8 Non-Neoplastic Pathology of the Large and Small Airways
The Conducting Airways
The trachea and conducting airways play a vital role in lung function. The diseases that affect these structures are commonly related to inhalation exposure but also may arise from developmental anomalies and systemic diseases (Box 8-1). In this section, topics are presented in anatomic order, beginning with the trachea and proceeding distally to the bronchi and bronchioles.
The Trachea
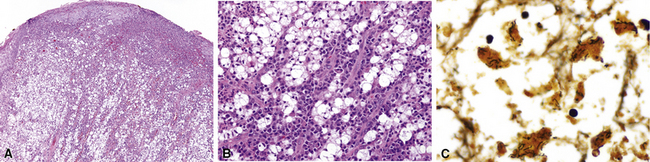
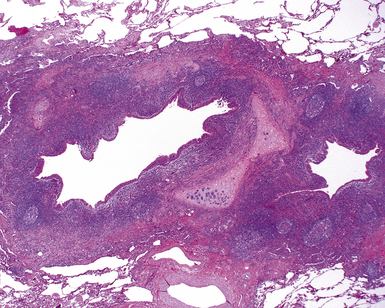
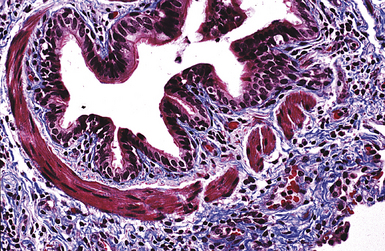
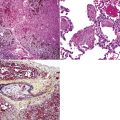
Non-neoplastic pathology of the trachea can be divided broadly into extrinsic and intrinsic diseases. Among extrinsic diseases are certain infections, such as rhinoscleroma (caused by Klebsiella rhinoscleromatis1,2) (Fig. 8-1), and systemic autoimmune diseases that affect cartilage (e.g., Wegener granulomatosis3,4). These diseases may cause tracheal obstruction or collapse and may also involve the larynx or the conducting airways, or both. Diseases intrinsic and unique to the trachea are few and mainly of congenital and/or metabolic origin. In the following section, we will describe three of these entities: tracheal amyloidosis, tracheobronchomalacia, and tracheobronchopathia osteochondroplastica. The latter may be rare but deserves mention as it can be encountered incidentally during bronchoscopy.
Tracheal Amyloidosis
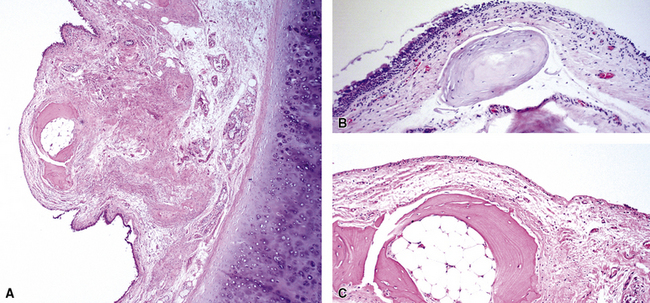
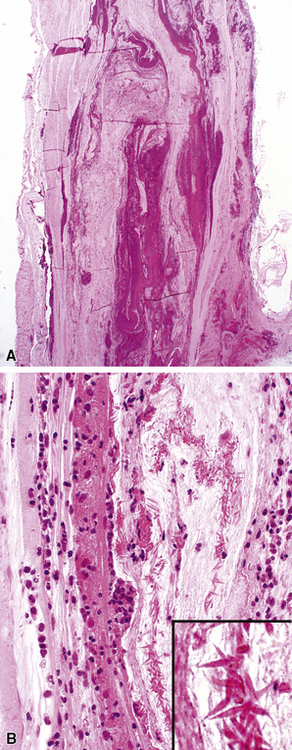
Tracheobronchial amyloidosis is an idiopathic disease characterized by amyloid deposition, often throughout the tracheobronchial tree5,6 but sometimes restricted to the larynx and trachea.7 Approximately 150 cases have been reported in the literature. Tracheobronchial amyloidosis most frequently produces dyspnea, wheezing (sometimes misdiagnosed as asthma), recurrent pneumonia, and atelectasis, which are believed to be sequelae of the narrowed airways. Pulmonary function tests usually show fixed obstruction. On histopathologic examination, amyloid deposits usually are seen in the submucosa and may involve the entire tracheal or bronchial wall, occurring in irregular masses or sheets (Fig. 8-2). Amyloid may be accompanied by multinucleate giant cells; calcification and ossification are frequent5,6,8,9 (Fig. 8-3). When amyloid is identified in bronchoscopic biopsy specimens, this typically is an organ-limited manifestation, similar to nodular lung parenchymal amyloid.10 Of the three major types of amyloid, AL, AA, and transthyretin, the AL type is the most common in tracheobronchial amyloid deposits. The prognosis is poor: Nearly one third of patients die within 7 to 12 years of diagnosis.6 Therapy is limited to debulking procedures and stent placement for localized lesions, although some evidence suggests that radiation therapy may provide more definitive treatment.11–14
Tracheobronchomalacia
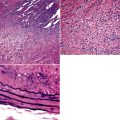
Tracheobronchomalacia (TBM) is a term used to encompass a number of conditions characterized by a decrease in the structural rigidity of the trachea and conducting airways. Such changes are manifested clinically by excessive expiratory collapse of the central airways owing to increased flaccidity or redundancy of the posterior membranous airway wall, or to weakness of the supporting cartilage (a recent in-depth review of the disease is available15). Although typically a disease process of the central airways, TBM has been shown to frequently have associated air trapping on computed tomography (CT) scan, an indicator of small-airway disease.16,17
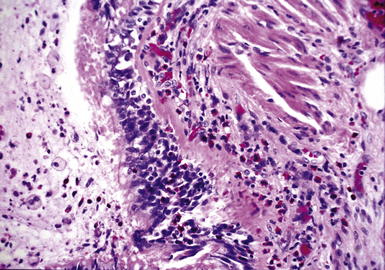
Both children and adults may be affected.18 In children, the disease most often is related to prematurity and the requirement for prolonged mechanical ventilation. In these patients, TBM appears to be self-limited.19,20 In adults, TBM is seen in the middle-aged and the elderly and is a progressive condition, commonly seen in association with COPD.16 Other reported associations in adults include prolonged intubation, vascular anomalies, and exposure to mustard gas.21 TBM may complicate amyloidosis and rheumatoid arthritis when these diseases affect the airways22 and may be related to relapsing polychondritis, a systemic disease affecting the cartilaginous tissue in several organs, including the airways.18,23–25 Pathologically, the tracheal wall is soft, as a result of inflammatory destruction of the cartilage (Fig. 8-4). Over time, the cartilage is replaced by fibrous tissue, accompanied by inflammatory infitration and reparative vascular proliferation.
Treatment is variable and depends on symptom severity, with conservative therapy appropriate for mildly symptomatic cases and more invasive therapy indicated for severely symptomatic cases, including tracheoplasty (for reduction of expiratory tracheal collapse and symptomatic improvement).26 In the absence of treatment, severely symptomatic TBM can lead to significant respiratory morbidity and rarely may be fatal.
Tracheobronchopathia Osteochondroplastica
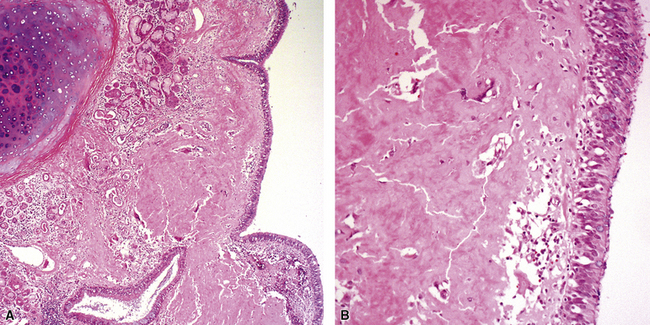
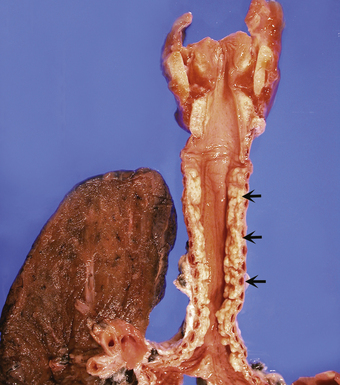
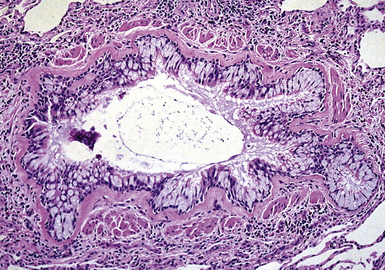
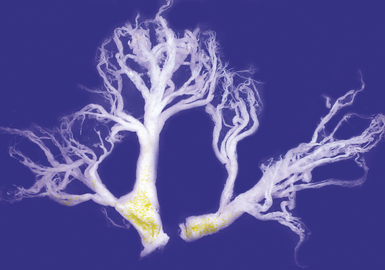
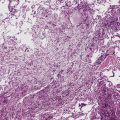
Tracheobronchopathia osteochondroplastica is a rare lung disease characterized by the presence of cartilaginous or osseous submucosal nodules that bulge into the lumen of the trachea and bronchi (Fig. 8-5), with sparing of the posterior membranous portion.27–30 The etiology and pathogenesis are unknown. The disease affects adults more commonly than children, with a predilection for males. Most cases are asymptomatic, and are most often diagnosed incidentally during intubation or bronchoscopy. A minority of patients may experience cough, dyspnea, and hemoptysis.
The bronchoscopic appearance alone is usually diagnostic, and biopsy is seldom if ever required.31 In the rare bronchoscopic biopsy showing the cartilaginous or ossified lesions of tracheobronchopathia osteochondroplastica (Fig. 8-6), the differential diagnosis includes ossified amyloid deposits. Other conditions associated with endoluminal nodular lesions include endobronchial sarcoidosis, endobronchial granulomatous infections, papillomatosis, and tracheobronchial calcinosis.
The Bronchi
The conducting airways begin at the carina and extend distally in the lung to the level of the noncartilaginous bronchioles. Although many diffuse lung diseases may involve the large airways secondarily, some primary diseases also may affect the bronchi and are presented here. Asthma-associated diseases, typically observed in the large airways, are discussed later in the final section of this chapter. Cystic fibrosis and ciliary disorders are not discussed here, because detailed expositions can be found in other sources.32,33
Bronchitis
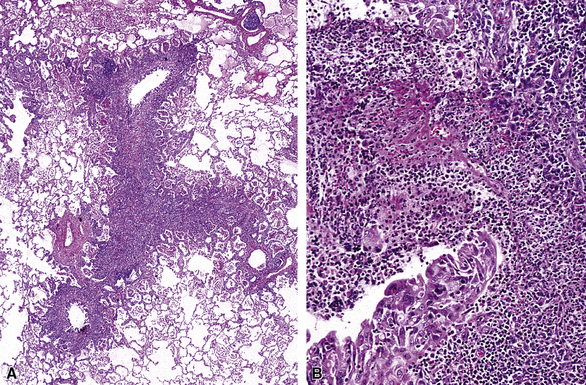
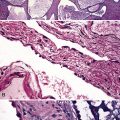
Infectious and inflammatory conditions of the large airways are particularly relevant to the surgical pathologist and the cytopathologist, given the prominence of inflammatory manifestations seen in biopsies obtained through the bronchoscope (endobronchial and transbronchial specimens). In practice, the presence of acute or chronic inflammation in endobronchial biopsies, in which bronchial mucosa and muscle or cartilage are evident, demands a descriptive diagnosis (Fig. 8-7). It is best to avoid the use of the term “chronic bronchitis” in reference to such inflammatory changes involving respiratory mucosa, because it carries a clinical implication regarding the diffuse nature of the disease and specific clinical manifestations. When bronchitis occurs as a direct result of respiratory infection, necrosis of the mucosa may be present (acute necrotizing bronchitis). In the postinfection period, residual chronic inflammatory changes may be the dominant findings. Unfortunately, the list of possible etiologic disorders associated with chronic inflammation in bronchial mucosa is quite long (Box 8-2) and sufficiently diverse as to not be useful in narrowing the scope of the clinical differential diagnosis. For this reason, a careful search for other, more specific findings is always in order, and these are worth mentioning as a list of pertinent negative findings (e.g., vasculitis, granulomas, necrosis, tumor).
Bronchiectasis
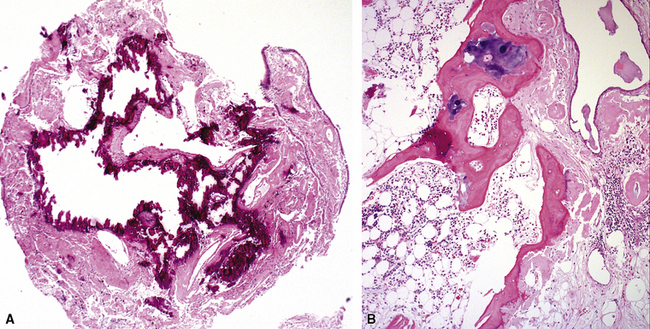
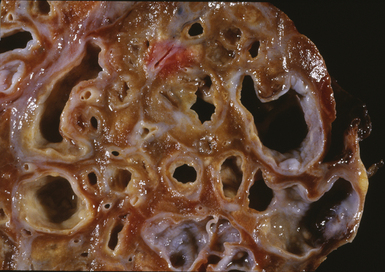
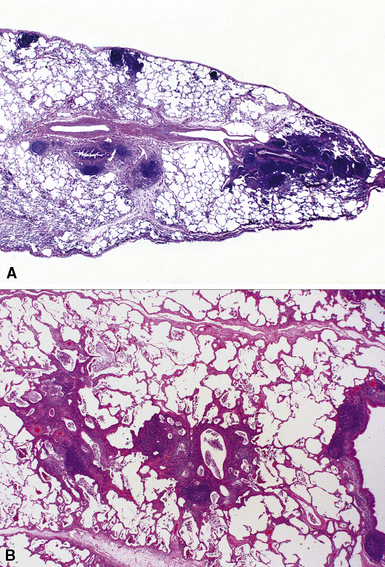
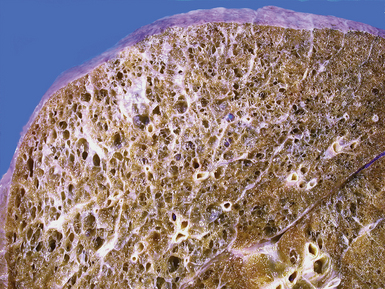
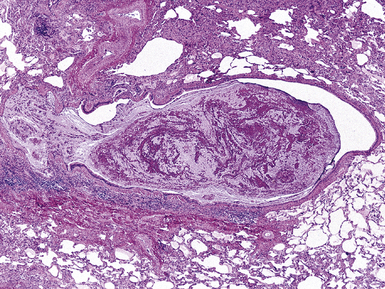
Bronchiectasis is defined as a permanent dilatation of the cartilaginous airways (bronchi), often attended by acute and chronic inflammation. Conceptually, bronchiectasis can be considered the end result of any number of conditions that damage the airway wall and result in weakening over time and dilatation.34 Primary causes are most commonly related to inherited abnormalities (ciliary dysfunction, abnormal mucus production), whereas secondary causes are numerous. In a review of multiple published series of bronchiectasis between 1935 and 1981, Barker and Bardana35 found a significant proportion of idiopathic cases (as much as 30% according to Nicotra and associates36). Among recognizable causes of bronchiectasis are infections, primary ciliary dyskinesia, immunodeficiency, cystic fibrosis, rheumatoid arthritis, inflammatory bowel disease,37 and graft-versus-host disease.38 An intriguing implication of Helicobacter pylori in the pathogenesis of bronchiectasis has been raised in some studies.39–41 In specific conditions such as cystic fibrosis, bronchiectasis may dominate the pulmonary pathology. Bronchiectasis, outside the setting of cystic fibrosis, often is perceived to be rare in Western societies but remains an important cause of chronic suppurative lung disease in the developing world. The decline in hospital admission rates for pediatric bronchiectasis in the Western countries has been noted since the 1950s and has been attributed to improvements in sanitation and nutrition, introduction of childhood immunization (particularly against pertussis and measles), and the early and frequent use of antibiotics.
Bronchiectasis is a radiologic diagnosis in the living patient but has distinctive features in resected lungs (Fig. 8-8) and lobes and sometimes in surgical biopsies. The proposed historical classification of bronchiectasis divided the process into three types:
Presenting signs and symptoms include cough with production of purulent sputum, fever, shortness of breath, and, occasionally, hemoptysis. No age or sex predilection has been noted, and the disease tends to run a course of recurrent exacerbation, sometimes with superimposed infection. Inflammatory changes in biopsies obtained with the bronchoscope correlate poorly with the presence or absence of bronchiectasis. Nevertheless, such specimens may be useful for ancillary laboratory studies (such as ultrastructural studies in search of ciliary abnormalities). On plain films of the chest, classic parallel linear opacities (referred to as “tram tracks”) correspond to thickened bronchial walls, whereas tubular opacities reflect mucus-filled bronchi. Today, most cases of bronchiectasis are diagnosed by HRCT.42,43 Overall, the sensitivity of HRCT in detecting bronchiectasis is quite high.44,45 The HRCT findings in bronchiectasis are summarized in Box 8-3.
Box 8-3 High-Resolution Computed Tomography Findings in Bronchiectasis
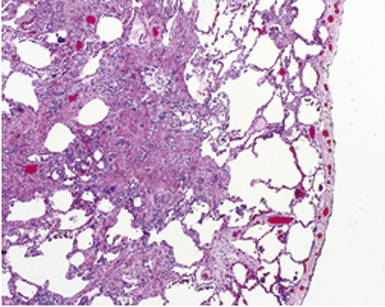
The gross findings in bronchiectasis have been well described in autopsy series.46 For the histopathologist, acute and chronic inflammatory changes dominate the bronchoscopic biopsy picture (Fig. 8-9). The presence of necrotic debris and mucus in airways may be a sign of coexistent bronchiectasis, but in most instances, even surgical lung biopsy findings only reflect “downstream” secondary manifestations of obstruction or infection occurring more proximally. Lymphoid hyperplasia surrounding the large airways may occur in bronchiectasis and bronchoscopically derived biopsies may sample such hyperplastic lymphoid tissue, raising concern for tumor on occasion (especially when a portion of a germinal center dominates the small biopsy specimen). Caution is always advisable in these settings to avoid the overdiagnosis of lymphoproliferative disease. Also, the necrotic granular debris that may accompany bronchiectasis may be sampled by bronchoscopic biopsy, thereby raising concern for necrotic neoplasm or abscess. Providing a broad differential diagnosis is the best approach here, to avoid confusion and possible misdirection of clinical management.
Middle Lobe Syndrome
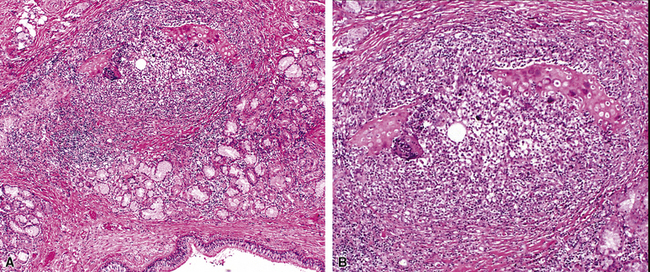
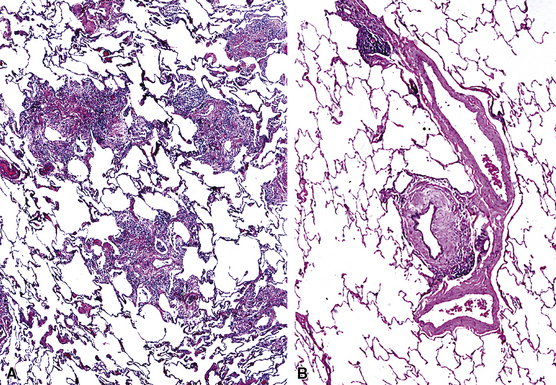
The concept of “right middle lobe syndrome” was highlighted in studies of children with chronic failure to thrive and right middle lobe abnormalities on chest radiographs. The proposed hypothesis was based on the notion that lymphoid hyperplasia occurring around the right middle lobe bronchus produced compression and narrowing of the bronchial lumen. Also, the position of the middle lobe (and the lingula of the left lung) relative to the remainder of the tracheobronchial tree makes it susceptible to obstruction and secondary chronic inflammatory changes in the lobe (long narrow bronchus). In clinical practice, the disease occurs more frequently in adults and often without an identifiable obstructing lesion.47 This population consists predominantly of middle-aged or elderly women, and the condition has been referred to as “Lady Windermere syndrome.”48–50 A role for Mycobacterium avium complex infection has been postulated in the pathogenesis of middle lobe syndrome, although it remains unclear whether this is an epiphenomenon related to the common occurrence of bronchiectasis in this condition. Consolidation of the right middle lobe, or lingula, attended by bronchiectasis, is typical.34,47
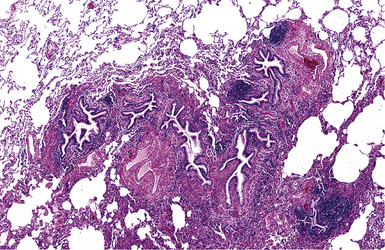
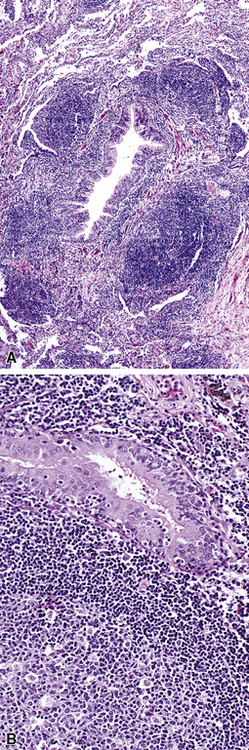
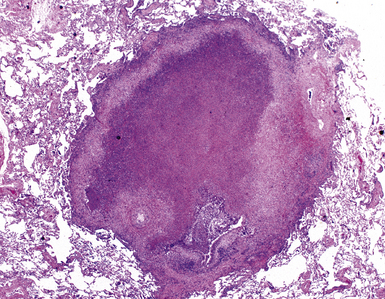
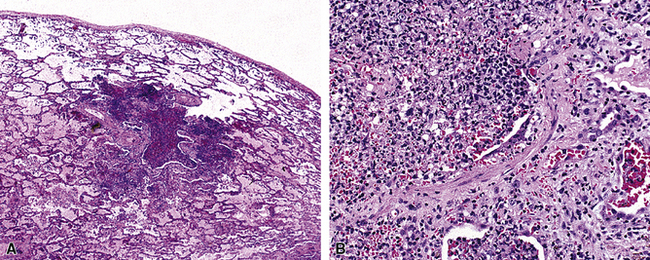
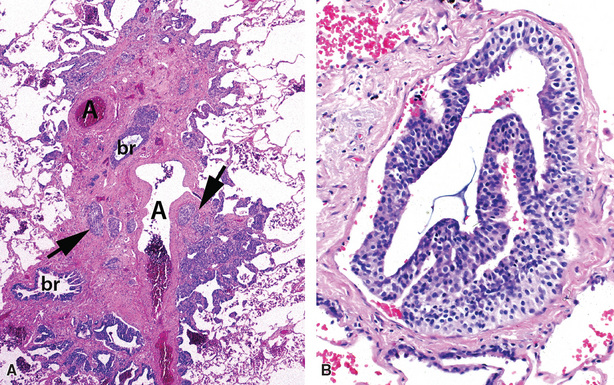
In surgical lung biopsies of the right middle lobe or lingula, advanced remodeling, granulomatous inflammation, extensive inflammatory infiltrates, and mucostasis, when present together, should always suggest the possibility of middle lobe syndrome (Fig. 8-10). The presence of granulomas in the context of middle lobe syndrome should raise suspicion for colonization by atypical mycobacterial species, and acid-fast organisms may be identified in granulomas.48 A number of inflammatory changes are observed in middle lobe syndrome, and these are listed in Box 8-4.47,51 Removal of any obstructing lesions, whether neoplasm or other, in early stages of the disease process may avoid the need for lobectomy.52
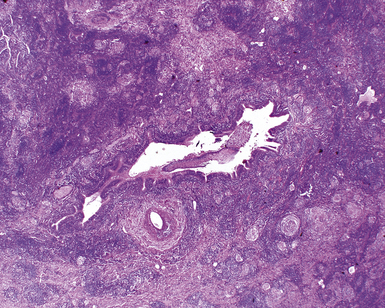
Figure 8-10 Middle lobe syndrome. The bronchial wall shows prominent lymphoid infiltration, with many germinal centers.
The Small Airways (Bronchioles and Alveolar Ducts)
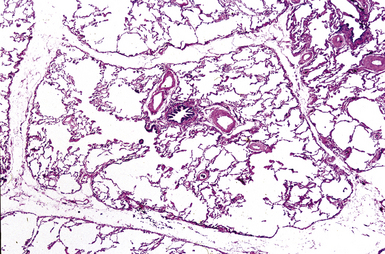
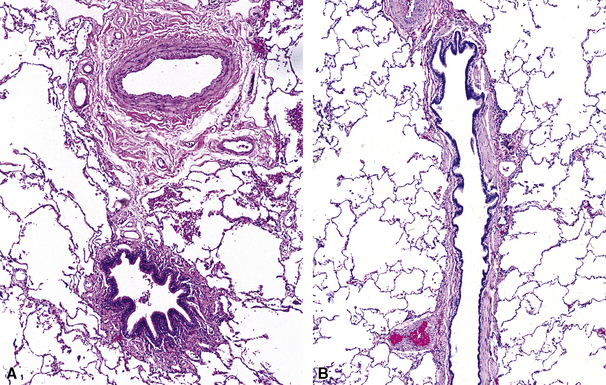
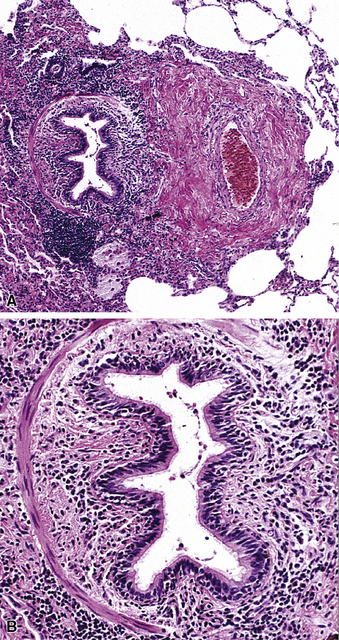
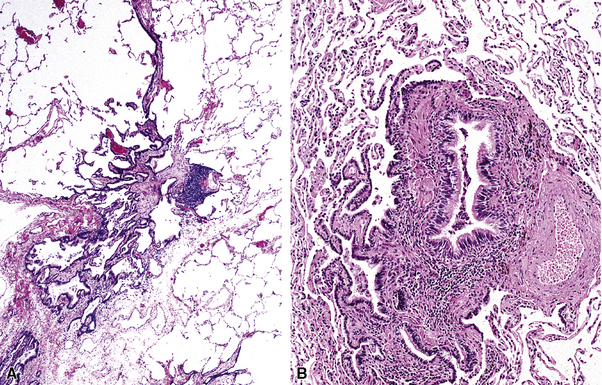
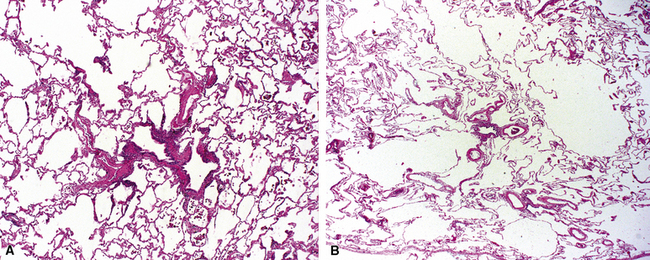
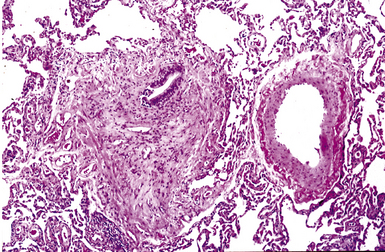
The small airways of the lung include the small bronchi, with a diameter less than 2 to 3 mm, and the membranous bronchioles (terminal and respiratory bronchioles). Terminal bronchioles have a diameter of less than 1 mm and are just proximal to the respiratory bronchioles, the first airways that have alveoli budding from their walls. The terminal bronchioles are located in the center of the pulmonary lobule and, like all conducting airways, are always accompanied by a pulmonary artery branch (Fig. 8-11). In cross-sectional views, their respective diameters are approximately equal (Fig. 8-12A). The lumen of the terminal bronchiole is uniform in longitudinal histologic sections, but minor variations in size can be seen (see Fig. 8-12B).
It is said that the small airways constitute the “Achilles heel” of the lung, because they play an important role in air distribution and flow but lack the rigid structure of the bronchi to protect them from collapse during exhalation, especially when affected by disease. The small airways can be affected secondarily by inflammatory diseases that involve primarily the bronchi or the alveoli, or both, or by primary diseases that selectively involve these delicate anatomic structures. No single classification of bronchiolar disorders has been widely accepted, although any number of classification schemes have been proposed based on cause and underlying diseases, radiologic features, histopathologic findings, or some combination of these parameters.53–56 As recently proposed by Ryu and colleagues, bronchiolar disorders can be divided into three groups: primary bronchiolar disorders, bronchiolar involvement in interstitial lung diseases, and bronchiolar involvement in large airway disease57,58 (Box 8-5). From a histopathologic point of view it should be pointed out that patients rarely come to lung biopsy with a preoperative diagnosis of “small airway disease.” The reasons for this are legion but mainly reflect the fact that disorders of the small airways are relatively “silent” clinically and radiologically. In addition, some of the small- airway lesions are quite subtle histologically and, at first glance, may be overlooked.59,60
Box 8-5 Classification of Bronchiolar Disorders
Adapted from Ryu JH. Classification and approach to bronchiolar diseases. Curr Opin Pulm Med. 2006;12(2):145–151; and Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168(11):1277–1292.
It is the rare lung biopsy that does not show some degree of histopathologic alteration in the small airways. Critical to the evaluation of such changes is knowledge of the clinical manifestations, the radiologic distribution of disease (whether localized or bilateral and diffuse), and the patient’s cigarette smoking history. Attempts to predict clinical disease based on isolated abnormalities of the small airways, as viewed through the microscope, often are unrewarding. The HRCT scan may provide valuable information regarding the possible pathologic pattern of disease, as shown in Table 8-1.
Table 8-1 High-Resolution Computed Tomography Abnormalities of the Small Airways
| Radiologic Finding | Pathologic Pattern |
|---|---|
| Centrilobular nodularity | Cellular bronchiolitis |
| Follicular bronchiolitis | |
| Respiratory bronchiolitis | |
| Peribronchial nodules | Follicular bronchiolitis |
| “Tree in bud” pattern | Cellular bronchiolitis |
| Panbronchiolitis | |
| Diffuse or “geographic” air-trapping | |
| Mosaic pattern | Constrictive bronchiolitis |
| Bronchioloectasis, bronchiectasis | Panbronchiolitis |
| Patchy ground-glass attenuation | Respiratory bronchiolitis |
| Ground-glass opacity | Follicular bronchiolitis |
Modified from Lynch DA. Imaging of small airways diseases. Clin Chest Med. 1993;14(4): 623–634.
Inflammatory Bronchiolitis
Inflammatory bronchiolitis, also known as cellular bronchiolitis, is a generic term that includes acute bronchiolitis, chronic bronchiolitis, and combined forms with variable amounts of both types of processes. All forms may be associated with bronchiolar fibrosis.60 An important point is that both acute and chronic forms of bronchiolitis may have significant overlap, with one form typically being dominant. Diseases of the airways rarely have histopathologic features sufficiently specific to point to a single etiologic disorder or a specific disease in the absence of some characteristic finding (e.g., viral inclusions, aspirated foreign material). When the surgical pathologist is confronted with isolated inflammatory changes in the small airways, it is important to invoke a differential diagnosis to stimulate clinicopathologic correlation, and thereby help to narrow considerations in the clinical differential diagnosis.
Acute Bronchiolitis
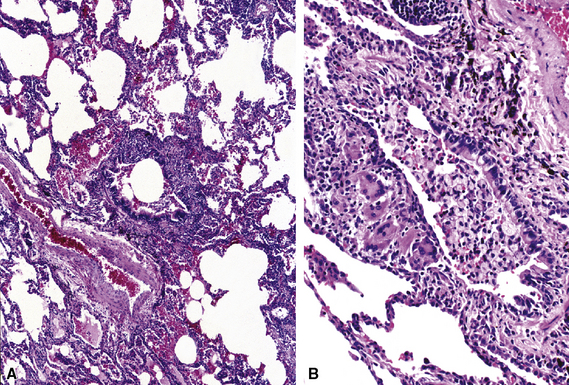
Florid acute bronchiolitis is characterized by predominantly acute inflammation of the small airways, with variable epithelial sloughing. Extension of acute inflammation into surrounding peribronchiolar parenchyma is typical (Fig. 8-13). Very often, acute bronchiolitis is associated with some degree of chronic inflammatory infiltration, as described below. Conditions associated with relatively pure acute bronchiolitis include the early phase of certain infections (most of which also produce epithelial necrosis).61,62 A careful search for viral inclusions is especially relevant in this scenario. Acute fume or toxic gas inhalation can also produce acute small-airway injury, with few if any chronic changes. Loose connective tissue polyps may develop as a consequence of basement membrane disruption with fibroblastic migration into the airway lumens from subepithelial regions (so-called Masson polyps). Acute aspiration can manifest occasionally as acute bronchiolitis (most commonly seen at autopsy, in cases in which aspiration may have been part of the terminal event). Acute bronchiolitis can be an uncommon manifestation of Wegener granulomatosis.63
Acute and Chronic Bronchiolitis
Acute and chronic inflammation of the small airways (Fig. 8-14) is one of the most common manifestations of so-called cellular bronchiolitis.64 This inflammatory process frequently is associated with other bronchiolocentric manifestations, including intraluminal polyp formation, constriction and obliteration, and presence of lymphoid follicles within the peribronchiolar sheath (follicular bronchiolitis).
When acute and chronic bronchiolitis are the primary manifestation in the lung biopsy, clinical manifestations may be accompanied by completely obstructive, completely restrictive, or a mixture of obstructive and restrictive pulmonary physiology. HRCT scans may show a predominantly nodular pattern, although reticulonodular infiltrates are more commonly observed. Box 8-6 lists the most frequent diseases associated with acute and chronic bronchiolitis.
Chronic Bronchiolitis
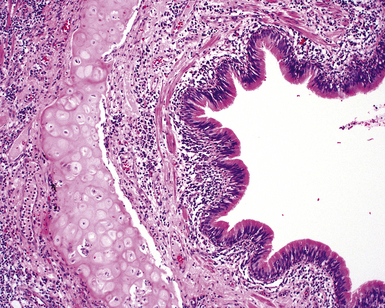
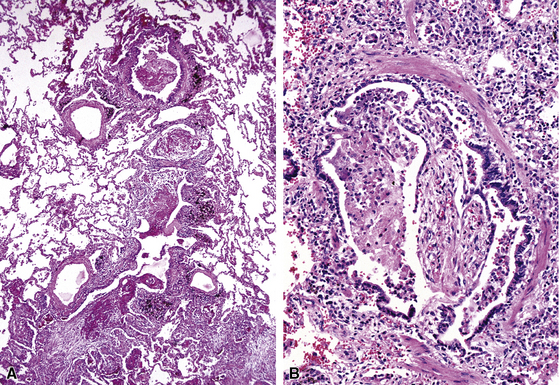
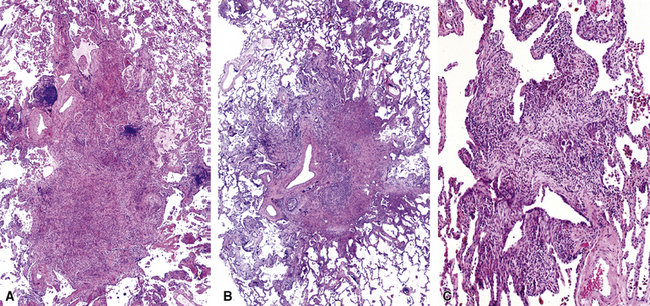
Chronic inflammation within and surrounding the walls of small airways (Figs. 8-15 and 8-16) can be seen in several conditions (Box 8-7). Chronic bronchiolitis can occur with or without lymphoid follicles or some degree of fibrosis. Follicular bronchiolitis is a specific subtype of chronic bronchiolitis, characterized by the presence of lymphoid follicles with well-formed germinal centers surrounding the bronchiolar walls, sometimes with lymphocytes migrating in the respiratory epithelium, either singly or in small clusters (Figs. 8-17 to 8-19). Follicular bronchiolitis may be seen in several connective tissue diseases.65–69 In cases with dense and extensive lymphoid infiltrates, especially if lymphoepithelial lesions are prominent, one should always consider the possibility of a lung lymphoma, most of which are low-grade B cell lymphomas of extranodal marginal zone type, arising from the mucosa-associated lymphoid tissue (MALT)70–72 (Fig. 8-20). Follicular bronchiolitis may also be seen in some occupational exposure settings, such as nylon flocking workers,73–77 and in immunodeficiency syndromes including the acquired immunodeficiency syndrome (AIDS) related to human immunodeficiency virus (HIV) infection.78
Granulomatous Bronchiolitis
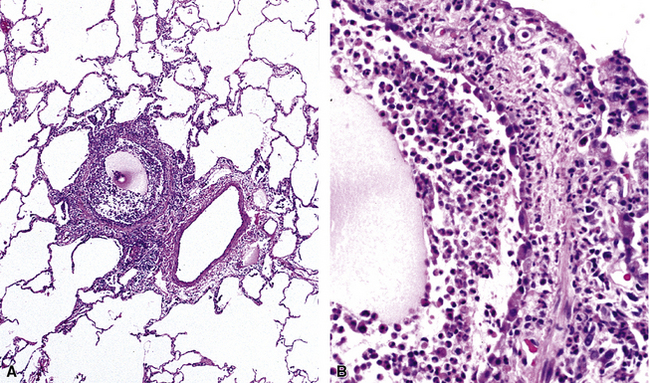
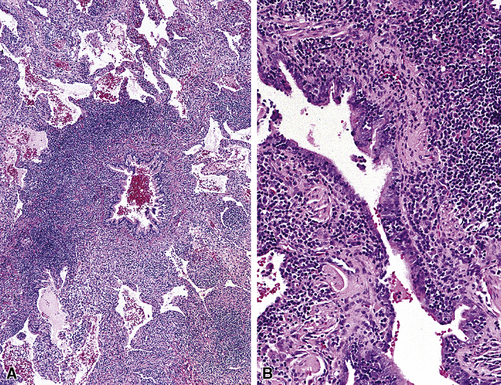
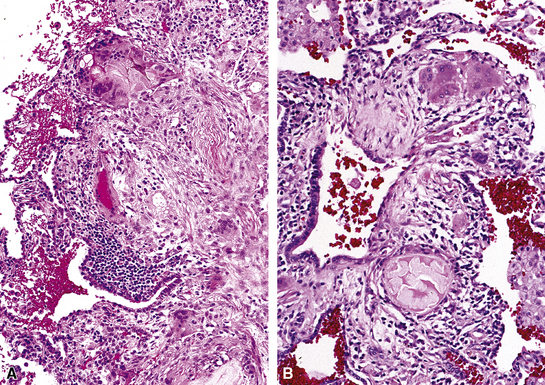
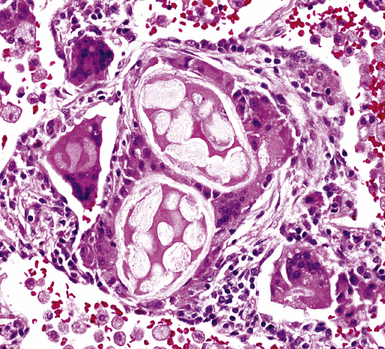
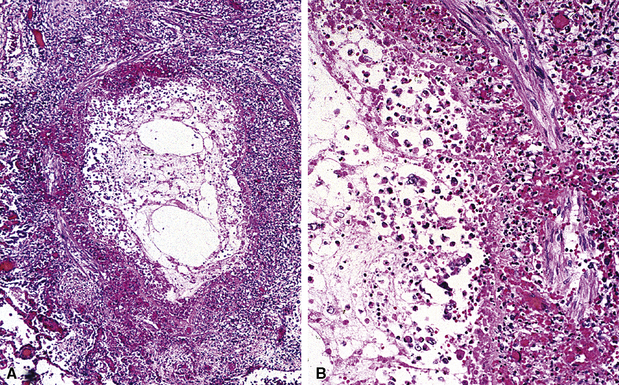
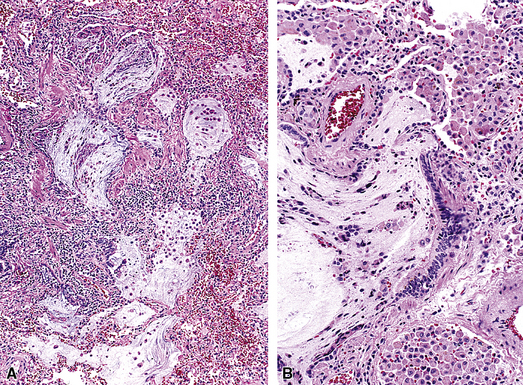
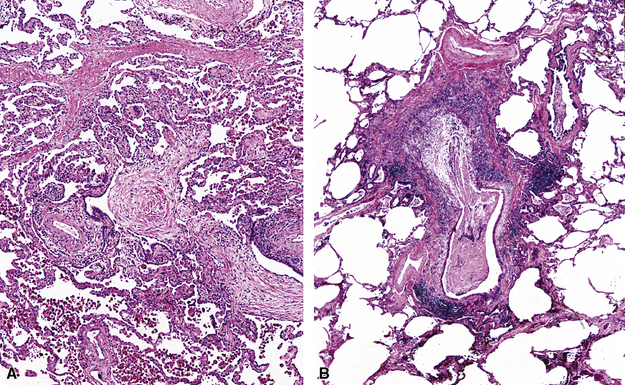
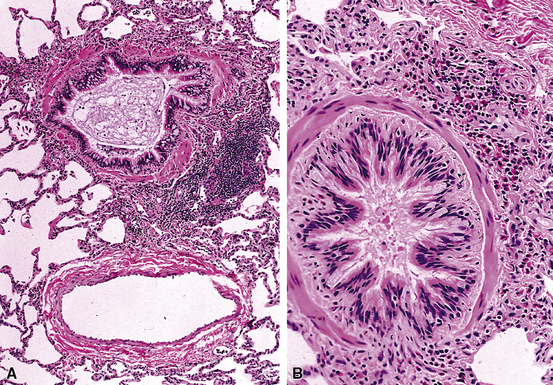
Isolated granulomatous bronchiolitis is unusual. Sarcoidosis is the leading consideration in the differential diagnosis in the absence of necrosis (Fig. 8-21). Granulomas of sarcoidosis are usually distributed along the lymphatic routes in the lung and therefore are frequently bronchiolocentric, allowing bronchoscopic biopsy to provide excellent diagnostic material. On the other hand, when necrosis is present, granulomas are usually present in the surrounding lung parenchyma as well, and herald the strong possibility of infection (especially infection caused by mycobacteria and fungi)79 (Fig. 8-22). Necrotizing granulomatous bronchiolitis also may be seen in the setting of middle lobe syndrome47 and chronic necrotizing forms of pulmonary aspergillosis.80 Even in the absence of necrosis, however, infection should still remain high in the differential diagnosis. Non-necrotizing granulomatous bronchiolitis can be seen in association with inflammatory bowel disease, such as Crohn disease, sometimes accompanied by microabscesses.81 Non-necrotizing peribronchiolar granulomas are also seen in a majority of patients with hypersensitivity pneumonitis (Fig. 8-23). Generally, granulomas in hypersensitivity are less well delimited and well formed than in infectious diseases and may take the form of loose aggregations of histiocytic epithelioid cells, with or without giant cells.
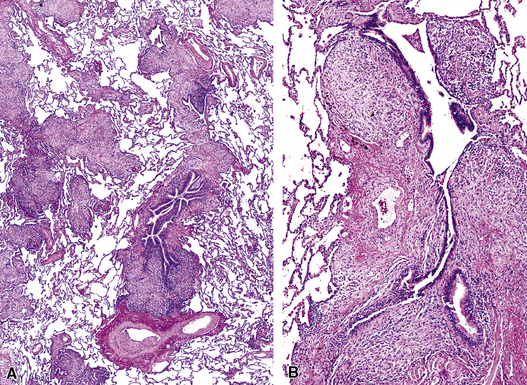
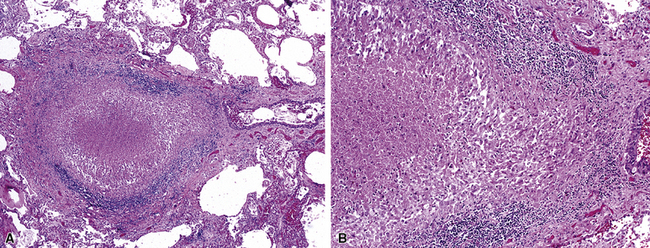
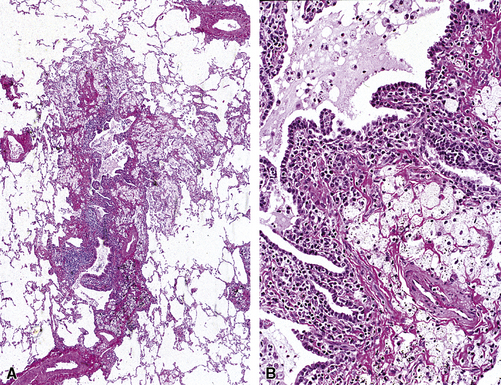
Figure 8-21 Sarcoidosis. A, At scanning magnification, the serpiginous outline of the bronchovascular bundle is accentuated by pale pink granulomas, collagen, and scant chronic inflammation. B, The bronchiole shows the typical granulomas and sclerotic background matrix of sarcoidosis, which involves the airway wall and impinges on the adjacent arterial wall. The granulomas are well formed, with a definite fibrous demarcation, and show a tendency toward coalescence. Inflammatory cells are scant, and the whole process has an eosinophilic appearance, which is clearly different from that of the lesions seen in granulomatous bronchiolitis due to infection (compare Fig. 8-22).
Another frequent cause of granulomatous bronchiolitis is aspiration pneumonia,64 which may be clinically occult and manifest as diffuse bronchiolar disease with bronchiolecentric granulomatous inflammation.82,83 It is worth noting that aspirated food particles are not usually birefringent under polarized light. The exceptions are talc and microcrystalline cellulose, both used as inert components of oral pharmaceutical tablets and susceptible to accidental aspiration. Talc aspiration also has been rarely reported in children related to the liberal use of baby powder on the skin.83 When isolated peribronchiolar multinucleated giant cells are the major finding, one should consider Wegener granulomatosis (Fig. 8-24), aspiration pneumonia (Figs. 8-25 and 8-26), and hard metal (cobalt) pneumoconiosis (so-called giant cell interstitial pneumonia of Liebow).
Bronchiolar Necrosis
Complete mucosal necrosis with epithelial sloughing and variable acute inflammation is typical of a limited number of lung diseases, mainly those of infectious origin (Box 8-8). In the presence of bronchiolar necrosis, a careful search for viropathic changes or microorganisms(bacterial and fungal) should be undertaken (Fig. 8-27 to 8-29). When the process is intensely suppurative with large nodular foci of necrosis, consideration should be given to the possibility of pyoderma gangrenosum lesions in the lung associated with inflammatory bowel disease (e.g., ulcerative colitis)84,85 or occurring de novo without inflammatory bowel disease.86–91
Respiratory (Smoker’s) Bronchiolitis
Respiratory bronchiolitis is a very special marker for smoking-related lung injury and is extremely common in lung biopsy and surgical specimens (cigarette smoking being a cause of, or associated with, many lung diseases).92–98 The histopathologic lesion of respiratory bronchiolitis is characterized by accumulation of lightly pigmented macrophages (smoker’s macrophages) within the lumens of the respiratory bronchioles as well as in the surrounding peribronchiolar alveoli. This macrophage accumulation is associated with mild distortions of the overall architecture of the bronchiole, slight inflammation, fibrosis, and smooth muscle hyperplasia of the bronchiole wall. Minimal inflammation and interstitial fibrosis variably extends to the surrounding alveolar walls (Figs. 8-30 and 8-31). Sometimes respiratory bronchiolitis can be sufficiently extensive to produce clinical and radiologic manifestations of diffuse interstitial lung disease, so-called respiratory bronchiolitis–associated interstitial lung disease (RBILD) (see Chapter 7).96,99,100 Desquamative interstitial pneumonia is also a consideration when this occurs. Histologically, RBILD is indistinguishable from respiratory bronchiolitis. A diagnosis of RBILD, as opposed to respiratory bronchiolitis, should be rendered only in the presence of clinically significant diffuse lung disease (shortness of breath, cough, mixed restrictive and obstructive physiology), if HRCT findings are compatible with the diagnosis (centrilobular micronodules, ground-glass opacities, and peribronchiolar thickening) and other causes of lung disease can be excluded with reasonable confidence.96 In rare instances, RBILD can also be associated with significant panlobular and subpleural alveolar septa fibrosis, which may simulate fibrotic nonspecific interstitial pneumonia (NSIP).101 Respiratory bronchiolitis is very commonly seen with Langerhans cell histiocytosis (pulmonary eosinophilic granuloma), because both have the same relationship to cigarette smoking.92,102,103
Bronchiolar Metaplasia (Lambertosis)
Bronchiolar metaplasia is a pathologic process in which bronchiolar epithelial cells extend beyond the respiratory bronchioles along alveolar septa, replacing the normal alveolar lining cells, and is usually accompanied by some degree of stromal fibrosis (Fig. 8-32). This lesion is often referred to as “Lambertosis,” because it is hypothesized that the metaplastic epithelium derives from the canals of Lambert that connect nonrespiratory bronchioles directly to adjacent alveoli. Lambertosis can be seen in a number of pathologic conditions, including COPD and constrictive bronchiolitis among others. In most cases the lesion is felt to be postinflammatory in origin and therefore could potentially be seen as a consequence of any number of inflammatory diseases involving the small airways. No specific cause is identified in some patients and the disease may be an idiopathic manifestation of an unidentified remote injury to the small airways. When this is the case, the disease may manifest clinically as an interstitial lung disease with restrictive physiology. The histopathology of bronchiolar metaplasia is one of variable extension of columnar, sometimes ciliated epithelium, beyond the alveolar ducts to involve alveolar walls. When the lesions are exuberant, they may appear as nodules 2 to 5 mm in diameter. These lesions must be distinguished from microscopic honeycombing, atypical adenomatous hyperplasia,104–108 and the micronodular pneumocyte hyperplasia of tuberous sclerosis complex.109–111
When prominent bronchiolar metaplasia is identified, along with some other significant pathology (e.g., in association with a lobectomy for carcinoma), the exact significance of this process relative to the patient’s clinical manifestations is unclear. In our experience, bronchiolar metaplasia in the setting of smoking-related lung disease may or may not have a clinical or radiologic correlation. Conversely, such changes in a nonsmoker are always significant. Diseases associated with prominent bronchiolar metaplasia are listed in Box 8-9.
Mucostasis
Mucostasis (also referred to as mucus stasis) is defined as visible amphophilic mucin within dilated terminal airways or in surrounding alveolar ducts and alveolar spaces64,112 (Fig. 8-33). Mucostasis is a common finding in cigarette smokers and can occur in a number of other settings, as detailed in Box 8-10. When mucus extrusion into alveolar spaces is extensive, a careful search for bronchioloalveolar carcinoma (BAC) is in order, especially in the absence of another specific pathologic entity in the biopsy. Correlation with imaging studies may be helpful in excluding BAC.
Bronchiolar Smooth Muscle Hyperplasia
Generalized thickening of the smooth muscle around conducting airways and alveolar ducts can be seen in a number of inflammatory lung diseases (Fig. 8-34A). Sometimes smooth muscle nodules formed of haphazard fascicles can be seen in smokers and are of unknown clinical relevance (see Fig. 8-34B). Smooth muscle hyperplasia associated with subpleural fibrosis is common in usual interstitial pneumonia (UIP) as well. The diseases associated with smooth muscle hyperplasia are listed in Box 8-11.
Fibrous Proliferations in and around Bronchioles
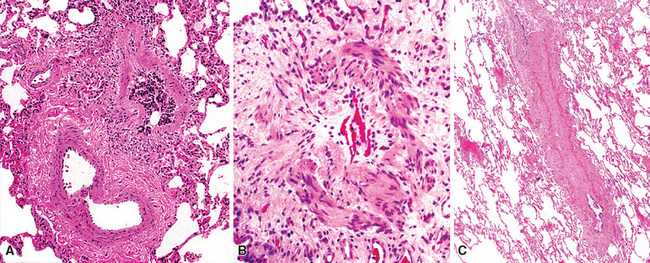
Increase of fibrous tissue in the lumen or wall of bronchioles and in immediately surrounding tissue may be seen in several clinicopathologic conditions, particularly those associated with acute inhalational injury (Figs. 8-35 and 8-36). The fibrous proliferation seen at the microscopic level may be of recent development, characterized by a loose fibroblastic intraluminar proliferation having a fibromyxoid stroma. Alternatively, fibrosis may be more advanced, with fewer fibrocytes, more abundant extracellular collagen, and usually more prominent distortion of bronchiolar morphology. More advanced fibrotic processes may have a predominant concentric growth pattern in and around the airway, resulting in narrowing or obliteration of the lumen. In other cases, fibrosis may have a predominant peribronchiolar-radiating pattern, involving the adventitia of the airway with variable extension into the adjacent lung parenchyma.
Among fibrous proliferations mainly involving the luminal portion of the airway are those processes characterized by loose fibroblastic polyps (Fig. 8-36A)—so-called Masson bodies.113 Rarely, bronchiolar intraluminal fibroblastic polyps may be the predominant finding in lung biopsies, such as in very acute fume inhalation. More frequently the polyps are located in the lumen of the bronchioles and, more conspicuously, in the surrounding distal air spaces representing a generalized parenchymal repair reaction. In these cases they are almost always associated with significant inflammatory changes in the airway wall and surrounding lung parenchyma, a feature now referred to as organizing pneumonia pattern (OP), or simply “air space organization.”114 When this type of fibroblastic proliferation has no apparent etiology, the condition is referred to clinically as cryptogenic organizing pneumonia (COP),115 formerly known as “idiopathic bronchiolitis obliterans organizing pneumonia” (BOOP).116 Intraluminal fibroblastic polyps can be seen in many lung disorders, as detailed in Box 8-12.
Box 8-12 Conditions Associated with the Organizing Pneumonia Pattern
Modified from Travis WD, et al. Non-neoplastic disorders of the lower respiratory tract. In: Kind DW, ed. Atlases of Nontumor Pathology. Washington: Armed Forces Institute of Pathology; 2002.
Bronchiolitis Obliterans Syndrome
Fibrous proliferation at the bronchiolar level may result in luminal constriction and ultimately complete obliteration of the the small airways. This process is best illustrated by chronic transplant rejection (see Chapter 12). In this setting the fibrotic process is referred to as obliterative bronchiolitis (OB)117,118 and corresponds to the clinical entity referred to as “bronchiolitis obliterans syndrome” (BOS).119–121 OB/BOS is not simply graft rejection but a complex multifactorial process involving immune-mediated and alloimmune dependent tissue injuries and aberrant reparative responses or remodeling.122 Histopathologically, the earliest stage is characterized by eccentric subepithelial fibrosis with scattered admixed chronic inflammatory cells. Concentric fibrosis then progresses over time until the lumen becomes markedly narrowed, or entirely obliterated. In other instances, the lumen may be initially occluded by loose fibrous tissue, presumably as a manifestation of active repair following an injury to the airway epithelium and basal lamina. Some investigators have distinguished these latter lesions as more typical of bronchiolitis obliterans—asserting that the outer dimensions of the bronchiole remain stable (i.e., not necessarily constricted).
In the non-lung transplant patient, the term constrictive bronchiolitis is less prone to misunderstanding than the term obliterative bronchiolitis, and refers to a small-airway disease characterized by variable narrowing or obliteration of the small airways. Constrictive bronchiolitis not associated with lung transplantation may be a consequence of injuries resulting from infection, toxic fume inhalation, drug reaction, chemical toxins, hematopoietic stem cell transplantation, or connective tissue disease (Table 8-2). In some cases no etiology is identified.60,64,68,123–133 It is worth noting that the pathologic findings in constrictive bronchiolitis may be quite subtle (see the introductory section “Pattern-Based Approach to Diagnosis,” Pattern 6, Minimal changes). When no explanation for a patient’s significant hypoxia is identifiable in lung biopsy sections, trichrome and elastic tissue stains may be helpful in highlighting the changes in small airways. Nevertheless, it is important to remember that some degree of subepithelial fibrosis in the small airways is almost a universal finding in heavy cigarette smokers. Without clinical manifestations of lung disease, such findings are most often irrelevant. Examples of the spectrum of constrictive airway injury are presented in Figures 8-37 to 8-39.
| Category | Specific Agents |
|---|---|
| Infections | Mycoplasma, viruses (cytomegalovirus, adenovirus, influenzavirus, parainfluenza virus, varicella virus) |
| Fumes | Nitrogen dioxide, sulfur dioxide, ammonia, phosgene, chlorine, fly ash, styrene, fire fumes, possibly volatile chemicals used as flavoring ingredients in food industry |
| Toxins | Ingested toxins from juice of the plant Sauropus androgynus132 |
| Massive dust exposure127 | World Trade Center Disaster “Ground Zero” dust exposure |
| Drugs | Penicillamine, cocaine, gold, chemotherapeutic agents (lomustine, 5-fluorouracil)177 |
| Paraneoplastic syndromes126,178 | Paraneoplastic pemphigus in cases of malignant lymphomas and Castleman disease |
| Stevens-Johnson syndrome130 | |
| Systemic connective tissue disease | Rheumatoid arthritis, mixed connective tissue disease |
| Idiopathic |
Data from Colby TV, Leslie KO. Small airway lesions. In: Cagle PT, ed. Diagnostic Pulmonary Pathology. New York: Marcel Dekker; 2000:231–249; King TE Jr. Bronchiolitis. In: Schwartz MI, King TE, eds. Interstitial Lung Disease. London: BC Decker; 1998:654–684; and Ryu JH. Classification and approach to bronchiolar diseases. Curr Opin Pulm Med. 2006;12(2):145–151.
Given the limited repertoire of airway repair after injury, it is probably best to think of constrictive and obliterative forms of bronchiolitis simply as a representation of a later evolution of the repair process, whereas organizing intraluminal polyps are a more subacute manifestation of injury.121 Why some injuries resolve with little permanent structural abnormalities, whereas others result in persistent damage, is unknown.
Fibrosis centered on the small airway, involving the wall of the bronchiole and surrounding lung parenchyma with or without any degree of inflammation, may be descriptively termed bronchiolocentric fibrosis (Fig. 8-40). This may be seen as a late manifestation of several conditions, most notoriously hypersensitivity pneumonitis. In some cases the process appears to be idiopathic.134–138
Terminal Airway Fibrosis with Dust Deposition (Pneumoconiosis-Associated Small Airway Disease)
Variable dust deposition around airways, accompanied by fibrosis, can be seen commonly in smokers. Under polarized light, small refractile silicate particles (typically aluminum and magnesium silicates) may be identified within areas of pigment deposition, either as a consequence of lifelong dust exposure in the older patient or as a common finding in cigarette smokers. In addition to occupational exposure to dust, a slight degree of airway remodeling with increased amounts of muscle and fibrous tissue can be seen in people exposed to high concentrations of ambient particulate matter, as can occur in some highly polluted urban areas.139
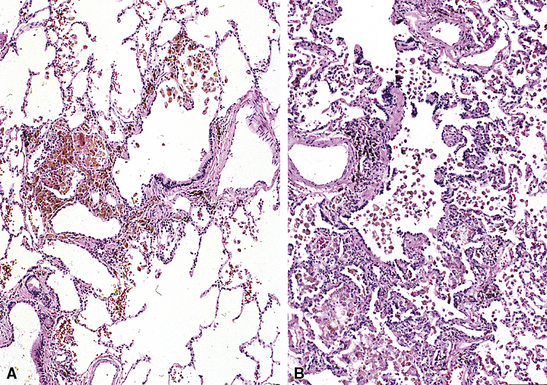
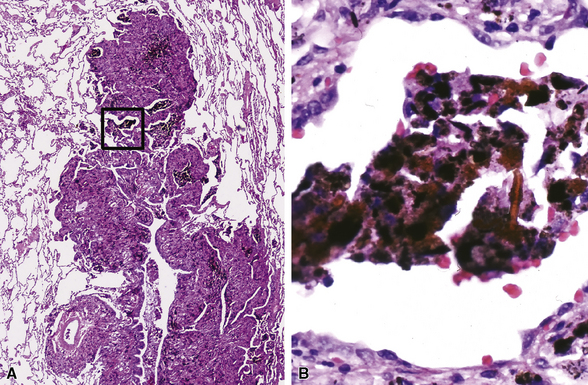
Small airway disease associated with exposure to a specific mineral dust, such as that from asbestos, aluminum oxide, iron oxide, silicates, or coal, is referred to as pneumoconiosis-associated small-airway disease.118 Pneumoconiosis-associated small-airway injury frequently demonstrates only minimally inflammatory changes (Figs. 8-41 and 8-42). The alveolar duct walls are thickened by fibrous tissue, and dust deposition occurs along the small airways. Clinical and radiographic correlation is essential before a pathologic diagnosis of clinically significant pneumoconiosis is made (see Chapter 9). When hemosiderin is present along with alveolar duct fibrosis and other pigment deposition, a careful search for asbestos bodies is in order. In this setting, iron stains (Prussian blue) may be helpful for identifying foci of iron deposition, thereby attracting the pathologist’s attention to areas in which asbestos bodies (ferruginous bodies) are more likely to be identified (see Fig. 8-42B). Not all ferruginous bodies are asbestos-related, so it is important to establish the presence of a delicate translucent core fiber—the characteristic feature of asbestos bodies.
Bronchiolocentric Nodules
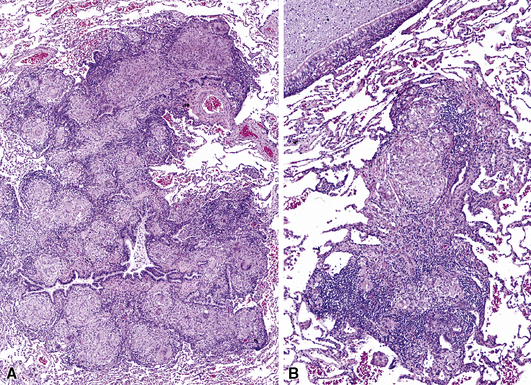
A number of diseases may produce nodular lesions centered on the small airways (bronchiolocentric nodules).60 The diseases that can produce bronchocentric nodules include primarily inflammatory lesions (e.g., panbronchiolitis—discussed further on), inflammatory and fibrotic lesions (e.g., pulmonary Langerhans cell histiocytosis) (Figs. 8-43 and 8-44), and miscellaneous disease processes (e.g., multiple carcinoid tumorlets, with or without constrictive bronchiolitis, or lymphangitic tumor, including malignant lymphoma).
Clinicopathologic Entities with Prominent Airway Manifestations
Asthma-Associated Airway Diseases
Asthma is a chronic inflammatory disease of the airways accompanied by bronchial hyperresponsiveness (the primary clinical manifestation). Affected individuals develop a number of well-characterized inflammatory changes in the large airways, including epithelial fragility, intraepithelial goblet cell hyperplasia, mural infiltration by eosinophils, smooth muscle hyperplasia, and enlargement of subepithelial mucous glands140 (Fig. 8-45 and Box 8-13). In a subset of asthmatic individuals also will develop chronic disease of the small airways.60,140–145 The large-airway pathology is seen mainly in lobectomy specimens removed for other causes and autopsy lungs from patients who die in status asthmaticus. The small-airway changes are more often observed in surgical biopsy specimens because these are taken from peripheral lung. These changes include chronic bronchiolitis, eosinophilic bronchiolitis, varicosity of bronchiolar lumens, bronchiolar metaplasia, constrictive bronchiolitis, and even features of bronchocentric granulomatosis (Figs. 8-46 to 8-49). These changes may also be appreciated in bronchoscopic biopsies. In addition to these bronchial and bronchiolar alterations, four distinctive, albeit partially overlapping, asthma-associated lung diseases are recognized: eosinophilic pneumonia, allergic bronchial pulmonary fungal disease, mucoid impaction of bronchi, and bronchocentric granulomatosis. Eosinophilic pneumonia is discussed in Chapter 5 (Acute Lung Injury) and Chapter 7 (Chronic Diffuse Lung Diseases); the other three asthma-associated diseases are described next.
Box 8-13 Pathologic Findings in Asthma
Data from Dunnill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysema. Thorax. 1969;24(2):176–179; Aikawa T, Shimura S, Sasaki H, et al. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101(4):916–921; and Messer JW, Peters GA, Bennett WA. Causes of death and pathologic findings in 304 cases of bronchial asthma. Dis Chest. 1960;38:616–624.
Allergic Bronchopulmonary Fungal Disease
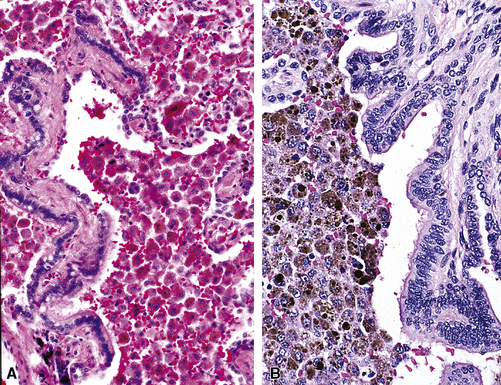
In certain predisposed individuals, Aspergillus and other fungi may colonize the mucus of the respiratory tract, resulting in a form of chronic inflammatory disease termed allergic bronchopulmonary fungal disease (ABFD).146–148 Other fungi implicated in the pathogenesis of this disorder include Pseudallescheria boydii, Bipolaris species, Torulopsis glabrata, Curvularia, and Lunata. The hallmark of ABFD is the presence of so-called allergic mucin.149 Allergic mucin is characterized by the presence of eosinophils and eosinophil cytoplasmic granular debris (Fig. 8-50). One or more additional manifestations are typical, such as mucoid impaction, bronchocentric granulomatosis, and eosinophilic pneumonia. Calcium oxalate crystals may be present in the granular debris of allergic mucin, similar to that seen with sinus hypersensitivity to Aspergillus and other fungi in asthmatic patients. When allergic mucin is present, silver stains for fungal organisms often are helpful in confirming the diagnostic impression of ABFD; however, the fungi often are focal and fragmented and can be easily overlooked. Because ABFD is considered an allergic manifestation, rather than a true infection, a course of oral corticosteroids is often the treatment of choice.
Mucoid Impaction of Bronchi
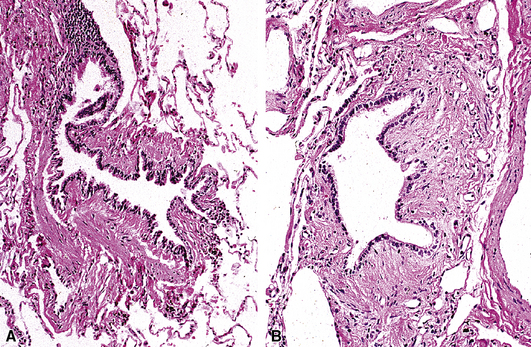
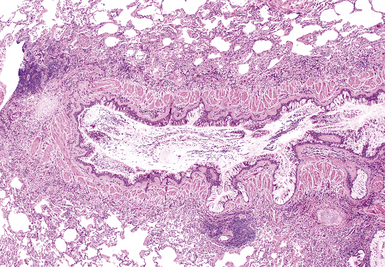
A distinctive clinicopathologic syndrome is characterized by the presence of extensive mucous plugging of the airways accompanied by airway dilatation.150–153 In this entity, termed mucoid impaction of bronchi (MIB), the mucus has the appearance of allergic mucin. MIB is considered to be one of the major manifestations of ABFD. Patients may be asymptomatic or may have obstructive pneumonia distal to the impaction. Radiologically, band-like or branching densities involving the upper lobes are characteristic findings (“gloved finger sign”).150 On occasion a solitary nodule may be seen. Branching strands of inspissated mucus may be coughed up by the patient (so-called plastic bronchitis) (Fig. 8-51), and bronchoscopy may identify clues to the disease with the presence of impacted airways containing mucus.
Microscopically, the affected bronchi are dilated and contain brownish to green mucus of tenacious consistency. The mucus has a laminated appearance (Fig. 8-52) with eosinophilic debris and Charcot-Leyden crystals (hexagonal brightly eosinophilic crystals); this appearance defines allergic mucin. Asthma-type changes may be present in bronchoscopic biopsies from more central airways. Fungal stains may demonstrate hyphae, so use of such stains is recommended whenever allergic mucin is found. Polarizable calcium oxalate crystals may also be present. Because mucous plugging can occur in a number of airway diseases, a distinctive laminated appearance should be present before the diagnosis is invoked.
Bronchocentric Granulomatosis
Bronchocentric granulomatosis is a distinctive form of granulomatous inflammation that surrounds the larger airways, replacing bronchial walls and mucosa.148,154–156 In bronchocentric granulomatosis, the lumen of the airway contains necrotic debris and palisaded histiocytes surround the lumen. Bronchocentric granulomatosis is not only confined to the larger bronchi but may also involve more distal bronchioles. Both infectious and noninfectious causes are described, and these are presented in Box 8-14. A useful distinguishing clinical feature for separating infectious and noninfectious bronchocentric granulomatosis is the presence or absence of asthma in the affected individual. In asthmatic patients, the disease may manifest as an exacerbation of the underlying airway disease, accompanied by wheezing, cough, and fever, or can be related to hypersensitivity and may be associated with mucoid impaction and ABFD. In nonasthmatic patients, infection should be the main consideration in the differential diagnosis, even when special stains are negative in tissue sections.
In the asthmatic patient, the radiographic findings are often accompanied by the changes of mucoid impaction (branching opacities in a bronchial distribution). In the nonasthmatic patient, given the propensity for infection as the etiology, the radiologic manifestations may be quite variable, ranging from localized consolidation to nodular parenchymal lesions on chest radiographs. Also, in the nonasthmatic patient, cavitation may be present in the nodules, attesting to the likelihood of an infectious etiology.157
The most prominent histologic manifestation of bronchocentric granulomatosis is the destruction of the airway wall (Fig. 8-53). This feature is helpful in distinguishing peribronchial granulomas that occur in a number of infectious and noninfectious conditions (e.g., sarcoidosis). As in all cases of necrotizing granulomatosis inflammation, special stains for organisms should always be performed, even in the asthmatic patient in whom a hypersensitivity disease process is suspected. Rarely, Wegener granulomatosis may be present in an exclusively airway-centered distribution, so clinical correlation and serologic studies may be useful. A feature helpful in excluding Wegener granulomatosis is the presence of well-formed granulomas without necrosis in the surrounding parenchyma (see Chapter 10, on vasculitis). When well-formed granulomas without necrosis are present, Wegener granulomatosis is an unlikely etiology. If the biopsy is obtained from the middle lobe or lingula, middle lobe syndrome should be considered as an alternative diagnosis. Aspiration pneumonia is also in the differential diagnosis, and a careful search for foreign material or foreign body giant cells is always advisable. Vegetable material with thick cellular walls or meat (skeletal muscle fragments) implicates aspiration when present.83 The presence of conchoidal calcifications, or calcium oxalate crystals in isolation, is not a sign of aspiration, but such bodies can occur in any granulomatous inflammatory reaction.
Chronic Obstructive Pulmonary Disease
COPD is a relatively common disease characterized by chronic airflow obstruction and small-airway abnormalities.158–160 The disease usually is related to cigarette smoking. The small airways in COPD are often subtly abnormal, and this is the most common situation in which minor abnormalities of the bronchioles are seen, reflecting the high prevalence of smoking in affected patients. Histologically, there is a minor degree of inflammation in the walls of bronchioles, including respiratory bronchioles, with variable occurrence of fibrosis, mucus stasis, loss of radial attachments, and bronchiolectasia (with dilatation and distortion). Emphysema and chronic bronchiolitis both occur in COPD but vary in proportion and severity from patient to patient.
Emphysema
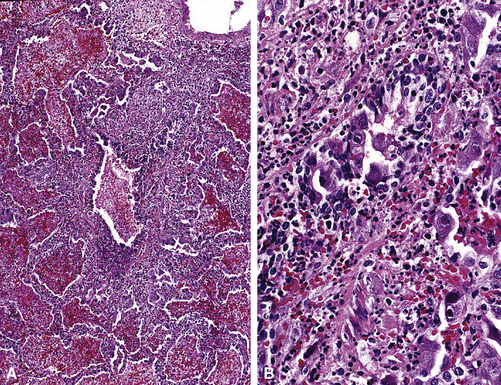
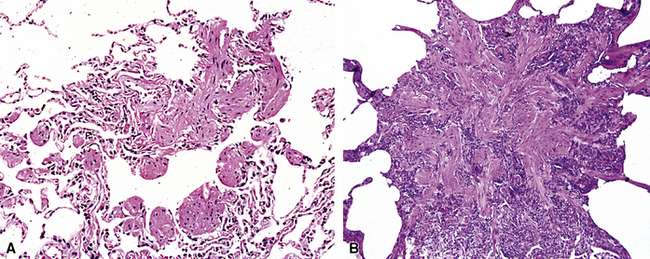
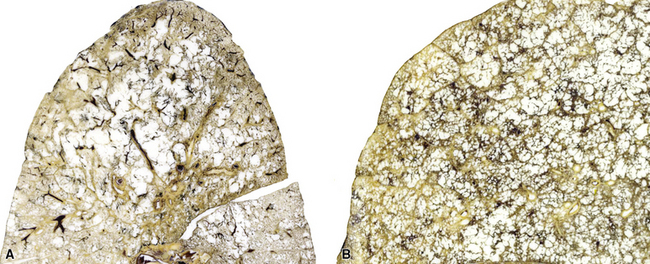
Emphysema may be classified according to the part of the lung acinus that is primarily affected.161 Proximal (centrilobular, centriacinar) emphysema (Fig. 8-54A) involves the proximal part of the lung acinus. This form of emphysema is strongly associated with smoking. The respiratory bronchioles are enlarged and destroyed, and the enlarged air spaces are seen at the center of the secondary acinus; respiratory bronchiolitis of variable degree may be present. Panacinar emphysema (see Fig. 8-54B) affects the whole acinus and is characteristic of α1-antitrypsin deficiency; the air spaces of the whole acinus are diffusely enlarged. Distal (septal, pleural, localized) emphysema affects the periphery of the acinus, most often beneath the pleura; it may be a cause of spontaneous pneumothorax in the young adult. Irregular emphysema may be seen in association with a lung scar, such as those due to healed Langerhans cell histiocytosis, granulomatous inflammation, dust deposits, or pulmonary infarcts. Although gross examination of the lungs allows distinction between centriacinar and panacinar emphysema,158 the surgical biopsy is not a reliable method for diagnosing pulmonary emphysema. Noting the presence of emphysema on surgical wedge biopsies is reasonable, but attempts to grade its severity are not advisable (Fig. 8-55). The associated small-airway and vascular changes may be prominent and include tortuosity.
Neuroendocrine Cell Hyperplasia with Occlusive Bronchiolar Fibrosis (Aguayo-Miller Disease)
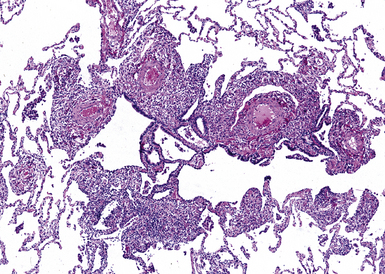
Diffuse idiopathic neuroendocrine cell hyperplasia (DIPNECH) of the bronchioles is a primary proliferation of neuroendocrine cells which can be associated with partial or total occlusion of airway lumens by fibrous tissue.162 DIPNECH is more frequent in women and is not related to cigarette smoke. DIPNECH may be an incidental finding or may present as a symptomatic disease, typically nonproductive cough, dyspnea, which usually is not progressive, and an obstructive lung function profile.163,164 HRCT scans may reveal evidence of small airway obstruction and coexistence of minute pulmonary nodules. Rare cases have been found in association with multiple endocrine neoplasia (MEN) type 1 syndrome or other endocrine disorders.163 Pathologically, the mildest lesions consist of linear zones of neuroendocrine cell hyperplasia with very focal subepithelial fibrosis. More obvious lesions consist of a plaque of eccentric fibrous tissue partially occluding the airway lumen. Sometimes the occlusion can be caused directly by the proliferation of neuroendocrine cells (Figs. 8-56 and 8-57) within the airway epithelium. The most severely involved bronchioles show total occlusion of their lumens by fibrous tissue, with few visible neuroendocrine cells.
Diffuse Panbronchiolitis
Diffuse panbronchiolitis (DPB), first described by Yamanaka and coworkers,165 is a distinctive inflammatory condition characterized by chronic bronchiolitis associated with prominent interstitial vacuolated or “foamy” histiocytes in a peribronchiolar distribution (Fig. 8-58). DPB is seen more commonly in natives of Japan, Korea, and China, and only rare cases have been described in non-Asian individuals.166,167 The age range for affected patients is 20 to 60 years. Men are twice as commonly affected as women. Chronic productive cough and dyspnea are the typical presenting complaints, and most patients have sinusitis.166,168–174 A genetic susceptibility has been well documented over the years, and recently has been identified as a human leukocyte antigen (HLA)-associated major susceptibility gene, probably residing within the HLA-B locus on the short arm of chromosome 6 (6p21.3). HLA-B54 is the haplotype reported in Japanese patients, and HLA-A11 is identified in those of Korean ancestry, suggesting that the DPB susceptibility locus is located between the HLA-B and HLA-A genes.175,176
A summary of the key morphologic findings related to diseases of the large and small airways is presented in Table 8-3.
Table 8-3 Key Morphologic Findings in Diseases of the Large and Small Airways
| Observation | Considerations |
|---|---|
| Small non-necrotizing granulomas in bronchial mucosa | Sarcoidosis, infection, aspiration, berylliosis, tracheobronchial amyloidosis |
| Small non-necrotizing granulomas around bronchioles | Hypersensitivity pneumonitis, infection, aspiration, Wegener granulomatosis, sarcoidosis, Crohn disease, Sjögren syndrome, primary biliary cirrhosis |
| Well-formed non-necrotizing granulomas | Sarcoidosis, berylliosis, infection |
| Well-formed necrotizing granulomas | Infection |
| Necrosis | Infection, Wegener granulomatosis, bronchocentric granulomatosis, relapsing polychondritis, pyoderma gangrenosum, fume exposure |
| Lymphoid follicles around bronchioles | Collagen vascular diseases, distal to bronchiectasis, lymphoproliferative diseases, inflammatory bowel diseases, as a minor component of a localized inflammation |
| Bronchiolar metaplasia | Healed bronchiolitis, chronic hypersensitivity pneumonitis, distal to bronchiectasis, as a component of constrictive bronchiolitis, as a focal incidental finding |
| Mucostasis | Bronchiolitis (of any cause), distal to bronchiectasis, allergic bronchopulmonary aspergillosis, as part of a localized inflammation, as an incidental finding (in the smoker) |
| Intra-alveolar foamy macrophages | Distal to bronchial obstruction (of any cause), hypersensitivity pneumonitis, cryptogenic organizing pneumonia, reactions to some drugs (e.g., amiodarone), some metabolic disorders |
| Interstitial foamy macrophages | Diffuse panbronchiolitis, inflammatory bowel diseases, distal to bronchiectasis |
| Lightly pigmented alveolar macrophages | Respiratory bronchiolitis (in smokers) |
| Densely pigmented alveolar macrophages | Chronic hemorrhage, asbestosis (if hemosiderin), dust exposure (when anthracotic with or without refractile material) |
| Conchoidal calcifications | Sarcoidosis, infection, hypersensitivity pneumonitis, berylliosis, aspiration |
| Neuroendocrine cell proliferation | Diffuse idiopathic pulmonary neuroendocrine hyperplasia (with or without partial or total occlusion of airway lumens by fibrous tissue), as an incidental finding frequently associated with bronchiectasis |
| Reduction in airway lumen diameter (sometimes associated with bronchioloectasia) | Constrictive/obliterative bronchiolitis as a consequence of infection, toxic fume inhalation, drug reaction, chemical toxins, connective tissue disease, transplantation |
| Mucous cell hyperplasia | Asthma |
| Smooth muscle hyperplasia | Asthma, constrictive bronchiolitis, fibrosing lung disease, localized inflammatory process, bronchiolar scarring/peribronchiolar metaplasia, incidental finding (especially in smoker), DIPNECH with occlusive bronchiolar fibrosis |
DIPNECH, diffuse idiopathic pulmonary neuroendocrine cell hyperplasia.
1 Al Jahdali H., Bamefleh H., Memish Z., et al. Upper airway obstruction due to rhinoscleroma: case report. J Chemother. 2001;13(suppl 1):69-72.
2 Verma G., Kanawaty D., Hyland R. Rhinoscleroma causing upper airway obstruction. Can Respir J. 2005;12(1):43-45.
3 Hellmann D., Laing T., Petri M., et al. Wegener’s granulomatosis: isolated involvement of the trachea and larynx. Ann Rheum Dis. 1987;46(8):628-631.
4 Schokkenbroek A.A., Franssen C.F., Dikkers F.G. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol. 2008;265(5):549-555.
5 Berk J.L., O’Regan A., Skinner M. Pulmonary and tracheobronchial amyloidosis. Semin Respir Crit Care Med. 2002;23(2):155-165.
6 O’Regan A., Fenlon H.M., Beamis J.F.Jr, et al. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine (Baltimore). 2000;79(2):69-79.
7 Ozyigit L.P., Kiyan E., Okumus G., Yilmazbayhan D. Isolated laryngo-tracheal amyloidosis presenting as a refractory asthma and longstanding hoarseness. J Asthma. 2009;46(3):314-317.
8 Papla B., Dubiel-Bigaj M. Tracheobronchial amyloidosis. Pol J Pathol. 1998;49(1):27-34.
9 Cordier J.F., Loire R., Brune J. Amyloidosis of the lower respiratory tract. Chest. 1986;90:827-831.
10 Howard M.E., Ireton J., Daniels F., et al. Pulmonary presentations of amyloidosis. Respirology. 2001;6(1):61-64.
11 Yang S., Chia S.Y., Chuah K.L., Eng P. Tracheobronchial amyloidosis treated with rigid bronchoscopy and stenting. Surg Endosc. 2003;17(4):658-659.
12 Kalra S., Utz J.P., Edell E.S., Foote R.L. External-beam radiation therapy in the treatment of diffuse tracheobronchial amyloidosis. Mayo Clin Proc. 2001;76(8):853-856.
13 Fukumura M., Mieno T., Suzuki T., Murata Y. Primary diffuse tracheobronchial amyloidosis treated by bronchoscopic Nd-YAG laser irradiation. Jpn J Med. 1990;29(6):620-622.
14 Herman D.P., Colchen A., Milleron B., et al. The treatment of tracheobronchial amyloidosis using a bronchial laser. Apropos of a series of 13 cases. Rev Mal Respir. 1985;2(1):19-23.
15 Carden K.A., Boiselle P.M., Waltz D.A., Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127(3):984-1005.
16 Loring S.H., O’Donnell C.R., Feller-Kopman D.J., Ernst A. Central airway mechanics and flow limitation in acquired tracheobronchomalacia. Chest. 2007;131(4):1118-1124.
17 Zhang J., Hasegawa I., Hatabu H., et al. Frequency and severity of air trapping at dynamic expiratory CT in patients with tracheobronchomalacia. AJR Am J Roentgenol. 2004;182(1):81-85.
18 Masaoka A., Yamakawa Y., Niwa H., et al. Pediatric and adult tracheobronchomalacia. Eur J Cardiothorac Surg. 1996;10(2):87-92.
19 Jacobs I.N., Wetmore R.F., Tom L.W., et al. Tracheobronchomalacia in children. Arch Otolaryngol Head Neck Surg. 1994;120(2):154-158.
20 Mair E.A., Parsons D.S. Pediatric tracheobronchomalacia and major airway collapse. Ann Otol Rhinol Laryngol. 1992;101(4):300-309.
21 Ghanei M., Moqadam F.A., Mohammad M.M., Aslani J. Tracheobronchomalacia and air trapping after mustard gas exposure. Am J Respir Crit Care Med. 2006;173(3):304-309.
22 Turkstra F., Rinkel R.N., Biermann H., et al. Tracheobronchomalacia due to amyloidosis in a patient with rheumatoid arthritis. Clin Rheumatol. 2008;27(6):807-808.
23 Nuutinen J. Acquired tracheobronchomalacia. Eur J Respir Dis. 1982;63(5):380-387.
24 Letko E., Zafirakis P., Baltatzis S., et al. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum. 2002;31(6):384-395.
25 Tsunezuka Y., Sato H., Shimizu H. Tracheobronchial involvement in relapsing polychondritis. Respiration. 2000;67(3):320-322.
26 Lee K.S., Ashiku S.K., Ernst A., et al. Comparison of expiratory CT airway abnormalities before and after tracheoplasty surgery for tracheobronchomalacia. J Thorac Imaging. 2008;23(2):121-126.
27 Abu-Hijleh M., Lee D., Braman S.S. Tracheobronchopathia osteochondroplastica: a rare large airway disorder. Lung. 2008;186(6):353-359.
28 Meyer C.N., Dossing M., Broholm H. Tracheobronchopathia osteochondroplastica. Respir Med. 1997;91(8):499-502.
29 Vilkman S., Keistinen T. Tracheobronchopathia osteochondroplastica. Report of a young man with severe disease and retrospective review of 18 cases. Respiration. 1995;62(3):151-154.
30 Chroneou A., Zias N., Gonzalez A.V., Beamis J.F.Jr. Tracheobronchopathia osteochondroplastica. An underrecognized entity? Monaldi Arch Chest Dis. 2008;69(2):65-69.
31 Prakash U.B. Tracheobronchopathia osteochondroplastica. Semin Respir Crit Care Med. 2002;23(2):167-175.
32 Cowan M.J., Gladwin M.T., Shelhamer J.H. Disorders of ciliary motility. Am J Med Sci. 2001;321(1):3-10.
33 O’Sullivan B.P., Freedman S.D. Cystic fibrosis. Lancet. 2009;373(9678):1891-1904.
34 Javidan-Nejad C., Bhalla S. Bronchiectasis. Radiol Clin North Am. 2009;47(2):289-306.
35 Barker A.F., Bardana E.J.Jr. Bronchiectasis: update of an orphan disease. Am Rev Respir Dis. 1988;137(4):969-978.
36 Nicotra M.B., Rivera M., Dale A.M., et al. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108(4):955-961.
37 Barker A.F. Bronchiectasis. N Engl J Med. 2002;346(18):1383-1393.
38 Tanawuttiwat T., Harindhanavudhi T. Bronchiectasis: Pulmonary manifestation in chronic graft versus host disease after bone marrow transplantation. Am J Med Sci. 2009;337(4):292.
39 Kanbay M., Kanbay A., Boyacioglu S. Helicobacter pylori infection as a possible risk factor for respiratory system disease: a review of the literature. Respir Med. 2007;101(2):203-209.
40 Tsang K.W., Lam S.K., Lam W.K., et al. High seroprevalence of Helicobacter pylori in active bronchiectasis. Am J Respir Crit Care Med. 1998;158(4):1047-1051.
41 Tsang K.W., Lam W.K., Kwok E., et al. Helicobacter pylori and upper gastrointestinal symptoms in bronchiectasis. Eur Respir J. 1999;14(6):1345-1350.
42 Kang E.Y., Miller R.R., Muller N.L. Bronchiectasis: comparison of preoperative thin-section CT and pathologic findings in resected specimens. Radiology. 1995;195(3):649-654.
43 Lucidarme O., Grenier P., Coche E., et al. Bronchiectasis: comparative assessment with thin-section CT and helical CT. Radiology. 1996;200(3):673-679.
44 Young K., Aspestrand F., Kolbenstvedt A. High resolution CT and bronchography in the assessment of bronchiectasis. Acta Radiol. 1991;32(6):439-441.
45 Cartier Y., Kavanagh P.V., Johkoh T., et al. Bronchiectasis: accuracy of high-resolution CT in the differentiation of specific diseases. AJR Am J Roentgenol. 1999;173(1):47-52.
46 Whitwell F. A study of the pathology and pathogenesis of bronchiectasis. Thorax. 1952;7(3):213-239.
47 Kwon K.Y., Myers J.L., Swensen S.J., Colby T.V. Middle lobe syndrome: a clinicopathological study of 21 patients. Hum Pathol. 1995;26(3):302-307.
48 Reich J.M., Johnson R.E. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
49 Bhatt S.P., Nanda S., Kintzer J.S.Jr. The Lady Windermere syndrome. Prim Care Respir J. 2009;18(4):334-336.
50 Reich J.M. Pathogenesis of Lady Windermere syndrome. Am J Respir Crit Care Med. 2009;179(12):1165. author reply 1165
51 Fujita J., Ohtsuki Y., Suemitsu I., et al. Pathological and radiological changes in resected lung specimens in Mycobacterium avium intracellulare complex disease. Eur Respir J. 1999;13(3):535-540.
52 Priftis K.N., Mermiri D., Papadopoulou A., et al. The role of timely intervention in middle lobe syndrome in children. Chest. 2005;128(4):2504-2510.
53 Colby T.V. Bronchiolar pathology. In: Epler G.R., editor. Diseases of the Bronchials. New York: Raven Press; 1994:77-100.
54 Couture C., Colby T.V. Histopathology of bronchiolar disorders. Semin Respir Crit Care Med. 2003;24(5):489-498.
55 Poletti V., Costabel U. Bronchiolar disorders: classification and diagnostic approach. Semin Respir Crit Care Med. 2003;24(5):457-464.
56 Lynch D.A. Imaging of small airways disease and chronic obstructive pulmonary disease. Clin Chest Med. 2008;29(1):165-179. vii
57 Ryu J.H. Classification and approach to bronchiolar diseases. Curr Opin Pulm Med. 2006;12(2):145-151.
58 Ryu J.H., Myers J.L., Swensen S.J. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168(11):1277-1292.
59 Yousem S. Small airways disease. Pathol Annu. 1991;26(part 2):109-143.
60 Colby T.V. Bronchiolitis. Pathologic considerations. Am J Clin Pathol. 1998;109(1):101-109.
61 Becroft D.M.O. Histopathology of fatal adenovirus infection of the respiratory tract in young children. J Clin Pathol. 1967;20:561-569.
62 Becroft D.M.O. Bronchiolitis obliterans, bronchiectasis and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol. 1971;24:72-79.
63 Travis W.D., Hoffman G.S., Leavitt R.Y., et al. Surgical pathology of the lung in Wegener’s granulomatosis. Review of 87 open lung biopsies from 67 patients. Am J Surg Pathol. 1991;15(4):315-333.
64 Colby T.V., Leslie K.O. Small airway lesions. In: Cagle P.T., editor. Diagnostic Pulmonary Pathology. New York: Marcel Dekker; 2000:231-249.
65 Howling S.J., Hansell D.M., Wells A.U., et al. Follicular bronchiolitis: thin-section CT and histologic findings. Radiology. 1999;212(3):637-642.
66 Wells A.U., du Bois R.M. Bronchiolitis in association with connective tissue disorders. Clin Chest Med. 1993;14(4):655-666.
67 Nicholson A.G., Colby T.V., Wells A.U. Histopathological approach to patterns of interstitial pneumonia in patients with connective tissue disorders. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19(1):10-17.
68 Leslie K.O., Trahan S., Gruden J. Pulmonary pathology of the rheumatic diseases. Semin Respir Crit Care Med. 2007;28(4):369-378.
69 Lee H.K., Kim D.S., Yoo B., et al. Histopathologic pattern and clinical features of rheumatoid arthritis–associated interstitial lung disease. Chest. 2005;127(6):2019-2027.
70 Nicholson A.G., Wotherspoon A.C., Diss T.C., et al. Reactive pulmonary lymphoid disorders. Histopathology. 1995;26(5):405-412.
71 Yousem S.A., Colby T.V., Carrington C.B. Follicular bronchitis/bronchiolitis. Hum Pathol. 1985;16(7):700-706.
72 Fiche M., Caprons F., Berger F., et al. Primary pulmonary non-Hodgkin’s lymphomas. Histopathology. 1995;26(6):529-537.
73 Boag A.H., Colby T.V., Fraire A.E., et al. The pathology of interstitial lung disease in nylon flock workers. Am J Surg Pathol. 1999;23(12):1539-1545.
74 Burkhart J., Jones W., Porter D.W., et al. Hazardous occupational exposure and lung disease among nylon flock workers. Am J Ind Med. 1999;1(suppl):145-146.
75 Eschenbacher W.L., Kreiss K., Lougheed M.D., et al. Nylon flock–associated interstitial lung disease. Am J Respir Crit Care Med. 1999;159(6):2003-2008.
76 Kern D.G., Kuhn C.3rd, Ely E.W., et al. Flock worker’s lung: chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129(4):261-272.
77 Kern D.G., Kuhn C.3rd, Ely E.W., et al. Flock worker’s lung: broadening the spectrum of clinicopathology, narrowing the spectrum of suspected etiologies. Chest. 2000;117(1):251-259.
78 Romero S., Barroso E., Gil J., et al. Follicular bronchiolitis: clinical and pathologic findings in six patients. Lung. 2003;181(6):309-319.
79 Khoor A., Leslie K.O., Tazelaar H.D., et al. Diffuse pulmonary disease caused by nontuberculous mycobacteria in immunocompetent people (hot tub lung). Am J Clin Pathol. 2001;115(5):755-762.
80 Yousem S.A. The histological spectrum of chronic necrotizing forms of pulmonary aspergillosis. Hum Pathol. 1997;28(6):650-656.
81 Casey M.B., Tazelaar H.D., Myers J.L., et al. Noninfectious lung pathology in patients with Crohn’s disease. Am J Surg Pathol. 2003;27(2):213-219.
82 Barnes T.W., Vassallo R., Tazelaar H.D., et al. Diffuse bronchiolar disease due to chronic occult aspiration. Mayo Clin Proc. 2006;81(2):172-176.
83 Mukhopadhyay S., Katzenstein A.L. Pulmonary disease due to aspiration of food and other particulate matter: a clinicopathologic study of 59 cases diagnosed on biopsy or resection specimens. Am J Surg Pathol. 2007;31(5):752-759.
84 Camus P., Piard F., Ashcroft T., et al. The lung in inflammatory bowel disease. Medicine (Baltimore). 1993;72(3):151-183.
85 Camus P., Colby T.V. The lung in inflammatory bowel disease. Eur Respir J. 2000;15(1):5-10.
86 Kar P.M., Aronoff G., Schuette P. Bronchopulmonary disease: an association with ulcerative colitis and pyoderma gangrenosum. J Ky Med Assoc. 1993;91(8):320-323.
87 Fukuhara K., Urano Y., Kimura S., et al. Pyoderma gangrenosum with rheumatoid arthritis and pulmonary aseptic abscess responding to treatment with dapsone. Br J Dermatol. 1998;139(3):556-558.
88 Wang J.L., Wang J.B., Zhu Y.J. Pyoderma gangrenosum with lung injury. Thorax. 1999;54(10):953-955.
89 Krüger S., Piroth W., Amo Takyi B., et al. Multiple aseptic pulmonary nodules with central necrosis in association with pyoderma gangrenosum. Chest. 2001;119(3):977-978.
90 Field S., Powell F.C., Young V., Barnes L. Pyoderma gangrenosum manifesting as a cavitating lung lesion. Clin Exp Dermatol. 2008;33(4):418-421.
91 Kitagawa K.H., Grassi M. Primary pyoderma gangrenosum of the lungs. J Am Acad Dermatol. 2008;59(suppl 5):S114-S116.
92 Myers J.L., Veal C.F.Jr, Shin M.S., Katzenstein A.L. Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases. Am Rev Respir Dis. 1987;135:880-884.
93 Yousem S., Colby T., Gaensler E. Respiratory bronchiolitis and its relationship to desquamative interstitial pneumonia. Mayo Clin Proc. 1989;64:1373-1380.
94 Moon J., du Bois R.M., Colby T.V., et al. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax. 1999;54(11):1009-1014.
95 Fraig M., Shreesha U., Savici D., Katzenstein A.L. Respiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am J Surg Pathol. 2002;26(5):647-653.
96 Wells A.U., Nicholson A.G., Hansell D.M., du Bois R.M. Respiratory bronchiolitis–associated interstitial lung disease. Semin Respir Crit Care Med. 2003;24(5):585-594.
97 Aubry M.C., Wright J.L., Myers J.L. The pathology of smoking-related lung diseases. Clin Chest Med. 2000;21(1):11-35. vii
98 Hartman T.E., Tazelaar H.D., Swensen S.J., Müller N.L. Cigarette smoking: CT and pathologic findings of associated pulmonary diseases. Radiographics. 1997;17(2):377-390.
99 Hansell D.M., Nicholson A.G. Smoking-related diffuse parenchymal lung disease: HRCT-pathologic correlation. Semin Respir Crit Care Med. 2003;24(4):377-392.
100 Caminati A., Harari S. Smoking-related interstitial pneumonias and pulmonary Langerhans cell histiocytosis. Proc Am Thorac Soc. 2006;3(4):299-306.
101 Yousem S.A. Respiratory bronchiolitis–associated interstitial lung disease with fibrosis is a lesion distinct from fibrotic nonspecific interstitial pneumonia: a proposal. Mod Pathol. 2006;19(11):1474-1479.
102 Yousem S., Colby T., Gaensler E. Respiratory bronchiolitis–associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. Mayo Clin Proc. 1989;64:1373-1380.
103 Vassallo R., Jensen E.A., Colby T.V., et al. The overlap between respiratory bronchiolitis and desquamative interstitial pneumonia in pulmonary Langerhans cell histiocytosis: high-resolution CT, histologic, and functional correlations. Chest. 2003;124(4):1199-1205.
104 Kitamura H., Kameda Y., Ito T., Hayashi H. Atypical adenomatous hyperplasia of the lung. Implications for the pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol. 1999;111(5):610-622.
105 Chapman A.D., Kerr K.M. The association between atypical adenomatous hyperplasia and primary lung cancer. Br J Cancer. 2000;83(5):632-636.
106 Mori M., Rao S.K., Popper H.H., et al. Atypical adenomatous hyperplasia of the lung: a probable forerunner in the development of adenocarcinoma of the lung. Mod Pathol. 2001;14(2):72-84.
107 Nakahara R., Yokose T., Nagai K., et al. Atypical adenomatous hyperplasia of the lung: a clinicopathological study of 118 cases including cases with multiple atypical adenomatous hyperplasia. Thorax. 2001;56(4):302-305.
108 Sartori G., Cavazza A., Bertolini F., et al. A subset of lung adenocarcinomas and atypical adenomatous hyperplasia–associated foci are genotypically related: an EGFR, HER2, and K-ras mutational analysis. Am J Clin Pathol. 2008;129(2):202-210.
109 Guinee D., Singh R., Azumi N., et al. Multifocal micronodular pneumocyte hyperplasia: a distinctive pulmonary manifestation of tuberous sclerosis. Mod Pathol. 1995;8(9):902-906.
110 Muir T.E., Leslie K.O., Popper H., et al. Micronodular pneumocyte hyperplasia. Am J Surg Pathol. 1998;22(4):465-472.
111 Myers J.L. Micronodular pneumocyte hyperplasia: the versatile type 2 pneumocyte all dressed up in yet another brand new suit!. Adv Anat Pathol. 1999;6(1):49-55.
112 Leslie K., Colby T., Swensen S. Anatomic distribution and histopathologic patterns in interstitial lung disease. In: Schwarz M., King T.J., editors. Interstitial Lung Disease. Hamilton, ON: BC Decker; 2002:31-50.
113 Lange W. Ueber eine eigenth atumliche erkrankung der kleinen bronchien und bronchiolen (bronchitis et bronchiolitis obliterans). Dtsch Arch Klin Med. 1901;70:342-364.
114 Davison A.G., Heard B.E., McAllister W.A., Turner-Warwick M.E. Cryptogenic organizing pneumonitis. Q J Med. 1983;52:382-394.
115 American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277-304.
116 Epler G.R., Colby T.V., McLoud T.C., et al. Bronchiolitis obliterans organizing pneumonia. N Engl J Med. 1985;312(3):152-158.
117 Colby T.V., Myers A.R. Clinical and histologic spectrum of bronchiolitis obliterans including bronchiolitis obliterans organizing pneumonia. Semin Respir Med. 1992;13:113-119.
118 Wright J.L., Cagle P., Churg A., et al. Diseases of the small airways. Am Rev Respir Dis. 1992;146(1):240-262.
119 Grossman E.J., Shilling R.A. Bronchiolitis obliterans in lung transplantation: the good, the bad, and the future. Transl Res. 2009;153(4):153-165.
120 Huang H.J., Yusen R.D., Meyers B.F., et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8(11):2454-2462.
121 Cordier J.F. Cryptogenic organising pneumonia. Eur Respir J. 2006;28(2):422-446.
122 Sato M., Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg. 2008;20(2):173-182.
123 Garg K., Lynch D.A., Newell J.D., King T.E.Jr. Proliferative and constrictive bronchiolitis: classification and radiologic features. AJR Am J Roentgenol. 1994;162(4):803-808.
124 Myers J.L., Colby T.V. Pathologic manifestations of bronchiolitis, constrictive bronchiolitis, cryptogenic organizing pneumonia, and diffuse panbronchiolitis. Clin Chest Med. 1993;14(4):611-622.
125 Kraft M., Mortenson R.L., Colby T.V., et al. Cryptogenic constrictive bronchiolitis. A clinicopathologic study. Am Rev Respir Dis. 1993;148(4 Pt 1):1093-1101.
126 Chin A.C., Stich D., White F.V., et al. Paraneoplastic pemphigus and bronchiolitis obliterans associated with a mediastinal mass: a rare case of Castleman’s disease with respiratory failure requiring lung transplantation. J Pediatr Surg. 2001;36(12):E22.
127 Mann J.M., Sha K.K., Kline G., et al. World Trade Center dyspnea: bronchiolitis obliterans with functional improvement: a case report. Am J Ind Med. 2005;48(3):225-229.
128 Akpinar-Elci M., Travis W.D., Lynch D.A., Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24(2):298-302.
129 Ghanei M., Tazelaar H.D., Chilosi M., et al. An international collaborative pathologic study of surgical lung biopsies from mustard gas–exposed patients. Respir Med. 2008;102(6):825-830.
130 Shah A.P., Xu H., Sime P.J., Trawick D.R. Severe airflow obstruction and eosinophilic lung disease after Stevens-Johnson syndrome. Eur Respir J. 2006;28(6):1276-1279.
131 White E.S., Tazelaar H.D., Lynch J.P.3rd. Bronchiolar complications of connective tissue diseases. Semin Respir Crit Care Med. 2003;24(5):543-566.
132 Wang J.S., Tseng H.H., Lai R.S., et al. Sauropus androgynus-constrictive obliterative bronchitis/bronchiolitis—histopathological study of pneumonectomy and biopsy specimens with emphasis on the inflammatory process and disease progression. Histopathology. 2000;37(5):402-410.
133 Mauad T., Dolhnikoff M. Histology of childhood bronchiolitis obliterans. Pediatr Pulmonol. 2002;33(6):466-474.
134 Fenton M.E., Cockcroft D.W., Wright J.L., Churg A. Hypersensitivity pneumonitis as a cause of airway-centered interstitial fibrosis. Ann Allergy Asthma Immunol. 2007;99(5):465-466.
135 Churg A., Myers J., Suarez T., et al. Airway-centered interstitial fibrosis: a distinct form of aggressive diffuse lung disease. Am J Surg Pathol. 2004;28(1):62-68.
136 Yousem S.A., Dacic S. Idiopathic bronchiolocentric interstitial pneumonia. Mod Pathol. 2002;15(11):1148-1153.
137 Takemura T., et al. Pathology of hypersensitivity pneumonitis. Curr Opin Pulm Med. 2008;14(5):440-454.
138 Churg A., Muller N.L., Flint J., Wright J.L. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30(2):201-208.
139 Churg A., Brauer M., del Carmen Avila-Casado M., et al. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect. 2003;111(5):714-718.
140 Hogg J.C. The pathology of asthma. APMIS. 1997;105(10):735-745.
141 Ueda T., Niimi A., Matsumoto H., et al. Role of small airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol. 2006;118(5):1019-1025.
142 Hogg J.C. Pathology of asthma. J Allergy Clin Immunol. 1993;92(1 Pt 1):1-5.
143 Hogg J.C. Bronchiolitis in asthma and chronic obstructive pulmonary disease. Clin Chest Med. 1993;14(4):733-740.
144 Bergeron C., Al-Ramli W., Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6(3):301-305.
145 Sumi Y., Hamid Q. Airway remodeling in asthma. Allergol Int. 2007;56(4):341-348.
146 Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135(3):805-826.
147 Cockrill B.A., Hales C.A. Allergic bronchopulmonary aspergillosis. Annu Rev Med. 1999;50:303-316.
148 Sulavik S.B. Bronchocentric granulomatosis and allergic bronchopulmonary aspergillosis. Clin Chest Med. 1988;9(4):609-621.
149 Aubry M.C., Fraser R. The role of bronchial biopsy and washing in the diagnosis of allergic bronchopulmonary aspergillosis. Mod Pathol. 1998;11(7):607-611.
150 Martinez S., Heyneman L.E., McAdams H.P., et al. Mucoid impactions: finger-in-glove sign and other CT and radiographic features. Radiographics. 2008;28(5):1369-1382.
151 Katzenstein A., Liebow A., Friedman P. Bronchocentric granulomatosis, mucoid impaction, and hypersensitivity reactions to fungi. Am Rev Respir Dis. 1975;111:497-537.
152 Tsai S.H., Jenne J.W. Mucoid impaction of the bronchi. Am J Roentgenol Radium Ther Nucl Med. 1966;96(4):953-961.
153 Sanerkin N.G., Seal R.M., Leopold J.G. Plastic bronchitis, mucoid impaction of the bronchi and allergic broncho-pulmonary aspergillosis, and their relationship to bronchial asthma. Ann Allergy. 1966;24(11):586-594.
154 Myers J.L. Bronchocentric granulomatosis. Disease or diagnosis? Chest. 1989;96(1):3-4.
155 Koss M.N., Robinson R.G., Hochholzer L. Bronchocentric granulomatosis. Hum Pathol. 1981;12(7):632-638.
156 Katzenstein A.L., Liebow A.A., Friedman P.J. Bronchocentric granulomatosis, mucoid impaction, and hypersensitivity reactions to fungi. Am Rev Respir Dis. 1975;111(4):497-537.
157 Ward S., Heyneman L.E., Flint J.D., et al. Bronchocentric granulomatosis: computed tomographic findings in five patients. Clin Radiol. 2000;55(4):296-300.
158 Wright J.L., Churg A. Advances in the pathology of COPD. Histopathology. 2006;49(1):1-9.
159 Gelb A.F., Hogg J.C., Müller N.L., et al. Contribution of emphysema and small airways in COPD. Chest. 1996;109(2):353-359.
160 Jeffery P.K. Histological features of the airways in asthma and COPD. Respiration. 1992;59(suppl 1(2)):13-16.
161 Cosio M.G., Cosio Piqueras M.G. Pathology of emphysema in chronic obstructive pulmonary disease. Monaldi Arch Chest Dis. 2000;55(2):124-129.
162 Aguayo S.M., Miller Y.E., Waldron J.A.Jr, et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med. 1992;327:1285-1288.
163 Davies S.J., Gosney J.R., Hansell D.M., et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax. 2007;62(3):248-252.
164 Adams H., Brack T., Kestenholz P., et al. Diffuse idiopathic neuroendocrine cell hyperplasia causing severe airway obstruction in a patient with a carcinoid tumor. Respiration. 2006;73(5):690-693.
165 Yamanaka A., Saiki S., Tamura S., Saito K. [Problems in chronic obstructive bronchial diseases, with special reference to diffuse panbronchiolitis.]. Naika. 1969;23(3):442-451.
166 Poletti V., Casoni G., Chilosi M., Zompatori M. Diffuse panbronchiolitis. Eur Respir J. 2006;28(4):862-871.
167 Aslan A.T., Ozcelik U., Talim B., et al. Childhood diffuse panbronchiolitis: a case report. Pediatr Pulmonol. 2005;40(4):354-357.
168 Iwata M., Colby T., Kitaichi M. Diffuse panbronchiolitis: diagnosis and distinction from various pulmonary diseases with centrilobular interstitial foam cell accumulations. Hum Pathol. 1994;25:357-363.
169 Nishimura K., Kitaichi M., Izumi T., Itoh H. Diffuse panbronchiolitis: correlation of high-resolution CT and pathologic findings. Radiology. 1992;184:779-785.
170 Randhawa P., Hoagland M., Yousem S. Diffuse panbronchiolitis in North America. Am J Surg Pathol. 1991;15:43-47.
171 Izumi T. Diffuse panbronchiolitis. Chest. 1991;100(3):596-597.
172 Kitaichi M. Pathology of panbronchiolitis from the viewpoint of differential diagnosis. In: Grassi C., Rizzato G., Pozzi E., editors. Sarcoidosis. Amsterdam: Elsevier Scientific; 1988:741-746.
173 Akira M., Kitatani F., Lee Y.S., et al. Diffuse panbronchiolitis: evaluation with high-resolution CT. Radiology. 1988;168:433-438.
174 Homma H., Yamanaka A., Tanimoto S., et al. Diffuse panbronchiolitis. Chest. 1983;83:63-69.
175 Keicho N., Ohashi J., Tamiya G., et al. Fine localization of a major disease-susceptibility locus for diffuse panbronchiolitis. Am J Hum Genet. 2000;66(2):501-507.
176 Matsuzaka Y., Tounai K., Denda A., et al. Identification of novel candidate genes in the diffuse panbronchiolitis critical region of the class I human MHC. Immunogenetics. 2002;54(5):301-309.
177 Valdivia-Arenas M.A., Soriano A.O., Arteaga R.B. Constrictive bronchiolitis after treatment of colon cancer with 5-fluorouracil. Chemotherapy. 2007;53(5):316-319.
178 Radzikowska E., Pawlowski J., Chabowski M., Langfort R. Constrictive bronchiolitis obliterans in patient with Castleman’s disease. Monaldi Arch Chest Dis. 2005;63(4):226-229.