CHAPTER 38 Non-penetrating surgery
Introduction
Trabeculectomy is the procedure of choice for glaucoma surgery thanks to its high success rate. Nevertheless, the procedure can be associated with major intraoperative and postoperative complications. In order to define a safer and more effective operation, during the last 20 years many alternatives to trabeculectomy have been proposed. Over the years, a growing body of evidence suggests that ‘non-penetrating glaucoma surgery’ (NPGS) is successful at lowering intraocular pressure and can be considered as a surgical option for glaucoma1,2. NPGS is represented by ‘deep sclerectomy’(DS) and by ‘Viscocanalostomy’(VC) (which was introduced by R. Stegmann in the early 1990); they are based on original studies by Krasnov (1972) and Zimmerman (1984) on ‘non-penetrating trabeculectomy’. Similarly, both procedures aim to lower intraocular pressure (IOP) by draining aqueous humor from the anterior chamber not through a patent scleral opening, but by slow percolation through the inner trabecular meshwork and/or Descemet’s membrane (the ‘sclerodescemetic membrane’) (Fig. 38.1). This avoids sudden IOP fall, hypotony, and a flat anterior chamber. The absence of anterior chamber opening and iridectomy limits inflammation and the risk of cataract and intraocular infection. Compared with deep sclerectomy, viscocanalostomy has significant advantages; it aims not only to be non-penetrating like deep sclerectomy, but also to restore the physiological outflow pathway, thus avoiding any external filtration. This would make the success of the procedure independent of conjunctival or episcleral scarring, a leading cause of failure in trabeculectomy, with fewer indications for wound healing modulation. Moreover, the absence of a filtering bleb avoids related ocular discomfort. In addition, the procedure can be carried out in any quadrant. A technical variation of viscocanalostomy, was recently introduced, named ‘canaloplasty’ (CP), aimed at a better and controlled dilation of Schlemm’s canal.
Deep sclerectomy
Fundamental principles
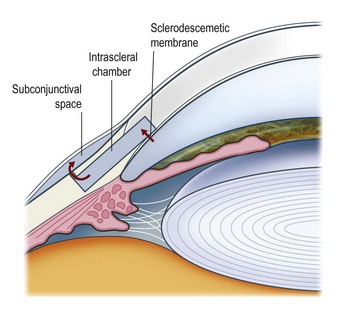
Deep sclerectomy (DS) aims to reduce intraocular pressure by external filtration of aqueous humor. Unlike trabeculectomy, aqueous exits the eye not through a patent hole, but by slow passage through the ‘sclerodescemetic membrane’ formed by the internal portion of the posterior and anterior trabecular meshwork and by the adjacent Descemet’s membrane. The membrane is created by removing the inner wall of Schlemm’s canal and juxtacanalicular trabeculum (sites of the increased outflow resistance in glaucoma) to expos the anterior trabecular meshwork and Descemet’s membrane. Percolating from the anterior chamber, aqueous fills an intrascleral ‘lake’ or ‘decompression chamber’, from where it drains into the subconjunctival space and/or is partially reabsorbed into the suprachoroidal space (Fig. 38.2).
Indications for surgery
Based on its high safety profile and on its mechanism of action, deep sclerectomy is indicated in primary open angle, pseudoexfoliative, and pigmentary glaucomas. Being non-penetrating, it can be useful particularly in aphakic eyes with vitreous in the anterior chamber, or in-patients where a sudden drop in IOP or long-lasting hypotony should be avoided, such as eyes with uncontrolled high pressure, eyes with high myopia, or even eyes with very advanced glaucoma. The procedure was also found to be effective in uveitic glaucoma3.
Operation technique
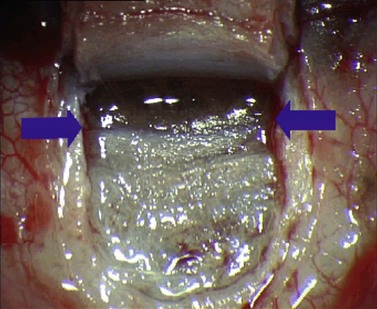
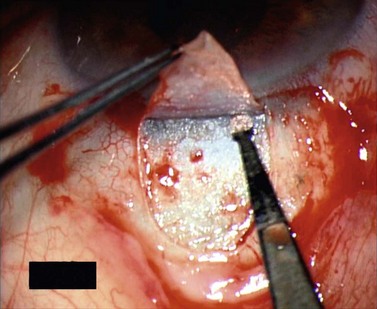
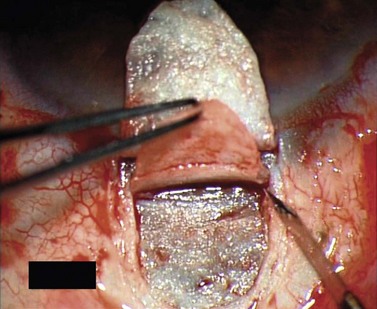
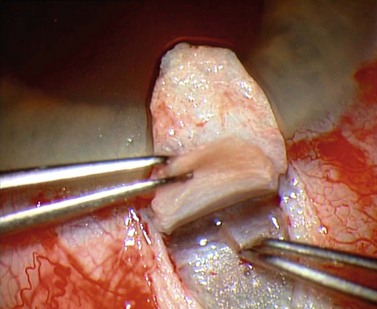
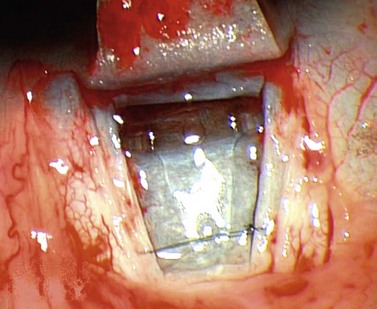
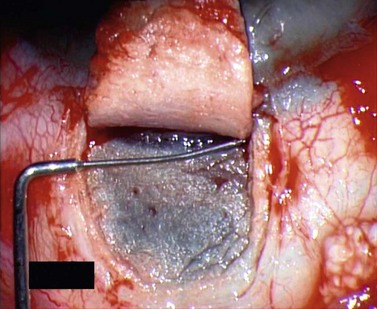
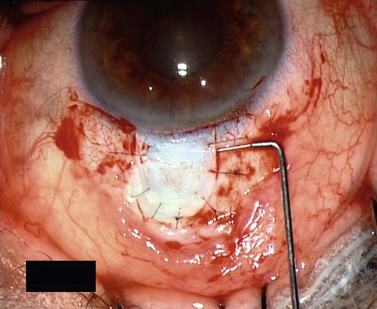
For good exposure of the surgical area, place a traction suture in the cornea or under the superior rectus. Raise a fornix-based conjunctival flap in the upper quadrant and lightly cauterize superficial blood vessels carefully, so as to preserve collector channels and to avoid scleral shrinkage and damage to Schlemm’s canal. Dissect a 5 × 5 mm superficial flap, approximately ⅓ scleral thickness, and advance anteriorly into clear cornea for about 1–1.5 mm (Fig. 38.3). To make the deep sclerokeratectomy, dissect a second 4 × 4 mm deep scleral flap. The dissection should be just deep enough to leave a thin layer of sclera (50–100 µm) over the choroid and the ciliary body with a dark reflex just visible below the scleral fibers. Start the deep sclerectomy posteriorly and carry it anteriorly until Schlemm’s canal is deroofed (Fig. 38.4). Advance the dissection into clear cornea to create the sclerodescemetic membrane, the site of aqueous filtration. Deepen the two lateral radial cuts and advance into clear cornea without touching the anterior trabeculum or Descemet’s membrane. By gently pulling the deep scleral flap with forceps and with counter traction on the bed of the canal using a triangular cellulose sponge, detach the anterior portion of the deep flap from the anterior trabecular meshwork and from Descemet’s membrane. Advance this 1–1.5 mm anteriorly. At this stage, there should be aqueous percolation through the membrane. To optimize outflow, peel the internal wall of Schlemm’s canal (the juxta-canalicular trabeculum and the canal endothelium) by grabbing it with thin forceps (‘external trabeculectomy’) (Fig. 38.5). This removes a homogeneous external trabecular membrane in one coherent plane and allows aqueous humor to egress through the remaining inner trabecular layers. Excise the deep flap by cutting it anteriorly.
To maintain the created space (‘intrascleral lake’ or ‘decompression chamber’) and to avoid postoperative scarring, different implants are used4. Absorbable porcine collagen implant (Aquaflow™, Staar surgical AG, Nidau, Switzerland), reticulate hyaluronic acid implant (HealaFlow™, Anteis, Geneve, CH), non absorbable implant (T Flux™, Ioltech Laboratoires, La Rochelle, France) or PMMA implant (Homdec SA, Belmont, Switzerland) can be sutured or positioned in the intrascleral space (Fig. 38.6).
Intraoperative complications
The two most common intraoperative complications are the inability to find Schlemm’s canal and perforation of the sclerodescemetic membrane. These complications are most common during the initial learning phase on the first 15–20 cases, where they can affect up to 30% of operated eyes. This falls to 3% with experience5. Inability to find the canal relates to an improper dissection of the deep scleral flap. Usually, fear of being too close to the choroid causes the surgeon to be too superficial, thus dissecting over the top of the canal. Generally, a careful deepening of the deep scleral flap suffices to reach and open the canal. Sclerodescemetic membrane ruptures can be as small as little holes or linear and transverse. Small ruptures without iris prolapse have no consequence. With shallow or flat anterior chambers, the external flap should be sutured tightly (consider releasable sutures). Long ruptures of Descemet’s membrane mostly occur at the junction with the anterior trabeculum, and are followed by iris prolapse. An iridectomy is always required with a conversion to trabeculectomy. This can be done by re-suturing into position the internal flap (in order to cover the rupture), and by doing a sclerectomy in its anterior portion. The superficial flap can then be sutured as in standard trabeculectomy. This complication is more common in eyes previously treated with argon laser trabeculoplasty.
Postoperative complications
Even if rare, blebitis can occur, but endophthalmitis has not yet been reported. Few cases of implant migration into the anterior chamber have occurred. This complication might occur in eyes with a perforated membrane and without, or with inadequate, fixation of the implant to the scleral bed. Cataract may occur late, but with a significantly lower incidence than that following trabeculectomy6,7. Scleral ectasia has been seen occasionally.
Results of surgery
Reported results vary from different follow-ups and techniques. In 2004, a meta-analysis of 29 articles found deep sclerectomy yielded an IOP <21 mmHg without medications in 69.7% of eyes without implant, 59.4% with collagen implant, and 71.1% with reticulated hyaluronic acid implant. No significant differences were found between the three techniques8. In 2008, a meta-analysis reported an IOP <21 mmHg in 68.7% of eyes after deep sclerectomy with implant, 48.6% after deep sclerectomy without implant, and 67.1% after deep sclerectomy with anti-metabolites (with or without implant)9. While this confirmed a higher success rate with a collagen implant, the role of anti-metabolites was confusing, in part because it included surgeries performed with different anti-metabolites (intraoperative mitomycin C, and intraoperative or postoperative 5-fluorouracil) and operations with and without implants. Randomized controlled studies comparing deep sclerectomy with and without mitomycin C (MMC) showed a better outcome when the anti-metabolite was used. Kozobolis et al. showed a success rate (IOP <22 mmHg without medications) of 72.5% without MMC and 95% with MMC, without significant different complication rates between the groups10. Neudorfer et al. reported a mean IOP decrease at 24 months of 48% and of 32% in the MMC and no-MMC groups respectively11.
When compared with trabeculectomy, randomized controlled studies, even if consistently showing a better safety profile for deep sclerectomy, do not agree on which procedure provides the better outcome. This relates to factors such as differences in technique, surgical experience, the use of anti-metabolites, and the use of goniopuncture. Overall, trabeculectomy provides lower IOP than deep sclerectomy , but when goniopuncture and/or anti-metabolites are added to DS, most studies show similar final IOP and success rates12–15.
Viscocanalostomy and canaloplasty
Fundamental principles
Similar to deep sclerectomy, the difference is to dilate Schlemm’s canal with injection of high molecular weight sodium hyaluronate through its opened ostia or through a probe inserted into the canal with a tensioning suture left inside canaloplasty. Schlemm’s canal dilation disrupts its inner and outer walls and disorganizes the juxta-canalicular zone, with resultant increase in conventional aqueous outflow facility, and uveoscleral outflow16. Unlike in deep sclerectomy, the superficial scleral flap is sutured watertight. This avoids external subconjunctival filtration: aqueous exits the decompression or intrascleral chamber mainly through the two ostia of Schlemm’s canal, and partly via the suprachoroidal space. Occasionally there is some external subconjunctival filtration.
Indications for surgery
Indications and contraindications are the same as in deep sclerectomy. In particular, for VC and CP to succeed, Schlemm’s canal and collector channels should not have been damaged by previous surgeries. Since the procedure provides final IOPs in the mid–high teens, it is mainly indicated in open angle glaucomas when target IOP is not very low. The absence (or very reduced) external filtration theoretically makes the technique safer, especially for eyes with chronic blepharitis, in contact lens wearers, or when the surgery has to be performed in the lateral or inferior quadrants. VC has been shown to be effective in uveitic glaucomas with well controlled inflammation17, in juvenile glaucomas18, and in congenital glaucomas19.
Operation techniques
Unlike in deep sclerectomy, dilation of Schlemm’s canal is the critical part of the procedure.
During viscocanalostomy, using the specific 165 µm cannula, high molecular weight sodium hyaluronate is slowly injected into Schlemm’s canal through the two ostia at the lateral edges of the inner flap (Fig. 38.7). To avoid damage to the canal endothelium, do not insert the cannula more than 1–1.5 mm from the ostia. The slow injection should be repeated 6–7 times on each side to avoid tears and ruptures of the canal.
During canaloplasty, a 200 µm microcatheter with a 250 µm distal tip incorporating an illuminating optical fiber is inserted into Schlemm’s canal through its open ostium. The catheter is gently pushed through the canal until its tip exits from the contralateral ostia (Fig. 38.8). A 10-0 prolene suture is tied to the distal tip and the catheter is withdrawn, pulling the suture into and along the canal. While withdrawing, 4–6 mg of sodium hyaluronate 1.4% should be injected every 2 hours. Once the suture has been pulled right around, it is cut from the catheter and tied to form a tight loop encircling Schlemm’s canal. This technique provides both viscodilation and a tensioning of the inner wall of the canal20.
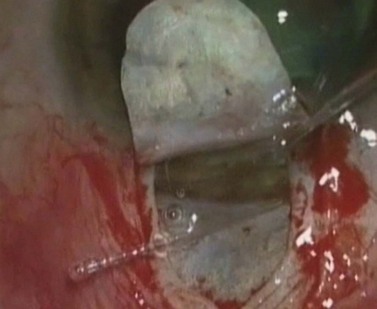
Fig. 38.8 During canaloplasty, a probe is passed through Schlemm’s canal.
Courtesy of P. Brusini M.D.
In contrast with DS, the superficial flap is sutured watertight to avoid external filtration. To seal the ‘intrascleral chamber’, the outer scleral flap is sutured with 6 or 7 10-0 nylon stitches. The different sizes of the two flaps allows a tight apposition of the external flap (Fig. 38.9). Finally, to minimize bleeding and to prevent collapse and scarring, high molecular weight sodium hyaluronate is injected underneath the flap (Fig. 38.10).
Intraoperative complications
As the viscocanalostomy surgical technique is very similar to deep sclerectomy, intraoperative complications are mostly the same. An excessive or too-rapid injection of sodium hyaluronate into Schlemm’s canal can rupture the trabecular meshwork with viscoelastic entering the anterior chamber. At the end of the procedure, an excessive amount of viscoelastic into the intrascleral chamber can break the sclerodescemetic membrane or detach Descemet’s membrane. During canaloplasty, complications include the inability to catheterize Schlemm’s canal in full, partial breaks in Schlemm’s canal with the creation of a false path, and micro or gross hyphemas21.
Postoperative complications
Viscocanaloplasty is safe with the few postoperative side effects mainly related to intraoperative technical complications. Postoperative management is less demanding with significantly less refractive change21 and eye discomfort than trabeculectomy22, as could be expected given the absence of a filtering bleb in most cases. Postoperative complications are similar to deep sclerectomy. Since viscocanalostomy is mostly independent of external filtration, excessive healing does not affect the outcome and does not require any specific intervention. Viscocanalostomy, due to its different mechanisms of action, requires fewer goniopunctures compared with deep sclerectomy.
Canaloplasty can be affected by suture extrusion through the trabecular meshwork into the anterior chamber20.
Results of surgery
As with deep sclerectomy, a direct comparison between different studies is difficult because criteria for success, length of follow-up, and techniques are different. Viscocanalostomy has been found effective to lower IOP with a good safety profile. In 2004, a meta-analysis of eight articles reported a success rate for viscocanalostomy (IOP <21 mmHg without medications) of 72.0%8. More recently, meta-analysis of 14 articles reported an IOP below 21 mmHg in 51.1% of the eyes at 25.6 months9. The mean final IOP at 20.4 months was 16.4 mmHg (range 12–20 mmHg). This meta-analysis was biased by inclusion of articles from a group operating within their learning curve, with a success at the beginning as low as 0%23, which increased to 30% by enlarging the sample size24, thus affecting the final result.
The only reported study on canaloplasty showed, at 24 months, a mean final pressure of 16.3 ± 3.7 mmHg (15.7 ± 3.1 mmHg in eyes with greater distension of the canal)25. An IOP <18 mmHg with or without medications in all postoperative visits from 6 months onward was found in up to 50% of the eyes.
When compared with trabeculectomy in randomized controlled trials, viscocanalostomy provided higher final mean IOPs and a lower success rate for IOPs in the low teens. In most of these studies, these differences did not reach statistical significance22,26–29.
No studies to date, have compared canaloplasty with trabeculectomy.
1 European Glaucoma Society: 3. Treatment principles and options. In Terminology and Guidelines for Glaucoma, 3rd edn, Savona: Editrice Dogma; 2008:155.
2 Carassa RG, Goldberg I. Non penetrating glaucoma drainage surgery. In: Weinreb RN, Crowston JG, editors. Glaucoma surgery. Open Angle Glaucoma. The Hague: Kugler; 2005:91-106.
3 Auer C, Mermoud A, Herbort CP. Deep sclerectomy for the management of uncontrolled uveitic glaucoma: preliminary data. Klin Monatsbl Augenheilkd. 2004;221:339-342.
4 Mendrinos E, Mermoud A, Shaarawy T. Nonpenetrating glaucoma surgery. Surv Ophthalmol. 2008;53:592-630.
5 Sanchez E, Schnyder CC, Mermoud A. Comparative results of deep sclerectomy transformed to trabeculectomy and classical trabeculectomy. Klin Monatsbl Augenheilkd. 1997;210:261-264.
6 Karlen ME, Sanchez E, Schnyder CC. Deep sclerectomy with collagen implant: medium term results. Br J Ophthalmol. 1999;83:6-11.
7 Shaarawy T, Karlen M, Schnyder C. Five-year results of deep sclerectomy with collagen implant. J Cataract Refract Surg. 2001;27:1770-1778.
8 Cheng JW, Ma XY, Wei RL. Efficacy of non-penetrating trabecular surgery for open angle glaucoma: a meta analysis. Chin Med J. 2004;117:1006-1010.
9 Hondur A, Onol M, Hasanreisoglu B. Nonpenetrating glaucoma surgery: meta-analysis of recent results. J Glaucoma. 2008;17:139-146.
10 Kozobolis VP, Christodoulakis EV, Tzanakis N, et al. Primary deep sclerectomy versus primary deep sclerectomy with the use of mitomycin C in primary open-angle glaucoma. J Glaucoma. 2002;11:287-293.
11 Neudorfer M, Sadetzki S, Anisimova S, et al. Nonpenetrating deep sclerectomy with the use of adjunctive mitomycin C. Ophthalmic Surg Lasers Imaging. 2002;35:6-12.
12 Mermoud A, Schnyder C, Sickenberg M, et al. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open angle glaucoma. J Cataract Refract Surg. 1999;25:323-331.
13 Wang N, Wu H, Ye T Chen X, et al. Analysis of intraoperative and early post-operative complications and safety in non penetrating trabecular surgery. Zhonghua Yan Ke Za Zhi. 2002;38:329-334.
14 Schwenn O, Springer C, Troost A, et al. Deep sclerectomy using hyaluronate implant versus trabeculectomy. A comparison of two glaucoma operations using mitomycin C. Ophthalmologe. 2004;101:696-704.
15 Cillino S, Di Pace F, Casuccio A, et al. Deep sclerectomy versus punch trabeculectomy: effect of low-dosage mitomycin C. Ophthalmologica. 2005;219:281-286.
16 Tamm ER, Carassa RG, Albert DM, et al. Viscocanalostomy in Rhesus monkeys. Arch Ophthalmol. 2004;122:1826-1828.
17 Miserocchi E, Carassa RG, Bettin P, et al. Viscocanalostomy in patients with uveitis: a preliminary report. J Cataract and Refr Surg. 2004;30:566-570.
18 Stangos AN, Whatham AR, Sunaric-Megevand G. Primary Viscocanalostomy for juvenile open-angle glaucoma. Am J Ophthalmol. 2005;140:490-496.
19 Noureddin BN, El-Haibi CP, Cheikha A, et al. Viscocanalostomy versus trabeculotomy ab externo in primary congenital glaucoma: 1-year follow-up of a prospective controlled pilot study. Br J Ophthalmol. 2006;90:1281-1285.
20 Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Interim clinical study analysis. J Cataract Refract Surg. 2007;33:1217-1226.
21 Egrilmez S, Ates H, Nalcaci S, et al. Surgically induced corneal refractive change following glaucoma surgery: nonpenetrating trabecular surgeries versus trabeculectomy. J Cat Refract Surg. 2004;30:1232-1239.
22 Carassa RG, Bettin P, Fiori M, et al. Viscocanalostomy versus Trabeculectomy in white adults affected by open-angle glaucoma: a 2-year randomized, controlled trial. Ophthalmology. 2003;110:882-887.
23 Jonescu-Cuypers C, Jacobi P, Konen W, et al. Primary viscocanalostomy versus trabeculectomy in white patients with open-angle glaucoma: A randomized clinical trial. Ophthalmology. 2001;108:254-258.
24 Luke C, Dietlein TS, Jacobi PC, et al. A prospective randomized trial of viscocanalostomy versus trabeculectomy in open-angle glaucoma: A 1-year follow-up study. J Glaucoma. 2002;11:294-299.
25 Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Two-year interim clinical study results. J Cataract Refract Surg. 2009;35:814-824.
26 O’Brart DSP, Rowlands E, Islam N, et al. A randomised, prospective study comparing trabeculectomy augmented with anti-metabolites with a viscocanalostomy technique for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol. 2002;86:748-754.
27 Yalvac IS, Sahin M, Eksioglu U, et al. Primary viscocanalostomy versus trabeculectomy for primary open-angle glaucoma: three-year prospective randomized clinical trial. J Cataract Refract Surg. 2004;30:2050-2057.
28 Yarangumeli A, Gureser S, Koz OG, et al. Viscocanalostomy versus trabeculectomy in patients with bilateral high tension glaucoma. Int Ophthalmol. 2004;25:207-213.
29 Gilmour DF, Manners TD, Devonport H, et al. Viscocanalostomy versus trabeculectomy for primary open angle glaucoma: 4-year prospective randomized clinical trial. Eye. 2009;23:1802-1807.