61 Non-Hodgkin’s Lymphomas
In 2009, approximately 66,000 new cases of NHL were diagnosed in the United States and accounted for almost 20,000 deaths. NHL is the fifth leading cause of cancer among men and women, representing almost 5% of new cancer cases. The incidence of NHL has increased by 150% since 1950. An increase of 50% was documented between 1973 and 1988 (a 3% to 4% increase each year). Over the last 2 decades, this increase has abated, and the fraction of 5-year survivors increased significantly from 48% in 1975-1977 to 65% in 1996-2004.1 Most of the increase in recent years is among patients in their sixth and seventh decades; as a result, many patients with NHL may have significant comorbidities that complicate treatment options, particularly with chemotherapy. Radiotherapy is an attractive option that could reduce the need for aggressive or long chemotherapy regimens in many patients.
Epidemiology
Pertinent epidemiologic facts about NHL include:
Classification
Over the last decades, lymphoma classification has changed multiple times. In the 1980s, the International Working Formulation (IWF) classified NHL into three major categories—low, intermediate, and high grade—based on the morphology and natural history. The Revised European-American Classification of Lymphoid neoplasm (REAL) was developed in 1994. It classified NHL based on the cell of origin (B, T, or NK) and included morphology, immunophenotype, and genetic and clinical features. The currently accepted World Health Organization (WHO) classification is a refinement of the REAL classification.2
The REAL/WHO classification of NHL includes several additional, newly identified entities not recognized by the IWF.2 After consideration of cell of origin (B, T, or NK) the classification subdivides lymphomas into those derived from precursor lymphocytes versus those derived from mature lymphocytes. The classification is further refined based on immunophenotype and genetic features. These considerations have aided in defining active treatment for specific subtypes of lymphoma.
Currently, a comprehensive description of the natural history and clinical features of all NHL diagnoses recognized by the WHO classification does not exist. However, the International Lymphoma Classification Project evaluated 1403 lymphoma cases and identified the 13 most common histologic types responsible for about 90% of cases of NHL in the United States.3 The findings were as follows: diffuse large B-cell (DLBCL), 31%; follicular lymphoma (FL), 22%; small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL), 6%; mantle cell lymphoma (MCL), 6%; peripheral T-cell lymphoma (PTCL), 6%; and marginal zone B-cell lymphoma (MZL), mucosa-associated lymphoid tissue (MALT) lymphoma, 5%. The remaining subtypes each occurred in less than 2% of cases. Composite lymphomas were not included in these distribution figures. Importantly, in the United States more than 50% of cases of lymphoma are either DLBCL or FL. In a study performed by the International T-cell Lymphoma Project, PTCL–not otherwise specified (PTCL-NOS) was the most common subtype of PTCL (29.3%).4
Cytogenetics and Molecular Biology
Several lymphomas are associated with nonrandom chromosomal abnormalities.5 These abnormalities often correlate with the histologic type, immunophenotype, and clinical behavior. For example, translocation t(14:18) (q32;q21) occurs commonly in follicular lymphoma. It results in the transposition of the bcl-2 oncogene of chromosome 18 to become adjacent to the heavy chain immunoglobulin gene on chromosome 14. The bcl-2 oncogene is essential for apoptosis or programmed cell death. The t(14:18) translocation interferes with normal senescence and death of the follicular center cell and is possibly engaged in its malignant transformation. Most patients with Burkitt’s lymphoma have translocation t(8:14) or one of its variants, and c-myc is the relevant translocated oncogene. The c-myc product is an important transcription factor, and its dysregulation is probably involved in the proliferative process. Translocation t(11:14) involving bcl-1 is characteristic for recognition of mantle cell lymphoma. The gene product of bcl-1, cyclin D, is directly involved in the regulation of cell division. Rearrangement of bcl-6 has been reported in a third of patients with diffuse large-cell lymphoma. In 25% to 40% of patients with MALT lymphoma, t(11:18) has been detected and indicates an unlikely response of MALT lymphoma of the stomach to antibiotic therapy of an associated H. pylori infection.
Pathologic Diagnosis
In Table 61-1, the more common histologies are listed by WHO classification and grouped as low grade (indolent), intermediate grade (aggressive), or high-grade (highly aggressive), a terminology that is still in clinical use.
Table 61-1 Abridged Version of the WHO Lymphoma Classification Organized by Clinical Groups
| Low Grade (Indolent) |
Staging and Prognostic Factors
Although histology is the predominant determinant of prognosis in this diverse group of lymphoid malignancies, stage remains important for selection of treatment strategy and predicting prognosis in each histologic category. The Ann Arbor Staging Classification system, developed originally for Hodgkin’s disease, has also been used for NHL. The staging system is described in Chapter 60, Hodgkin’s Disease, and reflects the number of sites of involvement and their relation to the diaphragm, existence of B symptoms, and the presence of extranodal disease. In NHL, most patients are assigned to a stage based on physical examination, imaging studies, and bone marrow biopsy. The Ann Arbor staging system is considered by many to be inadequate for NHL, a shortfall that has led to the incorporation of other prognostic factors to define initial therapy.
The International Prognostic Index
In addition to stage, other important prognostic factors have been identified in patients with NHL.6 They frequently include patient parameters such as age, performance status, and tumor burden indicators such as bulk, number of involved sites, presence of extranodal disease, and lactate dehydrogenase (LDH) level.
An international team developed a prognostic model to predict outcomes of patients with aggressive NHL6 (Table 61-2). The International Prognostic Index (IPI) identified five significant risk factors predictive of overall survival: age (<60 years versus >60 years), serum LDH (normal versus elevated), performance status (0 or 1 versus 2 to 4), stage (I or II versus III or IV), and number of extranodal sites involved (0 or 1 versus 2 to 4). These features were incorporated into a model that identified four groups of patients with significantly different outcomes following treatment. Risk groups were determined by the sum of adverse risk factors: low (0 to 1 factor), low-intermediate (2 factors), high-intermediate (3 factors), and high (4 to 5 factors). The predicted 5-year survival rates for these groups were 73%, 51%, 43%, and 26%, respectively. When only patients younger than 60 were evaluated, stage, performance status, and LDH levels remained significant for poor prognosis (age-adjusted IPI).
Table 61-2 International Prognostic Index for Aggressive NHL
| All Patients |
| IPI Score | 5-Year Survival (%) | |
|---|---|---|
| Low-risk | 0-1 | 73 |
| Low-intermediate | 2 | 51 |
| High-intermediate | 3 | 43 |
| High-risk | 4-5 | 26 |
| Patients ≤ 60 years | ||
| Serum LDH above normal | ||
| ECOG performance > 2 | ||
| Ann Arbor stage III or IV |
| Age-Adjusted IPI Score | 5-Year Survival (%) | |
|---|---|---|
| Low-risk | 0 | 83 |
| Low-intermediate | 1 | 69 |
| High-intermediate | 2 | 46 |
| High-risk | 3 | 32 |
| Patients With Early-Stage (I-II) Disease | ||
|---|---|---|
| Age > 60 | ||
| Serum LDH above normal | ||
| Stage II disease | ||
| ECOG performance status ≤2 |
| Modified IPI Score | 10-Year Survival (%) |
|---|---|
| 0 | 90 |
| 1-2 | 56 |
| 3 | 48 |
For follicular lymphoma, the Follicular Lymphoma International Prognostic Index (FLIPI) has been shown to be a valuable prognostic tool.7 It is depicted in Table 61-3.
Table 61-3 The International Prognostic Index for Follicular Lymphoma4
| Age | ≤60 years |
| Ann Arbor stage | III-IV |
| Hemoglobin level | <12 g/dL |
| Serum LDH level | >ULN (upper limit of normal) |
| Number of nodal sites | >5 |
| Risk Group According to FLIPI Chart | Number of Factors |
| Low | 0-1 |
| Intermediate | 2 |
| High | ≤3 |
FLIPI, Follicular Lymphoma International Prognostic Index.
Essential workup studies for staging and IPI based on National Comprehensive Cancer Network (NCCN) guidelines8 are detailed in Table 61-4.
Table 61-4 Essential Workup of Patients With Non-Hodgkin’s Lymphomas
| Essential and Useful Workup Studies |
Response Criteria
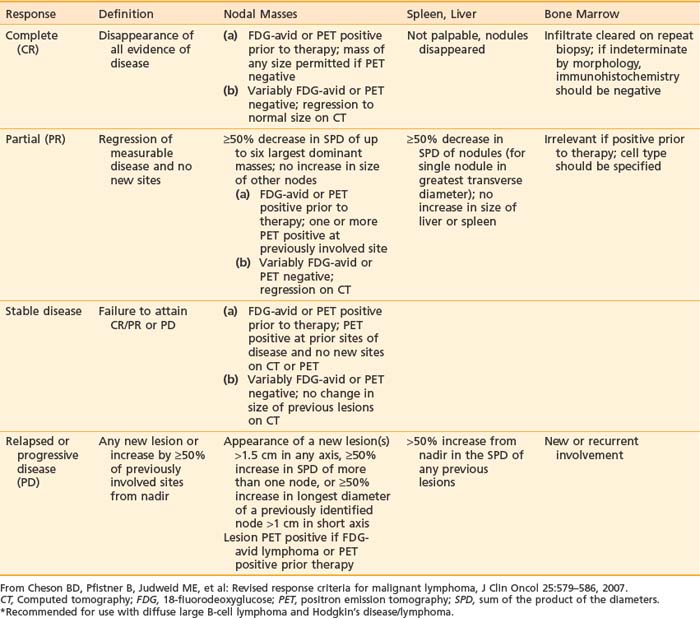
The International Working Group (IWG) published the guidelines for response criteria for lymphoma in 1999. These criteria are based on the reduction in size of the enlarged lymph nodes as measured by computed tomography (CT) scan, and the extent of bone marrow involvement, determined by bone marrow aspirate and biopsy.9 These guidelines were revised in 2007 by the International Harmonization Project to incorporate immunohistochemistry (IHC) flow cytometry and 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) scans in the definition of response. The category “complete response uncertain” (CRu) was essentially eliminated because residual masses were defined as a partial response (PR) or a complete response (CR) based on the result of the PET scan.10 Using the revised system, response is categorized as CR, PR, stable disease (SD), and relapsed disease or progressive disease (PD). However, the application of PET to responses is limited to histologies where there is reliable FDG uptake in active tumor. Response criteria for lymphoma are summarized in Table 61-5.
Common Lymphomas and the Role of Radiation Therapy
There are certain types of lymphomas that are more likely to require radiation oncology attention. Table 61-6 lists the types of HL and NHL most likely to benefit from radiation therapy.
Table 61-6 Indications for Radiotherapy in the Treatment of Non-Hodgkin’s Lymphomas
| Radiation Alone—Potentially Curative | Radiation Is Effective for Palliation and Local Control |
Low-Grade Lymphomas
Follicular Lymphomas
The disease involves predominantly lymph nodes, although the bone marrow is involved in approximately 40% of patients. Of particular interest to the radiation oncologist is that about 20% of FL patients present with localized (stage I and stage II) disease and are largely curable with radiation therapy.11 Although patients with advanced-stage disease may have an indolent course, there is no curative treatment, and there is an inexorable relapse rate of 15% to 20% per year. In many patients, follicular lymphomas12 eventually evolve into aggressive lymphomas manifested by histologic transformation to diffuse-pattern and large-cell morphology, clinical progression, and abnormal cytogenetic findings in addition to t(14;18).12 Diffuse large-cell lymphomas that evolve from follicular lymphomas lack the curability of diffuse large-cell lymphomas. A recent study disclosed a transformation risk of 28% in 10 years.12 The median survival after transformation was only 1.2 years.
The standard treatment for patients with localized stages (I and II) is regional or involved-field radiotherapy. Results from large series of patients with stage I and II indolent lymphomas who were treated with radiotherapy alone are summarized in Table 61-7. It should be noted that in past series, no clear distinction was made between early stages of follicular lymphomas and the less frequent currently recognized low-grade histologies of marginal zone and small lymphocytic lymphoma (SLL). Additionally, patients in series from more than 2 decades ago were staged less meticulously than today, and it is estimated that a significant number of them would be upstaged with current imaging technology (such as PET).
The report from Stanford5 of patients with stage I and II low-grade follicular lymphoma with long-term follow-up indicated that a substantial number of patients in this category have, indeed, been “cured” by radiotherapy. The median follow-up was 7.7 years, and observation has been maintained for up to 31 years. The median survival after radiotherapy was 14 years. The actuarial survival rates at 5, 10, 15, and 20 years were 82%, 64%, 44%, and 35%, respectively. Freedom from relapse (FFR) at 5, 10, 15, and 20 years was 55%, 44%, 40%, and 37%, respectively. Only 5 of 47 patients who reached 10 years without a relapse developed a late recurrence. There was no significant FFR difference between stage I and stage II or between nodal or extranodal disease. The survival of patients irradiated after the age of 60 years was significantly shorter than the survival of younger patients, but the decrease of survival in this age group was strongly affected by death from other causes.
Most relapses in early-stage patients occur in unirradiated sites during the first 5 to 6 years following therapy.14 In the Stanford series, administration of radiotherapy to nodal sites on both sides of the diaphragm was associated with a significantly better FFR compared with more localized treatment but did not translate into a clear survival benefit.11 Similar experience was reported from M.D. Anderson.15 In the absence of a clear survival benefit for total lymphoid irradiation in this setting, the common involvement of mesenteric nodes that may require whole abdominal irradiation, and in consideration of the fact that almost half the patients will eventually require chemotherapy for relapse, a limited field is preferred. Involved or regional field irradiation for these patients is currently the standard of care.
The optimal radiation dose for indolent lymphoma has not been determined in a prospective study. However, most current radiotherapy series for stage I and II indolent lymphomas usually employ 30 to 40 Gy, and in-field recurrences have been uncommon. Data from the Princess Margaret Hospital showed in-field disease control in 78% of patients treated with doses of less than 25 Gy and 91% control with doses greater than 25 Gy.16 At M.D. Anderson, excellent local control was achieved with 30 Gy to lesions smaller than 3 cm.10 A recent (as yet unpublished) prospectively randomized British study showed that 24 Gy provide response and local control rates similar to those obtained with 40 to 45 Gy.17 At Memorial Sloan-Kettering Cancer Center (MSKCC), a dose of 24 to 36 Gy to involved sites (36 Gy, if bulky) is the standard of care.
Several prospective randomized trials failed to demonstrate that the use of radiation followed by chemotherapy was superior to radiation alone in early-stage indolent lymphoma. The rare histology of early-stage nodal marginal zone lymphoma is treated (as is FL) with involved-field radiotherapy (IFRT) alone.8
Marginal Zone Lymphomas
Approximately 30% to 50% of patients with H. pylori–positive gastric MALT lymphoma (GML) will show persistent or progressing lymphoma even after eradication of H. pylori with antibiotic therapy. Even in complete responders, almost 15% will relapse within 3 years, suggesting that about half of patients with GML will eventually be considered for additional therapies.18 Most of those will still have disease limited to the stomach. In these patients and in those who present with no evidence of H. pylori infection, IFRT with relatively low radiation dose is the treatment of choice.8
Several institutions reported excellent results using IFRT of the stomach in H. pylori–independent GML patients who either failed antibiotic therapy or had no evidence for H. pylori infection.18–22 A recent update of the MSKCC experience included 51 patients with GML (stage I, 39; stage II, 10; stage IV, 2) who were either H. pylori–negative (30) or remained with persistent lymphoma after antibiotic therapies and adequate observation (21).19 All patients were treated with radiation to the stomach and perigastric nodes; the median total dose was 30 Gy in 4 weeks. All patients had regular follow-up endoscopic evaluations and biopsies, and 49 of 51 (96%) patients obtained a biopsy-proven complete response. Of three patients who relapsed, two were salvaged. Three patients died of other malignancies; all second tumors developed outside the radiation field. At a median follow-up of 4 years, freedom from treatment failure, overall survival and cause-specific survival were 89%, 83%, and 100%, respectively. Treatment was well tolerated, with no significant acute or chronic side effects. The experience from Toronto and Boston using the same radiation treatment approach was equally successful,20–22 supporting the notion that modest-dose IFRT is the treatment of choice for patients with persistent GML who have exhausted the antibiotic therapy approach or are unlikely to respond to it (H. pylori–negative patients).23,24 The techniques for treatment of gastric lymphoma have been described in detail.25
MALT lymphomas have also been described in various nongastric sites such as salivary glands, skin, orbit, conjunctiva, lung, thyroid, larynx, breast, kidney, liver, bladder, prostate, urethra, small intestine, rectum, pancreas, and even in the intracranial dura.24 The optimal management of nongastric MALT lymphomas has not yet been clearly established. Retrospective series included patients treated with surgery, radiotherapy, and chemotherapy, alone or in combination. Marginal zone lymphomas are exquisitely sensitive to relatively low doses of radiation. Specifically, MZLs in sites such as salivary glands, ocular structures, conjunctivae, thyroid, breast, and bladder have been successfully eradicated with IFRT encompassing the involved organ alone with a dose of 24 to 36 Gy.20,22 Even unusual sites (e.g., larynx, base of skull, urethra, prostate) not easily amenable to surgery have been well controlled by IFRT.
Radiation Therapy for Relapsed and Refractory Low-Grade Lymphomas
Several European groups showed that patients with low-grade lymphoma and persistent or relapsed disease following several regimens of chemotherapy responded to only two treatments of 2 Gy each (a total of 4 Gy). Using this schedule, a Dutch group reported on 109 patients (304 symptomatic sites), mostly with FL, with an overall response rate of 92% and a complete response rate of 61%.26,27 The 2-year actuarial freedom from local progression (FFLP) rate was 56%. Similarly, a French team reported an objective response of 81% of the sites, with 57% attaining a complete remission. The 2-year actuarial FFLP rate was 56%.28
Intermediate-Grade (Aggressive) Lymphomas
Mantle Cell Lymphoma
Mantle cell lymphoma presents as stage IV in most patients and is treated primarily with chemotherapy, but its exquisite sensitivity to radiation should be appreciated. A British Columbia Cancer Center study indicated that patients with MCL stages I and II benefited significantly from localized RT alone or RT combined with chemotherapy. The 5-year progression-free survival (PFS) was 68% for those receiving RT, compared to only 11% in patients not receiving RT (P < .002), 6-year overall survival was 71% and 25%, for RT versus no RT (P < .13).29
At MSKCC, we used low-dose RT for local control and palliation of 38 sites in 21 advanced-stage patients previously treated with chemotherapy. Local control with radiation was obtained in all sites, and a complete response was achieved in 64% of sites.30 In symptomatic patients, 94% obtained pain control with RT. Local progression occurred in 34% of patients at a median time to progression of 10 months. Since only a low dose of radiation is required (15 to 30 Gy) in MCL, large nodal sites may be treated with only minor side effects and without jeopardizing other future therapeutic options.
Diffuse Large B-Cell Lymphoma
Early-Stage Diffuse Large B-Cell Lymphoma
The question of whether the addition of radiotherapy improves the relapse-free and overall survival rate in patients with early-stage aggressive lymphoma who attained a complete response with chemotherapy is an issue marked by controversy.31,32
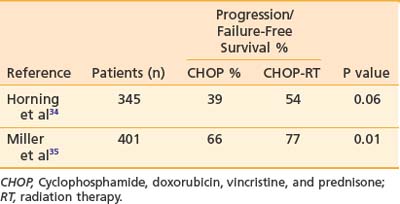
Adjuvant radiation therapy has become the standard of care in early-stage aggressive NHL following the analysis of two large randomized studies by the Eastern Cooperative Oncology Group (ECOG) and the Southwest Oncology Group (SWOG).33 These studies are summarized in Table 61-8. The ECOG study involved patients with bulky or extranodal stage I and stage II intermediate-grade NHL.34 All patients received 8 cycles of CHOP chemotherapy. Patients who attained only a partial response following chemotherapy received IFRT to 40 Gy, and 28% converted to complete-response status. Patients in complete response (61%) following chemotherapy alone were randomly assigned either to receive radiotherapy of 30 Gy to site pretreatment involvement or to observation alone. The recent 15-year update showed a statistically significant advantage to the adjuvant radiotherapy arm. The patients who received adjuvant radiotherapy attained a better failure-free survival rate than those receiving CHOP alone (54% versus 39%; P < .06).34 Overall survival that included all causes of death in this aging population was better in the irradiated group (60% versus 44%), but the difference was not statistically significant. Cause-specific survival was not reported.
The SWOG study enrolled patients with stage I and non-bulky stage II aggressive NHL.35 The patients were randomly assigned to receive either 8 cycles of CHOP chemotherapy alone or 3 cycles of CHOP followed by an involved field of 40 Gy (with an optional boost of up to 55 Gy). At a median follow-up of 4 years, the progression-free survival rate was significantly greater for the short-course CHOP plus radiotherapy group: 77% compared with 66% in the group receiving 8 cycles of CHOP with no radiotherapy. The combined-modality treatment also resulted in a superior overall survival rate (87% versus 75%; P < .01). Additionally, reversible toxicity occurring during therapy also favored the combined-modality arm.35 Recent analysis of the SWOG data suggested that patients with early-stage modified high IPI had inferior survival and may require more than 3 cycles of chemotherapy.36
The results of both randomized studies confirm the importance of adjuvant radiation therapy to the involved field in patients who attained a complete response following short (3 cycles) or long (8 cycles) chemotherapy. A relatively low dose of 30 Gy was adequate for patients who attained a complete response in the ECOG study (using 8 cycles of CHOP), while a higher dose (40 to 55 Gy) was used in the short chemotherapy arm of the SWOG study. At MSKCC, the standard consolidation dose is 30 to 36 Gy for patients who attained an unquestionable complete response following 3 to 6 cycles of chemotherapy. This is based on our and others’ excellent local control data with this dose range. A recent randomized study from England confirmed the adequacy of 30 Gy as well.17 At MSKCC, a higher dose (40 to 50 Gy) is still advised for uncertain complete responses or for patients that failed chemotherapy but are still potentially curable. For evaluating response, it is now recommended to obtain a PET-CT scan prior to and following chemotherapy, since a positive PET scan following chemotherapy may indicate an incomplete response that mandates a more aggressive approach.
Advanced-Stage Diffuse Large B-Cell Lymphoma
The standard treatment for patients with advanced-stage (III or IV) aggressive lymphoma is combination chemotherapy, and R-CHOP is the most commonly used combination.36 In North America, radiation therapy as consolidation to even bulky sites or incomplete responders is only occasionally considered, although supported by retrospective studies.37 Surprisingly, the data regarding the irrelevance of radiotherapy in these situations are scanty and/or indirect at best.38
Unfortunately, even though the superiority of combined modality over chemotherapy alone has been established for early stages, the concept and feasibility have not been tested in trials for advanced-stage NHL in the United States. It is of interest that recent randomized studies from other countries suggest that radiotherapy, particularly if administered to areas of originally bulky disease, may significantly improve the relapse-free and overall survival of patients who attained a CR with chemotherapy.39–42 Investigators from National Cancer Institute in Mexico conducted two consecutive randomized studies with a similar design. In the first study, 218 patients with stage IV diffuse large-cell lymphoma were included. Following chemotherapy, 155 patients (71%) achieved a complete response. Of the complete responders, 88 patients (56%) originally presented with bulky disease (>10 cm) and therefore were prospectively randomized to observation or to receive IFRT to a dose of 40 to 50 Gy. At 5 years, 72% of 43 patients randomized to receive radiotherapy were alive and disease-free, compared to only 35% of the 45 patients who were not irradiated (P < .01). Most relapses occurred in the original site. Overall survival was also improved for the irradiated patients (81% versus 55%; P < .01).39 In the more recent study, 341 patients with aggressive DLCL and presence of nodal bulky disease (tumor mass >10 cm) in pathologically proven complete response after intensive chemotherapy were randomized to either radiotherapy (involved fields, 40 Gy) or observation. The 5-year EFS and OS in irradiated patients were 82% and 87%, respectively, which were superior to the control group: EFS, 55% (P < .001) and OS, 66% (P < .01). Radiotherapy was considered “well tolerated,” and acute toxicity was “mild.”42
In Milan, 97 patients with stages III to IV diffuse large-cell lymphoma that were in CR after chemotherapy were either observed or received consolidation radiotherapy. At 5 years, patients with bulky disease (<10 cm) who received radiotherapy had a significantly longer time to relapse and a better overall survival (P < .05) compared with patients who were not irradiated. A multivariate analysis showed that the use of radiotherapy was an independent favorable prognostic factor for relapse (P < .001) and survival (P < .05).40
In Paris, patients with NHL who underwent high-dose chemotherapy with stem cell transplantation as upfront treatment or treatment for relapse and received posttransplantation radiotherapy had a better event-free survival compared with patients who had not received radiotherapy (P < .02 in multivariate analysis). Other studies have also supported the use of consolidation radiotherapy after high-dose chemotherapy and bone marrow transplantation.41
Primary Mediastinal Lymphoma
In most patients, primary mediastinal lymphoma is bulky and limited to the mediastinum. Consolidation with IFRT of the mediastinum after a complete, uncertain, or partial response with chemotherapy is a standard approach in most centers.43,44 Several large retrospective studies indicated the superiority of a combined-modality approach in primary mediastinal lymphoma over chemotherapy alone.45 Yet, prospective randomized studies evaluating the contribution of RT in mediastinal lymphoma have not been reported.
Radiation Fields
In almost all clinical scenarios, radiotherapy is given as an involved-field to either the involved organ or the involved lymph node group (see Chapter 60). This IFRT approach is recommended either for treatment with RT alone or when radiotherapy is used for consolidation or palliation.
1 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;97:225-249.
2 Jaffe ES, Harris NL, Stein H, Issacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384-4399.
3 Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780-2795.
4 Weisenburger DD, Wilson WH, Vose JM. Peripheral T-cell lymphoma, not otherwise specified: a clinicopathologic study of 340 cases from the international T-cell lymphoma project. Am Soc Hematol Annu Meet Abstr. 2006;108:2458.
5 Dunphy CH. Applications of flow cytometry and immunohistochemistry to diagnostic hematopathology. Arch Pathol Lab Med. 2004;128:1004-1022.
6 Shipp MA. Prognostic factors in aggressive non-Hodgkin’s lymphoma: who has “high-risk” disease? Blood. 1994;83:1165-1173.
7 Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258-1265.
8 Zelenetz AD, et al. NCCN physician guidelines: Non-Hodgkin Lymphoma 2009 v.1. www.nccn.org, 2009.
9 Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. Erratum published in J Clin Oncol 18:2351, 2000
10 Cheson BD, Pfistner B, Judweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-586.
11 MacManus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol. 1996;14:1282-1290.
12 Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007.
13 Vaughan Hudson B, Vaughan Hudson G, MacLennan KA, et al. Clinical stage 1 non-Hodgkin’s lymphoma: long-term follow-up of patients treated by the British National Lymphoma Investigation with radiotherapy alone as initial therapy. Br J Cancer. 1994;69:1088-1093.
14 Sutcliffe SB, Gospodarowicz MK, Bush RS, et al. Role of radiation therapy in localized non-Hodgkin’s lymphoma. Radiother Oncol. 1985;4:211-223.
15 Wilder RB, Jones D, Tucker SL, et al. Long-term results with radiotherapy for Stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys. 2001;51:1219-1227.
16 Bush RS, Gospodarowicz M, Sturgeon J, et al. Radiation therapy of localized non-Hodgkin’s lymphoma. Cancer Treat Rep. 1977;61:1129-1136.
17 Hoskin P. Radiation dose in non-Hodgkin’s lymphoma: preliminary results of UK NCRN randomized trial. Ann Oncol. 16, 2005.
18 Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916-1921.
19 Yahalom J, Portlock CS, Gonzales M, et al. H. pylori-Independent MALT lymphoma of the stomach: excellent outcome with radiation alone. Blood. 2002;100:160a.
20 Tsang RW, Gospodarowicz MK, Pintilie M, et al. Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50:1258-1264.
21 Fung CY, Grossbard ML, Linggood RM, et al. Mucosa-associated lymphoid tissue lymphoma of the stomach: long term outcome after local treatment. Cancer. 1999;85:9-17.
22 Hitchcock S, Ng AK, Fisher DC, et al. Treatment outcome of mucosa-associated lymphoid tissue/marginal zone non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2002;52:1058-1066.
23 Gospodarowicz MK, Pintilie M, Tsang R, et al. Primary gastric lymphoma: brief overview of the recent Princess Margaret Hospital experience. Recent Results Cancer Res. 2000;156:108-115.
24 Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol. 2001;80:B100-5.
25 Dell Bianca C, Hunt M, Furhang E, et al. Radiation treatment planning techniques for lymphoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;62:745-751.
26 Haas RL, Poortmans P, de Jong D, et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol. 2003;21:2474-2480.
27 Haas RL, Girinsky T. HOVON 47/EORTC 20013: chlorambucil vs 2×2 Gy involved field radiotherapy in stage III/IV previously untreated follicular lymphoma patients. Ann Hematol. 2003;82:458-462.
28 Girinsky T, Guillot-Vals D, Koscielny S, et al. A high and sustained response rate in refractory or relapsing low-grade lymphoma masses after low-dose radiation: analysis of predictive parameters of response to treatment. Int J Radiat Oncol Biol Phys. 2001;51:148-155.
29 Leitch HA, Gascoyne RD, Chhanabhai M, et al. Limited-stage mantle-cell lymphoma. Ann Oncol. 2003;14:1555-1561.
30 Rosenbluth BD, Yahalom J. Highly effective local control and palliation of mantle cell lymphoma with involved-field radiation therapy (IFRT). Int J Radiat Oncol Biol Phys. 2006;65:1185-1191.
31 Ng AK, Mauch PM. Role of radiation therapy in localized aggressive lymphoma. J Clin Oncol. 2007;25:757-759.
32 Longo DL. Combined modality therapy for localized aggressive lymphoma: enough or too much? J Clin Oncol. 1989;7:1179-1181.
33 Miller TP. The limits of limited stage lymphoma. J Clin Oncol. 2004;22:2982-2984.
34 Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group Study 1484. J Clin Oncol. 2004;22:3032-3038.
35 Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21-26.
36 Fisher RI, Miller TP, O’Connor O A. Diffuse aggressive lymphoma. Hematology (Am Soc Hematol Educ Program). 2004:221-236.
37 Schlembach PJ, Wilder RB, Tucker SL, et al. Impact of involved field radiotherapy after CHOP-based chemotherapy on stage III-IV, intermediate grade and large-cell immunoblastic lymphomas. Int J Radiat Oncol Biol Phys. 2000;48:1107-1110.
38 Shipp MA, Klatt MM, Yeap B, et al. Patterns of relapse in large-cell lymphoma patients with bulk disease: implications for the use of adjuvant radiation therapy. J Clin Oncol. 1989;7:613-618.
39 Aviles A, Delgado S, Nambo MJ, et al. Adjuvant radiotherapy to sites of previous bulky disease in patients stage IV diffuse large cell lymphoma. Int J Radiat Oncol Biol Phys. 1994;30:799-803.
40 Ferreri AJ, Dell’Oro S, Reni M, et al. Consolidation radiotherapy to bulky or semibulky lesions in the management of stage III-IV diffuse large B cell lymphomas. Oncology. 2000;58:219-226.
41 Fouillard L, Laporte JP, Labopin M, et al. Autologous stem-cell transplantation for non Hodgkin’s lymphoma: the role of graft purging and radiotherapy posttransplantation—results of a retrospective analysis on 120 patients autografted in a single institution. J Clin Oncol. 1998;16:2803-2816.
42 Aviles A, Fernandez R, Perez F, et al. Adjuvant radiotherapy in stage IV diffuse large cell lymphoma improves outcome. Leuk Lymphoma. 2004;45:1385-1389.
43 Aviles A, Garcia EL, Fernandez R, et al. Combined therapy in the treatment of primary mediastinal B-cell lymphoma: conventional versus escalated chemotherapy. Ann Hematol. 2002;81:368-373.
44 Zinzani PL, Martelli M, Bertini M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87:1258-1264.
45 Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer. 2004;90:372-376.