Chapter 96
Nonaortic Stents and Stent-Grafts
Benjamin Pearce, William Jordan
Endovascular therapy has changed the landscape of vascular surgical practice. A major thrust into catheter-based therapy was initiated in 1999 with U.S. Food and Drug Administration (FDA) approval of endografts for aneurysmorrhaphy. As this important technique became ubiquitous, surgeons began to utilize stents in other vascular beds both for initial therapy and as secondary treatment after failed vascular grafts. Endovascular therapy with stents is now utilized throughout the vascular system, including arteries and veins ranging from the intracranial circulation to the tibial arteries.
As the use of endovascular stents has undergone more critical study, it has become evident that various vascular beds react differently to stent placement.1–3 Furthermore, angioplasty and stenting alter the biology of the treated vessel, a finding that has implications for both short-term and long-term results. The nature of the lesion, as well as the vascular bed to be treated, dictate specific nuances that guide the surgeon in choosing an appropriate stent.
This chapter focuses on factors that relate to the choice of stents and stent-grafts for endovascular intervention in nonaortic vascular beds. The indications for stent use in conjunction with balloon angioplasty as well as the interaction between vessel and stent are reviewed. The multiple factors that influence the optimal stent choice in a given circumstance are considered, including device characteristics relating to the mechanics of stent deployment, cell design, precision, treatment length, deliverability, and adjunctive stent designs that may affect therapy.
Historical Background
Isolated dilatation of vessels using dilatation catheters was first introduced in 1964 by Dotter et al.4 This technique was refined in the 1970s by Gruentzig et al,5 who used smaller catheters with attached balloons that could be delivered through the vascular tree from a remote location. Balloon angioplasty became a popular technique in the 1980s but remained inferior to surgical reconstruction because of the high acute occlusion rate and intermediate restenosis rate. Acute technical failure occurred as a result of elastic recoil, vasospasm, plaque rupture, or dissection. Recurrent stenosis, caused by an intense hyperplastic response, was commonly seen within the first 2 years after intervention. In initial series of coronary angioplasty, interval restenosis occurred in 30% to 50% of treated lesions.6–8 Likewise, angiographic failure of isolated angioplasty in the renal, iliac, and femoropopliteal arteries occurred in up to 26%,9,10 32%,1 and 50%,11,12 of cases, respectively.
Stenting was introduced to improve results of angioplasty by ensuring an adequate lumen, maintaining flow, and reducing embolic load. Achieving optimal luminal diameter lessens the impact of in-stent neointimal formation. However, paradoxically, the stent itself has inherent properties that alter the development of normal vascular intimal formation and can lead to maladaptive remodeling through direct intimal damage related to the procedure and interaction between the arterial wall and the stent itself.
Stent-Vessel Interaction
Vessel Injury
The degree of intimal response to stent placement has been linked directly to the extent of vessel injury. Sullivan et al13 used an experimental stent with beveled struts to demonstrate this negative remodeling effect in vivo, utilizing a swine model. This experimental stent was designed to violate the internal elastic lamina. In comparison with Palmaz stents deployed in control animals, the experimental stent was associated with significantly greater neointimal formation. Furthermore, the extent of vessel injury demonstrated a linear effect on the absolute neointimal formation.
In addition, an inflammatory response to stent placement has been demonstrated histologically. In an early autopsy series, Farb et al14 demonstrated inflammatory cell infiltration in the area of the vessel directly adjacent to the stent struts. The absolute number of inflammatory cells present was significantly increased when stent struts violated the internal lamina and penetrated into the lipid core of the plaque. Subsequent analysis demonstrated that inflammatory mediators were present more than 6 months after implantation. Another study showed that in, addition to the effect of the stent itself, bacterial contaminants introduced to the vessel wall during stent delivery also play a role in neointimal formation.15
Fluid Dynamics
Endovascular stents also alter the fluid dynamics of the stented segment. The most widely studied impact of stent placement on flow and negative remodeling is the creation of areas of low (<5 dyne/cm2) wall shear stress (WSS). Alteration in WSS occurs both as a result of a change in luminal diameter of the treated vessel and secondary to the presence of the stent itself. In a computational flow model, LaDisa et al16,17 demonstrated several characteristics of stent placement that create a low shear stress environment. The factor that led to the most significant increase in the proportion of vessel wall exposed to a low shear environment was overdistention of the stented segment. Although some degree of stent oversizing is necessary for appropriate apposition of the stent to the vessel wall, LaDisa’s group demonstrated a 13-fold increase in the total native vessel exposed to low WSS with 20% stent oversizing over that with 10% oversizing. The low WSS can subsequently lead to greater neointimal hyperplasia and recurrent stenosis. This finding is validated clinically because most surgeons aim for a 10% oversizing when stenting infrainguinal occlusive lesions.
Strut Characteristics
The tolerances for stent construction are very strict, and minimal alteration of the strut height can have a significant impact on shear stress and neointimal formation. Intuitively, the area of the vessel wall adjacent to the stent struts has the greatest potential for negative remodeling. Eddy currents created as blood flows over the stent struts lead to regions of low shear. This effect results in a proportional change in shear stress with alterations of strut thickness. Formation of neointima in such areas of low shear stress has been reproduced in several models, and the thickness of the resultant neointima correlates with strut coverage, configuration, and thickness.18–20 Sprague et al21 demonstrated that positive remodeling, as measured by endothelial cell migration, is hampered in low WSS environments. In normal shear models, migration of endothelial cells increased 2.5-fold within 1 week after implantation of steel struts onto the endothelial surface. However, in stented models with low shear, this migration was delayed up to several months.22 The clinical effect of these findings was demonstrated by the angiographic restenosis rates of two nearly identical coronary stents that differed only by stent heights of 50 µm and 140 µm. The stent with the lower-profile design had less restenosis.23
Stent Composition
The material composition of the stent also plays a role in neointimal formation. The most common bare metal stent components are stainless steel, nitinol (nickel and titanium alloy), cobalt chromium alloy, and tantalum. Although the actual mechanism of vessel injury from stent components is unclear, corrosive products from alloys have been found within sections of vessel wall. In addition hypersensitivity of some patients to certain metals has been observed.24 Palmaz et al25 demonstrated that galvanic currents are created within stented arteries and lead to corrosion and subsequent vessel injury.
Stent Types and Characteristics
Each stent has intrinsic properties that determine whether or not it is suitable for any given lesion.25–31 On the basis of the biologic interaction between vessel and stent, an ideal stent would be easy to deliver through a small-caliber sheath, be readily visible on fluoroscopy, conform to the vessel upon deployment, prevent acute procedural failure, provide long-term resistance to negative remodeling, and be fracture-resistant.
Generally, stents are divided into two groups according to their construction and mode of deployment—self-expanding (SE) versus balloon-expandable (BE) (Table 96-1).
Table 96-1
Comparison of Balloon-Expandable and Self-Expanding Stents Based on Intrinsic Characteristics and Clinical Use
| Characteristic | Balloon Expandable | Self Expanding |
| Radial force | High | Low |
| Flexibility | Low | High |
| Requires delivery sheath | Yes | No |
| Radiopacity | High | Variable |
| Oversizing recommended | No | Yes |
| Treats lesions with variable diameter | No | Yes |
| Resistant to external compression/bending | No | Yes |
Important Characteristics
In a comparison of SE and BE stents, the major characteristics that determine suitability are radial force, flexibility, and precision of deployment. Both SE and BE stents can be covered with polytetrafluoroethylene (PTFE) or polyester and such stent-grafts combine the advantages of a stent with those of a graft.
Radial force is defined as the force required to produce a 50% reduction in the luminal diameter of the stent. The radial force of the stent maintains its apposition to the vessel wall and tacks down intimal flaps that may obstruct flow. It also provides the support to resist immediate vessel recoil and acute occlusion. This outward force may lead to a better technical result than that of balloon angioplasty alone. As the stent is incorporated into the vessel, the radial force resists deformation and negative remodeling to maintain luminal diameter over time.
Ultimately, radial force is a product of both stent design and composition. The original Palmaz stent has a stainless steel slotted/diamond design. The slotted configuration allows the stent to maintain a low profile for loading on the balloon. Once expanded, the slots become diamonds, resisting further conformational change and providing a high radial force. The Wallstent (Boston Scientific, Natick, Mass.) also has a diamond configuration, but is designed to change lengths with its diameter. Therefore, its radial force is related to both its design and the degree of endothelialization within the artery. As the stent becomes more securely anchored, it resists shortening and actually leads to increased radial force. Various balloon-expandable and self-expanding stents are shown in Figure 96-1.
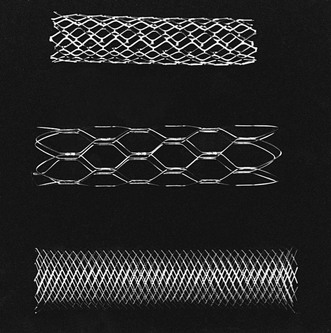
Figure 96-1 Comparison of stents. From top to bottom are the balloon-expandable Palmaz stent, the self-expanding nitinol Symphony stent, and the self-expanding stainless steel Wallstent.
Conversely, nitinol stents rely on the inherent nature of their metallic composition to provide resistance to deformation. The nitinol alloy assumes a predetermined configuration at a desired temperature. At low temperatures, the alloy exists in the martensite state (metallurgic property of shape-memory alloys in which the crystalline structure of the alloy is elongated or asymmetric at cooler temperatures), which is flexible and aids both mounting on the catheter shaft and deliverability. At higher temperatures, the alloy adopts a crystalline austenite state (metallurgic property of shape memory alloys in which the crystalline structure is symmetric) which makes the stent rigid, thereby providing more radial force.
Flexibility is determined by the same properties that govern radial force. BE stents require a force to change conformation. Thus these stents are less able to maneuver through tortuous vessels. BE stents are susceptible to deformation in mobile arteries because the torque required to maneuver them may enact a conformational change in the stent. In contrast, the nitinol stent, while in the martensite state, can be easily deformed and is therefore more suitable for arterial anatomy, which requires significant maneuverability to reach the target lesion. Interestingly, nitinol may change states not only in the setting of temperature change, but also in response to external compression. Such qualities afford nitinol stents improved flexibility in mobile arteries after implantation. Such is not the case for BE stents (Fig. 96-2).
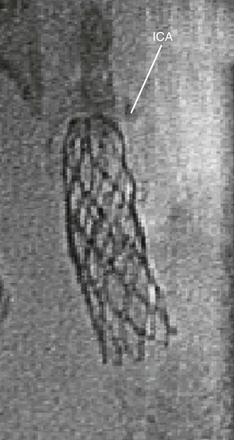
Figure 96-2 This balloon-expandable stent in the cervical portion of the carotid artery is deformed at the distal endpoint where the carotid artery is mobile and susceptible to external compression.
Radiopacity is a consideration important in stent deployment. The material used in stent construction is a major determinant of radiopacity. Stainless steel is more visible than nitinol; thus BE stents are, generally, more visible on radiographic imaging than SE stents. Improved visibility leading to increased accuracy of deployment must be considered in the choice of the appropriate stent for a given indication (Fig. 96-3).
Balloon-Expandable Stents
BE stents are optimal in clinical scenarios where high radial force and precise deployment are imperative. Unfortunately, the characteristics of BE stents may also limit deliverability to target lesions. Because they are mounted on balloons, BE stents are at risk for dislodgement both during transit to their target and while crossing the lesion. For this reason, it is recommended that a BE stent be delivered through a guiding catheter, or sheath, over a stiff guide wire. The guiding sheath should be advanced beyond the target lesion before the stent is delivered to the deployment site. Once the stent is positioned at the lesion, the sheath is retracted to allow for stent deployment. The BE stent is deployed as the balloon is inflated to nominal pressure. The balloon is designed to inflate at both ends simultaneously, so as to prevent a forward slippage (“watermelon seeding”) of the stent off the balloon before complete deployment.
The BE stent achieves a size corresponding to the degree to which the balloon is expanded. Because this stent is deployed by expansion, vigorous oversizing of the stent in relation to the vessel is not recommended. However, one advantage of the BE stent is that it can be further distended to a larger diameter if optimal vessel apposition is not obtained. Of note, aggressive oversizing of the stent with a larger balloon will foreshorten the stent and weaken its struts, leading to increased risk of stent failure.
Innate characteristics of BE stents, including minimal displacement with deployment, make them ideal for treating difficult lesions in which precise placement is paramount. Thus, BE stents are widely used to treat ostial lesions of aortic branch vessels, such as renal, common iliac, and subclavian arteries, where the lesions are anatomically fixed and thus have little potential for stent deformation with movement. In addition, plaque at bifurcations tend to be more calcific and prone to dissection. In our experience, deployment of “kissing” BE stents in such lesions results in a lower incidence of dissection than balloon angioplasty alone.
The construction of the BE stent also accounts for its major limitations. When the segment being treated transitions across a branch point from a larger into a smaller artery, the high radial force across arteries that have different radii make a BE stent inappropriate; examples of such branch points include the iliac and carotid bifurcations. In these settings, the rigid size constraints of the BE stent may not allow for adequate apposition to the entire treated segment. Furthermore, these transitions are frequently mobile and may lead to stent deformation. Additionally, the higher profile needed to deliver both stent and balloon may necessitate predilation for delivery of the stent to the desired location. In certain situations, such as treatment of renal and carotid stenoses, the consequences of embolization are severe. Predilation should be considered with small balloons to improve subsequent stent deliverability and to prevent embolism or dissection. Embolic protection devices can also be considered in territories at risk of embolic complications. Finally, a BE stent may fracture or retain a new conformation when stressed by outside forces. This risk of deformation affects the decision to use a BE stent across the inguinal ligament or joints. In these positions, the risk of stent compression with fracture and subsequent vessel occlusion is increased. Care must also be taken when one is operating on an artery treated with a BE stent. Clamping of such an artery may lead to a permanent conformational change or stent fracture that may require redilatation or surgical removal of the stent.
Self-Expanding Stents
Self-expanding stents are better suited for tortuous lesions or those traversing vessels of variable diameters. These stents are more flexible than BE stents (Fig. 96-4). Therefore, they can be delivered through vessels that create more torque within the catheter. To maintain the stent in its constrained form during transit to the lesion, the stent is covered by an outer sheath on the mounting catheter. When this sheath is withdrawn, the stent is allowed to take its natural shape. As such, SE stents do not require that guiding catheters or sheaths be advanced beyond the target lesion. Actual deployment of a SE stent requires sequential removal of the constraining sheath from the distal end of the catheter to its proximal end. During this maneuver, the stent can either inadvertently jump forward or be retracted by the operator. This potential jumping forward of the stent can lead to maldeployment and must be considered by the operator prior to final deployment maneuvers.
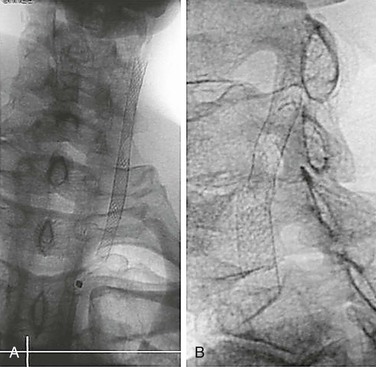
Figure 96-4 Plain radiographs of a stainless steel self-expanding stent (A) and nitinol self-expanding stent (B) show the improved visibility of the stainless steel stent.
Another factor to consider in the use of an SE stent is the need for adequate oversizing. As mentioned previously, flow dynamic models demonstrate optimal shear environments at a 10% oversizing. Nevertheless, it is essential to choose a stent that opposes the vessel wall on deployment because an SE stent cannot be overdilated. In the instance in which a self-expanding stent is undersized for a given vessel, a second, larger-diameter stent can be deployed within the initial stent to aid with stent fixation. A BE stent is the preferred choice in this circumstance because its increased radial force can overcome the nominal diameter of the SE stent.
Because SE stents have less radial force and, conversely, improved elasticity, they are ideal for lesions that cross tortuous vessels, joints, or vessel branches. Common locations where SE stents are used include the carotid bifurcation and the transition zone from the common to the external iliac artery. SE stents are used preferentially in infrainguinal arteries because they can accommodate movement within the limb with less likelihood of fracture and subsequent failure.
Cell Size: Open and Closed Cells
Stent cell size should be considered in the selection of the best stent for a given clinical situation. Cell size refers to the area outlined by connected metallic components within a stent. Stents with a large cell size are labeled “open cell” stents, and those with a small cell size are termed “closed cell” stents (see Figure 96-1). The cell size of a stent and the connection of the metallic wires to each other may influence performance.
A closed cell stent has consistent interconnection of all stent wires throughout its length. This consistency provides a fixed area of interstices within the stent and uniform coverage of the vessel wall. Such construction decreases the free cell area and, may trap fractured plaque at the time of deployment, limiting distal embolization. This configuration, however, makes a stent less flexible and conformable. Considering the previously mentioned advantage of the SE stent, the closed cell stent may have more difficulty matching the tortuosity and change in luminal diameter across a vessel length.
Conversely, in open cell stents, the wires are not interconnected throughout the entire stent. This feature allows for a greater range of movement between the stent components and leads to added flexibility and conformability. It also results in significantly more vessel wall exposure between stent struts and greater potential for debris to embolize during deployment or balloon dilatation. Free cell area can vary from 1.08 mm2 in the smallest closed cell stent to 11.48 mm2 in the largest open cell stent.26
The efficacy of cell design to lower embolic phenomena during stenting has yet to be determined. Stent cell size has received the most attention in the treatment of carotid lesions but may also have implications at other sites. Hart et al32 demonstrated a significant reduction in rates of stroke, transient ischemic attack (TIA), and death when closed cell stents were compared with open cell stents in a series of 701 carotid stents. The odds ratio was most significant in treatment of symptomatic lesions. A larger study combining patients from 10 European centers demonstrated no significant difference, even in symptomatic lesions, between the two stent designs.27 Because closed cell stents are less flexible, a bias may exist to use them in straighter, less complicated lesions. Ultimately, use of an open or closed cell stent remains the prerogative of the clinician because there is no convincing evidence of the superiority of one over the other. The operator should seek ideal stent characteristics based on both the nature of the lesion and its location.
Stent-Grafts (Covered Stents)
Stent-grafts, or covered stents, have expanded the use of endovascular technology beyond their contribution to treatment of aortic aneurysms. A covered stent may be considered the ultimate closed cell stent, with the inherent applications and limitations of complete coverage of the treatment area. Stent-grafts can be organized on the basis of graft material and deployment characteristics. Polyester (e.g., Wallgraft [Boston Scientific]) and polytetrafluroethylene stent-grafts (e.g., VIABAHN [W.L. Gore, Flagstaff, Ariz.], iCAST [Atrium USA, Hudson, N.H.], Jostent [Abbott, Abbot Park, Ill.], Fluency [Bard Peripheral Vascular, Inc., Tempe, Ariz.]) are currently available. Some stent-grafts are balloon-expandable (e.g., iCAST) whereas others are self-expanding (e.g.,VIABAHN, Jostent, Wallgraft). This stent-graft variety is further complicated by different deployment mechanisms and delivery characteristics that affect clinical utility. Even within the self-expanding category, deployment can be distal to proximal (Jostent, Wallgraft) or proximal to distal (VIABAHN). This variability allows stent-grafts to be utilized in a wide variety of lesions.
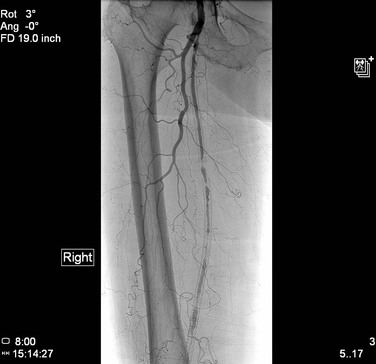
Stent-grafts provide the inherent advantage of continuous exclusion of the vessel wall from luminal flow. Historically, this feature allows complete exclusion of vessel defects, such as aneurysm, arteriovenous fistula, pseudoaneurysm, embologenic plaque, and frank perforation. Several case reports have demonstrated the utility of stent-grafts in acute vascular trauma. True aneurysms of peripheral arteries, such as popliteal or visceral artery aneurysms, are also amenable to treatment with covered stents.33 Stent-grafts have been deployed into the subclavian artery to treat aneurysmal degeneration, although special care should be taken to rule out an associated outlet syndrome in those patients who may require further surgical cervical or first rib excision.30
Stent-grafts can be utilized to “trap” debris within the treated lesion. This approach, in theory, lessens the risk of embolization during inflation. The embolic source can be trapped by inflation of a BE stent-graft, or an SE stent-graft can be deployed followed by post-deployment molding. Embolization has been shown in both ex vivo and in vivo models of angioplasty in several arterial beds.31,34,35 Therefore, use of stent-grafts may prove to be an effective treatment of renal, iliac, or subclavian lesions, in which embolization may add significant morbidity. However, the covered stent may have limited use in certain locations because it will exclude any branch points or collateral vessels covered by the stent during placement.
Another potential use for stent-grafts is to treat long lesions of the superficial femoral artery (SFA). In this position, the covered stent provides protection from embolization during deployment and theoretically provides a barrier to smooth muscle cell migration and neointimal growth within the treated segment. The SFA often has a large plaque burden that can hasten failure of a bare metal stent due to an aggressive neointimal response and ingrowth by medial smooth muscle cells (Fig. 96-5). In a randomized comparison, Kedora et al36 demonstrated equivalent rates of patency and limb salvage in a stent-graft and a prosthetic bypass for various lesions in the above-knee position, treating various Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC) lesions with comparable runoff. However, a rigorous randomized trial has not compared stent-graft placement with popliteal bypass from femoral to above the knee using vein conduit. A drawback of stent-graft use is the potential of exclusion of important branches or collaterals. When the stent-graft is deployed, collaterals may be covered within the treated segment and therefore subsequent stent thrombosis may lead to worse limb ischemia than in the original limb before treatment.
Selection of Stent
After consideration of the various stent characteristics, the selection of an appropriate stent depends on plaque morphology, external forces, anatomic location, and branch locations. Foremost, the stent must treat the primary lesion.
Plaque Morphology
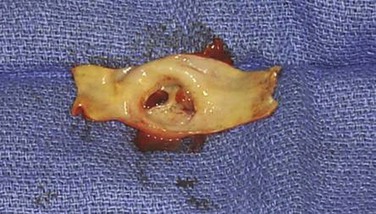
If there is concern about microembolization of debris with stent deployment, a covered stent or closed cell stent should be used. Procedural and early postprocedural embolization associated with carotid stenting can have important clinical sequelae (Fig. 96-6). Additional data about plaque morphology may help predict which lesions are more likely to embolize through the interstices of the stent. Some researchers have used ultrasound technology to evaluate gray-scale median to determine the embolization potential of a specific carotid plaque.37 Magnetic resonance or computed tomographic imaging may also provide morphologic plaque information about lipid content that can help the clinician choose a stent type.38
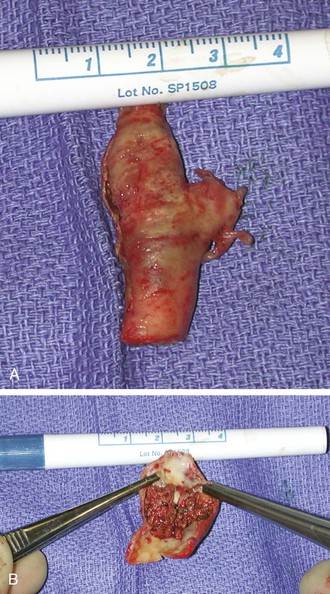
Figure 96-6 A and B, Photographs of an endarterectomized carotid plaque that had embolized and caused symptoms. This type of plaque is thought to be a greater risk for periprocedural embolization when an open cell stent is used to cover the stenosis.
Additionally, if stenting is indicated in the setting of possible fresh thrombus, one should consider selecting a covered stent. Although it may be difficult to differentiate stenotic plaque from organized thrombus, clinical or angiographic evidence of distal embolization in the lower extremity should raise concern about a proximal iliac, femoral, or popliteal lesion that is unstable and prone to repeated embolization during manipulation. In addition, an evaluation of the size and filling of collaterals at the level of occlusion may indicate a chronic rather than acute process. If a covered stent cannot be used, one should consider various embolization protection methods, such as filter wires, reversal of flow devices, and open arterial exposure, to flush debris away from the distal vascular bed.
External Forces
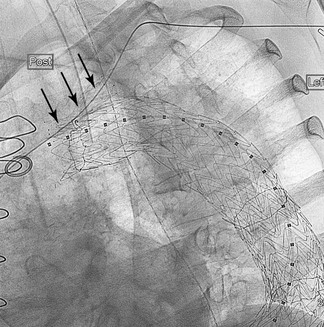
Once the inner surface of the artery and lesion-specific pathology have been considered, the clinician must review the potential impact of external forces on the stent in the arterial or venous circulation. Extrinsic forces affecting the origin of the supra-aortic trunks are minimal, but the extrathoracic carotid and subclavian arteries can be subjected to significant external compression forces. An example is a cervical rib compressing the subclavian artery. The axillary artery may be compressed by a hypertrophic pectoralis muscle or manipulation in the axilla. Lastly, a carotid stent can be compressed by neck rotation or vigorous external pressure, leading to occlusion that may cause a stroke (see Fig. 91-2). SE stents are more often used in these mobile areas because they are more resistant to external forces.
Anatomic Location
The celiac artery origin can be compressed by the median arcuate ligament. In our practice, treatment of this defect most often requires open surgical or laparoscopic division of the arcuate ligament followed by endovascular or open reconstruction of the celiac lesion.
In atherosclerotic lesions involving the celiac artery, BE stents are commonly used and are associated with good patency. Although access can be gained by either the brachial or femoral approach, the brachial approach is preferred because it allows for easier cannulation of the celiac artery. Careful angiographic evaluation in multiple planes is warranted to ensure exact deployment of the stent short of the main division of the celiac artery.
Superior mesenteric artery and renal ostial stenoses are typically treated with BE stents because disease affecting these ostia is typically associated with extensive atherosclerotic plaque in the aortic wall (Fig. 96-7). An adequate landing zone before the middle colic and inferior pancreaticoduodenal arteries must exist to avoid occlusion of these important branches. A covered BE stent may provide better embolic protection than a bare metal stent. There are some preliminary reports that covered stents may have more resistance to restenosis than bare metal stents in the mesenteric circulation.39 Late failure of a covered stent more likely would lead to thrombosis and, possibly, acute ischemic complications. Covered stents in mesenteric and renal arteries are commonly used as adjuncts in complex endovascular thoracoabdominal aneurysm repair.
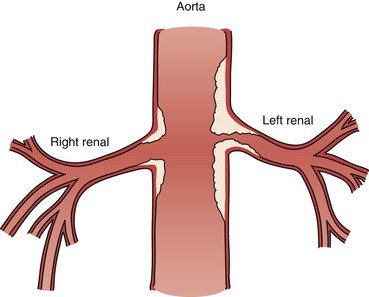
Figure 96-7 The atherosclerotic plaque of a renal artery stenosis actually originates in the aortic wall as the line drawing depicts. This type of plaque is susceptible to elastic recoil with angioplasty alone and is often better treated with a balloon-expandable stent that protrudes slightly into the aortic lumen to maintain an improved luminal flow.
SE stents may be superior for nonorificial lesions, particularly those associated with dissection. A covered SE stent can be ideal in nonatherosclerotic pathologies such as pseudoaneurysms associated with pancreatitis. More distal lesions of the mesenteric and renal circulations can often be treated with balloon angioplasty alone because of the high flow and small caliber of these vascular beds.
The left common iliac vein is another anatomic location where stents are often used to treat compression from the right common iliac artery (May-Thurner syndrome). Although most patients are asymptomatic, edema or deep venous thrombosis (DVT) may result (See Chapter 62). After lytic therapy for deep venous thrombosis, the underlying stenosis/compression may be exposed, leading to an indication for treatment. Most clinicians recommend utilizing an oversized SE stent in this anatomic location to provide the appropriate radial force and avoid stent migration. BE stents are more likely to cause perforation and are relatively contraindicated in venous disease.
The distal abdominal aorta and the iliac arteries are prone to extensive plaque formation that can cause ischemic symptoms. Treating a proximal common iliac stenosis often requires a “kissing stent” technique, in which stents are placed concurrently in both common iliac arteries to prevent the shift of the aortic bifurcation plaque into the contralateral lumen. The two stents are deployed simultaneously, matching their position in the distal aorta and proximal iliac arteries. Although both stent types are used in the proximal iliac arteries, the BE type are more often used for short lesions and the SE type for longer lesions that traverse the change in artery caliber past the iliac bifurcation. Lesions in the external iliac artery are usually treated by SE stents because of the usual tortuosity of the artery. Occasionally, the more distal segment of the external iliac can be better treated with a precisely placed, short, BE stent to avoid excessive material load in this smaller artery. Plaque characteristics that suggest a high risk of embolization would favor treatment with a closed cell or covered stent in the iliac arteries. Primary patency of endovascular repair falls short of open revascularization for complex aortoiliac pathology, but secondary patency rates are favorable.40 Published clinical series are showing promise for the use of covered, SE stents in the distal external iliac arteries extending into the common femoral arteries.41,42 Fewer fractures are noted with these stents in other vascular beds, such as the popliteal. The covered construction theoretically would make a stent fracture less relevant because the graft would prevent the stent from injuring the underlying vessel wall. Stent grafts in this position can also be sewn directly, allowing for a femoral patch angioplasty or interposition graft in the femoral artery if needed to obtain adequate outflow.
For the infrainguinal arteries, SE stents are utilized preferentially with a few exceptions. Stents in the common femoral arteries are generally contraindicated for two reasons. First, the stent could cover the origin of the profunda femoris artery and compromise flow into this vital artery. Although arteries often remain patent after they have been “jailed” (e.g., hypogastric artery, external carotid artery), the extent of disease in the common femoral artery and the potential consequences of occluding the profunda femoris have a more serious effect on the long-term salvage of the leg. Second, the common femoral artery remains a major access point for vascular procedures throughout the body, and a stent in this position may adversely affect subsequent access through that artery. In addition, the common femoral artery is easily accessible surgically, and repair or endarterectomy of the vessel can be performed with minimal risk.
Stenting of the infrainguinal arteries for occlusive disease is indicated for failure of primary angioplasty. YaJun et al43 demonstrated in a meta-analysis that acute results are better with adjunctive stenting but long-term patency is no different.
Dialysis outflow tracts are prone to treatment failure owing to the unique hemodynamic environment created by arteriovenous fistulae (AVFs) or AV grafts (AVGs). Stent-supported revascularization of the axillosubclavian veins and cephalic arch have been proven to improve access salvage over that with angioplasty alone.44 Because central stenosis often occurs in areas of significant mobility, careful evaluation for the contribution of thoracic outlet compression should be made before deployment of a stent. Given these considerations, a large self-expanding stent is recommended. The failure mode in these situations relates to neointimal hyperplasia, so the use of a stent-graft is not unreasonable. It is preferred in cases of venous disruption from high-pressure angioplasty. However, no significant data has demonstrated a benefit of covered over uncovered stents in treating central stenoses.
Much like venous anastomotic complications, the hemodynamics of the cephalic arch results in a common failure mode of cephalic vein–based access. Initial experience with bare metal stenting demonstrated a high incidence of recurrence. Self-expanding, covered stents have been shown to be superior to bare metal stents in the treatment of these lesions.47 (Care must be taken to avoid encroachment of the stent into the axillary vein.)
The venous anastomosis is the most common failure site of prosthetic arteriovenous grafts.45 The use of covered stents has been especially promising in treating these lesions. In a randomized trial, Haskal et al46 demonstrated the superiority of stent-grafts over angioplasty alone for the treatment of venous anastomotic stenosis. Covered stents change the hemodynamics of these anastomoses to a functional end-to-end anastomosis as well as retard the neointimal hyperplastic response. Stent-grafts in dialysis access commonly fail secondary to thrombosis. However, secondary patency of these grafts is promising, and they appear to be an appropriate first-line of therapy in venous anastomotic complications of arteriovenous grafts.
Branch Location
Once the external forces that may affect stent choice have been reviewed, the clinician must also consider the extent of the disease relative to branch points of the artery. When a plaque radiographically appears at the origin of a vessel, histologically it will extend into the “parent” artery (Fig. 96-8). For example, an ostial renal artery stenosis may appear isolated to the renal artery on subtraction angiography when, in fact, the majority of the plaque is in the wall of the aorta (Fig. 96-9). This extensive plaque requires a high–radial force stent with accurate deployment, and therefore the BE stent works best in this scenario. The BE stent is typically deployed with a small portion extending into the aorta so as to fully address the aortic portion of the plaque.
Failure Modes
Although stents are designed to maintain patency of the treated vessel, the presence of a foreign material in the arterial bed may actually lead to failure through neointimal hyperplasia and vessel restenosis or thrombosis. Studies have demonstrated that acute inflammatory changes occur with the disruption of the endothelial surface. After the acute phase, the arterial wall continues to react through a remodeling process that includes acute and chronic changes with a smooth muscle response that can be seen as neointimal hyperplasia.14 This chronic inflammatory change can actually lead to an obstructing lesion that can create a more recurrent stenosis or lead to in situ thrombosis.
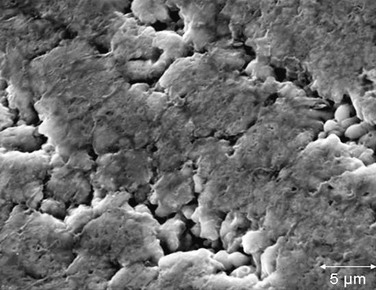
Stents are designed of various metallic components that have long but limited lifespans under the mechanical stress of the cardiac cycle. Stent fracture or failure may be related to metallic corrosion, which may in turn be related to the local vascular environment, external forces, and interaction of other intravascular material.48–52 Fig. 96-9 shows surface corrosion of an iliac stent that ultimately failed and was removed during the course of an aortobifemoral bypass. The figure shows pitting corrosion that may have contributed to device failure, recurrent stenosis, and eventual iliac artery thrombosis. In a common clinical scenario, when two overlapping stents are placed, the materials of the two stents can be additive in the corrosive process. Figure 96-10 shows two overlapping stents in the coronary circulation that ultimately fractured, likely as a result of galvanic corrosion between the stents. In this patient, a recurrent stenosis developed at an angulated area of a coronary vein graft.

Figure 96-10 Two overlapping coronary stents placed at a tortuous portion in the coronary artery created a stress point that led to stent fracture and recurrent stenosis. (Photo courtesy Dr. Britta Brott.)
Long (>200 cm) SE stents are now available. Use of an appropriate-length stent to avoid overlap may lead to fewer fracture-induced occlusions. In addition, overlap within tortuous segments seems to be especially prone to failure.53 Strategies to decrease occlusion within stented segments include use of drug-eluting stents in overlapped segments54 and telescoping of a covered stent inside a bare stent. The graft material theoretically inhibits the corrosive effect of metal-on-metal interaction.
Stents can also release ions into the local vascular environment that can adversely affect the treated lesion. Both stainless steel and nitinol stents have been shown to release cytotoxic agents that may contribute to smooth muscle necrosis, affecting the response of the artery to such a stent.55,56
New Developments
Drug-Eluting Technology
Many modalities have been investigated to control the hyperplastic response to an arterial implant. Specifically, stents have become a conduit to deliver medication to the local vascular environment in an effort to limit this response to injury. Three chemotherapeutic agents have been attached to BE stents for utilization in the coronary arteries: paclitaxel, sirolimus, and everolimus. These agents have been shown to aggressively inhibit the neointimal response and thereby improve patency rates. Paradoxically, drug-eluting stents (DESs) have created a new late failure mode of delayed stent thrombosis due to the lack of endothelialization and minimal incorporation into the associated vessel wall. The exposed raw surface of the stent carries a risk of stent thrombosis as high as 4% after 1 year.57 When evaluating the use of DESs in the peripheral circulation, one must consider that late stent thrombosis of the femoral artery carries a significantly different risk from that of a coronary artery.
Initial clinical trials of DESs in the SFA did not demonstrate better patency than with bare metal stents. The Sirolimus-Coated Cordis Self-Expandable Stent (SIROCCO) trial compared the results of a drug-eluting SE stent and a bare metal SE stent and found near-equivalent restenosis rates, 22.9% in for the DES and 22.1% for the bare metal SE stent at 2 years.58 A prospective industry-sponsored trial of a non-polymer, paclitaxel-coated SFA stent (Zilver PTX, Cook Medical, Bloomington, Ind.) has shown more promising results. The main arm of the study demonstrated noninferiority of the DES to primary angioplasty, although these data may be unreliable because of an overly high primary failure rate of percutaneous transluminal angioplasty. More interestingly, a separate arm directly compared bare metal stents to DESs in patients with failed percutaneous transluminal angioplasty and showed statistically significant better patency with DESs at 1 year.59 Enthusiasm for DES-supported intervention in the SFA on the basis of these results is hampered by the large number of short lesions and the high percentage of patients with claudication (90%) enrolled in the trial.
The PReventing Amputations using Drug eluting StEnts (PaRADISE) trial attempted to quantify the impact of DES technology on patients at greatest risk of amputation.60 In this noncontrolled, prospective study of DESs in tibial intervention, the major amputation rate was 6% at 3 years in patients presenting with critical limb ischemia. Maintenance of limb salvage required a re-intervention rate of 36% at a mean of less than 1 year, but overall survival, renal failure, and limb salvage rates were better than those in historical controls.
Considering the success of antiproliferative agents attached to stents, other investigators have delivered these medications to the treatment area with balloon catheters. Tepe et al61 reported a failure (defined as target vessel revascularization) rate of 4.4% in a clinical trial comparing balloon angioplasty, balloon angioplasty with a paclitaxel-coated balloon, and balloon angioplasty with paclitaxel in the contrast medium. Although there was no difference in amputation rate among the three groups, the angiographic restenosis rate was lower in the patients treated with a drug-coated balloon. This mechanism of delivery may enhance overall vessel response to angioplasty by delivering a high dose of medication without sustained systemic exposure and without long-term inhibition of positive remodeling and subsequent late stent thrombosis.
Absorbable Stents
The long-term interaction between stent and vessel can result in late stenosis and occlusion. One approach to mitigate this effect is to create stents composed of biodegradable material. Bioabsorbable stents theoretically provide scaffolding for healing of the vessel before they are eventually absorbed. Although lower extremity data are not available, absorbable stents in the coronary arteries have shown a 30% mass reduction of neointimal hyperplasia at 12 months and 60% reduction at 18 months.62 Tamai et al63 reported the use of a poly-L-lactic acid (PLLA) bioabsorbable stent in the coronary arteries of 15 patients with a restenosis rate of 10.5% at 6 months. Even with imaging at 4 years, these investigators found some hyperplasia related to the local stent reaction, but there were no late stent thromboses in these patients.64 Using intravascular ultrasound and coronary angiographic imaging at 6 months, this group found that the biodegradable stents were still present, but the data suggested less neointimal hyperplasia than with bare metal stents.
The clinical effect of this expected degradation may lead to some late events, such as embolization with resulting small vessel ischemia. However this clinical phenomenon has not been well documented. After the initial reports of success with the PLLA stent, Ormiston et al62 reported the success of attaching everolimus to the PLLA stent to obtain the benefit of a medicated stent that reduces the intimal response while potentially removing the nidus for late stent thrombosis. These investigators found a decreased neointimal response and reduced cardiac event rate (3.3%) in a series of 30 patients. Although stent absorption was not complete, no patient suffered late stent thrombosis. These investigations are certainly important in the clinical application of vascular stents, but the fine balance between modulating the hyperplastic response with medication and promoting the degradation of a vascular foreign body has yet not been achieved.
Future Developments
The progressive miniaturization of revascularization technology remains a persistent goal for clinicians treating vascular disease. Considering that open surgery has been refined over the last 50 years, the endovascular era is still relatively young. Simple dilatation of diseased vessels has had limited clinical success, thus leading to intensive efforts to improve those results with intravascular devices such as stents and with antiproliferative medications. As with any new technology, new problems have been created. The alteration of hemodynamic variables, such as vessel compliance and shear stress, continues to limit the patency of endovascular intervention. Fortunately, this alteration appears to come at the cost of repeat interventions rather than limb loss. Stents have improved some of these catheter-based treatments, but further modifications are necessary to provide more effective therapy for the patient with vascular disease.
Selected Key References
Dyet JF, Watts WG, Ettles DF, Nicholson AA. Mechanical properties of metallic stents: how do these properties influence the choice of stent for specific lesions? Cardiovasc Intervent Radiol. 2000;23(1):47–54.
LaDisa JF Jr, Olson LE, Guler I, Hettrick DA, Audi SH, Kersten JR, et al. Stent design properties and deployment ratio influence indexes of wall shear stress: a three-dimensional computational fluid dynamics investigation within a normal artery. J Appl Physiol. 2004;97(1):424–430.
Mauri L, Hsieh W, Massaro JM, Ho KL, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029.
Palmaz JC, Bailey S, Marton D, Sprague E. Influence of stent design and material composition on procedure outcome. J Vasc Surg. 2002;36(5):1031–1039.
Sullivan TM, Ainsworth SD, Langan EM, Taylor S, Snyder B, Cull D, et al. Effect of endovascular stent strut geometry on vascular injury, myointimal hyperplasia, and restenosis. J Vasc Surg. 2002;36(1):143–149.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Lee ES, et al. Comparing patency rates between external iliac and common iliac artery stents. J Vasc Surg. 2000;31:889–894.
2. Ward MR, et al. Response to balloon injury is vascular bed specific: a consequence of de novo vessel structure? Atherosclerosis. 2000;151:407–414.
3. Schillinger M, et al. Inflammatory response to stent implantation: differences in femoropopliteal, iliac, and carotid arteries. Radiology. 2002;224:529–535.
4. Dotter CT, et al. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654–670.
5. Gruentzig AR. Percutaneous transluminal coronary angioplasty. Semin Roentgenol. 1981;16(2):152–153.
6. Gruentzig AR, et al. Long-term follow-up after percutaneous transluminal coronary angioplasty: the early Zurich experience. N Engl J Med. 1987;316:1127–1132.
7. Nobuyoshi M, et al. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol. 1988;12:616–623.
8. Nobuyoshi M, et al. Restenosis after percutaneous transluminal coronary angioplasty: pathologic observations in 20 patients. J Am Coll Cardiol. 1991;17:433–439.
9. Isles CG, et al. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM. 1999;92:159–167.
10. Kandarpa K, et al. Transcatheter interventions for the treatment of peripheral atherosclerotic lesions: part II. J Vasc Interv Radiol. 2001;12:807–812.
11. Gray BH, et al. High incidence of restenosis/reocclusion of stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J Vasc Surg. 1997;25:74–83.
12. Schillinger M, et al. Restenosis after percutaneous transluminal angioplasty in the femoropopliteal segment: the role of inflammation. J Endovasc Ther. 2001;8:477–483.
13. Sullivan TM, et al. Effect of endovascular stent strut geometry on vascular injury, myointimal hyperplasia, and restenosis. J Vasc Surg. 2002;36:143–149.
14. Farb A, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999;99:44–52.
15. Whelan DM, et al. Foreign body contamination during stent implantation. Cathet Cardiovasc Diagn. 1997;40:328–332.
16. LaDisa JF Jr, et al. Stent design properties and deployment ratio influence indexes of wall shear stress: a three-dimensional computational fluid dynamics investigation within a normal artery. J Appl Physiol. 2004;97:424–430.
17. LaDisa JF Jr, et al. Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2465–H2475.
18. Sommer CM, et al. Impact of stent design on in-stent stenosis in a rabbit iliac artery model. Cardiovasc Intervent Radiol. 2010;33(3):565–575.
19. Garasic JM, et al. Stent and artery geometry determine intimal thickening independent of arterial injury. Circulation. 2000;101(7):812–818.
20. Rogers C, et al. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation. 1995;91(12):2995–3001.
21. Sprague EA, et al. Human aortic endothelial cell migration onto stent surfaces under static and flow conditions. J Vasc Interv Radiol. 1997;8:83–92.
22. Palmaz JC, et al. Influence of surface topography on endothelialization of intravascular metallic material. J Vasc Interv Radiol. 1999;10:439–444.
23. Kastrati A, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–2821.
24. Shih CC, et al. The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J Biomed Mater Res. 2000;52:395–403.
25. Palmaz JC, et al. Influence of stent design and material composition on procedure outcome. J Vasc Surg. 2002;36:1031–1039.
26. Flueckiger F, et al. Strength, elasticity, and plasticity of expandable metal stents: in vitro studies with three types of stress. J Vasc Interv Radiol. 1994;5:745–750.
27. Dyet JF, et al. Mechanical properties of metallic stents: how do these properties influence the choice of stent for specific lesions? Cardiovasc Intervent Radiol. 2000;23:47–54.
28. Grenacher L, et al. Resistance to hoop stress in balloon expandable stents: evaluation in an ex vivo model. Invest Radiol. 2003;38:65–72.
29. Grenacher L, et al. In vitro comparison of self-expanding versus balloon-expandable stents in a human ex vivo model. Cardiovasc Intervent Radiol. 2006;29:249–254.
30. Schoder M, et al. Elective and emergent endovascular treatment of subclavian artery aneurysms and injuries. J Endovasc Ther. 2003;10:58–65.
31. Edwards MS, et al. Atheroembolism during percutaneous renal artery revascularization. J Vasc Surg. 2007;46:55–61.
32. Hart JP, et al. Do device characteristics impact outcome in carotid artery stenting? J Vasc Surg. 2006;44(4):725–730.
33. Sfyroeras GS, et al. Flow-diverting stents for the treatment of arterial aneurysms. J Vasc Surg. 2012;56(3):839–846.
34. Lam RC, et al. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46:1155–1159.
35. Rapp JH, et al. Subclinical embolization after carotid artery stenting: new lesions on diffusion-weighted magnetic resonance imaging occur postprocedure. J Vasc Surg. 2007;45:867–872.
36. Kedora J, et al. Randomized comparison of percutaneous Viabahn stent grafts vs prosthetic femoral-popliteal bypass in the treatment of superficial femoral arterial occlusive disease. J Vasc Surg. 2007;45:10–16.
37. Biasi GM, et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. J Vasc Surg. 2005;41:370.
38. Altaf N, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008;47:337–342.
39. Oderich G, et al. Comparison of covered stents vs. bare metal stents for treatment of chronic atherosclerotic mesenteric arterial disease. Presented at Society of Vascular Surgery Annual Meeting, Plenary Session, National Harbor, Md., June 7-9, 2012.
40. Jongkind V, et al. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg. 2010;52:1376–1383.
41. Calligaro KD, et al. Results of polytetrafluoroethylene-covered nitinol stents crossing the inguinal ligament. J Vasc Surg. 2013;57:421–426.
42. Chang RW, et al. Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease. J Vasc Surg. 2008;48(2):362–367.
43. He N, et al. Percutaneous transluminal angioplasty (PTA) alone versus PTA with balloon-expandable stent placement for short-segment femoropopliteal artery disease: a metaanalysis of randomized trials. J Vasc Interv Radiol. 2008;19(4):499–503.
44. Kakisis JD, et al. Balloon angioplasty vs nitinol stent placement in the treatment of venous anastomotic stenoses of hemodialysis grafts after surgical thrombectomy. J Vasc Surg. 2012;55:472–478.
45. Naoum JJ, et al. The use of covered nitinol stents to salvage dialysis grafts after multiple failures. Vasc Endovascular Surg. 2006;40:275–279.
46. Haskal ZJ, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362:494–503.
48. Coury AJ, et al. Degradation of materials in the biological environment. Ratner BD, et al. Biomaterials science: an introduction to materials in medicine. ed 2. Elsevier Academic Press: San Diego, CA; 2004:411–454.
49. Rondelli G, et al. Localized corrosion behaviour in simulated human body fluids of commercial Ni-Ti orthodontic wires. Biomaterials. 1999;20:785–792.
50. Shih CC, et al. Electrochemical and SEM characterization of gold-coated stents in vitro. J Electrochem Soc. 2007;154:C326–C330.
51. Thomas KA, et al. Tissue reaction to implant corrosion in 38 internal fixation devices. Orthopedics. 1988;11:441–451.
52. Cook SD, et al. The in vivo performance of 250 internal fixation devices: a follow-up study. Biomaterials. 1987;8:177–184.
53. Kapnisis KK, et al. Stent overlapping and geometric curvature influence the structural integrity and surface characteristics of coronary nitinol stents. J Mech Behav Biomed Mater. 2013;20:227–236.
54. Aoki J, et al. Chronic arterial responses to overlapping paclitaxel-eluting stents: insights from serial intravascular ultrasound analyses in the TAXUS-V and -VI trials. JACC Cardiovasc Interv. 2008;1:161–167.
55. Eliades T, et al. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am J Orthod Dentofacial Orthop. 2004;125:24–29.
56. Shih CC, et al. The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J Biomed Mater Res. 2000;52:395–403.
57. Mauri L, et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029.
58. Duda SH, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther. 2006;13:701–710.
59. Dake MD, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. [Zilver PTX Investigators] Circ Cardiovasc Interv. 2011;4:495–504.
60. Feiring AJ, et al. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol. 2010;55:1580–1589.
61. Tepe G, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–699.
62. Ormiston JA, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907.
63. Tamai H, et al. Initial and 6-month results ofbiodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399–404.
64. Tsuji T, et al. Four year follow up of the biodegradable stent (IGAKI-TAMAI Stent). Circ J. 2004;6(Suppl I):135.