CHAPTER 353 Nonatherosclerotic Carotid Lesions
Carotid Body Tumors
In 1743, von Haller1 reported the anatomy of the carotid body. He described the carotid body as a nodule about the size of a kernel of wheat at the bifurcation of the common carotid artery (CCA). Anatomic dissection revealed that the structure was contained in a dense meshwork at the base of the carotid bifurcation.2 Believing that the structure was nerve tissue, he called it the “intercarotid ganglion.” In 1862, Luschka, as discussed by Balfour and Wildner,2 characterized the carotid body microscopically after examining hundreds of specimens. He described it as 5 to 7 mm long, 2.5 to 4 mm wide, and 1.5 mm thick and as having the primary histologic characteristics of a gland. Years later, Kohn defined the carotid body as chromaffin cells embedded in the sympathetic nerve fibers about the carotid artery and coined the term paraganglion.3
Lahey and Warren4 reported that Marchand attempted the first surgical resection of a carotid body tumor in 1880. The report detailed an aggressive tumor resection with division of the carotid artery; jugular vein; and vagus, hypoglossal, and sympathetic nerves. The patient died 3 days after surgery. Lahey and Warren4 also noted that in 1886 Maydl reported excising a carotid body tumor with division of the common, internal, and external carotid arteries. The patient developed aphasia and hemiplegia. These early attempts included ligation and resection of the carotid artery bifurcation but no attempts at reconstruction. It was not until 1889 that Albert first resected a carotid body tumor (the size of an apple) by dissecting the tumor, preserving the internal carotid artery (ICA), and ligating the external carotid artery (ECA). The tumor recurred as its original site within a year and required re-excision, as reported by Keen and Funke5 and Paltauf.6 In 1917, Lund7 described the first successful resection of bilateral carotid tumors.
By the 1960s and 1970s, several hundred cases of carotid body tumors had been reported, but the complication rates associated with surgical treatment were still high. The larger series reported mortality rates of 5% to 15% with surgical excision, cerebrovascular complications in 8% to 20% of patients, and postoperative cranial nerve injuries in 32% to 44% of patients.8 These reports, however, preceded the widespread use of intraoperative electroencephalography (EEG) and cerebral blood flow monitoring techniques to determine the usefulness of shunting during carotid cross-clamping. Today, early diagnosis with gadolinium-enhanced magnetic resonance imaging (MRI) of the neck, improved surgical and endovascular techniques, and radiotherapy adjuncts have considerably reduced the morbidity rates associated with treating these tumors.
Anatomy
The carotid body is a light-brown structure within the adventitial layer of the dorsal aspect of the CCA bifurcation. The vascular supply is from the carotid bifurcation, and the primary innervation is the carotid sensory branch of the glossopharyngeal nerve. This branch is also known as the carotid sinus nerve or the nerve of Hering.9 The carotid body is connected to the CCA bifurcation by a fibrovascular band called the ligament of Mayer. Embryologically, the carotid body may have constituents of both the third branchial arch mesoderm and neural elements of the neural crest ectoderm.10
Carotid bodies are part of a group of neuroepithelium-derived aggregates known as paraganglia. Tumors of these cells have also been known as paragangliomas, glomus tumors, and chemodectomas. Tumors of similar histology arise in several locations, including the ciliary body of the orbit, aortic-pulmonary paraganglia, carotid body (glomus caroticum), middle ear (glomus tympanicum), ganglion nodosum of the vagus nerve (glomus vagale), jugular body (glomus jugulare), and adrenal medulla (pheochromocytoma). Although most glomus tumors appear to be composed of nonchromaffin cells that are physiologically silent, some contain secretory granules that may secrete catecholamines similar to pheochromocytomas.10
Histology
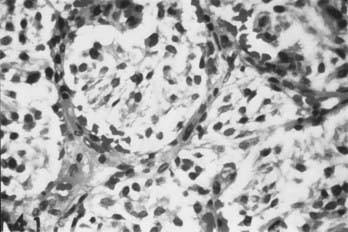
Carotid body tumors consist of epithelioid cells grouped into cords or clusters, also known as Zellballen. Typically, the cells are polyhedral with granular cytoplasm (Fig. 353-1). The stroma is composed of a fine mesh of reticulin fibers.11 Intertwined is a rich network of capillaries, giving the general appearance of a glomerulus. Several reports have detailed the chromaffin-positive nature of the secretory granules, indicating that the carotid body and tumors of it may be capable of secreting catecholamines such as norepinephrine and dopamine.12,15
The angiogenic nature of carotid body tumors is a central issue in the pathophysiology of these lesions, and ultimately it dictates many aspects of their management. Vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PD-ECGF) are expressed in carotid body tumors and may contribute to the extreme vascularity of these tumors.16
The influence of estrogens is another environmental factor that may accelerate the development of carotid body tumors. In high-altitude populations, carotid body tumors show a marked female preponderance, as high as 12 : 1,17 and even at sea level, the predominance of carotid body tumors in women is still apparent, at a ratio of 2 : 1.18 This suggests an interaction between hypoxic and estrogenic factors in the development of carotid body tumors. This interaction might occur at the level of VEGF or PD-ECGF synthesis, both of which are regulated by hypoxia as well as estrogen level.19–22
Genetics
A genetic cause of carotid body tumors is highly suggested from examples of familial occurrence. In the sporadic form, the rate of bilateral tumor occurrence is 5%. An autosomal dominant expression of bilateral tumors, however, occurs in 32% of patients with a familial occurrence.23,24 Comorbid cervical paragangliomas (glomus vagale, glomus jugulare) are relatively common in patients with carotid body tumors. Gardner and coworkers25 reviewed 11 patients with carotid body tumors, 3 of whom had other head and neck paragangliomas.
Seminal genetic studies have indicated an increased expression of the oncogenes c-myc, bcl-2, and c-jun in most carotid body tumor specimens studied.26 Wang and colleagues27 suggested that the expression of the oncoprotein bcl-2 may deregulate apoptosis, thus suppressing programmed cell death, a critical step in the tumorigenesis of carotid body tumors.
Clinical Presentation
The most common manifestation of carotid body tumors is a palpable neck mass in the high cervical region. Less common initial presentations include cranial nerve deficits such as laryngeal dysfunction (hoarseness), difficulty swallowing, and unilateral tongue atrophy or weakness. These symptoms reflect the tumor’s proximity to the vagus and hypoglossal nerves. There have also been reports of patients who became symptomatic with Horner’s syndrome28 or carotid sinus syndrome.29
There is no reported sex predominance in carotid body tumors. Typically, diagnosis is between 30 and 60 years of age. The youngest reported patient was 7 years old.30,32
Radiographic and Medical Evaluation
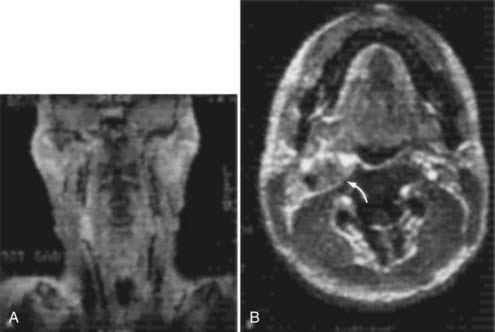
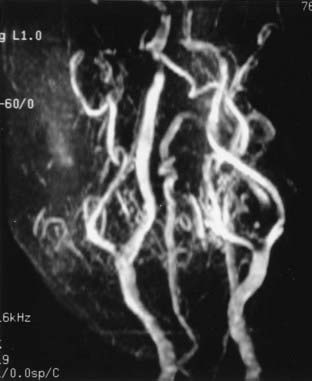
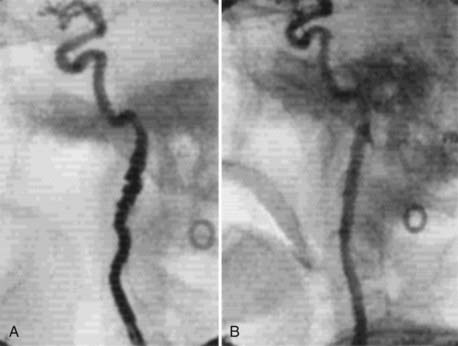
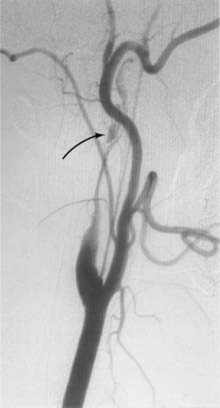
Cervical MRI with and without gadolinium can help differentiate carotid body tumors from other lesions that arise in the same region: carcinomas, enlarged lymph nodes, branchial cleft cysts, and leiomyomas.29,33 MRI demonstrates a brightly enhancing, well-circumscribed mass at the cervical carotid bifurcation (Fig. 353-2). Magnetic resonance angiography can be useful by displaying the hypervascular nature of the tumor and the characteristic splaying of the ECA and ICA (Fig. 353-3).
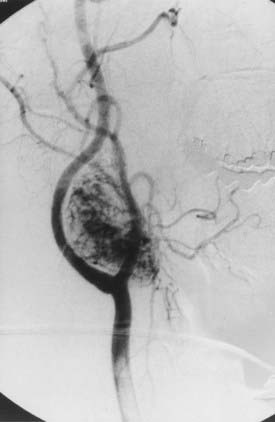
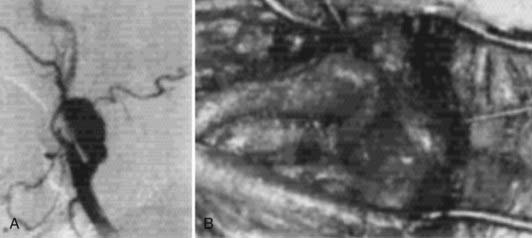
Angiography can help confirm the diagnosis. These tumors are extraordinarily vascular (Fig. 353-4). The ascending pharyngeal artery often provides most of the vascular supply, and the superior thyroid artery almost always provides a minority contribution. The blood supply of the tumor can also be derived from the ICA, vertebral artery, and thyrocervical trunk. If angiography is planned, a concomitant session to embolize the tumor can be coordinated. Studies have shown that preoperative embolization significantly reduces blood loss during resection of a carotid body tumor.34,35
An evaluation of the endocrine system may be warranted, particularly in patients who have elevated blood pressure or tachycardia. Clinicians must be aware of the potential comorbidity of a pheochromocytoma and that some carotid body tumors secrete catecholamines. In such patients, α-adrenergic blockade must be induced pharmacologically 2 weeks before surgery. Once this blockade is established, β-adrenergic blockade is recommended to control heart rate and cardiac arrhythmias.29 Careful preoperative assessment of blood pressure may indicate the need for urine metanephrine analysis.
Characteristics of Tumor Growth
In an effort to assess the preoperative risks of tumor resection, Shamblin and associates36 revised a classification scheme for carotid body tumors. Group I tumors are restricted to the space between the ICA and ECA. Generally, these tumors dissect easily from the surrounding structures. Neither the carotid vessels nor adjacent nerves travel through the tumor. Group II tumors partially involve the ECA and ICA but do not encase the arteries completely. Tumor involving the adventitia of the artery can still be dissected free. Finally, group III tumors totally encase the ICA and invade the adventitia into the muscularis. Dissecting group III tumors from the artery requires reconstruction or sacrifice of the ICA. In this challenging group, the superior laryngeal and hypoglossal nerves may traverse the tumor.
Metastasis
Most carotid body tumors are benign. They are problematic only in their local invasion of vascular and nervous structures and, occasionally, the oropharynx. Staats and coworkers31 reviewed 500 patients and found a 6.4% incidence of malignancy with local or distant metastases. Distant metastases have been reported in lymph nodes, bone, lung, liver, pancreas, thyroid, kidney, brain, and breast.37–39
Surgical Treatment
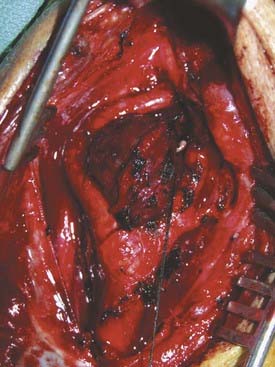
The initial incision primarily reflects the size and extent of the tumor. For example, a small group I tumor may be accessed through a transverse incision along a cervical skin crease, giving the best cosmetic result. Larger tumors with group II or III extension require a larger exposure to control the proximal and distal carotid artery for possible shunting. Here, an oblique incision along the medial border of the sternocleidomastoid muscle is indicated. As in a standard exposure for carotid endarterectomy, the common facial vein is ligated and cut to mobilize the jugular vein laterally. The hypoglossal and vagus nerves are identified. Frequently, the hypoglossal nerve may be displaced superiorly and posteriorly by the tumor8; consequently, care must be exerted during dissection of the superior aspect of the tumor to avoid damaging this nerve (Fig. 353-5). In the more challenging group III tumors, nerves may actually traverse the tumor, making dissection difficult and tedious. In rare cases, the vagus nerve can be involved with the tumor. Tumor invasion into the carotid arterial wall requires temporary occlusion with, or without shunting, to dissect the tumor and, if necessary, top patch the arterial wall defect with a graft.8
Patetsios and colleagues40 reported that in their 30 years of experience with carotid body paragangliomas (29 patients with 34 tumors), complications included two arterial thromboses (7%), five permanent cranial nerve deficits (17%), and one death (3%). There were no strokes. Vascular reconstruction was necessary in eight cases (28%).
Preoperatively, the patient’s blood pressure should be monitored closely, particularly immediately after surgery. Labile blood pressure, presumed to be secondary to baroceptor failure from loss of carotid sinus function, has been reported after resection of a carotid body tumor.41,42 Netterville and coworkers42 reviewed 30 patients with 46 carotid body tumors and found baroreceptor failure in 10 patients. Interestingly, first bite pain occurred in 10 of 25 patients assessed postoperatively.
The role of radiotherapy in managing carotid body tumors remains unclear. Radiotherapy is not used as the sole primary treatment of carotid body tumors, but it is the preferred therapy in recurrent or residual tumor with intracranial extension.8,35,43 A retrospective study by Krych and coworkers44 reported that in 33 patients with paragangliomas who were treated with external-beam radiotherapy or stereotactic radiosurgery, the 10-year tumor control rate was 92%. Radiosurgery may be useful for a metastasis or intracranial extension, however, no significant series of this relatively rare pathology has been reported to date. It has been shown that radiation therapy does not eradicate the tumor cells and probably exerts its major effects by inducing a vasculitis within tumor vessels.45
Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD) can be a unifocal or multifocal disease that manifests as arteriopathy of medium to larger arteries. In 1938, Leadbetter and Burkland46 reported the first clear description of FMD in the pathologic findings of a patient who had hypertension secondary to FMD of the renal artery. In fact, most cases of FMD in the literature involve the renal artery. In 1964, Palubinskas and Ripley47 provided the first angiographic description of FMD of the extracranial ICA in an addendum to their report of FMD in extrarenal arteries. Several reports of cephalocervical FMD followed in the 1960s and 1970s, particularly of the extracranial ICA and vertebral artery.48–53 Subsequent reported cases of intracranial artery involvement with FMD have expanded our understanding of the disease process.54,55 Two major institutional reviews of cerebral angiography, one reviewing 6100 angiograms, the other 13,955, found 0.53% and 0.6% incidences of FMD, respectively.56,57
Histologic studies indicate that the arterial dysplasias present as three primary pathologies: intimal fibroplasia, medial fibroplasias, and subadventitial hyperplasia.58 In medial fibroplasias, also known as fibromuscular hyperplasia, the expression of the disease ranges from fibrodysplasia limited to the outer media to complete involvement of the entire media. Collagen and ground substance accumulate in the inner media in the milder form of the disease, separating in the disorganized smooth muscle cells. In the severe form, the media is profoundly disorganized, with fibroblasts and collagen replacing the smooth muscle.59
Etiology
The exact cause of FMD is becoming better defined as we begin to understand the multifactorial pathology, with a spectrum of phenotypic expressions. Its prevalence in women is much higher than in men, with an approximate 9 : 1 ratio. Stanley and colleagues59 suggested that progestin and estrogen play a role in the development of the disease because 94% of the patients they reviewed were women. Laboratory studies have demonstrated that sex hormones may contribute to the formation of FMD directly by local alterations of the vessel wall (endothelial proliferation, intimal thickening, and focal nodular thickening of the intima, media, and adventitia) or indirectly through an immune response.60,63 However, in a case-control study of 33 patients, endogenous or exogenous sex hormones did not appear to play a role in the etiology of FMD, whereas cigarette smoking and genetics did appear to be linked to the development of FMD.64 Mettinger’s familial analysis of patients with FMD suggests that is an inheritable dominant trait with limited penetrance in men.65,66
Mechanical and anatomic features have also been evaluated in the development of FMD. Stanley and colleagues59 implied that mechanical stresses on the vessel walls play a role in the development of FMD. Likewise, the disease appears to involve primarily arteries with a paucity of vasa vasorum, indicating that arterial wall ischemia may contribute to disease progression. Long-term follow-up of some cases has indicated definite progression of the disease in some patients with carotid FMD.52
Clinical Presentation
Patients with cervical carotid FMD typically present in their 50s to 60s. On physical examination, about two thirds of symptomatic patients have a cervical bruit that is audible on ausculation.56 Symptoms of cerebral ischemia have been reported in 18% to 56% of patients.67 In such cases, the sequential arrangement of fibromuscular rings reduces blood flow while increasing turbulence within the vessel. In addition to ischemic symptoms, FMD has been associated with spontaneous carotid artery dissection,68,69 aneurysm formation,70,71 carotid-cavernous fistula,72,73 and thromboembolism.56 In Mettinger’s review of 284 patients with aortocranial FMD, a surprising 21% had associated intracranial aneurysms.66
Angiographic Findings
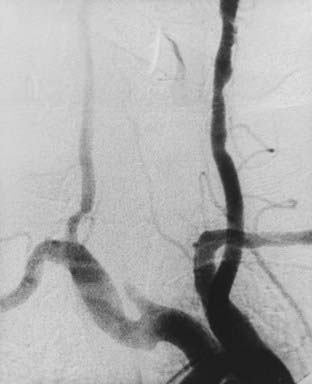
In 1977, Osborn and Anderson58 described the angiographic appearance of carotid FMD variants in a summary report. The classic angiographic appearance, present in the more than 80% of patients, is the “string of beads”—multiple, irregularly spaced arterial constrictions with normal or ectatic intervening segments. Angiographically, it is similar to stationary arterial waves or circular spastic contractions, although these show more regularly spaced constrictions with no associated dilated segments. The second variant is that of smooth, concentric tubular stenosis. Angiographically, this variant can be distinguished from Takayasu’s arteritis, which affects the proximal segments of the aortic branches. In contrast, FMD typically spares the origins. The third variant, termed atypical FMD by Houser and Baker,48 usually involves one wall of the affected segment, creating a smooth or corrugated outpouching of the vessel.
Treatment
A report by DeBakey’s group in 1968 described the surgical luminal dilation of the ICA in 12 patients and of the vertebral artery in 1 patient.74 This report is interesting from a historical perspective, but it also documents intraluminal pressure at the proximal and distal ends of the affected segments in 2 patients. Before dilation, they found pressure before 25 and 50 mm Hg; after surgical treatment, there were no discernible gradients. These findings demonstrated that multiple tandem rings, as seen in FMD, significantly reduce arterial pressure and blood flow, although individual stenotic rings do not narrow the carotid artery significantly.
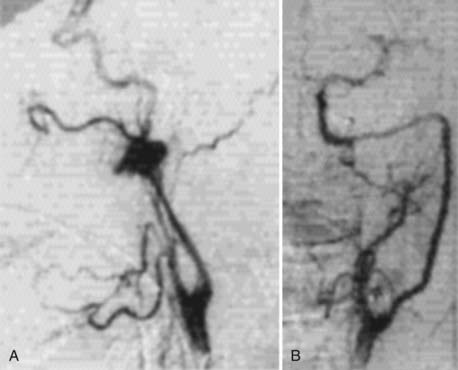
Contemporary treatment has used the endovascular techniques of balloon angioplasty and stenting for medically refractory cervical FMD lesions (Fig. 353-6). In the early 1980s, several reports documented the successful treatment of FMD by percutaneous balloon angioplasty.75,79 By the mid-1980s, the limitations of this technology were revealed in reports of complications of percutaneous transluminal angioplasty of FMD, including dissection80 and aneurysm formation.81 The transfemoral access route has significantly reduced the morbidity of endovascular procedures. Subsequently, endoluminal stenting, performed in coordination with angioplasty for cervical FMD, has become increasingly more prominent as the primary treatment method in medically refractory cases.
Extracranical Carotid Artery Aneurysms
Although aneurysms of the extracranial carotid artery were first described in 1687,82 they are exceedingly rare. Interestingly, successful surgical treatment of an extracranial carotid aneurysm was reported in 1836 by Cooper, who used hunterian ligation of the proximal CCA.83
Early treatment, which was effective to a degree, consisted of proximal ligation but no attempt at resecting the aneurysm, reestablishing circulation, or bypassing the diseased segment. However, Winslow’s84 1926 review of a series of patients with extracranial ICA aneurysms stressed the importance of treatment. He reported a 71% mortality rate related to the aneurysm in untreated patients, compared with a 30% mortality in patients who underwent hunterian ligation.
Treatment of these aneurysms evolved to attempts to preserve the cerebral circulation and to avoid hunterian ligation. In 1952, Dimtza85 first successfully excised an extracranial carotid aneurysm and reanastomosed the ICA to reestablish circulation. In the ensuing years, it became clear that operating on aneurysms in this location held just a slightly higher risk than a standard carotid endarterectomy when proper vascular techniques were used.86
Clinical Presentation
The clinical symptoms associated with extracranial carotid aneurysms vary according to their location and size. Large aneurysms usually present as pulsatile cervical or parapharyngeal masses that may or may not be tender.87 Rarely, patients become symptomatic with hemorrhage, which may result in a neck hematoma or epistaxis, depending on the location of the aneurysm. Some patients may present with transient ischemic attacks (TIAs) related to emboli produced by the turbulent blood flow associated with a larger, tortuous aneurysm.88,89
These aneurysms may be caused by trauma, infection, or iatrogenic causes such as previous carotid surgery. Most true aneurysms of the cervical carotid artery, however, are caused by atherosclerotic disease of the artery.90 There have been reports of extracranial or skull base carotid aneurysms in which the cause was related to genetic vascular diseases, such as Marfan’s syndrome or progeria, but these are the minority of cases.91,92
The treatment of the extracranial carotid artery aneurysms depends on the size, shape, cause, and location of the aneurysm. The primary goals of treatment should be exclusion of the aneurysm and preservation of adequate cerebral blood flow. Therefore, the hunterian ligation employed by Cooper for this pathology is no longer applicable to most cases. The results of small contemporary operative series involving preservation of the carotid artery have been good. Painter and coworkers93 reviewed the literature on extracranial carotid aneurysms treated surgically over a 10-year period by methods other than ligation (e.g., segmental resection aneurysmorrhaphy). Of the 61 operative cases they found, three patients (5%) had permanent neurological deficits from the surgery, and one died (1.6%). In the Serbian Multicentric Study of 91 extracranial carotid artery aneurysms treated by aneurysmectomy with arterial reconstruction, there were five patients (5.5%) who had postoperative strokes (two of the stroke patients died postoperatively) and two patients (2.2%) who had transient cranial nerve injuries. Three patients died more than 5 years after the operation from myocardial infarction.94
If an aneurysm is in the low to mid cervical region, it can be approached directly. Even fusiform aneurysms (Fig. 353-7) can be excised surgically, with primary reanastomosis of the proximal and distal artery. When primary reanastomosis is not feasible, a Dacron or saphenous vein patch graft can be used to reconstruct the carotid artery. Temporary clipping of the CCA or ICA is necessary during the procedure. Therefore, intraoperative EEG monitoring, pentobarbital burst suppression, and intravenous heparin are recommended. Should the EEG change during the procedure, intraoperative shunting may be necessary, as in a carotid endarterectomy procedure.
In aneurysms of the high cervical ICA, a direct approach is not feasible without extensive dissection of the skull base or upper neck. Coffin and associates95 described 14 patients with high cervical carotid aneurysms resulting from spontaneous dissection, blunt trauma, FMD, or atheroma. With all aneurysms extending to the C1 level or higher, they were able to revascularize 12 arteries and ligate 3 (one after an extracranial-intracranial bypass). Overall, their results were good. After surgery, only one patient had a TIA, and four patients had lower facial nerve palsies (from the extended cervical approach). In such cases, trapping the diseased segment and performing a high-flow vein bypass constitute the preferred treatment (Fig. 353-8). Aneurysms of the high-cervical segment may be related to parapharyngeal infection or iatrogenic trauma. In such cases, the field is contaminated, and primary direct repair of the carotid artery may not be feasible. Likewise, endovascular stents may not be indicated because placing a foreign body, such as a stent or stent graft, in an infected region may result in chronic implant infection. Carotid occlusion is a reasonable alternative if the patient passes a balloon test occlusion. If the patient fails balloon test occlusion, bypass occlusion with carotid occlusion is the preferred long-term strategy.
If the affected site is not infected, an endoluminal stent may be considered. One advantage of a stent is preservation of the ICA. It may be particularly useful in the high cervical region, which is difficult to access surgically. Likewise, if the contralateral carotid artery is diseased, preservation of the ipsilateral carotid artery may be preferable to relying on bypass patency. Hurst and colleagues96 reported a few cervical ICA aneurysms treated with stents alone; the aneurysms were thrombosed, and the parent artery was preserved.
Spontaneous Carotid Artery Dissection
The first case of spontaneous dissection of the ICA was reported by Jentzer97 in 1954. Spontaneous carotid dissections have been reported in association with arteriopathies secondary to FMD, cystic medial necrosis, and Marfan’s syndrome, but the cause of most cases is unknown. Likewise, a few familial cases have been reported, but they are rare.98–100
Clinical Presentation
Typically, spontaneous dissection of the cervical carotid artery manifests with symptoms of headache and TIA or stroke. Meissner and Mokri101 reviewed 80 patients with spontaneous ICA dissection. Headache was the most common symptom, occurring in 83% of patients. The headache manifested as frontal or periorbital pain in some and as periauricular pain in others. Strangely, neck pain was comparatively infrequent, occurring in 21% of patients. Focal cerebral ischemic symptoms, manifesting as TIAs, strokes, or both, occurred in 60% of these patients, and atypical Horner’s syndrome was seen in half the patients.
It is yet to be established whether males or females are more susceptible to spontaneous carotid artery dissections. However, one study analyzing gender differences in 696 patients with spontaneous carotid artery dissections reported a higher incidence in men (n = 399; P < .0001). Women with carotid artery dissections were younger and had a higher incidence of multiple dissections. Outcome and mortality were similar in both sexes.102 The age of onset is typically younger than 50 years, although younger individuals have been affected. In contrast, stroke tends to affect elderly patients more frequently.
Preliminary data suggest that a deficiency in type III collagen may predispose patients to spontaneous carotid dissection by compromising the integrity of the arterial wall. Van den Berg and colleagues103 performed protein analysis of type I and type II collagen in 16 patients with spontaneous cervical arterial dissections and in 41 healthy controls. Although they found no type III collagen gene mutations by single-stranded conformation polymorphism-heteroduplex analysis in patients with dissections, a few patients had low type III–to–type I collagen ratio.
Recent studies have shown that nutritional status may also be a risk factor for carotid artery dissections. Specifically, three separate studies, each with at least 30 patients, have reported an increased incidence of spontaneous carotid artery dissections in patients who have mild hyperhomocysteinemia and low folate levels.104–106 Mutations of the methylenetetrahydrofolate reductase (MTHFR) gene (the MTHFR TT genotype), which result in elevated homocysteine levels, demonstrated a significant association with spontaneous carotid artery dissections.106
Additionally, an underlying extracellular matrix defect is suspected in patients with spontaneous carotid artery dissections. These patients have higher plasma levels of proteases, particularly matrix metalloproteinase 2. The association becomes stronger in patients with multiple dissections.107
Finally, this patient population may also have a functional abnormality of the carotid wall, which may predispose them to dissections. In a case-control study using color duplex sonography, Baumgartner and colleagues108 demonstrated that spontaneous and endothelial-independent (nitroglycerin) vasodilation is impaired in patients with spontaneous carotid dissection. Vasodilation abnormalities may be a predisposing factor for dissections.
The physical examination of patients with spontaneous carotid dissection should accurately assess neurological deficits from any presenting or evolving TIA or stroke. Interestingly, the oculosympathetic palsy caused by an ICA dissection is not associated with the classic triad of Horner’s syndrome (i.e., ptosis, miosis, and anhidrosis). Typically, these patients have only ptosis and miosis. Facial sweating is seldom affected, a pattern that reflects the anatomy of the sympathetic fibers. The sympathetic fibers leading to the eyes travel along the ICA and are affected in dissection. In contrast, the sympathetic fibers leading to the facial sweat glands travel with the ECA and are seldom affected.100
A thorough examination should assess for the presence of a carotid bruit, which was present in more than 40% of Meissner and Mokri’s patients.101 Patients rarely become symptomatic, and lower cranial nerve examination is warranted before any treatment is instituted.
A few authors have recommended following patients with ICA dissection by measuring their retinal artery pressure with oculopneumoplethysmograhpy.100 Increasing retinal artery pressures and the resolution of the luminal stenosis related to arterial dissection are reliably correlated.
Angiographic Findings
The classic description of carotid arterial dissection is a long, tapered narrowing of the ICA just distal to the carotid bulb, also known as the string sign.109 Dissecting aneurysms also occur in the affected segment (Fig. 353-9). Of course, complete occlusion of the ICA is also possible with these lesions. In patients with complete occlusions from spontaneous dissections who were treated with anticoagulation therapy, the long-term recanalization rate was 47% in the report by Pozzati and coworkers110,111 and 43% in that by Bogousslavsky and associates.112
Treatment
An endovascular option is available even in patients with high cervical dissections. Angioplasty and stenting remain viable options, even as primary treatment considerations. In complex cases or dissections resulting in a double lumen, the difficulty lies in identifying which is the true lumen and which is the false one. Provided the stent is well apposed to the lumen wall, small pseudoaneurysms or vessel irregularities outside the stent typically thrombose with time, even when patients are receiving antiplatelet medical therapy. In a series of 26 consecutive patients with carotid dissection treated with angioplasty and stenting, Kadkhodayan and coworkers113 reported that dissection-induced stenosis was reduced from 71% ± 18% to no significant stenosis in 95% of patients. The procedural TIA rate was 3 of 29 procedures (10.3%), whereas there were no procedural strokes. The 30-day occlusion and death rate was 2 of 29 procedures (6.9%).
Radiation Stenosis
Radiation is commonly used to treat many vascular pathologies, including arteriovenous malformations, arteriovenous fistulas, cavernous malformations, and vascular tumors. In some cases, however, radiation to the normal artery can induce pathology.114–117 Histologic studies have demonstrated that radiation has variable effects, depending on the arterial size and radiation dose.118
Not long after the discovery of x-rays by Roentgen, the adverse effects of radiation became known. In 1899, Gassman described histologically the small vessel endothelial proliferation associated with radiodermatitis.119 In 1909, Wolbach120 noted that this endothelial proliferation also caused obliteration or thrombosis of the capillary lumina. In 1944, Sheehan121 reported a more detailed study in which he described the development of plaque-like thickenings in the intima of small arteries secondary to the accumulation of foam cells, hyaline, and fibrin in the endothelial lining and internal elastic membrane. Working with a radiation injury model in the carotid artery of rabbits, Konings and coworkers122 determined that endothelial cells damaged by irradiation do not function properly as a barrier to plasma lipoprotein infiltration in the arterial wall. This dysfunction leads to lysosomal activation and cellular proliferation within the vessel wall, resulting in intimal plaque formation.
With the expanded use of radiation in the treatment of neck and mediastinal malignancies, reports of radiation changes in larger arteries became more prevalent. In 1940, Cade123 briefly mentioned a case of presumed radiation injury to the aorta of a patient treated for esophageal cancer. Thomas and Forbus,124 however, are credited with the first clinical report of a patient with radiation injury in a large artery. In 1959, they detailed the course of a 19-year-old patient who underwent radiotherapy for mediastinal lymphoma.
Clinical Evaluation
Imaging studies are indicated for patients who developed symptoms of cerebral ischemia or emboli after radiotherapy to the head, neck, or mediastinum. Although radiation changes in large arteries look very similar to the atherosclerotic changes seen in the carotid bulb in atherosclerotic vaso-occlusive disease, the spectrum of radiation stenosis is more varied. First, the arterial changes associated with radiation stenosis can occur in regions typically unaffected by atherosclerosis, such as the CCA.125 Second, the surrounding tissues in radiation stenosis can be severely affected, requiring myocutaneous flaps after surgical intervention. Third, some authors speculate that the carotid arterial wall is damaged, making carotid endarterectomy in these patients more dangerous than a standard carotid endarterectomy.126 A carotid duplex scan should give sufficient information about most of the cervical carotid artery, but it is a poor choice for the evaluation of the proximal CCA and the distal cervical ICA. Magnetic resonance angiography of the arteries can give a more complete view of the arterial origins in high cervical segments127 but may not be obtainable in some patients. Cervical and cerebral angiography remains the “gold standard” for evaluating potential radiation stenosis. Figure 353-10 shows an aortic arch angiogram of a 46-year-old woman with a history of radiation for lymphoma who presented with a right hemisphere stroke. The angiogram demonstrated an occlusion of the right CCA, near the origin, in addition to an occlusion of the left vertebral artery. The figure shows the type of changes seen in radiation-associated atherosclerosis over a diffuse segment of the left CCA.
Treatment
Some authors have reported that it is more difficult to dissect plaques caused by radiation stenosis than to dissect atherosclerotic plaques because the former are more adherent to the intima.126 Patients with radiation-associated carotid atherosclerosis were previously thought to be unacceptable candidates for surgical carotid endarterectomy (CEA), but their surgical risks are actually comparable to those of patients undergoing standard CEA. In 1999, Kashyap and associates128 reported 24 patients who received a mean dose of 6300 cGy and who underwent 26 carotid artery operations. In this series, 58% of patients had cerebral or monocular TIAs, 27% had asymptomatic high-grade stenosis, 12% had prior stroke, and 4% had tumor invasion in the carotid artery. Two thirds of the patients had severe scarring or fibrosis of the skin of the neck, and 4 patients had permanent tracheostomies. Complications were limited to two restenoses, two wound infections, and four cranial nerve palsies. Based on carotid artery backpressure or EEG monitoring, selective intraoperative shunting was performed in 31% of the cases. A patch graft arterial repair was necessary in 79% of the surgically treated patients.
Endovascular treatment is also a viable option in the treatment of radiation stenosis of the carotid arteries. Because of concerns associated with soft tissue fibrosis, operating through an area of a previous myocutaneous flap, and more proximal arterial involvement, endovascular angioplasty stenting has emerged as the primary treatment for radiation stenosis. The long-term efficacy of stenting in this setting has yet to be analyzed, but the short-term results are promising.129,130 Kim and coworkers131 reported that in 16 patients with a total of 19 carotid arteries treated with angioplasty and stenting, the procedural stroke rate was 1 of 23 (4%) and the procedural TIA rate was 0 of 23 (0%). The 30-day postprocedure complication rate was 0 of 23 (0%), and no new neurological symptoms were reported. With a mean follow-up time of 28 months (range, 5 to 78 months), 15 of the 19 vessels (79%) developed no new stenosis, 2 of 19 (11%) had repeat angioplasty and stent placement, and 1 of 19 (5%) had a repeat angioplasty.
Finally, preliminary results from controlled, prospective randomized trials of carotid angioplasty and stenting in high-surgical-risk patients appear promising. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial has suggested that angioplasty and stenting with a cerebral protection device, when compared with CEA, is associated with a comparable or lower incidence of 30-day adverse clinical events, defined as a combined end point of stroke, death, and myocardial infarction. Only patients at high risk were enrolled in the SAPPHIRE trial, which included patients with radiation stenosis.132 Further subgroup analysis in these patients is warranted.
Bogousslavsky J, Despland PA, Regli F. Spontaneous carotid dissection with acute stroke. Arch Neurol. 1987;44:137-140.
Corrin LS, Sandok BA, Houser OW. Cerebral ischemic events in patients with carotid artery fibromuscular dysplasia. Arch Neurol. 1981;38:616-618.
Gardner P, Dasling M, Weisberger E, et al. Carotid body tumors, inheritance, and a high incidence of associated cervical paragangliomas. Am J Surg. 1996;172:196-199.
Hurst RW, Haskal ZJ, Zager E, et al. Endovascular stent treatment of cervical internal carotid artery aneurysms with parent vessel preservation. Surg Neurol. 1998;50:313-317.
Kadkhodayan Y, Jeck DT, Moran CJ, et al. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. Am J Neuroradiol. 2005;26:2328-2335.
Kim PH, Kadkhodayan Y, Derdeyn CP, et al. Outcomes of carotid angioplasty and stenting for radiation-associated stenosis. Am J Neuroradiol. 2005;26:1781-1788.
Krych AJ, Foote RL, Brown PD, et al. Long-term results of irradiation for paragangliomas. Int J Radiation Oncology Biol Phys. 2006;65:1063-1066.
Leonetti JP, Donzelli JJ, Littooy FN, et al. Perioperative strategies in the management of carotid body tumors. Otolaryngol Head Neck Surg. 1997;117:111-115.
Litle VR, Reilly LM, Ramos TL. Preoperative embolization of carotid body tumors: when is it appropriate? Ann Vasc Surg. 1996;10:464-468.
Mettinger KL. Fibromuscular dysplasia and the brain. II. Current concept of the disease. Stroke. 1982;13:53-58.
Meyer FB, Sundt TMJr, Pearson BW. Carotid body tumors: a subject review and suggested surgical approach. J Neurosurg. 1986;64:377-385.
Mokri B, Piepgras DG, Sundt TMJr, et al. Extracranial internal carotid artery aneurysms. Mayo Clin Proc. 1982;57:310-321.
Mokri B, Sundt TMJr, Jouser OW, et al. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol. 1986;19:126-138.
Osborn AG, Anderson RE. Angiographic spectrum of cervical and intracranial fibromuscular dysplasia. Stroke. 1977;8:617-626.
Pollock M, Jackson BM. Fibromuscular dysplasia of the carotid arteries. Neurology. 1971;21:1226-1230.
Pryse-Davies J, Dawson IM. Some morphological histochemical, and chemical observations on chemodectomas and the normal carotid body, including a study of the chromaffin reaction and possible ganglion cell elements. Cancer. 1964;17:185-202.
Radak D, Davidovic L, Vukobratov V, et al. Carotid artery aneurysms: Serbian multicentric study. Ann Vasc Surg. 2007;21:23-29.
Sandok BA, Houser OW, Baker WL. Fibromuscular dysplasia: neurological disorders associated with disease involving the great vessels in the neck. Arch Neurol. 1971;24:462.
Shamblin WR, ReMine WH, Sheps SG, et al. Carotid body tumor (chemodectoma): clinicopathologic analysis of ninety cases. Ann J Surg. 1971;122:732-739.
Teitelbaum GP, Lefkowitz MA, Giannota SL. Carotid angioplasty and stenting in high-risk patients. Surg Neurol. 1998;50:300-311.
1 von Haller A. Elementa physiologia corporis humani. 1743;iv:1766.
2 Balfour DC, Wildner F. The intercarotid paraganglion and its tumors. Surg Gynecol Obstet. 1914;18:203-213.
3 Kohn A. Die paraganglien. Arch Miker Anat. 1903;62:263-265.
4 Lahey FH, Warren KW. A long term appraisal of carotid body tumors with remarks on their removal. Surg Gynecol Obstet. 1941;85:281-288.
5 Keen WW, Funke J. Tumors of the carotid gland. JAMA. 1906;47:469-479.
6 Paltauf. Ziegler’s Beitr z path Anat u alleg Path, xi. 1892.
7 Lund FB. Tumors of the carotid body. JAMA. 1917;69:348-352.
8 Meyer FB, Sundt TMJr, Pearson BW. Carotid body tumors: a subject review and suggested surgical approach. J Neurosurg. 1986;64:377-385.
9 Williams PL, Warwick R, Dyson M, et al. Gray’s Anatomy, 37th ed. London: Churchill, Livingstone; 1989.
10 Pryse-Davies J, Dawson IM. Some morphological histochemical, and chemical observations on chemodectomas and the normal carotid body, including a study of the chromaffin reaction and possible ganglion cell elements. Cancer. 1964;17:185-202.
11 Byrne JJ. Carotid body and allied tumors. Am J Surg. 1958;95:371-384.
12 Rios P, Russo F, Paganelli C, et al. Carotid chemodectoma: a clinical case report and review of the literature [Italian]. G Chir. 1995;15:21-28.
13 Ikejiri K, Muramori K, Takeo S, et al. Functional carotid body tumor: report of a case and review of the literature. Surgery. 1996;119:222-225.
14 Hirano S, Shoji K, Kojima H, et al. Dopamine-secreting carotid body tumor. Am J Otolaryngol. 1998;19:412-416.
15 Levit SA, Sheps SG, Espinosa RE, et al. Catecholamine-secreting paraganglioma of glomus-jugulare region resembling pheochromocytoma. N Engl J Med. 1969;281:805-811.
16 Jyung RW, LeClair EE, Bernat RA, et al. Expression of angiogenic growth factors in paragangliomas. Laryngoscope. 2000;110:161-167.
17 Saldana MJ, Salem LE, Travezan R. High altitude hypoxia and chemodectomas. Hum Pathol. 1973;4:251-263.
18 Parry DM, Li FP, Strong LC, et al. Carotid body tumors in humans: genetics and epidemiology. J Nat Cancer Inst. 1982;68:573-578.
19 Griffiths L, Dachs GU, Bicknell R, et al. The influence of oxygen tension and pH on the expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human breast tumor cells grown in vitro and in vivo. Cancer Res. 1997;57:570-572.
20 Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845.
21 Shweiki D, Itin A, Neufeld G, et al. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993;91:2235-2243.
22 Osuga Y, Toyoshima H, Mitsuhashi N, et al. The presence of platelet-derived endothelial cell growth factor in human endometrium and its characteristic expression during the menstrual cycle and early gestational period. Hum Reprod. 1995;10:989-993.
23 Grufferman S, Gillman MW, Pasternak LR, et al. Familial carotid body tumors. Case report and epidemiologic review. Cancer. 1980;46:2116-2122.
24 Rush BFJr. Familial bilateral carotid body tumors. Ann Surg. 1963;157:633-636.
25 Gardner P, Dasling M, Weisberger E, et al. Carotid body tumors, inheritance, and a high incidence of associated cervical paragangliomas. Am J Surg. 1996;172:196-199.
26 Wang DG, Barros D’Sa AA, Johnston CF, et al. Oncogene expression in carotid body tumors. Cancer. 1996;77:2581-2587.
27 Wang DG, Johnston C, Barros D’Sa AA, et al. Expression of apoptosis-suppressing gene bcl-2 in human carotid body tumors. J Pathol. 1997;183:218-221.
28 Sankar NM, Munene J, Arumugam SB, et al. Benign carotid body tumor presenting with Horner’s syndrome: a case report. Tex Heart Inst J. 1996;23:180-182.
29 Sampson JH, Wilkins RH. Paragangliomas of the carotid body and temporal bone. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1996:1559-1571.
30 Fletcher WE, Arnold JH. Carotid body tumor: a review of the literature and report of an unusual case. Am J Surg. 1954;87:617-623.
31 Staats EF, Brown RL, Smith RR. Carotid body tumors, benign and malignant. Laryngoscope. 1966;76:907-916.
32 Harrington SW, Clagett OT, Dockerty MD. Tumors of the carotid body. Clinical and pathological considerations of twenty tumors affecting nineteen patients (one bilateral). Ann Surg. 1941;114:820-833.
33 Reiner SA, Medina J, Minn KW. Vascular leiomyoma of the carotid sheath stimulating a carotid body tumor. Am J Otolaryngol. 1998;10:127-129.
34 Litle VR, Reilly LM, Ramos TL. Preoperative embolization of carotid body tumors: when is it appropriate? Ann Vasc Surg. 1996;10:464-468.
35 Liapis C, Gougoulakis A, Karydakis V, et al. Changing trends in management of carotid body tumors. Am Surg. 1995;61:989-993.
36 Shamblin WR, ReMine WH, Sheps SG, et al. Carotid body tumor (chemodectoma): clinicopathologic analysis of ninety cases. Ann J Surg. 1971;122:732-739.
37 Romanski R. Chemodectoma (non-chromaffinic paraganglioma) of the carotid body with distant metastases. Am J Pathol. 1954;3:1-9.
38 Rangwala AF, Sylvia LC, Becker SM. Soft tissue metastasis of a chemodectoma: a case report and review of the literature. Cancer. 1978;42:2865-2869.
39 Kawai A, Healey JH, Wilson SC, et al. Carotid body paraganglioma metastatic to bone. Report of two cases. Skeletal Radiol. 1998;27:103-107.
40 Patetsios P, Gable DR, Garret WV, et al. Management of carotid body paragangliomas and review of a 30-year experience. Ann Vasc Surg. 2002;16:331-338.
41 Boyle JR, London NJ, Tan SG, et al. Labile blood pressure after bilateral carotid body tumor surgery. Eur J Vasc Endovasc Surg. 1995;9:346-348.
42 Neterville JL, Reilly KM, Robertson D, et al. Carotid body tumors: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105:115-126.
43 Leonetti JP, Donzelli JJ, Littooy FN, et al. Perioperative strategies in the management of carotid body tumors. Otolaryngol Head Neck Surg. 1997;117:111-115.
44 Krych AJ, Foote RL, Brown PD, et al. Long-term results of irradiation for paragangliomas. Int J Radiation Oncology Biol Phys. 2006;65:1063-1066.
45 Spector GJ, Compagno J, Perez CA, et al. Glomus jugulare tumors: effects of radiotherapy. Cancer. 1975;35:1316-1321.
46 Leadbetter WF, Burkland CE. Hypertension in unilateral renal disease. J Urol. 1938;39:61-626.
47 Palubinskas AJ, Ripley HR. Fibromuscular hyperplasia in extrarenal arteries. Radiology. 1964;82:451-455.
48 Houser OW, Baker HLJr. Fibromuscular dysplasia and other uncommon diseases of the cervical carotid artery. Angiographic aspects. Am J Roentgenol Radium Ther Nucl Med. 1968;104:201-212.
49 Bergan JJ, MacDonald JR. Recognition of cerebrovascular fibromuscular hyperplasia. Arch Surg. 1969;98:332-335.
50 Momose KJ, New PF. Non-atheromatous stenosis and occlusion of the internal carotid artery and its main branches. AJR Am J Roentgenol. 1973;118:550-556.
51 Pollock M, Jackson BM. Fibromuscular dysplasia of the carotid arteries. Neurology. 1971;21:1226-1230.
52 Galligioni F, Iraci G, Martin G. Fibromuscular hyperplasia of the extracranial internal carotid artery. J Neurosurg. 1971;34:647-651.
53 Sandok BA, Houser OW, Baker WL. Fibromuscular dysplasia: neurological disorders associated with disease involving the great vessels in the neck. Arch Neurol. 1971;24:462.
54 Frens DB, Petajan JH, Anderson R, et al. Fibromuscular dysplasia of the posterior cerebral artery: report of a case and review of the literature. Stroke. 1974;5:161-166.
55 Iosue A, Kier EL, Ostrow D. Fibromuscular dysplasia involving the intracranial vessels. Case report. J Neurosurg. 1972;37:749-752.
56 So EL, Toole JF, Moody DM, et al. Cerebral embolism from septal fibromuscular dysplasia of the common carotid artery. Ann Neurol. 1979;6:75-78.
57 Corrin LS, Sandok BA, Houser OW. Cerebral ischemic events in patients with carotid artery fibromuscular dysplasia. Arch Neurol. 1981;38:616-618.
58 Osborn AG, Anderson RE. Angiographic spectrum of cervical and intracranial fibromuscular dysplasia. Stroke. 1977;8:617-626.
59 Stanley JC, Gewertz BL, Bove EL, et al. Arterial dysplasia: histopathologic character and current etiologic concepts. Arch Surg. 1975;110:561-566.
60 Irey NS, Norris HJ. Intimal vascular lesions associated with female reproductive steroids. Arch Pathol. 1973;96:227-298.
61 Irey NS, Manion WC, Taylor HB. Vascular lesions in women taking oral contraceptives. Arch Pathol. 1970;89:1-8.
62 Vessey MP. Is the pill safe enough to continue using? N Engl J Med. 1978;238:906.
63 Grossman CJ. Interactions between gonadal steroids and the immune system. Science. 1985;227:257-261.
64 Sang CN, Whelton PK, Hamper UM, et al. Etiological factors in renovascular fibromuscular dysplasia. Hypertension. 1989;14:472-479.
65 Mettinger KL, Ericson K. Fibromuscular dysplasia and the brain. I. Observations on angiographic, clinical and genetic characteristics. Stroke. 1986;13:46-52.
66 Mettinger KL. Fibromuscular dysplasia and the brain. II. Current concept of the disease. Stroke. 1982;13:53-58.
67 Hopkins LN, Budny JL. Fibromuscular dysplasia. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw Hill; 1996:2169-2172.
68 Andersen CA, Collins GJJr, Rich NM, et al. Spontaneous dissection of the internal carotid artery associated with fibromuscular dysplasia. Am Surg. 1980;46:263-266.
69 Ringel SP, Harrison SH, Norenberg MD, et al. Fibromuscular dysplasia: multiple “spontaneous” dissecting aneurysms of the major cervical arteries. Ann Neurol. 1977;1:301-304.
70 Ehrenfeld WK, Wylie EJ. Fibromuscular hyperplasia of the internal carotid artery. Arch Surg. 1974;109:676-681.
71 Bergentz SE, Ericsson BF, Linell F, et al. Bilateral fibromuscular hyperplasia of the internal carotid arteries with aneurysm formation. Acta Chir Scand. 1976;142:501.
72 Zimmerman R, Leeds NE, Naidich TP. Carotid-cavernous fistula associated with intracranial fibromuscular dysplasia. Radiology. 1977;122:725-726.
73 Laufman HH, Lind TA, Mullan S. Spontaneous carotid cavernous fistula with fibromuscular dysplasia. Acta Neurochir (Wien). 1978;40:123-129.
74 Morris GCJr, Lechter A, DeBakey ME. Surgical treatment of fibromuscular disease of the carotid arteries. Arch Surg. 1968;96:636-643.
75 Garrido E, Montoya J. Transluminal dilatation of internal carotid artery in fibromuscular dysplasia: a preliminary report. Surg Neurol. 1981;16:469-571.
76 Hasso AN, Bird CR, Zinke DE, et al. Fibromuscular dysplasia of the internal carotid artery: percutaneous transluminal angioplasty. AJR Am J Roentgenol. 1981;136:955-960.
77 Dublin AB, Baltaxe HA, Cobb CA3rd. Percutaneous transluminal carotid angioplasty in fibromuscular dysplasia: case report. J Neurosurg. 1983;59:162-165.
78 Starr DM, Lawrie GM, Morris GCJr. Fibromuscular disease of carotid arteries: long term results of graduated internal dilatation. Stroke. 1981;12:196-199.
79 Wilms GE, Smits J, Bart AL, et al. Percutaneous transluminal angioplasty in fibromuscular dysplasia of the internal carotid artery: one year clinical and morphological follow-up. Cardiovasc Intervent Radiol. 1985;8:20-23.
80 Jooma R, Bradshaw JR, Griffith HB. Intimal dissection following percutaneous transluminal carotid angioplasty for fibromuscular dysplasia. Neuroradiology. 1985;27:181-182.
81 Lord RSA, Graham AR Benn IV. Radiologic control of operative carotid dilatation. Aneurysm formation following balloon dilation. J Cardiovasc Surg (Torino). 1986;27:158-162.
82 Mokri B, Piepgras DG, Sundt TMJr, et al. Extracranial internal carotid artery aneurysms. Mayo Clin Proc. 1982;57:310-321.
83 Cooper A. Account of the first successful operation performed on the common carotid artery for aneurysm. Guys Hosp Rep. 1836;1:53-59.
84 Winslow N. Extracranial aneurysm of the internal carotid artery: history and analysis of the cases registered up to August 1, 1925. Arch Surg. 1926;13:689-729.
85 Dimtza A. Aneurysms of the carotid arteries: report of two cases. Angiology. 1956;7:218-227.
86 Taylor SP, Langan EM3rd, Snyder BA, et al. Nonendarterectomy procedures of the carotid artery. A five year review. Am Surg. 1999;65:323-327.
87 Ekestrom S, Bergdahl L, Huttunen H. Extracranial carotid and vertebral artery aneurysms. Scand J Thorac Cardoiovasc Surg. 1983;17:135-139.
88 Petrovic P, Avramov S, Pfau K, et al. Surgical management of extracranial carotid artery aneurysms. Ann Vasc Surg. 1991;5:506-509.
89 McCollum CH, Wheeler WG, Noon GP, et al. Aneurysms of the extra cranial carotid artery: twenty one years’ experience. Am J Surg. 1979;137:196-200.
90 Kaupp HA, Haid SP, Jurayj MN, et al. Aneurysms of the extracranial carotid artery. Surgery. 1972;72:946-952.
91 Ohayama T, Ohara S, Momma F. Aneurysm of the cervical internal carotid artery associated with Marfan’s syndrome—case report. Neurol Med Chir (Tokyo). 1992;32:965-968.
92 Green LN. Progeria with the carotid artery aneurysms: report of a case. Arch Neurol. 1981;38:659-661.
93 Painter TS, Hertzer NR, Beven EG, et al. Extracranial carotid aneurysms: report six cases and review of the literature. J Vasc Surg. 1985;2:312-318.
94 Radak D, Davidovic L, Vukobratov V, et al. Carotid artery aneurysms: Serbian multicentric study. Ann Vasc Surg. 2007;21:23-29.
95 Coffin O, Maiza D, Galateau-Salle F, et al. Results of surgical management of internal carotid artery aneurysm by the cervical approach. Ann Vasc Surg. 1997;11:482-490.
96 Hurst RW, Haskal ZJ, Zager E, et al. Endovascular stent treatment of cervical internal carotid artery aneurysms with parent vessel preservation. Surg Neurol. 1998;50:313-317.
97 Jentzer A. Dissecting aneurysm of the left internal carotid artery. Angiology. 1954;5:232.
98 Friedman A. Arterial dissections. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1996:2173-2176.
99 Mokri B, Piepgras DG, Wiebers DO, et al. Familial occurrence of spontaneous dissection of the internal carotid artery. Stroke. 1987;18:246-251.
100 Mokri B, Sundt TMJr, Jouser OW, et al. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol. 1986;19:126-138.
101 Meissner I, Mokri B. Vascular diseases of the cervical carotid artery. Cardiovasc Clin. 1992;22(3):161-188.
102 Arnold M, Kappeler L, Georgiadis D, et al. Gender differences in spontaneous cervical artery dissection. Neurology. 2006;67:1050-1052.
103 Van den Berg JS, Limburg M, Kappelle LJ, et al. The role of type III collagen in spontaneous cervical arterial dissections. Ann Neurol. 1998;43:494-498.
104 Arauz A, Hoyos L, Cantu C, et al. Mild hyperhomocysteinemia and low folate concentrations as risk factors for cervical artery dissection. Cerebrovasc Dis. 2007;24:210-214.
105 Gallai V, Caso V, Paciaroni M, et al. Mild hyperhomocysteinemia: a possible risk factor for cervical artery dissection. Stroke. 2001;32:714-718.
106 Pezzini A, Del Zotto E, Archetti S, et al. Plasma homocysteine concentrations, C677T MTHFR genotype, and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombolic stroke. Stroke. 2002;33:664-669.
107 Guillon B, Peynet J, Bertrand M, et al. Do extracellular-matrix regulating enzymes play a role in cervical artery dissection? Cerebrovasc Dis. 2007;23:299-303.
108 Baumgartner RW, Lienhardt B, Mosso M, et al. Spontaneous and endothelial-independent vasodilation are impaired in patients with spontaneous carotid dissection. A case-control study. Stroke. 2007;38:405-406.
109 Petro GR, Witwer GA, Cacayorin ED, et al. Spontaneous dissection of the cervical internal carotid artery: correlation of arteriography, CT, and pathology. AJR Am J Roentgenol. 1987;148:393-398.
110 Pozzati E, Giuliani G, Acciarri N, et al. Long-term follow-up of occlusive cervical carotid dissection. Stroke. 1990;21:528-531.
111 Pozzati E, Gaist G, Poppi M. Resolution of occlusion in spontaneously dissected carotid arteries. Report of two cases. J Neurosurg. 1982;56:857-860.
112 Bogousslavsky J, Despland PA, Regli F. Spontaneous carotid dissection with acute stroke. Arch Neurol. 1987;44:137-140.
113 Kadkhodayan Y, Jeck DT, Moran CJ, et al. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328-2335.
114 Nardelli E, Fiaschi A, Ferrari G. Delayed cerebrovascular consequences of radiation to the neck: a clinicopathologic study of case. Arch Neurol. 1978;35:538-540.
115 Murros KE, Toole J. The effect of radiation carotid arteries: a review article. Arch Neurol. 1989;46:449-455.
116 Dubec JJ, Munk PL, Tsang V, et al. Carotid artery stenosis in patients who have undergone radiation therapy for head and neck malignancy. Br J Radiol. 1998;71:872-875.
117 Elerding SC, Fernandez RN, Grotta JC, et al. Carotid artery disease following external cervical irradiation. Ann Surg. 1981;194:609-615.
118 Fonkalsrud EW, Sanchez M, Zerubavel R, et al. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet. 1977;145:395-400.
119 Gassman A. Zur Histologic der Rontgenulcera Forstchr Geb Roentgenstr. 1899;2:199-207.
120 Wolbach SB. The pathologic history of chromic x-ray dermatitis and early x-ray carcinoma. J Med Res. 1909;21:415-449.
121 Sheehan JF. Foam cell plaques in the intima of irradiated arteries. Arch Pathol. 1944;37:297-307.
122 Konings AW, Hardonk MJ, Wieringa RA, et al. Initial events in radiation-induced atheromatosis. I. Activation of lysosomal enzymes. Strahlentherapie. 1975;150:44-448.
123 Cade S. Malignant Disease and its Treatment by Radium. Baltimore: Williams & Wilkins; 1940. 248
124 Thomas E, Forbus WD. Irradiation injury to the aorta and the lung. Arch Pathol. 1959;67:256-263.
125 Chung TS, Yousem DM, Lexa FJ, et al. MRI of carotid angioplasty after therapeutic radiation. J Comput Assist Tomogr. 1994;18:533-538.
126 Silverberg GD, Britt RH, Goffinet DR. Radiation induced carotid artery disease. Cancer. 1978;41:130-137.
127 Atkinson JL, Sundt TMJr, Dale AJ, et al. Radiation-associated atheromatous disease of the cervical carotid artery. Report of seven cases and review of the literature. Neurosurgery. 1989;14:171-178.
128 Kashyap VS, Moore WS, Quinones-Baldrich WJ. Carotid artery repair for radiation-associated atherosclerosis is a safe and durable procedure. J Vasc Surg. 1999;29:90-99.
129 Melliere D, Becquemin JP, Berrahal D, et al. Management of radiation-induced occlusive arterial disease. A reassessment. J Cardiovasc Surg (Torino). 1997;38L:261-269.
130 Teitelbaum GP, Lefkowitz MA, Giannota SL. Carotid angioplasty and stenting in high-risk patients. Surg Neurol. 1998;50:300-311.
131 Kim PH, Kadkhodayan Y, Derdeyn CP, et al. Outcomes of carotid angioplasty and stenting for radiation-associated stenosis. AJNR Am J Neuroradiol. 2005;26:1781-1788.
132 Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.