CHAPTER 67 Nonimplantation Salvage of Severe Elbow Dysfunction
INTRODUCTION
Today, several salvage options exist for severe elbow dysfunction following trauma or failed prior intervention. If the joint is stiff and destroyed, interposition arthroplasty is recommended (see Chapter 69). If strength is required in a young person, especially if the joint is infected, fusion may be considered (see Chapter 70). If the injury is to the brachial plexus, strategies for management are discussed in Chapter 71. In this chapter, we review resection arthroplasty and allograft reconstruction for massive bone loss.
RESECTION ARTHROPLASTY
TECHNIQUE
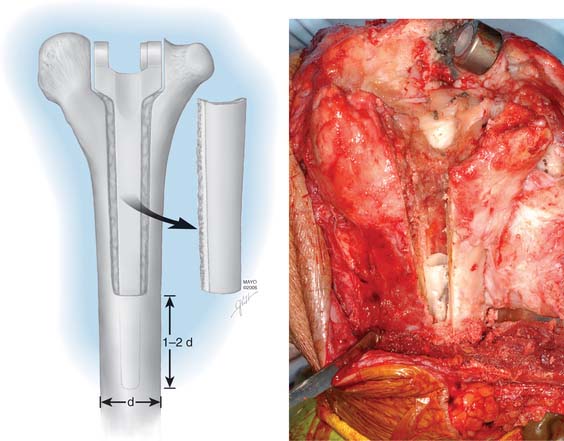
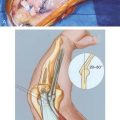
To remove a well-fixed implant, a trapezoid-shaped cortical window is removed from the posterior aspect of the distal humerus (Fig. 67-1). Care is taken to preserve the epicondyles (Fig. 67-2). The olecranon is positioned between the two columns. We use a No. 3 PDS suture to help stabilize this relationship.
AFTERCARE
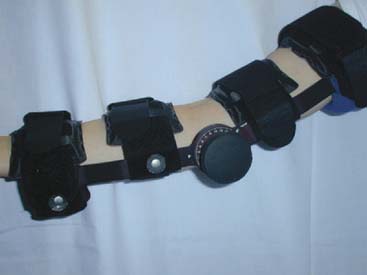
A cast is applied for 6 weeks. Protected motion is begun using a Mayo Elbow Brace (Donjoy International, Vista, CA) and continued for an additional 6 to 8 weeks (Fig. 67-3).
RESULTS
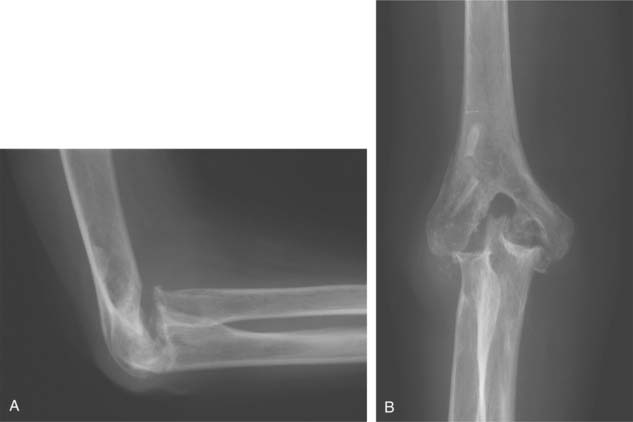
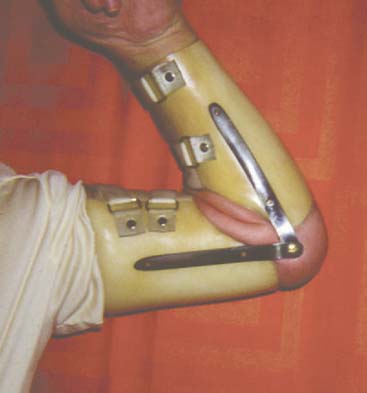
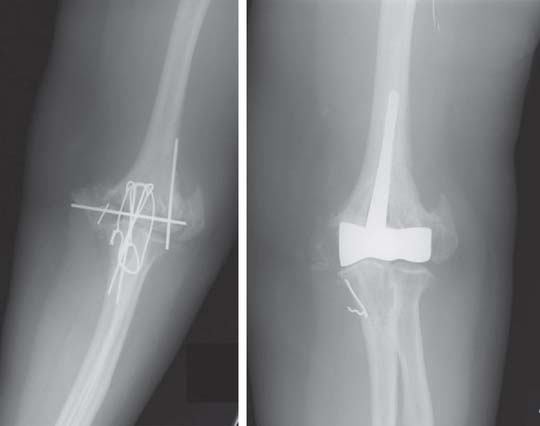
We have just completed an assessment of 59 resected elbows after failed total elbow arthroplasty (TEA) for sepsis at a mean time of 12 years after surgery. Although the study is still under way, preliminary analysis demonstrates a clear distinction between those with “contained” articulation (Fig. 67-4) having medial and lateral condyles doing markedly better than those with more extensive resection preventing any opportunity for a fulcrum effect (Fig. 67-5). Custom orthotic braces are occasionally prescribed, but infrequently worn on a regular basis (Fig. 67-6).
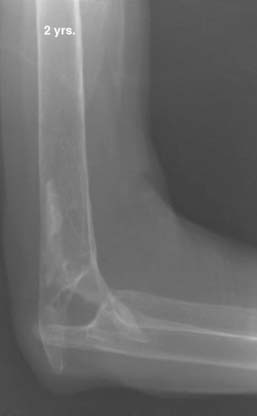
FIGURE 67-4 Two years after resection arthroplasty, notice the maintenance of the medial and lateral columns.
SEGMENTAL BONE LOSS
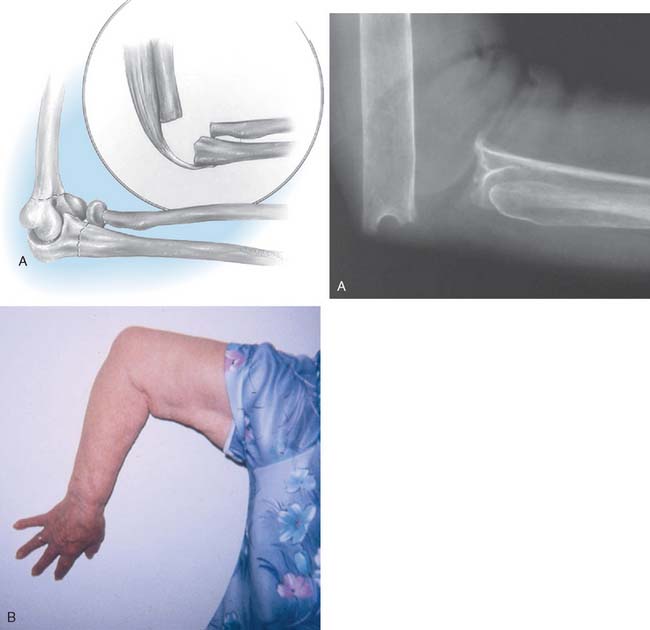
The unstable or “flail” elbow with segmental bone loss, that is, complete loss of articulation and ligament attachments, is difficult to manage. Treatment with a primary replacement is discussed in Chapters 59 and 66. This problem can be a sequel of trauma, infection, failed elbow arthroplasty, or tumor resection. Bone may be lost at the original injury following a severe open fracture, or it may be removed subsequent to infection, particularly if the fragment is avascular. In treating recalcitrant union of supracondylar fractures, surgeons have in the past (and disastrously) succumbed to the temptation to remove the condylar fragment of the humerus and treat the problem by inserting an endoprosthesis.
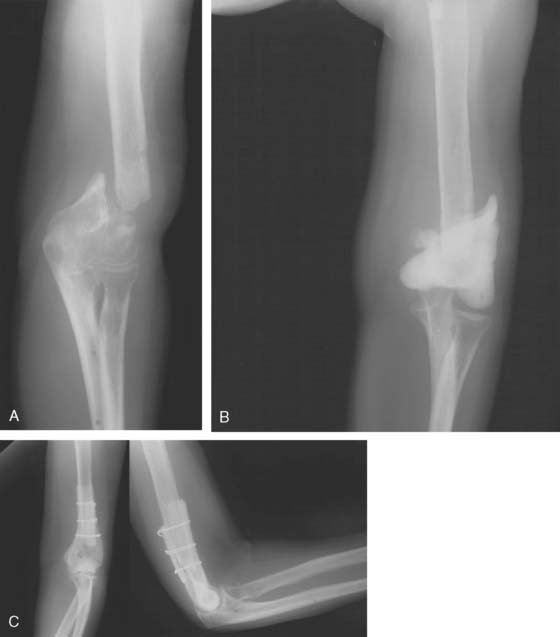
If the problem is limited to just the articular region of the humerus, a hemireplacement may be effective (Fig. 67-7). If, however, all stability and distal contour of the humerus is lost, today, a linked replacement is performed for such patients (see Chapter 59).
On the other hand, in the situation of a flail elbow, particularly in a young patient, arthrodesis may be considered (see Chapter 70). However, failure of fusion, even under optimal conditions, has been high in some series2,14,16 and successful arthrodesis is unlikely in patients with large bone stock deficiencies. Reconstruction procedures, such as those involving reattachment of muscles more proximally on the humerus or interposing a tongue of triceps muscle into the gap, are unlikely to be successful. Muscle transfer procedures can improve flexion in cases of flail elbow associated with paralysis, but in these cases, the problem is of a different nature. A successful outcome will occur only if a stable fulcrum is provided and muscles are restored to their normal functioning length.
ALLOGRAFT REPLACEMENT
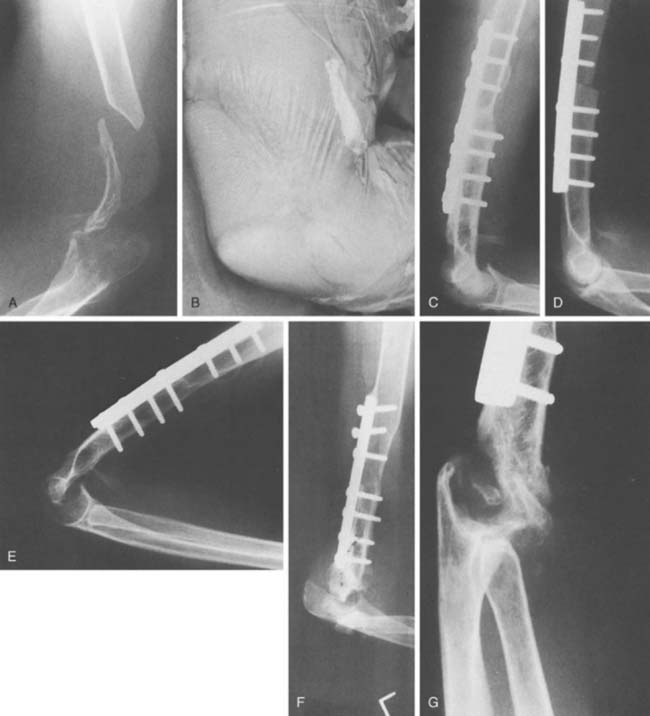
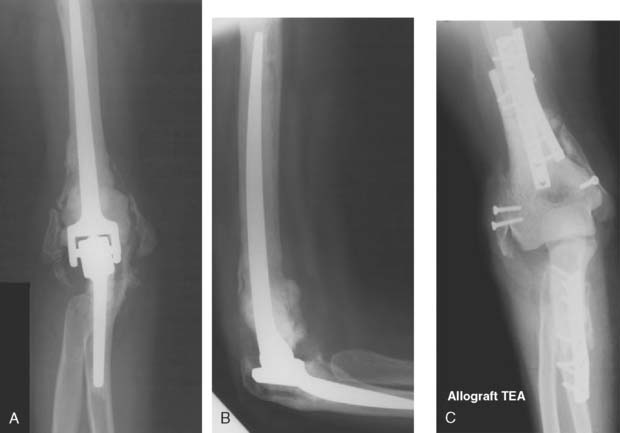
For the younger patient, at this time, allograft replacement of the missing bone segment might be considered.17 Autografts such as vascularized fibula18 and iliac crest8 have been used in the upper limb, but lack of articular surface and donor site morbidity limit their application. Lexer10 is credited with the first report of an allograft transplantation as early as 1908. Renewed interest has been sparked by a series of publications in the late 1960s and 1970s.11–13 Ottolenghi12 believed that partial allograft joint transplantation gave better results than replacement of the whole joint. Considerable success has been documented in replacing isolated segments of the humerus.4 The use of distal humeral allograft or proximal ulnar allograft requires careful stabilization of the joint by reconstructing both the medial and lateral collateral ligament. For bone loss distal to the humeral collateral attachments, we use a hemiprosthetic device (see Fig. 67-7) (see Chapter 52). With more extensive bone loss, a hemiallograft replacement is employed (Fig. 67-8).
Animal studies indicate that a major cause of failure in total joint allograft replacement is delayed or incomplete union and failure of revascularization.6 Thus, allograft reconstruction is a viable choice for short-term restoration of function in this difficult group of patients. It provides a stable joint without a requirement for associated complex soft tissue reconstruction. It is important, if good stability and function are to be achieved, that the allograft match as nearly as possible the size of the lost segment of bone and its articular contour.4,15 Radiographs of the normal side are valuable for planning purposes. When inserting the allograft, we take care to achieve the correct degree of muscle tension. This precaution not only guarantees good functional restoration but also improves joint stability, and ligamentous reconstruction is unnecessary. Before any reconstructive procedure, there must be a careful assessment of the quality of all soft tissues, including the muscle as well as skin and soft tissues. The soft tissues must be able to accommodate a large allograft and still permit closure. Vascularized free flaps of soft tissue may be required (see Chapter 36).
LONG-TERM CONSIDERATIONS
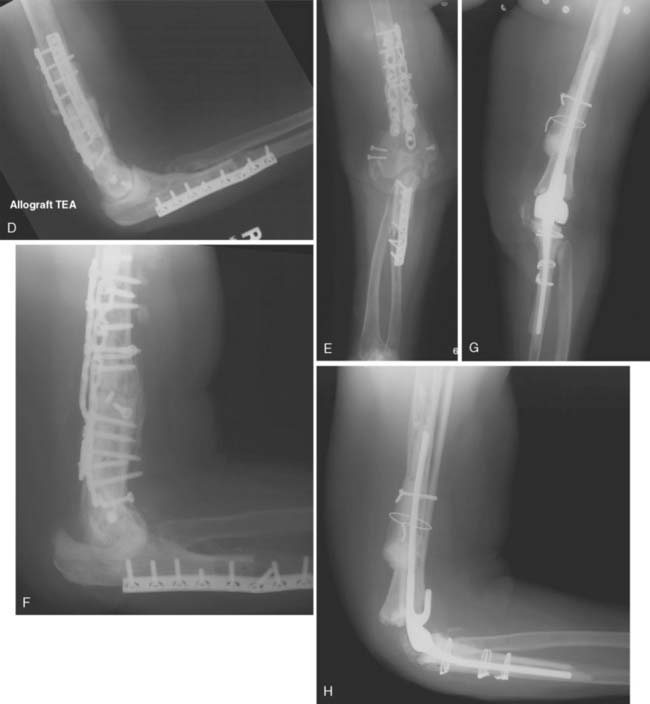
The fate of implanted allograft material depends on many factors, some of which are not fully understood. Large segmental allografts must unite with host bone (by a process of osteoconduction) if they are to succeed.7 This union occurs frequently, provided that the fixation to the host is technically satisfactory (Fig. 67-9). The standard AO plating technique is usually effective. Considerable loss of bone is observed to occur with time in that portion of the graft remote from the donor host junction. The exact nature of this neurotrophic type response is unknown at this time. One of the advantages of the method is that this gradual loss of allograft over time appears to allow some functional adaptation of muscles so that joint stability sufficient to permit continuing good function remains.3 Subluxation may also occur. Even if further reconstruction is required on some future occasion, it is facilitated, not prejudiced, by the augmentation of the patient’s bone stock that is achieved by the allograft. In fact, if we perform their procedure, it is considered a staged bone augmentation procedure that facilitates elbow replacement should this be necessary.
BICOMPATIBILITY
The immune response is not universally deleterious to the outcome of these transplants because satisfactory and full incorporation of the allograft into the host’s skeleton does occur. Graft incorporation depends on the mechanical environment and the type of graft as well as immune factors that are complicated and poorly understood.7,9 The immunogenicity of allografts is reduced by freezing or freeze drying, which renders them more suitable for clinical use. However, even preserved allografts provoke recognizable cellular and humoral antibody responses. Donor-specific anti—human leukocyte antigen (HLA) antibodies and cellular antibodies have been identified in frozen and freeze-dried grafts.5 The major reactivity appears to be cellular because viable bone marrow cells and, to a lesser extent, bone cells are highly immunogenic and induce both a cell-mediated and a humoral response. Antigens in the extracellular matrix (collagen and the noncollagenous proteins) seem to be of much less significance in the clinically observed allograft response.9 At this time, it has not been possible to identify the specific criteria that define an immune rejection of these grafts, as opposed to failure caused by some other process. Any infection inevitably spells failure for this procedure. As more becomes known of the nature of the rejection phenomenon, it may be possible to favorably manipulate the response, perhaps by tissue typing and immunosuppression of the host.
RESULTS
Urbaniak15 found total joint transplantation to be successful in six of eight cases, with a relatively short follow-up period of up to 6 years. Subsequent surveillance has tempered this enthusiasm due to predictable deterioriation of the articulation.3
Others have reported somewhat similar results. A French report described patient satisfaction in five of seven patients with TEA replacements.1 In five of six patients, progressive joint resorption occurred. In our limited experience, ultimate joint deterioration universally occurs, but can be readily managed by a joint replacement (Fig. 67-10). Otherwise, there is little information in the peer-reviewed literature regarding this treatment option.
1 Allieu Y., Marck G., Chammas M., Desbonnet P., Raynaud J.P. Total elbow joint allograft for long-term posttraumatic osteoarticular loss. Follow-up results at 12 years. Rev Chir. Orthop. Reparatrice Appar. Mot. 2004;90:319.
2 Carnesale P.G., Stewart M.J.. Complications of arthrodesis surgery. Epps C.H., editor. Complications in Orthopaedic Surgery, Vol. 2. J. B. Lippincott Co., Philadelphia, 1978;1109.
3 Dean G.S., Holliger E.H.4th, Urbaniak J.R. Elbow allograft for reconstruction of the elbow with massive bone loss. Long term results. Clin. Orthop. Relat. Res. 1997;341:12.
4 Dee R. Reconstructive surgery following total elbow endoprosthesis. Clin. Orthop. Relat. Res. 1982;170:196.
5 Friedlaender G.E. Immune responses to osteochondral allografts. Current knowledge and future directions. Clin. Orthop. Relat. Res. 1983;174:58.
6 Goldberg V.M., Heiple K.G. Experimental hemijoint and whole-joint transplantation. Clin. Orthop. Relat. Res. 1983;174:43.
7 Goldberg V.M., Stephenson S., Schaffer J.W. Biology of allografts and autografts. In: Friedlaender G.E., Goldberg V.M., editors. Bone and Cartilage Allografts: Biology and Clinical Applications. Chicago: American Academy of Orthopedic Surgery Publications, 1991.
8 Gschwend N. Salvage procedure in failed elbow prosthesis. Arch. Orthop. Trauma Surg. 1983;101:95.
9 Horowitz M.C., Friedlaender G.E. The immune response to bone grafts. In: Friedlaender G.E., Goldberg V.M., editors. Bone and Cartilage Allografts: Biology and Clinical Applications. Chicago: American Academy of Orthopedic Surgery Publications, 1991.
10 Lexer E. Die Verwendung der freien Knochenplastik nebst Versuchen über Gelenkversteifung und Gelenk-transplanation. Arch. Lin. Chir. 1908;86:939.
11 Mankin H.J., Doppelt S., Tomford W. Clinical experience with allograft implantation. Clin. Orthop. Relat. Res. 1983;174:69.
12 Ottolenghi C.E. Massive osteo- and osteo-articular bone grafts. Clin. Orthop. Relat. Res. 1972;87:156.
13 Parrish F.F. Allograft replacement of all or part of the end of a long bone following excision of a tumor. J. Bone Joint Surg. 1973;55A:1.
14 Stewart M. Arthrodesis. In: Edmonson A.S., Crenshaw A., editors. Campbell’s Operative Orthopaedics. St. Louis: C. V. Mosby Co.; 1980:1137.
15 Urbaniak J.R., Black K.E. Cadaveric elbow allografts: a six-year experience. Clin. Orthop. Relat. Res. 1985;197:131.
16 Van Gorder G.W., Chen C.M. The central-graft operation for fusion of tuberculosis of knees, ankles, and elbows. J. Bone Joint Surg. 1959;41A:1029.
17 Wavreille G., Dos Remedios C., Chantelot C., Limousin M., Fontaine C. Anatomic bases of vascularized elbow joint harvesting to achieve vascularized allograft. Surg. Radiol. Anat. 2006;28:498.
18 Weiland A., Moore J.R., Daniel R.K. Vascularized bone autografts. Clin. Orthop. Relat. Res. 1983;174:87.