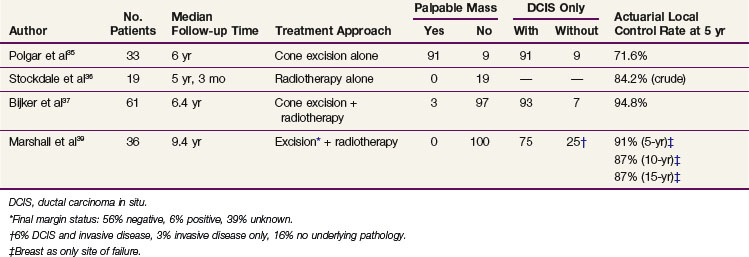
The appropriate management of Paget’s disease remains unsettled; however, in concordance with physician and patient preference, attempts have been made to investigate the role of breast-conservation therapy. Because mastectomy was initially shown to be effective, the transition to breast-conserving treatment has been cautious due to the inability to accumulate a significant number of uniform patients similarly treated.29,30 Limited series have described results with various forms of breast-conserving treatment, including limited surgical resection alone, radiotherapy alone, and limited resection followed by radiotherapy34–39 (Table 60-2).
The combination of limited surgical resection and postoperative radiotherapy appears to be the most successful breast-conservation approach. Two collaborative studies have shown the successful use of breast-conservation therapy in Paget’s disease of the nipple. The European Organization for Research and Treatment of Cancer (EORTC) Study 10873 was a multi-institutional registration study reporting a 5-year local recurrence rate of 5.2%.37 In this study, a complete excision with tumor-free margins of the nipple-areolar complex and underlying breast tissue was followed by whole-breast radiotherapy to 50 Gy. The median follow-up time was 6.4 years, and the majority of patients were found to have an underlying DCIS without a palpable mass.
The second study, a seven-institution collaborative review of 36 patients, included patients with Paget’s disease without a palpable mass or mammographic density.39 The median follow-up time was 9.4 years, and all had at least 12 months of follow-up. Patients underwent complete (69%) or partial (25%) excision of the nipple-areolar complex and underlying breast tissue, with 6% reported as a biopsy only. The final margin status was documented as negative in 56%, positive in 6%, and unknown in 39%. All received whole-breast irradiation at a median dose of 50 Gy, and the majority received an additional boost dose to the tumor bed. Actuarial rates of local in-breast failure as the only site of first recurrence were 9% at 5 years and 13% at both 10 years and 15 years. Despite the variation of clinical, pathologic, and treatment factors, statistical evaluation did not identify any factors that significantly predicted for risk of local recurrence.
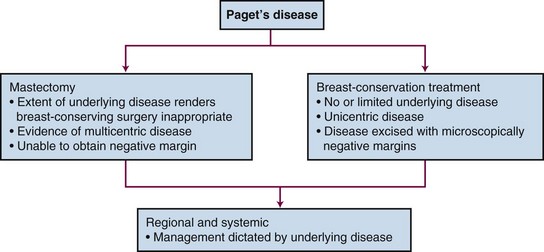
On presentation, the workup and evaluation should include a bilateral breast examination, mammography, and biopsy to confirm the diagnosis of Paget’s disease and fully evaluate the extent of the underlying malignant disease. An individual’s prognosis is not dependent on the diagnosis of Paget’s disease but rather depends on the associated underlying malignant disease. Therefore local treatment decisions as well as systemic and regional nodal disease risk management should be based on the underlying disease (Fig. 60-2). Standard breast-conservation therapy principles governing patient selection, surgical resection, and radiotherapy should be applied. Complete surgical resection of the disease process (i.e., nipple-areolar complex excision in conjunction with any underlying disease) with a negative microscopic surgical margin is then followed by standard whole-breast irradiation.
Ductal Carcinoma in Situ
Epidemiology
Ductal carcinoma in situ (DCIS) is represented by a heterogeneous spectrum of histologic appearances that all arise within and are confined to the ductal lumens of the breast. This clonal proliferation does not breach the epithelial basement membrane, and there is no evidence of invasion into the adjacent breast stroma.40 DCIS lacks the ability to metastasize; the rarely reported axillary nodal metastasis or distant metastasis has been attributed to the probable presence of an undetected component of invasive carcinoma.40
Before 1980, the incidence of DCIS was low (i.e., only 1.4% of all breast biopsies and only 5% of all breast malignant tumors).41,42 With the increased use of mammography and as pathologists began to recognize that DCIS was a distinct pathologic entity, the incidence of DCIS dramatically increased.43,44 The incidence of DCIS increased from 4800 cases reported in 1983 to more than 50,000 cases reported in 2003; this represents a 10-fold increase in only 20 years.40,45 Of the estimated 192,370 new breast cancers diagnosed in 2009, 62,280 will be noninvasive, of which 85% will be DCIS.46 With this increase, there has been a corresponding change in the presentation of DCIS lesions from predominantly palpable to more than 90% of new lesions being nonpalpable.45 Studies have shown that the rate of DCIS lesions detected by screening increases with age, whereas DCIS accounts for a progressively smaller percentage of the total cancers detected (i.e., invasive cancers plus DCIS).47,48 The rate of DCIS detection has been shown to increase from 0.56 per 1000 mammograms among women aged 40 to 49 years to 1.07 per 1000 mammograms among women aged 70 to 84 years.48
Associated risk factors for the development of DCIS are similar to risk factors for invasive disease and include older age, benign breast disease, a family history of disease, and reproductive factors, including nulliparity or older age of pregnancy.40,49–51
Prevention and Early Detection
Although successful treatment options are in use, improved outcomes can be realized through both prevention of DCIS development and the early detection of new lesions. The role of tamoxifen in the prevention of disease development has been studied by the NSABP in a protocol that randomized patients at high risk for the development of breast cancer to either tamoxifen or placebo.23 Patients eligible included those who were either 60 years of age or older, had a history of LCIS, or were between the ages of 35 and 59 years with a 5-year predicted risk for breast cancer, using the Gail model, of at least 1.66%. The 5-year follow-up data reveal that for noninvasive breast cancer, tamoxifen use was associated with a 50% reduction in risk. The average annual rate of noninvasive breast cancer per 1000 women was 2.68% in the placebo group compared with 1.35% in the tamoxifen group, yielding a relative risk (RR) of 0.50 (95% CI, 0.33 to 0.77).23
Mammography plays an essential role in the early detection of noninvasive lesions, providing an opportunity for intervention early in the course of disease development. The distinctive mammographic feature of DCIS is the presence of microcalcifications, which has been reported in 84% to 98% of cases.52,53 Seventy-two percent to 76% of DCIS lesions have microcalcifications as the sole mammographic finding, and an additional 12% have microcalcifications in combination with other mammographic abnormalities.52,53
Beyond detection, several attempts have been made to correlate the mammographic appearance of the lesion with the histologic type, grade, and extent of disease. Linear branching microcalcifications have been reported to be associated with high-grade or comedo-type DCIS. Heterogeneous granular calcification is commonly associated with moderately differentiated DCIS and fine granular microcalcifications more typically with low-grade, noncomedo DCIS.48,52,53 Although there is a degree of correlation between appearance and histologic type, there is considerable overlap, which limits the use of this information in clinical decision making. Microcalcifications are not always present throughout the histologic abnormality, and the estimated mammographic size and extent of disease frequently underestimate the true pathologic extent of the disease process. Although the mammographic extent can be used to guide the extent of surgical excision, caution should be exercised because the size is typically underestimated by 1 to 2 cm.54,55 In patients presenting with nipple discharge with a negative mammographic evaluation, galactography may be helpful in distinguishing the presence of a papilloma from an underlying DCIS.56
Biologic Characteristics and Molecular Markers
DCIS is a precursor lesion to invasive ductal carcinoma and exists along an evolutionary continuum that starts with benign breast tissue and ends with an invasive breast lesion.57 This concept has been verified in several ways. For years, pathologists have recognized and documented confirmation of a histologic progression from benign breast cells to invasive breast cancer and realized that this histologic evidence was more commonly present where an invasive lesion was discovered. The evolutionary concept is further supported by the acknowledged association between the presence of DCIS and the subsequent increased risk of developing an invasive breast cancer.40,58,59 In some series, a 10-fold risk of developing an invasive lesion has been reported. The presence of shared identical genetic abnormalities between DCIS and synchronous invasive breast cancer demonstrates a clonal relationship of biologic progression.40,58–60
Documented genetic and molecular differences can differentiate DCIS from normal breast tissue. Genetic alterations have been evaluated with an analysis of LOH that has demonstrated gain or loss of multiple loci.58–60 LOH is not seen in normal breast tissue but is present with an increasing frequency that correlates with histologic progression from benign to malignant tissue. In hyperplasias from noncancerous breasts, LOH is rarely documented; however, it is more commonly present (in 42% to 50% of patients) in atypical ductal hyperplasia. Among specimens harvested from cancerous breasts, 77% of noncomedo and 80% of comedo DCIS lesions share LOH with the synchronous invasive lesion in at least one locus.60 Molecular markers have been studied and are found to have a heterogeneous distribution of expression.40 The ER is present in 70% of DCIS lesions, but the rate of expression is high (90%) in low-grade lesions and is significantly less (25%) in high-grade lesions. This trend is reversed with the HER2/neu proto-oncogene and the TP53 tumor suppression gene. About 50% of DCIS lesions exhibit overexpression of the HER2/neu gene, with the TP53 tumor suppressor gene mutated in 25% of cases. Both of these molecular markers are noted in less than 20% of low-grade lesions, but they are found in up to two thirds of high-grade lesions.
Pathologic Characteristics and Pathways of Spread
The primary goal of pathologic assessment is to differentiate DCIS from invasive cancer because the presence of invasive disease alters the focus of treatment from the breast only to possible regional and distant metastases. Once DCIS has been identified, pathologic classification follows in an attempt to predict the clinical outcome and direct the treatment management. Historically, an architectural classification system has been used, dividing tumors into the five classic subtypes of comedo, solid, cribriform, papillary, and micropapillary cancers.61–63 Less common subtypes have been described and include apocrine, neuroendocrine, signet-ring cell cystic, and hypersecretory carcinomas and clinging DCIS.64 The difficulty with an architectural classification method is that there can be a mixture of architectural subtypes identified within the same lesion and that the reproducibility of classification is unreliable because pathologists apply various criteria.55,65,66 Additionally, the value of an architectural classification system is questionable because the architectural subtypes do not correlate well with clinical behavior.67 Although this was not an issue when mastectomy was routinely used, the ability to predict clinical behavior has become important now that breast-conserving approaches are being more commonly applied.
In an attempt to better predict clinical behavior, several classification systems have been proposed.56,68,69 These are based on nuclear grade of the tumor cells and presence of comedo necrosis with and without architectural features. These characteristics appear to correlate with the risk of local recurrence.50 In 1997, an international consensus conference was convened to discuss several pathologic aspects concerning DCIS, with the goal of reaching a consensus where possible.70 Conference participants recognized that several classification systems had been proposed, and although a specific system was not endorsed, they recommended that four features be routinely described: nuclear grade, necrosis, polarization, and architectural pattern. They also recommended that several additional features be documented in the pathology report, including the margin status, size of the lesion, location of microcalcification in relationship to the DCIS lesion, and correlation of characteristics of the tissue specimen with radiographic and mammographic findings.
Primary Therapy
All presentations of DCIS can be successfully managed with total mastectomy. This is supported by reports from mastectomy series showing disease control rates that approach 100% and cancer-specific mortality rates of less than 4%.71–74 However, it is recognized that the availability of mammographic screening has shifted the most common presentation of DCIS from one of a palpable mass with advanced involvement to one of early-stage and limited-extent of disease for which a total mastectomy would intuitively appear to be excessive. To promote mastectomy in the management of noninvasive disease would be in opposition to the increasing use of breast-conservation therapy for invasive disease. Although a phase III trial comparing total mastectomy with breast-conservation therapy for DCIS has never been done, the psychological benefits of preserving the breast and the acceptable rates of local control achieved with breast-conserving therapy support the use of total mastectomy only when necessary. Total mastectomy is most commonly reserved for patients presenting with multicentric or diffuse disease, for patients in whom the use of radiotherapy is contraindicated, when an unacceptable cosmetic result is anticipated following appropriate surgical excision, or for salvage in the event of in-breast recurrence following standard breast-conservation therapy.75
Retrospective analyses of lumpectomy and whole-breast irradiation have reported 5-year cause-specific survival rates that approach 100% and local control rates that range from 85% to 95%.76–82 Prospective randomized trials have been completed evaluating the role of postoperative radiotherapy and tamoxifen in breast-conserving management of DCIS (Tables 60-3 and 60-4). In a Cochrane Database review, postoperative radiotherapy for DCIS of the breast identified and focused on four prospective randomized trials87 (see Table 60-3). This meta-analysis confirmed a statistically significant benefit from the addition of radiotherapy for all ipsilateral breast events (hazard ratio [HR], 0.49; p <.00001), for DCIS (HR, 0.61; p = .03), and for invasive recurrence (HR, 0.50; p = .0001). This benefit was demonstrated in all of the subgroups evaluated, which included patients who underwent complete excision versus those who underwent incomplete excision, patients older than 50 years versus those younger than 50 years, patients with comedo necrosis versus those without comedo necrosis, and patients with lesions of 1 cm or larger versus those with lesions of less than 1 cm.
TABLE 60-3 Randomized Clinical Trials Evaluating the Role of Postoperative Radiotherapy in the Management of DCIS
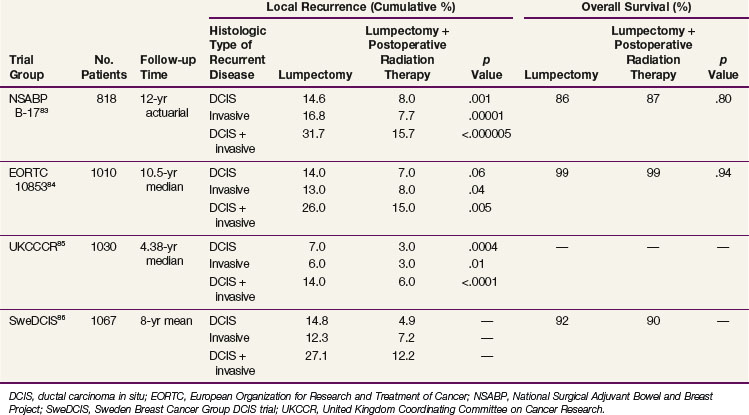
The first of these four prospective randomized trials is the National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-17, designed to evaluate the role of postoperative irradiation.83,88–90 All 814 evaluable patients enrolled were initially diagnosed with DCIS and underwent lumpectomy achieving clear surgical margins (i.e., inked specimen margins that were tumor free on histologic testing). Patients were subsequently randomized to receive postoperative whole-breast radiotherapy or no radiotherapy. Randomization was stratified using age (≤49 years or >49 years), tumor type (DCIS or DCIS with LCIS), method of detection (mammographic or clinical detection, or both), and use of axillary dissection (or not). The radiotherapy guidelines used consisted of whole-breast tangential fields treated to 50 Gy as per previous NSABP studies; a boost to the surgical bed was not included. With 12 years of follow-up, the rate of in-breast tumor recurrence with lumpectomy only was 31.7%, as compared with 15.7% when irradiation was delivered (p <.000005). Nine pathologic features were evaluated for their ability to predict for in-breast recurrence: only comedo necrosis remained as a significant predictor for recurrence. The average annual hazard rates for IBTR were lower for all nine pathologic characteristics when radiotherapy was added.
In 1996, the European Organization for Research and Treatment of Cancer (EORTC) completed a randomized phase III trial (10853).84,91 This trial also evaluated the role of radiotherapy after complete local excision of DCIS. Five hundred patients were treated and followed in the excision only group, and 502 underwent excision plus irradiation. All patients had histologically confirmed tumor-free margins, defined as no DCIS at the inked margin, and the prescribed radiotherapy consisted of whole-breast tangential fields treated to 50 Gy in 25 fractions. A boost dose was not advised, and only 5% received a boost to the surgical bed. At a median follow-up interval of 10.5 years, researchers reported a 26% in-breast failure rate with excision only and a 15% in-breast failure rate with excision plus radiotherapy. Risk factors for recurrence were evaluated, and the relative benefit from the addition of radiotherapy was equivalent in all subgroups. It should be noted that there were two groups identified with an exceptionally low risk of recurrence of less than 10%. These groups were defined as those with well-differentiated DCIS with either a clinging or micropapillary growth pattern. The relative benefit of radiotherapy remained consistent in these patient groups, but the absolute benefit was greatly reduced.84
The United Kingdom Coordinating Committee on Cancer Research (UKCCCR) DCIS Working Group has also conducted a randomized trial investigating the role of adjuvant irradiation.85 Within a more complex 2 × 2 factorial protocol design, the aim was to compare four treatment regimens: excision alone, excision plus tamoxifen, excision plus radiotherapy, and excision plus radiotherapy and tamoxifen. Tamoxifen was prescribed as 20 mg/day, and radiotherapy was delivered through whole-breast tangential fields to a total dose of 50 Gy. A boost was not recommended. A total of 1030 patients were enrolled. Reports after 52.6 months of follow-up showed that the crude incidence rate of local recurrence was 14% in the 508 patients who were treated with excision only and was reduced to 6% in the 522 patients who were treated with excision followed by radiotherapy. The addition of tamoxifen to radiotherapy offered minimal benefit toward the overall ipsilateral local control rates; however, it did appear to reduce the ipsilateral recurrence rate of DCIS in the absence of radiotherapy.85
The SweDCIS study from the Swedish Breast Cancer Group enrolled 1067 patients from 1987 to 1999, randomizing between lumpectomy followed by radiotherapy and lumpectomy only for treatment of DCIS.86 Patients underwent a sector resection with the aim of achieving a 1-cm gross surgical margin; microscopic clear resection was not required. The majority of patients received 50 Gy in 25 fractions to a whole-breast treatment target. A split course of 54 Gy in 2-Gy fractions delivered in two treatment series separated by a 2-week break was allowed. No boost dose was delivered. At a mean follow-up time of 8 years, the in-breast failure risk reduction was 16% at 10 years (95% CI, 10.3 to 21.6) with a relative risk of 0.40 (95% CI, 0.30 to 0.54). Subgroup analysis by age, lesion size, focality, completeness of excision, and the presence of a lesion detected by screening confirmed that all groups benefited in risk reduction with the addition of radiotherapy.86
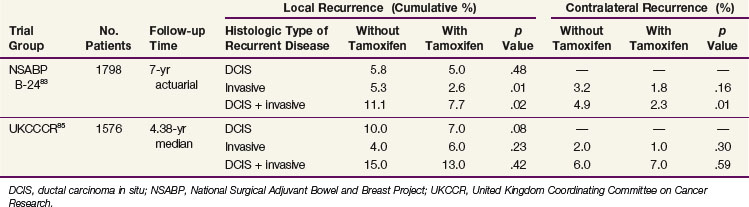
In a trial asking a similar question of the role of tamoxifen and building on the results from B-17, the NSABP initiated a trial to determine if the addition of tamoxifen to lumpectomy and postoperative radiotherapy would be more effective than lumpectomy and postoperative radiotherapy alone.83,92 Women with DCIS or DCIS and LCIS were eligible; patients included women with one or more masses or calcification clusters that were totally excised. Women with microscopically positive margins for DCIS or LCIS were also included, representing 15% of each treatment arm. All of the 1804 women underwent excision and were randomized to whole-breast irradiation and placebo (n = 902) or whole-breast irradiation followed by tamoxifen (n = 902). Patients were stratified according to age (≤49 years or >49 years), tumor type (DCIS or DCIS and LCIS), and method of detection (by mammography or clinical examination, or both). Eighty percent of patients presented with nonpalpable lesions that were measured as 1 cm or less. The postoperative radiotherapy was delivered with standard tangential fields to a total dose of 50 Gy. The placebo or tamoxifen (10 mg twice daily) was continued for 5 years. At 7 years of follow-up, the in-breast failure rate following lumpectomy, radiotherapy, and placebo was 11.1%, and it was reduced to 7.7% when tamoxifen was added to lumpectomy and radiotherapy (p = .02). The occurrence rate of cancer in the contralateral breast was also reduced with the addition of tamoxifen from 4.9% to 2.3% (p = .01).
Risk factor assessment has been performed with the goals of determining which patients are ideal for breast-conservation therapy, which patients would be better treated with mastectomy, and which patients could be successfully managed with lumpectomy only. Single-institution studies and randomized studies have identified factors that predict for an increased risk of recurrence.* Tumor size, the presence of comedo necrosis, the nuclear grade, young patient age, and the margin status have all been identified as factors associated with a higher risk of in-breast recurrence. Although not all publications are consistent, most series report higher local recurrence rates in younger women judged as less than 40, 45, or 50 years old.98 It is therefore appropriate to take age into consideration when determining appropriateness for breast-conservation therapy and when presenting options to the patient.93,97 Whether this increased risk of local failure could be offset with an increase in the boost dose or a wider resection margin is uncertain. However, the addition of radiation therapy reduces the risk of recurrence in all cases; only when diffuse disease, signified by mammographic appearance or the inability to achieve clear surgical margins, is encountered is mastectomy the preferred method of surgical management.
In the absence of these high-risk factors, the prevalent question is whether a less comprehensive treatment approach (i.e., wide excision only or lumpectomy and accelerated partial breast irradiation) is sufficient. Although failure pattern data and retrospective analysis exist to confirm the validity of these more directed treatment approaches, confirmation by an appropriately designed clinical trial is lacking.83,88,89,95,99 The Van Nuys Prognostic Index is a proposed method of identifying patients whose risk of in-breast failure following lumpectomy is so low that adjuvant radiotherapy would be of minimal benefit.100 This scoring index is based on tumor grade, tumor size, patient age, and surgical margin width. Achieving a clear margin of more than 1 cm has been determined to be an important predictor of this index. It is important to note that this determination is characterized by a comprehensive, exhaustive pathologic evaluation assuring that a 1-cm clear surgical margin is achieved in all directions. The scoring index was developed and evaluated at a single institution through retrospective analysis and has yet to be consistently independently validated.101
In a prospective single-arm study at the Dana-Farber/Harvard Cancer Center evaluating wide excision alone, 158 patients with grade I or II DCIS lesions of 2.5 cm or less and treated with wide excision with final surgical margins of 1 cm or more were identified as low-risk patients and enrolled without further treatment.102 Treatment with tamoxifen was not permitted. Presently accepted standard practice approaches to surgical resection and pathologic assessment were applied. The median follow-up time was 40 months. Accrual was prematurely closed after 13 patients developed local recurrence at an unacceptably high in-breast failure rate corresponding to a 5-year rate of 12%.
In an intergroup trial run by the Eastern Cooperative Oncology Group and North Central Cancer Treatment Group, wide excision only in a population of conservatively selected patients with DCIS was evaluated.103 Eligible patients included those with low- or intermediate-grade DCIS lesions measuring 2.5 cm or less or high-grade DCIS lesions measuring 1 cm or less. The microscopic margin width was required to be 3 mm or more with no residual calcifications on postoperative mammograms. Despite the eligibility requirements, it should be noted that the patients entered in the trial had more favorable characteristics; the median lesion size was 6 mm; most lesions had margins of more than 5 mm and approximately 50% had margins of more than 10 mm; and the median age was 60 years and less than 10% were younger than 45 years.104 Five hundred and sixty-five patients were evaluable, and with a median follow-up of 6.2 years, the 5-year rate of ipsilateral in-breast failure was 6.1% in the low- to intermediate-grade group and 15.3% in the high-grade group.
In 2005, the NSABP and RTOG jointly launched a phase III accelerated partial breast irradiation trial that randomized patients between standard whole-breast irradiation following lumpectomy versus accelerated partial breast irradiation to determine if in-breast control rates were comparable.105 Because the in-breast failure patterns for DCIS suggest that treatment directed to the primary lesion plus a 2-cm margin should achieve local control rates that are equal to whole-breast treatment approaches, patients with pure DCIS or DCIS and LCIS are eligible for stratified randomization. Small studies evaluating the role of accelerated partial breast irradiation in the treatment of DCIS have been reported; however, conclusions that would influence treatment practices await the results of the larger NSABP/RTOG phase III trial.106,107
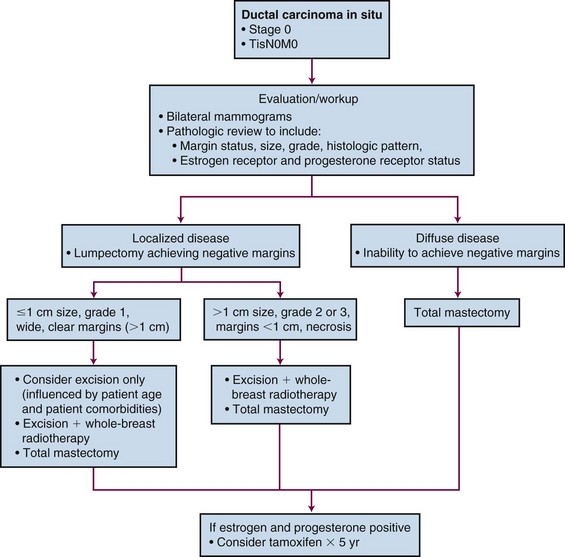
In summary, DCIS is a noninvasive malignant tumor that is managed successfully with treatment directed toward the breast only (Fig. 60-3). Treatment approaches addressing the whole breast are considered the standard of care, although partial breast treatment (i.e., wide excision only vs. lumpectomy plus partial breast irradiation) is being evaluated and is dependent on selection criteria. Although mastectomy can always be considered a treatment option, breast-conservation therapy is the preferred treatment approach. When considering a patient for standard breast-conservation therapy, it is important to first ensure that clinical and mammographic information confirms that the lesion is unicentric. When surgical excision of the lesion is performed, negative pathologic margins should be established and an acceptable cosmetic result achieved. Postoperative irradiation should be delivered with whole-breast tangential fields to a homogeneous dose of 46 to 50 Gy in 1.8 to 2 Gy per fraction. An additional dose to the surgical bed plus the tumor margin (1 to 2 cm) typically follows, so that the total cumulative dose to the surgical bed is 60 to 66 Gy.
1 Foote FW, Stewart FW. Lobular carcinoma in situ—a rare form of mammary cancer. Am J Pathol. 1941;17:491-495.
15 Abner AL, Connolly JL, Recht A, et al. The relationship between the presence and extent of lobular carcinoma in situ and the risk of local recurrence for patients with infiltrating carcinoma of the breast treated with conservative surgery and radiation therapy. Cancer. 2000;88:1072-1077.
16 Moran M, Haffty B. Lobular carcinoma in situ as a component of breast cancer. The long-term outcome in patients treated with breast-conservation therapy. Int J Radiat Oncol Biol Phys. 1998;40:353-358.
17 Jolly S, Kestin LL, Goldstein NS, et al. The impact of lobular carcinoma in situ in association with invasive breast cancer on the rate of local recurrence in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2006;66:365-371.
18 Sasson AR, Fowble B, Hanlon AL, et al. Lobular carcinoma in situ increases the risk of local recurrence in selected patients with stages I and II breast carcinoma treated with conservative surgery and radiation. Cancer. 2001;91:1862-1869.
19 Ciocca RM, Li T, Freedman G, et al. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann Surg Oncol. 2008;15:2263-2271.
20 Ben-David MA, Kleer CG, Paramagul C, et al. Is lobular carcinoma in situ as a component of breast carcinoma a risk factor for local failure after breast-conserving therapy? Results of a matched pair analysis. Cancer. 2006;106:28-34.
21 Fisher ER, Costantino J, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) protocol B-17—five-year observations concerning lobular carcinoma in situ. Cancer. 1996;78:1403-1416.
22 Fisher ER, Land SR, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project—twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100:238-244.
23 Fisher B, Costantino J, Wickerham DL, et al. Tamoxifen for prevention of breast cancer. Report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
24 Velpeau S. A Treatise on Diseases of the Breast and Mammary Region. London: Sydenham Society; 1856.
34 Kawase K, DiMaio DJ, Tucker SL, et al. Paget’s disease of the breast. There is a role for breast-conserving therapy. Ann Surg Oncol. 2005;12:1-7.
35 Polgar C, Orosz Z, Kovacs T, et al. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:904-1905.
36 Stockdale AD, Brierly JD, White WF, et al. Radiotherapy for Paget’s disease of the nipple. A conservative alternative. Lancet. 1989;2:664-666.
37 Bijker N, Rutgers EJT, Duchateau L, et al. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2001;91:472-477.
38 Pierce LJ, Haffty BG, Solin LJ, et al. The conservative management of Paget’s disease of the breast with radiotherapy. Cancer. 1997;80:1065-1072.
39 Marshall JK, Griffith KA, Haffty BG, et al. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
46 American Cancer Society. Cancer Facts and Figures 2009. Atlanta, Georgia: American Cancer Society; 2009.
83 Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ. An update of the National Surgical Adjuvant Breast and Bowel Project Experience. Semin Oncol. 2001;28:400-418.
84 Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ. Ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC breast cancer cooperative group and EORTC radiotherapy group. J Clin Oncol. 2008;24:3381-3387.
85 Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand. Randomized controlled trial. Lancet. 2003;362:95-102.
86 Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247-1252.
87 Goodwin A, Parker S, Ghersi D, Wilcken N: Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev 3:CD000563, 2009.
88 Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581-1586.
89 Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer. Findings from National Surgical Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441-452.
90 Fisher ER, Costantino JP, Fisher B, et al. Pathologic findings from the National Surgical Breast and Bowel Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). Cancer. 1995;75:1310-1319.
91 Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ. Analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263-2271.
92 Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer. National Surgical Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993-2000.
95 Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455-1461.
97 Vicini FA, Kestin LL, Goldstein NS, et al. Impact of young age on outcome in patients with ductal carcinoma in situ treated with breast conserving therapy. J Clin Oncol. 2000;18:296-306.
98 Turaka A, Freedman GM, Li T, et al. Young age is not associated with increased local recurrence for DCIS treated by breast-conserving surgery and radiation. J Surg Oncol. 2009;100:25-31.
100 Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267-2274.
102 Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031-1036.
103 Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast. A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319-5324.
104 Harris JR, Morrow M. Clinical dilemma of ductal carcinoma in situ. J Clin Oncol. 2009;27:5303-5305.
105 Vicini F, White J, Arthur D, et al. NSABP Protocol B39/RTOG Protocol 0413. A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer, December 8. http://208.251.169.72/members/protocols/0413/0413.pdf, 2004. Available at
106 Keisch M, Vicini F, Beitsch P, et al. American Society of Breast Surgeons MammoSite Radiation Therapy System Registry Trial. Ductal carcinoma-in-situ subset analysis—4-year data in 194 treated lesions. Am J Surg. 2009;198:505-507.
107 Benitez PR, Streeter O, Vicini F, et al. Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ. A phase II clinical study. Am J Surg. 2006;192:427-433.
1 Foote FW, Stewart FW. Lobular carcinoma in situ—a rare form of mammary cancer. Am J Pathol. 1941;17:491-495.
2 Rosen PP, Lieberman PH, Braun DW, et al. Lobular carcinoma in situ of the breast. Am J Surg Pathol. 1978;2:225-251.
3 Page DL, Kidd TE, Dupont WE, et al. Lobular neoplasia of the breast. Higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22:1232-1239.
4 Schnitt SJ, Morrow M. Lobular carcinoma in situ. Current concepts and controversies. Semin Diagn Pathol. 1999;16:209-223.
5 Wheeler JE, Enterline HT, Roseman JM, et al. Lobular carcinoma in situ of the breast—long term follow-up. Cancer. 1974;34:554-563.
6 Tavassoli FA. Lobular neoplasia. In: Tavassoli FA, editor. Pathology of the Breast. 2nd ed. New York: Elsevier; 1999:373-400.
7 Albonico G, Querzoli P, Ferretti S, et al. Biological profile of in situ breast cancer investigated by immunohistochemical technique. Cancer Detect Prev. 1998;22:313-318.
8 Bur ME, Zimarowski MJ, Schmitt SJ, et al. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9 Acs G, Lawton TJ, Rebbeck TR, et al. Differential expression of e-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol. 2001;115:85-98.
10 Goldstein NS, Kestin LL, Vicini FA. Clinicopathologic implications of e-cadherin reactivity in patients with lobular carcinoma in situ of the breast. Cancer. 2001;92:738-747.
11 Andersen JA. Lobular carcinoma in situ of the breast—an approach to rational treatment. Cancer. 1977;39:2597-2602.
12 Georgian-Smith D, Lawton TJ. Calcifications of lobular carcinoma in situ of the breast. Radiologic-pathologic correlation. AJR Am J Roentgenol. 2001;176:1255-1259.
13 Cohen MA. Cancer upgrades at excisional biopsy after diagnosis of atypical lobular hyperplasia or lobular carcinoma in situ core-needle biopsy. Some reasons why. Radiology. 2004;231:617-621.
14 Foster MC, Helvie MA, Gregory NE, et al. Lobular carcinoma in situ or atypical lobular hyperplasia at core-needle biopsy: is excisional biopsy necessary? Radiology. 2004;231:813-819.
15 Abner AL, Connolly JL, Recht A, et al. The relationship between the presence and extent of lobular carcinoma in situ and the risk of local recurrence for patients with infiltrating carcinoma of the breast treated with conservative surgery and radiation therapy. Cancer. 2000;88:1072-1077.
16 Moran M, Haffty B. Lobular carcinoma in situ as a component of breast cancer. The long-term outcome in patients treated with breast-conservation therapy. Int J Radiat Oncol Biol Phys. 1998;40:353-358.
17 Jolly S, Kestin LL, Goldstein NS, et al. The impact of lobular carcinoma in situ in association with invasive breast cancer on the rate of local recurrence in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2006;66:365-371.
18 Sasson AR, Fowble B, Hanlon AL, et al. Lobular carcinoma in situ increases the risk of local recurrence in selected patients with stages I and II breast carcinoma treated with conservative surgery and radiation. Cancer. 2001;91:1862-1869.
19 Ciocca RM, Li T, Freedman G, et al. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann Surg Oncol. 2008;15:2263-2271.
20 Ben-David MA, Kleer CG, Paramagul C, et al. Is lobular carcinoma in situ as a component of breast carcinoma a risk factor for local failure after breast-conserving therapy? Results of a matched pair analysis. Cancer. 2006;106:28-34.
21 Fisher ER, Costantino J, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) protocol B-17—five-year observations concerning lobular carcinoma in situ. Cancer. 1996;78:1403-1416.
22 Fisher ER, Land SR, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project—twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100:238-244.
23 Fisher B, Costantino J, Wickerham DL, et al. Tamoxifen for prevention of breast cancer. Report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
24 Velpeau S. A Treatise on Diseases of the Breast and Mammary Region. London: Sydenham Society; 1856.
25 Paget J. On the disease of the mammary areola preceding cancer of the mammary gland. St Bartholomew Hosp Rep. 1874;10:87-89.
26 Jamali FR, Ricci A, Deckers PJ. Paget’s disease of the nipple-areola complex. Surg Clin North Am. 1996;76:365-381.
27 Kohler S, Rouse RV, Smoller BR. The differential diagnosis of pagetoid cells in the epidermis. Mod Pathol. 1998;11:79-92.
28 Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742-749.
29 Ashikari R, Park K, Huvos AG, et al. Paget’s disease of the breast. Cancer. 1970;26:680-685.
30 Sakorafas GH, Blanchard K, Sarr MG, et al. Paget’s disease of the breast. Cancer Treat Rev. 2001;27:9-18.
31 Paone JF, Baker RR. Pathogenesis and treatment of Paget’s disease of the breast. Cancer. 1981;48:825-829.
32 Markpoulos Ch, Gogas H, Sampalis F, et al. Bilateral Paget’s disease of the breast. Eur J Gynaec Oncol. 1997;18:495-496.
33 Hayes R, Cummings B, Miller RAW, et al. Male Paget’s disease of the breast. J Cutan Med Surg. 2000;4:208-212.
34 Kawase K, DiMaio DJ, Tucker SL, et al. Paget’s disease of the breast. There is a role for breast-conserving therapy. Ann Surg Oncol. 2005;12:1-7.
35 Polgar C, Orosz Z, Kovacs T, et al. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2002;94:904-1905.
36 Stockdale AD, Brierly JD, White WF, et al. Radiotherapy for Paget’s disease of the nipple. A conservative alternative. Lancet. 1989;2:664-666.
37 Bijker N, Rutgers EJT, Duchateau L, et al. Breast-conserving therapy for Paget disease of the nipple. Cancer. 2001;91:472-477.
38 Pierce LJ, Haffty BG, Solin LJ, et al. The conservative management of Paget’s disease of the breast with radiotherapy. Cancer. 1997;80:1065-1072.
39 Marshall JK, Griffith KA, Haffty BG, et al. Conservative management of Paget disease of the breast with radiotherapy. Cancer. 2003;97:2142-2149.
40 Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430-1441.
41 Rosner D, Bedwani RN, Vana J, et al. Noninvasive breast carcinoma, Results of a national survey by the American College of Surgeons. Ann Surg. 1980;192:139-147.
42 Wilson RE, Donegan WL, Mettlin C, et al. The 1982 national survey of carcinoma of the breast in the United States by the American College of Surgeons. Surg Gynecol Obstet. 1984;159:309-318.
43 Lenhard RE. Cancer statistics. A measure of progress. CA Cancer J Clin. 1996;46:3-7.
44 Schnitt SJ, Silen W, Sadowsky NL, et al. Ductal carcinoma in situ (intraductal carcinoma) of the breast. N Engl J Med. 1988;318:898-903.
45 Ernster VL, Barclay J, Kerlikowske K, et al. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913-918.
46 American Cancer Society. Cancer Facts and Figures 2009. Atlanta, Georgia: American Cancer Society; 2009.
47 Kopans DB. Re detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2003;95:487.
48 Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546-1554.
49 Trentham-Dietz A, Newcomb PA, Storer BE, et al. Risk factors for carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2000;9:697-703.
50 Kerlikowske K, Molinaro A, Cha I. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692-1702.
51 Kerlikowske K, Barclay J, Grady D, et al. Comparison of risk factors for ductal carcinoma in situ and invasive breast cancer. J Natl Cancer Inst. 1997;89:76-82.
52 Tabar L, Fagerberg CJG, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Lancet. 1985;1:829-832.
53 Tabar L, Gad A, Parsons WC, et al. Mammographic appearances of in situ carcinomas. In: Silverstien MJ, editor. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002:87-104.
54 Holland R, Hendriks J, Verbeek A, et al. Extent, distribution and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990;335:519-522.
55 Satake H, Shimamoto K, Sawaki A, et al. Role of ultrasonography in the detection of intraductal spread of breast cancer. Correlation with pathologic findings, mammography and MR imaging. Eur Radiol. 2000;10:1726-1732.
56 Patchefsky AS, Schwartz GF, Finkelstein SD, et al. Heterogeneity of intraductal carcinoma of the breast. Cancer. 1989;63:731-741.
57 Allred DC, Mohsin SK, Fuqua SAW. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8:47-61.
58 Radford DM, Phillips NHJ, Fair KL, et al. Allelic loss and the progression of breast cancer. Cancer Res. 1995;55:5180-5183.
59 Stratton MR, Collins N, Lakhani SR, et al. Loss of heterozygosity in ductal carcinoma in situ of the breast. J Pathol. 1995;175:195-201.
60 O’Connell P, Pekkel V, Fuqua SA, et al. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J Natl Cancer Inst. 1998;90:697-703.
61 Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. Edinburgh: Churchill Livingstone; 1987.
62 Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992;23:1095-1097.
63 Rosen PP. Rosen’s Breast Pathology. Philadelphia: Lippincott Raven; 1997. pp 237-245
64 Lagios MD. Ductal carcinoma in situ: controversies in diagnosis, biology, and treatment. Breast J. 1995;1:68-78.
65 Schnitt SJ, Connolly JL, Tavasolli FA, et al. Interobserver variability in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992;16:1133-1143.
66 European Commission Working Group on Breast Screening Pathology. Causes of inconsistency in diagnosing and clarifying intraductal proliferation of the breast. Eur J Cancer. 2000;36:1769-1772.
67 Bavde S, A’Hern RP, Ward AM, et al. Prediction of local recurrence of ductal carcinoma in situ of the breast using five histological classifications. A comparative study with long follow-up. Hum Pathol. 1998;29:915-923.
68 Silverstein MJ, Poller DN, Waisman JR, et al. Prognostic classification of breast ductal carcinoma in-situ. Lancet. 1995;345:1154-1157.
69 Holland R, Peterse JL, Millis RR, et al. Ductal carcinoma in situ. A proposal for a mew classification. Semin Diagn Pathol. 1994;11:167-180.
70 Consensus conference on the classification of ductal carcinoma in situ. Cancer. 1997;80:1798-1802.
71 Sunshine JA, Moseley MS, Fletcher WS, et al. Breast carcinoma in situ. A retrospective review of 112 cases with a minimum 10-year follow-up. Am J Surg. 1985;150:44-51.
72 Silverstein MJ, Cohlan BF, Gierson ED, et al. Duct carcinoma in situ. 228 cases without microinvasion. Eur J Cancer. 1992;28:630-634.
73 Schuh ME, Nemoto T, Penetrante R, et al. Intraductal carcinoma. Analysis of presentation, pathologic findings, and outcome of disease. Arch Surg. 1986;121:1303-1307.
74 Arnesson LG, Smeds S, Fagerberg G, et al. Follow-up of two treatment modalities for ductal carcinoma in situ of the breast. Br J Surg. 1989;76:672-675.
75 Solin L, Fourquet A, Vicini FA, et al. Salvage treatment for local recurrence after breast-conserving surgery and radiation as initial treatment for mammographically detected ductal carcinoma in situ of the breast. Cancer. 2001;91:1090-1097.
76 McCormick B, Rosen PP, Kinne D, et al. Duct carcinoma in situ of the breast. An analysis of local control after conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1991;21:289-292.
77 Haffty BG, Peschel RE, Papdopoulus D, et al. Radiation therapy for ductal carcinoma in situ of the breast. Conn Med. 1990;54:482-484.
78 Van Zee KJ, Liberman L, Samli B, et al. Long term follow-up of women with ductal carcinoma in situ treated with breast conserving surgery. The effect of age. Cancer. 1999;86:1757-1767.
79 Sneige N, McNeese MD, Atkinson EN, et al. Ductal carcinoma in situ treated with lumpectomy and irradiation. Histological analysis of 49 specimens with emphasis on risk factors and long term results. Hum Pathol. 1995;26:642-649.
80 Solin LJ, Kurtz J, Fourquet A, et al. Fifteen-year results of breast conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ (intraductal carcinoma of the breast). J Clin Oncol. 1996;14:754-763.
81 Amichetti M, Caffo O, Richetti A, et al. Ten-year results of treatment of ductal carcinoma in situ (DCIS) of the breast with conservative surgery and radiotherapy. Eur J Cancer. 1997;33:1559-1565.
82 Mirza NQ, Vlastos G, Meric F, et al. Ductal carcinoma-in situ. Long-term results of breast conserving therapy. Ann Surg Oncol. 2000;7:656-664.
83 Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ. An update of the National Surgical Adjuvant Breast and Bowel Project Experience. Semin Oncol. 2001;28:400-418.
84 Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ. Ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC breast cancer cooperative group and EORTC radiotherapy group. J Clin Oncol. 2008;24:3381-3387.
85 Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand. Randomized controlled trial. Lancet. 2003;362:95-102.
86 Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247-1252.
87 Goodwin A, Parker S, Ghersi D, Wilcken N: Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev 3:CD000563, 2009.
88 Fisher B, Costantino J, Redmond C, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581-1586.
89 Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer. Findings from National Surgical Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441-452.
90 Fisher ER, Costantino JP, Fisher B, et al. Pathologic findings from the National Surgical Breast and Bowel Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). Cancer. 1995;75:1310-1319.
91 Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ. Analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263-2271.
92 Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer. National Surgical Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993-2000.
93 Fowble B. Overview of conservative surgery and radiation therapy ductal carcinoma in situ. In: Silverstein MJ, Recht A, Lagios MD, editors. Ductal Carcinoma in Situ of the Breast. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002:287-302.
94 Eusebi V, Feubale E, Foschini MP, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994;11:223-235.
95 Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455-1461.
96 Ottensen GL, Graversen HP, Blichert-Toft M, et al. Ductal carcinoma in situ of the female breast. Short-term results of a prospective nationwide study—The Danish Breast Cancer Cooperative Group. Am J Surg Pathol. 1992;16:1183-1196.
97 Vicini FA, Kestin LL, Goldstein NS, et al. Impact of young age on outcome in patients with ductal carcinoma in situ treated with breast conserving therapy. J Clin Oncol. 2000;18:296-306.
98 Turaka A, Freedman GM, Li T, et al. Young age is not associated with increased local recurrence for DCIS treated by breast-conserving surgery and radiation. J Surg Oncol. 2009;100:25-31.
99 Kuske RR. Should all ductal carcinoma in situ patients receive radiation therapy and is partial breast irradiation an option? In: Silverstein MJ, Recht A, Lagios MD, editors. Ductal Carcinoma in situ of the Breast. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002:287-302.
100 Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267-2274.
101 de Mascarel I, Bonichon F, MacGrogan G, et al. Application of the Van Nuys prognostic index in a retrospective series of 367 ductal carcinomas in situ of the breast examined by serial macroscopic sectioning. Practical considerations. Breast Cancer Res Treat. 2000;61:151-159.
102 Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031-1036.
103 Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast. A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319-5324.
104 Harris JR, Morrow M. Clinical dilemma of ductal carcinoma in situ. J Clin Oncol. 2009;27:5303-5305.
105 Vicini F, White J, Arthur D, et al. NSABP Protocol B39/RTOG Protocol 0413. A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer, December 8. http://208.251.169.72/members/protocols/0413/0413.pdf, 2004. Available at
106 Keisch M, Vicini F, Beitsch P, et al. American Society of Breast Surgeons MammoSite Radiation Therapy System Registry Trial. Ductal carcinoma-in-situ subset analysis—4-year data in 194 treated lesions. Am J Surg. 2009;198:505-507.
107 Benitez PR, Streeter O, Vicini F, et al. Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ. A phase II clinical study. Am J Surg. 2006;192:427-433.