27 New Approaches to the Surgical Treatment of End-Stage Heart Failure
Epidemiology, pathophysiology, and limitations of current management
Scope of the Problem
According to the American Heart Association, approximately 6 million people in the United States have congestive heart failure (CHF). Available statistics indicate that the incidence of CHF in the population approaches 15.2 per 1000 after age 65, 31.7 per 1000 after age 75, and 65.2 per 1000 after age 85, with 1,106,000 hospital discharges for heart failure (HF) in 2006 alone.1 HF is the leading cause of hospitalization in patients older than 65,2 with a reported associated cost of $24 to $50 billion annually.1–4 On a global scale, HF reportedly affects 0.4% to 2.0% of the adult population.5
Despite great advances in the understanding of the pathophysiology of HF and the development of medications that can potentially attenuate the progression of that pathophysiology, morbidity and mortality from this disease remain high. The incidence rate of hospitalization for HF increased by 70% during the 1990s,2 and patients with New York Heart Association (NYHA) Class IV symptoms currently have a reported 1-year mortality rate of 30% to 50%.6 By comparison, the corresponding rates for NYHA Class I-II patients and Class II-III patients are 5% and 10% to 15%, respectively. (Table 27-1 defines the NYHA symptomatic classes.7) Thus, one of the major goals in the management of HF is the prevention of progression to advanced stages.
TABLE 27-1 New York Heart Association (NYHA) Functional Capacity
| Functional Capacity | Objective Assessment |
|---|---|
| Class I. Patients with cardiac disease but without resulting limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, dyspnea, or anginal pain. | A. No objective evidence of cardiovascular disease |
| Class II. Patients with cardiac disease resulting in slight limitation of physical activity. They are comfortable at rest. Ordinary physical activity results in fatigue, palpitation, dyspnea, or anginal pain. | B. Objective evidence of minimal cardiovascular disease |
| Class III. Patients with cardiac disease resulting in marked limitation of physical activity. They are comfortable at rest. Less than ordinary activity causes fatigue, palpitation, dyspnea, or anginal pain. | C. Objective evidence of moderately severe cardiovascular disease |
| Class IV. Patients with cardiac disease resulting in inability to carry on any physical activity without discomfort. Symptoms of heart failure or the anginal syndrome may be present even at rest. If any physical activity is undertaken, discomfort is increased. | D. Objective evidence of severe cardiovascular disease. |
From The Criteria Committee of the New York Heart Association: Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th edition. Boston: Little, Brown & Co, 1994, pp 253–256.
Brief Review of the Pathophysiology
The current understanding of the pathophysiology of chronic HF maintains that initial increases in end-diastolic ventricular volume and pressure trigger the release of endogenous natriuretic peptides that promote diuresis.8–10 Concurrent activation of the sympathetic nervous system causes peripheral vasoconstriction and increases the inotropic state of the myocardium. Initially, these mechanisms act to decrease excessive preload (which restores wall tension to normal) and maintain cardiac output (CO) and arterial blood pressure (BP) in the face of mildly depressed ventricular function. Eventually, however, the carotid, ventricular, and aortic arch baroreceptors are activated by the relative hypovolemia, which leads to further activation of the sympathetic nervous system (via the medullary vasomotor regulatory center), as well as the renin-angiotensin-aldosterone axis, and the release of vasopressin. The resultant peripheral vasoconstriction, mild fluid retention, and further increases in heart rate and inotropy will again compensate for the failing heart. Ultimately, however, chronic sympathetic stimulation causes myocardial β1-adrenergic receptors to downregulate, and as ventricular function deteriorates, left ventricular (LV) end-diastolic volumes and pressures again increase, resulting in increased ventricular wall tension. During this time, increased levels of angiotensin II result in adverse myocardial remodeling. Remodeling is a key event in the progression of HF and refers to changes in not only ventricular geometry (e.g., dilation) but also myocardial composition (e.g., myocyte hypertrophy, lengthening, hyperplasia, fibrosis). In addition, increased circulating levels of angiotensin II may enhance myocyte apoptosis (programmed cell death) via a protein kinase C–mediated increase in cytosolic calcium levels.11
Modern medical management of chronic CHF, therefore, uses agents that have been shown to decelerate the progression to severe failure, reduce adverse myocardial remodeling, and enhance survival (e.g., angiotensin-converting enzyme inhibitors,12–14 β-blockers,15–18 and aldosterone antagonists19,20), in combination with other agents that improve the symptoms but have not been shown to improve long-term survival alone (e.g., diuretics, digoxin). (See Chapter 10.)
Limitations of Current Medical Management
Given that there are currently between 300,000 and 800,000 patients in the United States who have progressed to NYHA Class III and IV status despite modern medical management,21 it appears that current treatment strategies have significant limitations. Part of the failure of medical management alone to control the progression of the disease may be that treatment has traditionally focused on systolic dysfunction. Four classic stages of HF have been described: (1) an initial cardiac injury or insult, (2) activation of specific neurohormonal axes with resultant cardiac remodeling, (3) compensatory fluid retention and peripheral vasoconstriction, and (4) ultimate contractile failure.22 In contrast with this traditional conception, it is now known that diastolic dysfunction (decreased lusitropic function) is the primary problem in an estimated 30% to 50% of patients with HF. Pharmacologic interventions aimed at improving diastolic dysfunction and attenuating (if not reversing) remodeling currently are the subject of randomized trials worldwide.
Surgical options for heart failure
A growing number of surgical procedures exist (or have been developed) to relieve CHF symptoms and arrest the progression of the disease through correction of abnormal myocardial depolarization, enhancement of myocardial blood supply, improvement in ventricular loading conditions, and restoration of more normal ventricular geometry. Box 27-1 provides a list of current surgical interventions for CHF.
Cumulative worldwide experience with the interventions listed in Box 27-1 suggests that these procedures not only relieve symptoms but may attenuate or possibly arrest the progressive myocardial remodeling that accompanies chronic HF.23–27 In some cases, partial reversal of the adverse myocardial remodeling has been demonstrated, and combination therapy (surgical intervention with targeted pharmacologic treatment) intended to enhance reverse remodeling is actively being investigated.28
Revascularization
Coronary artery disease has become the most common cause of HF.29 Of those patients currently listed for heart transplantation, 36% carry a primary diagnosis of ischemic heart disease, and 31% of those transplanted in 2007 had ischemia as their primary indication. Commonly used terms describing the extent of myocardial injury are defined in Table 27-2.
TABLE 27-2 Commonly Used Terms Describing the Extent of Myocardial Injury and the Potential for Recovery
| Term | Definition |
|---|---|
| Ischemic | Insufficient oxygen supply to meet myocardial oxygen demand |
| Stunned | Acute myocardial dysfunction after an ischemic event with potential for full recovery |
| Hibernating | Chronically ischemic, dysfunctional myocardium with potential for full recovery |
| Maimed | Dysfunctional myocardium on the basis of ischemia that does not fully recover |
| Infarcted | Myocardial necrosis caused by ischemia with no potential for recovery |
Where viable myocardium and feasible targets exist, revascularization of chronically ischemic, hibernating myocardium can improve ventricular function, downgrade NYHA functional class, and improve prognosis.30,31 Although the primary benefit of revascularization appears to be functional improvement of the left ventricle, reducing ischemic substrate for arrhythmias and retarding adverse myocardial remodeling are important secondary benefits.24
Despite an increased perioperative risk for morbidity and mortality in this population, the world’s literature reports current survival rates between 57% and 75% at 5 years, with in-hospital mortality rates between 1.7% and 11%.31 A review reported an 83.5% survival rate at 2 years after revascularization compared with only 57.2% survival in patients with CHF who were not revascularized.30 In general, morbidity and mortality tend to correlate inversely with EF and directly with NYHA functional class. Additional factors predisposing patients to greater morbidity and mortality include advanced age, female sex, hypertension, diabetes, and emergent operations32 (see Chapter 18). The decreases in morbidity and mortality after revascularization in this high-risk population in recent years are at least partially attributable to improvements in surgical technique and myocardial protection, but the concurrent performance of mitral valve repair and ventricular reshaping address the adverse ventricular loading conditions present and also may contribute to improved outcomes. The results of ongoing clinical trials evaluating combinations of surgical procedures (e.g., revascularization plus ventricular reshaping vs. revascularization alone) are discussed in detail later.
The importance of determining the viability of myocardium in the area to be revascularized cannot be overstated because the potential for recovery of function depends on residual contractile reserve, integrity of the sarcolemma, and metabolically preserved cellular function.31 Methods to detect viable myocardium include dobutamine stress echocardiography, single-photon emission computed tomography, positron emission tomography, and cardiac magnetic resonance imaging (see Chapters 1 and 2). Although dobutamine stress echocardio-graphy often has been shown to have the greatest predictive accuracy,33 important limitations need to be taken into account. Dobutamine stress echocardiography does not demonstrate viability directly, but improvement in mechanical contraction under pharmacologic stimulation. An example for a false-negative result can be seen if there is loss of contractile proteins in the presence of preserved function of the muscle fiber membrane.31 Some centers report using only the intraoperative assessment of myocardial wall thickness and contractility to determine potential viability with revascularization of the target region.34 Regardless of the specific method used, the important point is that there needs to be viable tissue to revascularize, and the best results will be obtained in properly selected individuals. Overwhelmingly, encouraging results worldwide suggest that, when feasible, revascularization is of benefit and provides survival advantage to patients with significant ventricular dysfunction.
Correction of Mitral Regurgitation
The mitral valve is a complex apparatus consisting of the anterior and posterior leaflets, the mitral annulus, the chordae tendineae, the papillary muscles, and the wall of the left ventricle. The posterior portion of the annulus is only rudimentarily developed and flexible. This explains why this portion of the annulus is prone to dilation during pathologic volume-overloaded states and mandates some form of mechanical stabilization when surgical valve repair is undertaken. The normal annulus has a three-dimensional (3D) saddle shape that is exacerbated during systole because of apical displacement of the commissures.35 In addition, the aortic root bulges posteriorly during systole. These dynamic phenomena lead to the ability of the annulus to change its shape during the cardiac cycle. During systole, an elliptical shape is assumed that facilitates coaptation of the leaflets. During diastole, a more circular form increases orifice dimensions, decreasing resistance to LV inflow.36 Consequently, the LV free wall, papillary muscles, and chordae play an important role in the competence of the valve, as well as in LV function during systole.
The mechanism responsible for mitral regurgitation can best be understood by utilizing the Carpentier classification (Table 27-3). This classification describes the motion of the mitral leaflets and position of the coaptation zone relative to the annular plane. The mitral regurgitation seen in patients with CHF is most often functional, primarily because of apical displacement of the papillary muscles resulting in tethering of the leaflets leading to systolic restriction of leaflet motion (type IIIb).
TABLE 27-3 Carpentier Classification of Mitral Regurgitation
| Carpentier Class | Leaflet Motion | Typical Pathology |
|---|---|---|
| Type I | Normal leaflet motion | Annular dilation, leaflet perforation |
| Type II | Excessive leaflet motion | Leaflet prolapse or flail, chordal rupture or elongation |
| Type IIIa | Restricted leaflet motion | Rheumatic leaflet(s), thickened or fused leaflets or chordae |
| Type IIIb | Restricted leaflet motion | Papillary muscle displacement/dysfunction (dilated cardiomyopathy or ischemic) |
From Carpentier A, Chauvaud S, Fabiani JN, et al: Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J Thorac Cardiovasc Surg 79:338, 1980.
Historically, many physicians considered mitral regurgitation advantageous for the failing left ventricle. It was believed that a low-pressure atrial “pop off” allowed the failing ventricle to protect itself from the high afterload of the systemic circulation and gave the illusion that the heart had a better overall contractile state than really existed. This misconception was “supported” by the fact that surgical replacement of the mitral valve was associated with a very high mortality rate in patients with depressed LV function.37
Romano and Bolling’s38 work disproved this misconception, showing that despite increased operative risk, mitral valve repair or replacement was beneficial to patients with severely depressed LV function, CHF, and mitral regurgitation. Operative mortality rates of 5% were reported, with 1- and 2-year survival rates of 80% and 70%, respectively.38 Not only was long-term mortality reduced, but the increase in LV systolic function (on average by 10%) enabled a downgrading of NYHA class and resulted in an improved quality of life (see Chapter 19).
Although the majority of patients with end-stage HF will exhibit functional mitral regurgitation (as discussed earlier), there may be additional concurrent valvular pathology present in a given patient. An intraoperative transesophageal echocardiography (TEE) evaluation of the valvular anatomy, the mechanism of the mitral regurgitation, and direct surgical inspection will determine the feasibility of repair. Data obtained from patients suffering from organic mitral valve regurgitation have been extrapolated to the functional mitral regurgitation group in the belief that valve repair is preferable to valve replacement, because there are demonstrated hemodynamic advantages associated with preservation of the subvalvular apparatus,39 and long-term anticoagulation is not required. However, review of the literature cannot unequivocally support this assumption. Magne et al40 were unable to show a survival advantage between MV repair and replacement in patients with ischemic mitral regurgitation. Gillinov et al41 showed that a survival benefit could be obtained by repairing the mitral valve as opposed to replacing it, especially in lower-risk patients. However, in high-risk patients, defined as patients with extremely low EF and dilated ventricles, this survival benefit was lost, prompting them to recommend MV replacement.41 Braun et al42 showed that end-diastolic diameter larger than 65 mm was associated with poorer survival in 108 patients undergoing restrictive mitral annuloplasty and CABG. Reverse remodeling was seen in all patients with end-diastolic diameter smaller than 65 mm; however, it was seen in only 25% with end-diastolic diameter larger than 65 mm, making them question whether MV repair/CABG is justified in grossly dilated ventricles. Most likely a ventricular solution will need to be considered in this subset of patients.
A common dilemma that is encountered by the perioperative team is what to do with a patient with ischemic cardiomyopathy and moderate mitral regurgitation. In the setting of severe mitral regurgitation, most would agree to perform concurrent mitral regurgitation repair, and in the setting of mild mitral regurgitation, to leave the mitral valve dysfunction unaddressed. Penicka et al’s43 study potentially could help shed light on this topic. They found improvement in moderate ischemic mitral regurgitation only in patients in whom viable myocardium in the region of the papillary muscles could be identified by preoperative single-photon emission computed tomographic testing. Patients with nonviable myocardium and dyssynchrony between the papillary muscles showed postoperative worsening of ischemic mitral regurgitation. Consequently, the authors argued to perform annuloplasty in all patients with nonviable myocardium and perform isolated CABG in patients with viable myocardium.43
The primary repair technique in the setting of type IIIb dysfunction consists of downsized annuloplasty. Although much debate exists regarding whether the annuloplasty should be performed with the aid of a complete semirigid ring or band, most experts agree that the annulus needs to be remodeled and stabilized. Although this technique results in excellent short-term results, most surgeons realize that addressing a ventricular problem at the annular level cannot represent the ideal solution. Magne et al44 showed that the use of annuloplasty rings eliminated severe mitral regurgitation; however, this was at the cost of leaving patients with functional mitral stenosis. One valvular disorder could be exchanged for another by undersizing the annulus (see Chapter 19).
After the procedure, TEE is used to assess the adequacy of the repair and potential improvement of overall cardiac function. Often, hemodynamic improvement is not immediately apparent, and TEE is used to optimize preload and guide pharmacologic interventions (see Chapters 12 to 14, 32, and 34).
Left Ventricular Restoration
In 1985, building on work by Cooley, Jatene, and others,45–47 Dor et al introduced a ventricular reshaping procedure intended to improve systolic performance by excluding akinetic/dyskinetic and aneurysmal portions of the left ventricle with a circular stitch at the transitional zone between contractile and noncontractile myocardium. A small patch was used within the ventricular cavity as needed to reestablish ventricular wall continuity at the level of the purse-string suture.48
This procedure generally was performed in conjunction with revascularization (and included mitral repair/replacement as needed) and thus helped establish important principles for a surgical ventricular restoration: revascularization of ischemic myocardium, decreasing of ventricular volume, and the restoration of ventricular shape. A subsequent publication by the same group described the results of this procedure in 130 patients (35% of whom had “heart failure” as the indication for the operation) and reported a 6% in-hospital mortality rate and a 3% late mortality rate because of recurrence of cardiac failure.49
In 1996, Batista et al50 introduced a procedure for the reshaping of the nonischemic, dilated, and failing left ventricle of NYHA Class IV patients through resection of a wedge of normal myocardium from the LV apex to the base (laterally, between the papillary muscles). This “partial left ventriculectomy” restored more normal ventricular geometry and decreased wall tension. Functional mitral regurgitation also was addressed during the Batista procedure by a mitral valve replacement or repair. Although many patients did benefit initially from this procedure (reduction of NYHA functional class to NYHA Class I in 57% and NYHA Class II in 33.3%),51 perioperative mortality was high (20% in both Batista’s own series and in the large Cleveland Clinic experience).51,52 In addition, the experience of several centers was that many patients required rescue mechanical circulatory assistance after the procedure, and many patients experienced a redilatation of their left ventricle resulting in a return to NYHA Class IV status.53–55 Thus, despite the short-lived period of initial enthusiasm in the mid- to late-1990s, the Batista procedure essentially has been abandoned. The concept of ventricular reshaping, however, remains of interest.
The modified Dor procedure (endoventricular circular patch plasty) has been used successfully to reshape large, dilated, spherical left ventricles of patients who have had an anterior wall myocardial infarction (MI) with resulting aneurysm and akinesis/dyskinesis. Essentially, a Dacron patch is placed within the LV cavity to exclude the large akinetic/dyskinetic area of the anterior wall. This restores LV geometry to a more normal elliptical shape and improves systolic function. When performed concurrently with CABG, significant early and late improvements in both NYHA functional class and EF have been demonstrated, with an in-hospital mortality rate of 12%.56,57 A trial of 439 patients undergoing this procedure found an improved in-hospital mortality rate of 6.6% and an 18-month survival rate of 89.2%. In this series, CABG was performed concurrently in 89%, mitral valve repair in 22%, and mitral valve replacement in 4%.58
Surgical ventricular restoration is the modern name for a modified Dor procedure, in which, in addition to exclusion of the akinetic aneurysmal segment of the anterior and/or septal wall and revascularization, a sizing balloon (or other sizer) is used to create an elliptical left ventricle of 30% to 40% smaller volume than baseline. Despite a worldwide surgical ventricular restoration database already in place including more than 5000 surgical ventricular restoration patients, a randomized, controlled trial was designed named “The Surgical Treatment for Heart failure (STICH),” with the intention to scientifically prove the efficacy of this procedure as an adjunct to revascularization. The results of this important trial have been eagerly awaited for years. Unfortunately, STICH did not demonstrate that the addition of surgical ventricular restoration to revascularization was associated with a greater improvement in symptoms or exercise tolerance, or with a reduction in the rate of death or hospitalization for cardiac causes when compared with revascularization alone.59 The negative results from this trial have caused much controversy and the validity questioned.60 Noted areas of concern revolve around participant eligibility for anatomic reasons, lack of standardized volumetric assessments in large numbers of participants, and alteration of the primary objective after trial commencement. In essence, the expert opinion is that the STICH trial was “well conceived, but poorly executed.” At the time of this writing, surgical ventricular restoration continues to be performed for true LV aneurysms but has fallen out of favor as a routine adjunct for the dilated end-stage left ventricle.
Cirillo’s61 recent publication described a further modification of the Dor concept wherein the shape and orientation of the patch, as well as the manner of suturing, ostensibly maintained more physiologic orientation of the myocardial fibers. Named the “KISS” procedure (Keep fIbers orientation with Strip patch reShaping), this methodology reportedly allowed for a return of normal apical rotation and ventricular torsion to optimize systolic performance. Long-term results of this new approach await further study.
Cardiac Resynchronization Therapy and Implantable Cardioverter-Defibrillators
The progression of disease resulting in advanced cardiac failure is typically accompanied by conduction defects and arrhythmias, and pacemakers and implantable cardioverter-defibrillators (ICDs) are common in this population. In addition to the well-known defects in sinus or atrioventricular node function, intraventricular conduction defects delay the onset of RV or LV systole in 30% to 50% of patients with advanced HF.62,63 This lack of coordination of LV and RV contractions further impairs CO64–67 and has been reported to increase the risk for death in this population68,69 (see Chapters 4 and 25).
Studies have shown that atrial-synchronized biventricular pacing (pacing the left and right ventricles in a carefully timed manner) can resynchronize RV and LV contraction, improving CO and overall hemodynamics. This enhances these patients’ ability to exercise, which improves their NYHA functional class, and decreases the length and frequency of their hospitalizations, which improves their quality of life.70–74
Sudden death from ventricular fibrillation accounts for approximately 350,000 deaths annually in the United States.1 Patients with advanced HF experience ventricular fibrillation with a frequency six to nine times that of the general population,1 and ventricular fibrillation causes 40% of all deaths in this population even in the absence of apparent disease progression based on symptoms.75 Thus, ICDs commonly are indicated for patients with advanced cardiac failure. An ICD is a device capable of arrhythmia detection and automatic defibrillation. ICDs successfully terminate ventricular fibrillation in greater than 98% of episodes, and studies have demonstrated that an ICD increases survival and decreases the risk for sudden death in patients with ischemic cardiomyopathy and decreased LV function.76–78
The COMPANION trial (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure) studied 1500 patients with NYHA Class III/IV HF, a QRS interval of greater than 120 milliseconds, PR interval greater than 150 milliseconds, and an LVEF less than or equal to 35%. Compared with optimal pharmacologic therapy alone, CRT decreased the risk of the combined end point of death from or hospitalization for HF by 34%. The combination of CRT and ICD implantation reduced these risks by 40%.79
The CARE-HF (Cardiac Resynchronization in Heart Failure) trial enrolled 800 patients with NYHA Class III/IV HF, a QRS interval of more than 150 milliseconds, a QRS interval of more than 120 milliseconds with echocardiographic evidence of dyssynchrony, and an LVEF of 35% or less. Compared with optimal medical therapy alone, CRT (without ICD functionality) reduced all-cause mortality by 36%. In addition, CRT showed significant improvement of cardiac dyssynchrony, ventricular function, and mitral regurgitation based on echocardiographic criteria.68
Most recent guidelines give a Class I recommendation for placement of a CRT device with or without ICD in patients with LVEF ≤ 35%, QRS ≥ 120 milliseconds, presence of sinus rhythm, and NYHA Class III/IV symptoms on optimal medical therapy.80 However, two recent trials have investigated the effects of CRT on patients with NYHA Class I/II HF. The REVERSE trial (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) randomized 610 NYHA Class I/II patients with QRS ≥ 120 milliseconds and LVEF ≤ 40% to receive a CRT device (±ICD) that was either active (CRT-ON) or disabled (CRT-OFF). This study showed a significant delay in time to first hospitalization and improvement in measures of LV remodeling in the CRT-ON group.81 The MADIT-CRT trial (Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy) randomized 1820 NYHA Class I/II patients with QRS ≥ 130 milliseconds and LVEF ≤ 30% to receive CRT and ICD or ICD alone. The CRT-ICD group had a significant 41% decrease in risk for first HF event, significant reduction in LV volumes, and increase in LVEF as compared with the ICD only group.82 Thus, although CRT previously has been about reduction in symptoms, it is anticipated that results such as those reported from MADIT-CRT will lead to an increased utilization of CRT in relatively asymptomatic patients with developing HF to prevent the progression of the disease.
Mechanical circulatory support
Ventricular Assist Devices: Implementation of Support
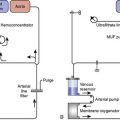
VADs can be used to take over the pumping function of the failing ventricle and provide effective CO to the arterial circulation downstream from the failing ventricle. In the case of the failing right ventricle, a right ventricular assist device (RVAD) can divert the deoxygenated venous return to the heart and pump it directly to the pulmonary arterial circulation. In the case of the failing left ventricle, a left ventricular assist device (LVAD) can divert the oxygenated blood returning to the left side of the heart and pump it directly into the aorta. Figure 27-1 demonstrates common cannulation strategies in the heart and great vessels by which MCS is implemented. Although there are a few notable exceptions (discussed later), these basic cannulation strategies are common to all manufacturers’ devices currently in use, regardless of the type of output they produce.
Mechanical Circulatory Support: Modern Practice
In prior years, VADs were regarded as either a last-ditch hope for recovery after an acute cardiac event that resulted in refractory cardiogenic shock (“bridge-to-recovery”), or as a “bridge-to-transplantation” for patients with chronic HF who were doing poorly. This has all changed now. More widespread acceptance of MCS, the recognition that outcomes are better when the support is instituted earlier rather than later, the development and use of risk scores to appropriately select patients and support strategies,83–94 the demonstration that there is often significant improvement in multiorgan function during the time spent on VAD support,95–97 the advent of new and improved devices, and a substantially decreased rate of VAD-associated complications98–101 have allowed for an increased and an improved clinical utilization of this life-saving technology in the management of both acute and chronic HF.
Short-Term Ventricular Assist Device Use
Early experience also demonstrated that specific patient conditions or comorbidities (Table 27-4) potentially increased the risk for complications during VAD support or made support difficult to implement. It also was proposed that patients who were not potential transplant candidates should probably not be considered for VAD support. Finally, it commonly was believed for many years that patients unlikely to survive regardless of the reestablishment of effective systemic perfusion should probably not even be considered for temporary VAD support. However, society currently demands that everyone (no matter how critically ill) be given an opportunity to recover, and the new and improved MCS technology is often now used with the understanding that improvement in clinical status will allow for a continuation of support, whereas a worsening of the clinical picture will prompt a discontinuation of support. Nevertheless, it is the advancements of the technology and an increased patient management experience that have brought a new flexibility to the arena of short-term MCS.
TABLE 27-4 Conditions or Comorbidities That Make Ventricular Assist Device Placement or Use Difficult, Make the Patient More Likely to Have Major Complications, or Make Meaningful Recovery Unlikely
BSA, body surface area; ESLD, end stage liver disease; ESRD, end stage renal disease.

Figure 27-2 The Tandem Heart percutaneous Ventricular Assist Device (pVAD).
(Courtesy of CardiacAssist Inc., Pittsburgh, PA.)

Figure 27-5 The Thoratec pVAD (percutaneous ventricular assist device).
(Reproduced from Thoratec Corporation, Pleasanton, CA.)
Bridge-to-Immediate Survival
Since the late 1960s, the IABP has been the most commonly used “bridge-to-immediate survival” because it simultaneously increases myocardial O2 supply and decreases O2 demand, interrupting the otherwise inexorable cycle leading to ventricular failure. It is estimated that 5% to 10% of patients will experience development of cardiogenic shock after an acute MI, and early survival rates for these patients are on the order of 5% to 21%.102 However, 75% of such patients who are unresponsive to pharmacologic interventions will exhibit hemodynamic improvement with IABP therapy alone,103 and early survival rates in these patients are reported to approach 93% when treated with IABP counterpulsation.104
Although a balloon pump can improve the output from an acutely stunned ventricle, it only can augment forward CO by about 25% to 30% at maximum depending on the afterload,105,106 and it is clear that there will be no augmentation of forward CO if there is a complete absence of LV function; thus, by itself, an IABP cannot be expected to rescue a patient from catastrophic myocardial failure (see Chapter 32).
The TandemHeart pVAD
Centrifugal pumps long have been used as MCS via both intrathoracic and percutaneous femoral cannulation strategies.107 Although standard intrathoracic cannulations require sternotomy, percutaneous femoral arterial and venous cannulations can be performed outside of an operating room setting. A disadvantage of femoral venous cannulation, however, is that ventricular decompression is often inadequate to substantially reduce myocardial oxygen demand.
With this device, a 21-French venous inflow cannula is percutaneously advanced retrograde from the femoral vein through the right atrium and across the interatrial septum into the left atrium (see Figure 27-2); 2.5 to 5 L/min of continuous, nonpulsatile outflow from the centrifugal device is returned to the femoral artery to support the circulation. Heparinization to an ACT of 180 to 200 seconds is used during support. Although a theoretical downside to cannulation of the femoral artery for device outflow is retrograde arterial perfusion through the potentially diseased aorta of a patient with atherosclerosis, cerebral embolism has not been reported as a significant problem.
The primary use of this innovative device has thus far been as a margin of safety in high-risk patients undergoing a variety of high-risk percutaneous coronary interventions.108–110 Recent publications document the utility of the TandemHeart as a “bridge-to-immediate survival” from cardiogenic shock after acute MI,111 after postcardiotomy failure to wean from CPB,112 and in cases of acute myocarditis.113 With respect to outcomes, a prospective comparison of the TandemHeart and the IABP in 41 patients with cardiogenic shock after acute MI found that, although hemodynamic and metabolic indices were significantly improved by the TandemHeart (with respect to the IABP), complications (including bleeding and limb ischemia) were more frequent with the VAD. There was no significant difference in 30-day mortality between the two groups.114
The utility of the TandemHeart as active hemodynamic support during off-pump coronary artery bypass surgery has also been reported.115 Thus, the TandemHeart can serve as a “bridge-to-next decision,” a “bridge-to-recovery,” a “bridge-to-a-bridge,” and even as a “bridge-to-transplantation”116 (if the wait time is very short for a donor organ). Currently, the TandemHeart holds a CE mark (Conformité Européene) in Europe and is FDA-cleared in the United States for up to 6 hours of use.
Impella
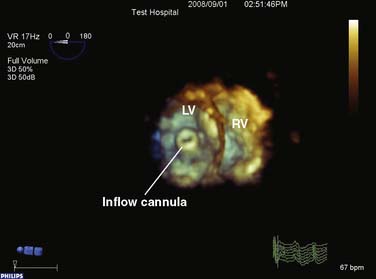
The Impella Pump System (Abiomed) is a recently introduced family of axial flow devices that can be used to support the left, right, or both ventricles. Although the directly implantable Impella LD and RD remain the subject of clinical trials in the United States at the time of this writing, the Impella LP 2.5 has been FDA-cleared since June 2008 to provide partial circulatory support for periods up to 6 hours. The LP 2.5 is a catheter-based miniaturized axial flow pump that can provide up to 2.5 L/min of flow. Like the Hemopump available in the late 1980s and early 1990s, the LP 2.5 is inserted percutaneously into the femoral artery and then passed retrograde up the aorta and across the aortic valve into the left ventricle. Oxygenated blood is then impelled from the left ventricle to the ascending aorta (see Figure 27-3).
Early clinical experience with the Impella LP 2.5 in patients with cardiogenic shock reported significantly increased CO, decreased pulmonary capillary wedge pressure, and decreased lactate levels by 6 hours of support117; 68% of the patients studied were successfully weaned from support, although only 38% survived. Among the observed complications were clinically significant hemolysis in 38% of the patients and one instance of pump displacement.
When compared with an IABP, a prospective study of 26 patients with cardiogenic shock caused by AMI reported that the use of a percutaneously placed LVAD (Impella LP 2.5) is feasible and safe and provides superior hemodynamic support compared with standard treatment using an IABP.118
Though potentially useful as a bridge-to-immediate-survival, the main use of the Impella thus far has been as an extra margin of safety in patients undergoing high-risk percutaneous coronary intervention. The safety and feasibility of the Impella LP 2.5 for hemodynamic support for this indication were demonstrated in the recently published PROTECT I trial.119 The LP 2.5 also may be of use in high-risk patients undergoing off-pump coronary artery bypass surgery or as a margin of safety in high-risk patients undergoing noncardiac surgery.120
Lifebridge (Lifebridge AG, Munich, Germany)
The Lifebridge represents one of the new generation of devices specifically designed for “bridge-to-immediate survival.” This compact device essentially provides a maximum of 3.5 L/min of ECMO in a “plug-and-play” manner via percutaneous femoral arterial and venous cannulations. The 18-kg unit consists of a disposable patient module (which contains a CPB circuit, including an oxygenator), a centrifugal pump, a control module containing a driving motor, and an automated gas detection and bubble removing system. In situations of cardiac arrest, a heat exchanger can be configured in line with the bypass circuit to provide protective cooling of the patient. The safety and efficacy of this device were recently discussed in a publication from Germany, where this novel device was successfully used in the resuscitation and management of a patient with post-MI cardiogenic shock.121
Bridge-to-Recovery, Bridge-to-Next Decision
It is now well-known that the success of “bridge-to-recovery” with a VAD hinges on appropriate patient selection and prompt intervention. If the myocardium is going to recover after an acute insult, it generally tends to do so within a week or two (although the process may take longer than a month in some patients), and the patient may then be weaned from MCS. Although dismal in the past, rates of successful weaning from short-term support have improved. In the postcardiotomy cardiogenic shock population, survival rates approaching 50% have been reported with the Abiomed BVS5000 and AB5000 ventricle in “experienced centers with well-defined protocols for patient selection and timing of intervention.”122,123 Survival from acute MI cardiogenic shock has been reported at 42% with the AB5000 ventricle (mean number of days supported, 25.4) and 27% with the BVS 5000 (mean number of days supported, 5.2).124 Limited information is available at the time of this writing regarding current rates of recovery from short-term support with the new devices (discussed later), but the strategies of support have changed dramatically in recent years and current data may no longer be directly comparable with the pure “bridge-to-recovery” strategy used in prior decades.
Early information125 based on the experience with the Abiomed BVS5000 indicated that the best outcomes tended to occur in the following situations:
Poor outcomes occur in the following situations:
Additional considerations revolve around whether univentricular or biventricular support is required, but often this is a decision now made in retrospect in the arena of short-term support, once LVAD support has been engaged and the clinical situation is reassessed. Severe RV dysfunction has been reported to occur in up to 30% of LVAD-supported patients126,127 because of unfavorable alterations in RV geometry (e.g., leftward shift of the interventricular septum) resulting in increased RV compliance and decreased RV contractility in the presence of increased RV preload128 and potentially increased RV afterload. Although the overall incidence of RV failure in the setting of LVAD support appears to be decreased with continuous flow not intended to completely decompress the left ventricle during support, the perioperative management of RV preload and afterload continues to play an enormous role in the potential for RV dysfunction after implementation of LVAD support. The issue of RV failure during LVAD support is more of an issue during long-term support and is discussed in more detail later.
When severe cardiopulmonary failure is present, and there is uncertainty about the recoverability of the situation (or the neurologic status of the patient), ECMO again has become a popular circulatory support strategy.129 ECMO has significant disadvantages, however, including a somewhat limited potential duration of support, the need for dedicated personnel to manage the flows and the anticoagulation, and a high incidence of complications as the duration of support increases.
The CentriMag
The CentriMag (Thoratec Laboratories; see Figure 27-7) is a small centrifugal pump with a magnetically levitated impeller. As with other short-term devices, the pump head itself remains paracorporeal during support, connected to cannulae in the heart and great vessels.
The impeller of the CentriMag is magnetically levitated and hydrodynamically suspended in the patient’s blood; there is no central bearing (which has advantages), and without a bearing, there is less heat produced and potentially less thrombus formation.130 There is also less hemolysis associated with the design of the CentriMag,131 and, therefore, potentially less inflammatory response and less peripheral vasoconstriction from the plasma-free hemoglobin. The derangement of liver function tests generally seen after a few days with a standard biohead are reportedly not seen with the CentriMag (Monique Boshell, Thoratec Corporation, personal communication).
Published clinical experiences with the CentriMag have reported the safety and efficacy of this device as a bridge-to-immediate survival, a bridge-to-next decision, a bridge-to-a-bridge, a bridge-to-recovery, and a bridge-to-transplantation for patients with acute cardiogenic shock of causative factors ranging from acute MI cardiogenic shock to postcardiotomy cardiogenic shock (including failed heart transplantation) to RV failure while on LVAD support.132–134
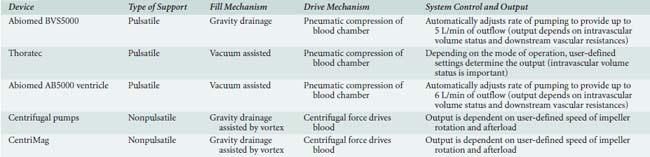
Table 27-5 describes the basic characteristics of the devices currently used for short-term support, and Box 27-2 provides common clinical scenarios in which short-term VAD use may be indicated.
TABLE 27-5 Basic Characteristics of the Devices Currently Used for Short-Term Support (e.g., Bridge-to-Recovery)
Long-Term Ventricular Assist Device Use
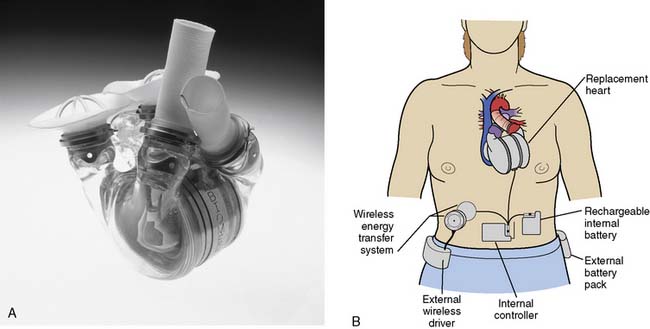
Permanent replacement of the failing heart was the original intent of the research and development in the field of mechanical circulatory assistance, and this dream is alive and well in the latest version of the total artificial heart, the AbioCor Implantable Replacement Heart (Abiomed; Figure 27-8), but most long-term VAD use has been as a bridge-to-transplantation.
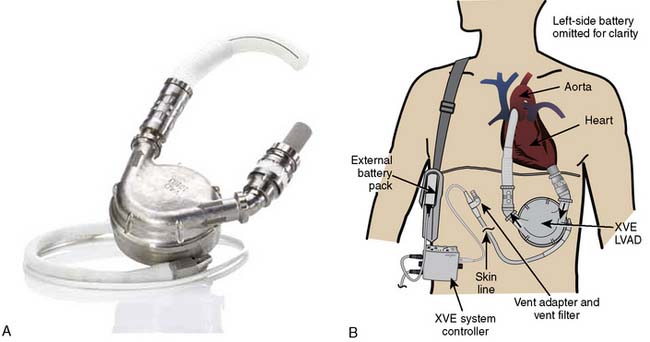
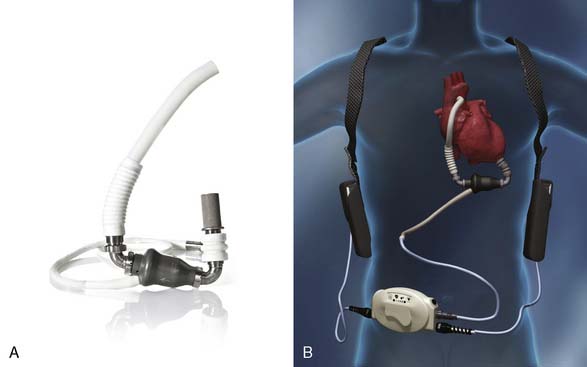
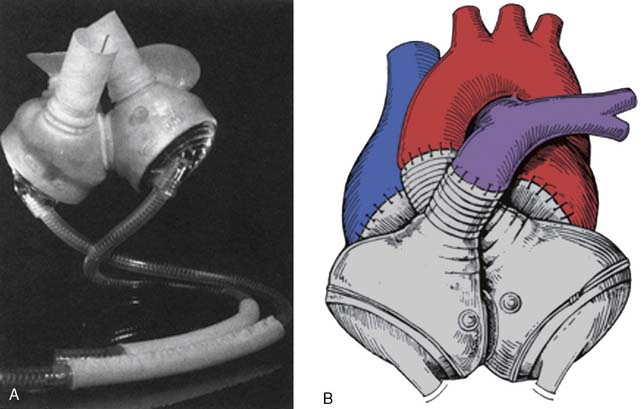
Current FDA-approved devices used as a bridge-to-transplantation in the United States include the Thoratec pVAD (Thoratec Laboratories; see Figure 27-5), the HeartMate XVE (Thoratec Corporation, Woburn, MA; Figure 27-9), the IVAD (implanted vascular assist device; Thoratec Laboratories; Figure 27-10), the HeartMate II (Thoratec Laboratories; Figure 27-11), and the CardioWest TAH (total artificial heart; SynCardia Systems, Tucson, AZ; Figure 27-12). Currently, the HeartMate XVE and the HeartMate II are the only devices FDA approved in the United States for DT. The Novacor LVAS (World Heart, Ottawa, Canada) was approved as a bridge-to-transplantation in 1998 but is no longer being implanted. Despite arguably superior engineering regarding device longevity in comparison with other devices available at the time, as well as comparable rates of successful bridging to transplantation, the Novacor was associated with a high incidence of thromboembolic complications. Table 27-6 summarizes the basic characteristics of devices used for long-term support.

Figure 27-10 The IVAD (implanted vascular assist device).
(From Thoratec Corporation, Pleasanton, CA.)
Intermediate- or long-term VAD support potentially is indicated as a bridge-to-transplantation in situations in which no myocardial recovery is expected (e.g., end-stage cardiomyopathy) or when an acutely stunned or infarcted left ventricle fails to recover despite support with a short-term VAD. By providing effective CO in place of the failed native heart, this technology can stave off the end-organ damage resulting from a rapidly deteriorating CO and allows severely decompensated transplant-eligible patients to potentially survive long enough to receive a donor heart. An additional benefit of this application of VADs is an improved quality of life, often as an outpatient, while awaiting a new heart. Further, significant improvements in multiorgan function have been demonstrated during the time spent on VAD support,95–97 and it is unusual nowadays for a patient to present for heart transplantation without an LVAD in situ.
The HeartMate I (World Heart, Oakland, CA; see Figure 27-9) has heretofore been the most commonly used bridge-to-transplantation device for patients with advanced LV failure. The original pneumatically powered HeartMate IP was FDA-approved for this indication in 1994, and the electrically powered version (the VE, vented electric) was approved in 1998. Data from prior years indicated a 67% success rate of bridging-to-transplantation with the HeartMate VE.135 The major advantage of the pulsatile HeartMate LVAS always has been the “antithrombogenic” lining of its blood chamber that obviated the need for formal anticoagulation with warfarin once a neointima was established. The major disadvantages of the HeartMate included infections of the large percutaneous lead and in the preperitoneal pocket where the device was implanted, as well as a limited durability beyond 18 months.
The current incarnation (the XVE) is the result of improvements to the device, and it has been in use since approximately 2002. According to the manufacturer, more than 4500 patients have been implanted with the HeartMate XVE in 186 centers worldwide. The average age has been 51 years old, with a range from 8 to 74, and the longest duration of support (ongoing patient on one device) has been 1854 days.136
Patients with biventricular failure generally have been bridged to transplantation with the Thoratec pVAD (FDA-approved for this indication in 1995) or, potentially, the IVAD (the implantable, titanium-coated version of the Thoratec device). According to the manufacturer, more than 4000 patients have been supported by the Thoratec pVAD at more than 240 medical centers in 26 countries. The longest duration of support is reportedly 1204 days, with 858 of those patients discharged to home, and the rate of successful bridge-to-transplantation is reported at 69%.136 For the IVAD, more than 500 patients reportedly have been implanted at 95 medical centers in 9 countries, with the longest duration of support being 979 days,136 and a reported rate of successful bridge-to-transplantation of 69%.137
The CardioWest total artificial heart (SynCardia Systems; see Figure 27-12) is available in select centers internationally as a bridge-to-transplantation for patients with biventricular failure, and a resurgence of interest in this device has been seen recently. A successful bridge-to-transplantation rate of 79% was observed in prior years.138
Complications such as infection of percutaneous drivelines, perioperative bleeding, RV failure, sepsis, and multisystem organ failure were always an enormous issue with the first-generation, pulsatile VADs. Thromboembolism was less prevalent with the HeartMate because of the antithrombogenic lining of its blood chamber, but it did occur. A review of 228 patients on long-term support with the Thoratec, Novacor, and HeartMate as a bridge to transplantation reported cerebral embolism in 24%, 39%, and 16%, respectively, despite adherence to recommended anticoagulation protocols.139
The HeartMate II
The HeartMate II (see Figure 27-11) is a small, nonpulsatile axial flow pump that has demonstrated a significantly decreased incidence of complications and a significantly improved durability compared with its predecessor. According to the manufacturer, more than 5000 patients worldwide have been implanted with the HeartMate II, with the longest duration of support (ongoing patient on one device) greater than 5 years, and 79% of patients successfully transplanted, recovered, or supported to 18 months.136
The smaller size of the HeartMate II has simplified implantation, and the rates of common complications have been shown to be significantly decreased by comparison with the pulsatile HeartMate I. Published series using identical definitions of certain complications have reported a 64% decrease in reoperation for bleeding, a 10-fold decrease in percutaneous lead infections, a 55% decrease in stroke, and a 70% decrease in RV failure after implementation of HeartMate II LVAD support.140–142 The reason for the decreased incidence of these specific complications is likely multifactorial (e.g., improved surgical techniques and lack of a need for a large preperitoneal pocket, the routine use of antifibrinolytics during device implantation in the modern era, a smaller percutaneous lead, improved perioperative care protocols and patient management, improved anticoagulation protocols).
The decreased incidence of severe RV failure requiring RVAD seen with the HeartMate II143 may be because of the nature of the type of flow it produces. The first generation of LVADs (e.g., the HeartMate VE and the XVE) produced pulsatile flow and, therefore, had to capture the entire potential output from the left ventricle to eject a physiologic stroke volume with each pump cycle. Consequently, the left ventricle frequently was emptied to the point where the interventricular septum was displaced significantly to the left during support, resulting in decreased function of the interventricular septum144 and overall RV dysfunction from the change in its overall geometry. The nonpulsatile next generation of LVADs (whether axial or centrifugal) produce continuous flow and do not fully decompress the left ventricle during support, theoretically preserving RV function, at least in part, because the interventricular septum is not significantly displaced to the left. In contrast with the reported 25% to 30% rates of RV failure in the past with first-generation pulsatile devices, the rate of RV failure with the HeartMate II recently was reported at 5%.145
Permanent Ventricular Assist Device Use: Destination Therapy
The REMATCH trial (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) established that the use of a left-sided VAD was not only an effective tool to treat patients with advanced HF but resulted in more than twice the survival rate and an improved quality of life in comparison with optimal medical management.146 Based on the results of REMATCH, the FDA approved the HeartMate VE in November 2002 for transplant-ineligible patients as DT, but improvements to the HeartMate device resulted in the HeartMate XVE by the time FDA approval was granted, and it is the XVE that has been implanted for DT patients in the United States. Although use of the XVE quickly was associated with fewer adverse events and a better survival rate than in the REMATCH trial,147 increased experience with patient management likely also played a role in the observed improved outcomes.
To date, DT has been indicated only for transplant-ineligible patients, but it may be anticipated that this indication may be broadened going forward because although cardiac transplantation remains the gold standard therapy for end-stage disease, the number of donor organs is severely limited in comparison with the number of patients who would benefit, and most patients with end-stage cardiac disease cannot realistically expect to be transplanted. Another factor that may lead to a potential broadening of the indication is the FDA approval of the HeartMate II for DT in January 2010, after the completion of the HeartMate II DT pivotal clinical trial.148 In this prospective, randomized trial, the HeartMate II was pitted against the HeartMate XVE on a 2:1 basis. Not only was the incidence of adverse events, including infection, sepsis, right-heart failure, and mechanical problems, significantly lower in HeartMate II patients compared with patients implanted with the XVE, but HeartMate II patients experienced shorter hospital stays. After approval of the HeartMate II for DT, the number of patients implanted with the XVE as DT has dropped off significantly, though the VXE may remain useful for patients who cannot be anticoagulated.
New Risk Scores: Optimizing Survival while Minimizing Risk during Long-Term Ventricular Assist Device Support
Lietz–Miller Risk Score
Lietz et al149 described the outcomes of nearly the entire U.S. DT population in the post-REMATCH era from the fall of 2002 through December 2006 and identified the most important determinants of in-hospital mortality. In this landmark study,149 the main preoperative determinants of mortality in this population were shown to be poor nutrition (resulting in subsequent sepsis), hematologic abnormalities (resulting in subsequent stroke), RV dysfunction (resulting in postimplantation RV dysfunction), lack of inotropic support, and preexisting end-organ dysfunction (resulting in ultimate multisystem organ failure), though it should be noted that people did die of other things, including technical problems with their LVAD. Approximately 25% either required LVAD replacement or died as a result of pump failure or complications, but the death of only 6% was directly attributable to device failure. Of concern was the reported probability of device exchange or fatal device failure, which was approximately 18% at 1 year and 73% at 2 years with the HeartMate XVE.
Furthermore, Lietz et al149 were able to stratify patients into risk categories based on a risk score calculated from these predictors that correlates well with survival. According to their analysis, the highest-risk patients have severe deterioration in their medical condition (as evidenced by poor nutritional status with low serum albumin), impaired renal function, and markers of significant right-heart failure such as low PA pressures or congestive levels of hepatic enzymes. Probable infection, as evidenced by increased white blood cell counts and anemia and coagulation abnormalities such as declining platelet counts and increased international normalized ratio, worsen the chance of operative survival. Thus, if operative risk derives from comorbidities, then initially high risk because of correctable factors should not dissuade physicians from considering LVAD therapy in certain cases because intensive medical treatment can convert high-risk patients to acceptable candidates.
INTERMACS Profile
INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) is a relatively new registry of patients supported by FDA-approved “durable” MCS devices for DT, bridge-to-transplantation, and/or recovery. Analysis of the growing registry has allowed for the development of a classification scheme (INTERMACS “profile”) that helps guide medical and surgical decision making regarding the timing of interventions and therapies (e.g., VAD insertion), provides a conventional frame of reference that facilitates communication between practitioners, and allows for an understanding of risks associated with various interventions.150
The INTERMACS profile describes the status of the HF patient who might benefit from MCS. INTERMACS stratifies HF patients into seven levels of clinical acuity, defined as 1 (“critical cardiogenic shock”), 2 (“progressive decline despite inotropes”), 3 (“stable but inotrope-dependent”), 4 (“recurrent advanced HF”), 5 (“exertion-intolerant”), 6 (“exertion-limited”), and 7 (“advanced NYHA III”). The assigned profile is modifiable by the coexistence of arrhythmias, the need for temporary circulatory support, and the frequency of requisite hospitalization.151
It also is anticipated that the INTERMACS registry will allow for the collection of outcomes data, as well as assess the efficacy of specific therapeutic interventions. A recent analysis of the INTERMACS registry data reported that cardiogenic shock, advanced age, and severe right-heart failure manifested as ascites or increased bilirubin are predictors of death in patients supported by MCS devices. Furthermore, although biventricular HF patients who require biVAD support have a transplant rate similar to that of LVAD-only patients, an increased mortality is seen at 6 and 12 months.152
Seattle Heart Failure Model
The Seattle Heart Failure Model153 was developed to predict survival in patients with HF. This multivariate risk score uses 21 parameters (age, sex, NYHA class, weight, EF, systolic BP, presence of ischemic cardiomyopathy, daily furosemide-equivalent dose, inotrope use, statin use, allopurinol use, angiotensin-converting enzyme use, β-blocker use, angiotensin-receptor blocker use, potassium-sparing diuretic use, ICD use, hemoglobin, lymphocyte percent on complete blood count differential, serum uric acid, serum cholesterol, and serum sodium). The model subsequently was modified for LVAD patients by adding two variables: IABP-implanted or ventilated, or both, and inotrope therapy.154 Overall, the Seattle Heart Failure Model reportedly provides an accurate estimate of mean, 1-, 2-, and 3-year survival, and allows estimation of effects of adding medications or devices to a patient’s regimen. A recent comparison of available risk indices (including the Lietz–Miller risk score, the Columbia risk score, APACHE II, and INTERMACS) found the Seattle score to best predict mortality in continuous-flow LVAD patients.155
Ventricular Assist Device Use and the Potential for Myocardial Recovery
Although echocardiographic and histologic support for this general premise has been available since the mid 1990s,156 the underlying biochemical mechanisms of remodeling (and its reversal) are only now being elucidated. Patten et al showed that therapy with a VAD normalizes inducible nitric oxide synthase expression in association with decreased cardiomyocyte apoptosis.156 Decompression of the left ventricle by an LVAD has been reported to allow for normalization of LV geometry, regression of myocyte hypertrophy,157 favorable changes in LV collagen content,158 and normalized expression of genes controlling excitation-contraction coupling and the calcium content of the sarcoplasmic reticulum.159 It has been reported that maximum structural reverse remodeling is complete after around 40 days of LV decompression, with reversal of some of the molecular aspects of remodeling by 20 days.160
Abiocor Implantable Replacement Heart
The first TAH was a pneumatically driven biventricular pump developed by Dr. Domingo Liotta and colleagues in the 1960s. This device (the Liotta TAH) was implanted in a 47-year-old patient with severe HF by Dr. Denton Cooley on April 4, 1969, and was used for 64 hours as a bridge-to-heart transplantation.160 The patient died of Pseudomonas pneumonia 32 hours after his transplantation, but the Liotta heart proved that a mechanical device could be successfully used clinically to sustain a patient.
The second human implantation of a TAH also was performed by Dr. Cooley. In July 1981, the Akutsu III TAH was used successfully for 55 hours as bridge-to-transplantation in a 36-year-old patient with end-stage HF.161 The Jarvik-7 TAH was first implanted as a permanent replacement heart in August 1985 in a 61-year-old man with primary cardiomyopathy and chronic obstructive pulmonary disease.162 Although the patient survived only 112 days, the duration of his survival was encouraging. Since 1991, the Jarvik-7 has been known as the CardioWest TAH. As discussed earlier, this device is still in use today as a bridge-to-human heart transplantation in selected centers in the United States, France, and Canada.
The AbioCor Implantable Replacement Heart (see Figure 27-8) represents a major advance in artificial heart technology because it is truly totally implantable; there are no percutaneous cables, conduits, or wires. The device is motor driven, so a source of compressed air to drive the pumping action is not required, allowing patients complete mobility. The device itself weighs approximately 2 pounds and is orthotopically implanted. Transcutaneous energy transfer is used (in lieu of a percutaneous cable) to supply the motor-driven hydraulic pumping of the artificial ventricles with power and system control. Artificial unidirectional valves within the device mandate anticoagulation during support.
Cardiac transplantation
Cardiac transplantation remains the ultimate surgical intervention for advanced HF and greatly impacts the lives of those patients who receive a new heart, but considering the massive scope of this public health issue, this management strategy is epidemiologically and biostatistically small because of the extremely limited number of donor organs available each year. Although it is estimated that at least 100,000 patients could meet the transplant criteria at any given time,21 only 3153 are currently listed to potentially receive one.163 Furthermore, it is apparent that the number of available donor hearts is limited to approximately 2200 each year in the United States. Thus, transplantation simply is not a realistic expectation for the majority of patients with advanced end-stage HF. Although survival varies slightly by blood type (AB > B > A > O), patients fortunate enough to get a donor organ can currently expect a 1-year survival rate of approximately 87%, a 3-year survival rate of approximately 78%, and a 5-year survival rate of approximately 73%163 (see Chapter 23).
New therapies
Cellular Transplantation into the Myocardium
The use of cell transplantation is not a novel approach to treating disease. Skin and bone- marrow transplantations were the first replacement therapies described. These were followed by embryologic neuron transplantation in patients with Parkinson’s disease and islet cell transplantation for the treatment of diabetes mellitus.164
Skeletal muscle cells, however, are histologically different from native cardiomyocytes. Adhesion molecules, which are found in native cardiomyocytes, are not found in skeletal myocytes (N-cadherin and connexin-43).104 These adhesion molecules are important for adhesion to the extracellular matrix and for intercellular communication.
Clinically, a Phase I study from Poland, the POZNAN trial,165 showed that myoblasts can be implanted safely via a percutaneous route into a scarred region of the left ventricle. Only 10 patients were enrolled into this study. Nine were transplanted. One could not be transplanted because of technical reasons. Six patients of the nine were followed up. Improvement of NYHA class and EF were observed in all of them. Similar results were seen by Menasche et al.164 A frequently encountered occurrence in the phase I studies was the fact that many patients had episodes of ventricular tachycardia after the procedure. They were successfully treated with amiodarone or electric cardioversion. Phase II studies are now in progress in the United States and Europe. Many other cell types are now being experimentally injected into the myocardium or given intravenously in an attempt to regrow cardiac myocytes.166–168 These cells include adult bone marrow stem cells, embryonic stem cells, and cardiac progenitor cells found mainly in the atrium. They have been injected alone or with multiple growth factors such as granulocyte-macrophage colony-stimulating factor, vasoactive endothelial growth factor, and angiopoietan-1, which mobilize progenitor cells and induce new cell growth. The transplanted cells may morph into new cardiac muscle cells, or they may improve cardiac function by boosting the growth of new blood vessels, or releasing other growth factors that encourage cell proliferation and survival. Any of these effects could explain some of the early positive results seen to date.
Gene Transfer in Cardiac Myocytes
Gene therapy has received much interest by the media. Multiple research groups are trying to cure or at least alleviate symptoms brought on by CHF through gene therapy. It is necessary to inoculate the cell with DNA to change the genetic programming of a cell. Vectors are being used to achieve this goal. Vectors commonly used range from plasmid DNA to different virus types (e.g., adenovirus, herpes virus). The two cellular pathways that are being targeted are the sarcoplasmic reticulum and the β-adrenergic pathway.169 The targets of gene therapy are to increase β-adrenergic function, adenylyl cyclase, the V2 vasopressin receptor, and sarcoplasmic reticulum Ca2+ ATPase (SERCA2a), while also decreasing phospholamban and β-adrenoreceptor kinase (BARK 1). All of these new therapies hold great promise, and many trials are under way.170
Anesthetic considerations in the patient with severely impaired cardiac function
Anesthetic Agents and Technique
Traditionally, a technique based on high-dose opioid (e.g., total fentanyl dose, 50 to 100 μg/kg, or total sufentanil dose, 5 to 10 μg/kg), together with a neuromuscular blocking agent, has been used for patients with severely depressed cardiac function. Although such a technique likely will result in many hours of hemodynamic stability, potential disadvantages of this technique are that amnesia may not be adequate and the bradycardia and initial chest wall rigidity that typically accompany such an induction must be pharmacologically countered (see Chapter 9).
Central venous access and pulmonary artery catheterization (PAC) are extremely useful (if not mandatory) in this patient population for several reasons. First, pharmacologic interventions are frequently necessary, and potent inotropic and vasoactive agents are preferably administered to the circulation through a central route. Second, despite recent controversy regarding the usefulness and potentially increased morbidity and mortality with a PAC in critically ill patients, the ability to follow and optimize trends of CO and other hemodynamic indices, as well as the ability to assess the efficacy of pharmacologic interventions to manipulate pulmonary vascular resistance, cannot be overlooked.171 The studies critical of routine PAC use do not directly address the cardiac surgical population and thus cannot reasonably be used to exclude this patient population from their use. Third, an extraordinarily useful monitor for evaluating the adequacy of oxygen delivery is measurement of mixed venous oxygen saturation (see Chapter 14). It might be argued that a central venous catheter alone can be used to estimate central filling pressures, and Mangano et al172 demonstrated the ability to assess LV filling pressures using a central venous catheter, but only if the EF was greater than 40%. The population in question, however, will (by definition) present to the operating room with severely depressed LV function, justifying the use of a PAC in the majority of cases.
Nowhere is TEE a more invaluable intraoperative tool than during surgical procedures intended to improve cardiac function, because the success of many of these procedures depends on specific information provided by the echocardiographer. For example, TEE visualization of the precise mechanism and location of mitral regurgitation often determine the feasibility of valve repair. TEE is used to assess the anatomy of the valve overall, as well as to specifically evaluate the leaflets for abnormal thickening, calcification, mobility, and points of coaptation with respect to the annular plane. Doppler analyses and color-flow mapping complement the 2D evaluation and may provide additional information. The use of a mechanistic classification of mitral regurgitation greatly facilitates communication with the surgeon. The Carpentier classification of mitral regurgitation (see Table 27-3) is often used because it mechanistically distinguishes valves with normal leaflet motion (type I), excessive leaflet motion (type II), and restricted leaflet motion (type III).173
Transesophageal Echocardiography and Ventricular Assist Devices
Regardless of the specific manufacturer’s device attached to the inflow and outflow cannulae, TEE is an invaluable tool before, during, and after the placement of VADs (Table 27-7).
TABLE 27-7 Common Perioperative Echocardiographic Assessment of Patients Undergoing Left Ventricular Assist Device Insertion
| Preoperative LVAD Assessment (Patient Screening) | Intraoperative and Postoperative LVAD Assessment |
|---|---|
| Intracardiac shunts | Intracardiac shunts |
| Intracavitary thrombus | Deairing (left ventricle and device) |
| Atherosclerosis or severe calcifications of the aortic arch | Aortic dissection |
| Aortic regurgitation/mitral stenosis | Aortic regurgitation (valve opening) |
| Right ventricular function (tricuspid regurgitation) | Positioning and flow dynamics of both cannulae |
| Ventricular (apical) scars or aneurysms | Left ventricular unloading |
| Right ventricular function (tricuspid regurgitation) Assessment of cardiac tamponade |
LVAD, left ventricular assist device.
From Castillo JG, Anyanwu AC, Adams DH, et al: Real-time 3-dimensional echocardiographic assessment of current continuous-flow rotary left ventricular assist devices. J Cardiothorac Vasc Anesth 23:702–710, 2009, Table 3.
Before LVAD placement, TEE is used to detect specific anatomic pathologies that will:
During LVAD placement, TEE is used to:
After LVAD placement, TEE is used to:
For a more detailed description of the utility of TEE before and during VAD placement and in the perioperative period, the interested reader is referred to the many published reviews available in the literature and major textbooks of echocardiography.174–178 (See Chapters 12 and 13.)
Three-dimensional Transesophageal Echocardiography and Ventricular Assist Devices
Volume assessment by 3DE has been shown to be rapid, accurate, and superior to conventional standardized 2D methods. Ventricular volume and mass obtained by 3DE have even compared favorably with those obtained from studies with magnetic resonance imaging, further demonstrating advantages in efficacy and accuracy in assessing volumes in remodeled ventricles after MI.179–181
Left and Right Ventricles
RV function is a major concern after LVAD implantation, and it has been the world’s experience that up to 30% of patients will develop RV dysfunction after LVAD implantation. A subset of these will require the implantation of an RVAD.182–184
Inflow Cannula
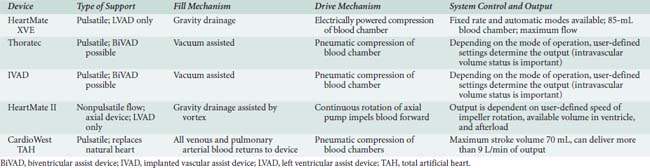
3DE has made it substantially easier to inspect and visualize the orientation of the inflow cannula, commonly entering the left ventricle from the apex. The echocardiographic examination of the inflow cannula position and orientation using 2DE required at least two orthogonal views (four-chamber and two-chamber long axis). A dataset can be acquired and spatially oriented when using 3DE so that the imager views the mitral valve en face from the left atrial perspective. The cropping tool can now be used to edit away the mitral valve and basal regions of the left ventricle, enabling the echocardiographer to obtain an en face view of the outflow cannula as it enters the LV apex. The cannula orifice should be centrally located entering the apex of the ventricle, aligned to the LV inflow tract (mitral valve orifice), not abutting any ventricular structures (Figure 27-13). Often the cannula ends up being slightly angled toward the anteroseptal ventricular wall. As long as the deviation is less than 30 degrees, no hindrance of ventricular drainage should be encountered. Figure 27-14 depicts the proper positioning of the inflow cannula of the Impella VAD entering the left ventricle in a retrograde fashion through the aortic valve.
In conjunction with color-flow Doppler, the echocardiographer can check for unidirectional laminar flows through the ventricle to the device. The presence of abnormal high-velocity turbulent flows or an aliasing flow at the cannula orifice suggests cannular obstruction. The differential diagnosis can include hypovolemia, a thrombotic episode, malalignment with partial obstruction by ventricular walls, or compression of the interventricular septum (“suck-down” effect; Figure 27-15). Because the treatment for these disorders is different, it is important to have excellent echocardiographic imaging capabilities. In the scenario of malalignment, the surgeon may be able to reposition the cannula by moving the flexible conduit and reassessing the LVAD hemodynamics, routinely done at the time of chest closure. A “suck-down” effect is treated by primarily reducing device flows and volume loading of the patient, as well as potentially providing RV support.
Conclusions
The preceding sections have summarized the advances made in surgical therapy of CHF. As one of the fastest growing segments of the population of patients with heart disease, CHF patients will be the subject of many ongoing investigations. As the biotechnology industry and practitioners make further advances, the interested reader must pay close attention to the medical literature to remain current on this subject.185–187
1 American Heart Association. Heart disease and stroke statistics—2010 update. Dallas, TX: American Heart Association, 2010.
2 Lloyd-Jones D.M. The risk of congestive heart failure: Sobering lessons from the Framingham Heart Study. Curr Cardiol Rep. 2001;3:184.
3 O’Connell J.B., Bristow M.R. Economic impact of heart failure in the United States: Time for a different approach. J Heart Lung Transplant. 1994;13(Suppl):S107.
4 Zeltsman D., Acker M.A. Surgical management of heart failure: An overview. Annu Rev Med. 2002;53:383.
5 Vitali E., Colombo T., Fratto P., et al. Surgical therapy in advanced heart failure. Am J Cardiol. 2003;91(Suppl):88F.
6 Dec G.W. Management of heart failure: Crossing boundary over to the surgical country. Surg Clin N Am. 2004;84:1.
7 The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, ed 9, Boston: Little, Brown & Co; 1994:253-256.
8 Francis G.S., Tang W.H., Sonnenblick E.H. Pathophysiology of heart failure. In: Furster V., Alexander R.W., O’Rourke R.A., et al, editors. Hurst’s the heart. ed 11. New York: McGraw-Hill; 2004:697-722.
9 Massie B.M. Pathophysiology of heart failure. In: Goldman L., Ausiello D., editors. Cecil textbook of medicine. ed 22. Philadelphia: WB Saunders; 2005:291-299.
10 Colucci W.S., Braunwald E. Pathophysiology of heart failure. In: Braunwald E., editor. Heart disease: a textbook of cardiovascular medicine. ed 7. Philadelphia: WB Saunders; 2005:509-538.
11 Kajstura J., Cigola E., Malhotra A., et al. Angiotensin II induces apoptosis of adult ventricular myocytes in-vitro. J Mol Cell Cardiol. 1997;29:859.
12 The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429.
13 The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293.
14 Pfeffer M.A., Braunwald E., Moye L.A., on behalf of the SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. N Engl J Med. 1992;327:669.
15 Packer M., Bristow M.R., Cohn J.N., for the US Carvedilol Heart Failure Study Group. The effect of carvedilol on mortality and morbidity in patients with chronic heart failure. N Engl J Med. 1996;334:1349.
16 Leizorovicz A., Lechat P., Cucherat M., et al. Bisoprolol for the treatment of chronic heart failure: A meta-analysis on individual data of two placebo-controlled studies. CIBIS and CIBIS II. Am Heart J. 2002;143:301.
17 McGavin J.K., Keating G.M. Bisoprolol: A review of its use in chronic heart failure. Drugs. 2002;62:2677.
18 Wikstrand J., Hjalmarson A., Waagstein F., et al. Dose of metoprolol CR/XL and clinical outcome in patients with heart failure. Analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure. J Am Coll Cardiol. 2002;40:491.
19 Pitt B., Zannad F., Remme W.J., for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709.
20 Farquharson C.A.J., Struthers A.D. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594.
21 Westaby S., Narula J. Preface: Surgical options in heart failure. Surg Clin N Am. 2004;84:15.
22 Packer M. Management of heart failure. In: Goldman L., Ausiello D., editors. Cecil textbook of medicine. ed 22. Philadelphia: WB Saunders; 2005:300-310.
23 Mickleborough L., Merchant N., Ivanov J., et al. Left ventricular reconstruction: Early and late results. J Thorac Cardiovasc Surg. 2004;128:27.
24 Kumpati G.S., McCarthy P.M., Hoercher K.J. Surgical treatments for heart failure. Cardiol Clin. 2001;19:669.
25 Elefteriades J., Edwards R. Coronary bypass in left heart failure. Semin Thorac Cardiovasc Surg. 2002;14:125.
26 Bolling S.F. Mitral reconstruction in cardiomyopathy. J Heart Valve Dis. 2002;11(Suppl 1):S26.
27 Tolis G.A.Jr, Korkolis D.P., Kopf G.S., et al. Revascularization alone (without mitral valve repair) suffices in patients with advanced ischemic cardiomyopathy and mild-moderate mitral regurgitation. Ann Thorac Surg. 2002;74:1476.
28 Hon J.K.F., Yacoub M.H. Bridge to recovery with the use of left ventricular assist device and clenbuterol. Ann Thorac Surg. 2003;75:S36.
29 Miller W.L., Tointon S.K., Hodge D.O., et al. Long-term outcome and the use of revascularization in patients with heart failure, suspected ischemic heart disease, and large reversible myocardial perfusion defects. Am Heart J. 2002;143:904.
30 Liao L., Cabell C.H., Jollis J.G., et al. Usefulness of myocardial viability or ischemia in predicting long-term survival for patients with severe left ventricular dysfunction undergoing revascularization. Am J Cardiol. 2004;93:1275.
31 Vitali E., Colombo T., Fratto P., et al. Surgical therapy in advanced heart failure. Am J Cardiol. 2003;91(Suppl):88F.
32 Trachiotis G.D., Weintraub W.S., Johnston T., et al. Coronary artery bypass grafting in patients with advanced left ventricular dysfunction. Ann Thorac Surg. 1998;66:1632.
33 Carstensen S. Dobutamine-atropine stress echocardiography. Heart Drug. 2005;5:101.
34 Mickleborough L.L., Carson S., Tamariz M., et al. Results of revascularization in patients with severe left ventricular dysfunction. J Thorac Cardiovasc Surg. 2000;119:550.
35 Salgo I.S., Gorman J.H.3rd, Gorman R.C., et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711-717.
36 Geha A.S., El-Zein C., Massad M.G. Mitral valve surgery in patients with ischemic and nonischemic dilated cardiomyopathy. Cardiology. 2004;101:15.
37 Phillips H.R., Levine F.H., Carter J.E., et al. Mitral valve replacement for isolated mitral regurgitation: Analysis of clinical course and late postoperative left ventricular ejection fraction. Am J Cardiol. 1981;48:647.
38 Romano M.A., Bolling S.F. Mitral valve repair as an alternative treatment for heart failure patients. Heart Fail Monit. 2003;4:7.
39 Reese T.B., Tribble C.G., Ellman P.I., et al. Mitral repair is superior to replacement when associated with coronary artery disease. Ann Surg. 2004;239:671-675.
40 Magne J., Girerd N., Sénéchal M., et al. Mitral repair versus replacement for ischemic mitral regurgitation: Comparison of short-term and long-term survival. Circulation. 2009;120(11 Suppl):S104-S111.
41 Gillinov A.M., Wierup P.N., Blackstone E.H., et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122:1125-1141.
42 Braun J., van de Veire N.R., Klautz R.J., et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85:430-436. discussion 436–437
43 Penicka M., Linkova H., Lang O., et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474-1481.
44 Magne J., Sénéchal M., Mathieu P., et al. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol. 2008;51:1692-1701.
45 Cooley D. Ventricular endoaneurysmorrhaphy: A simplified repair for extensive postinfarction aneurysm. J Cardiac Surg. 1989;4:200-205.
46 Loop F.D., Effler D.B. Left ventricular aneurysm. In Gibbon’s surgery of the chest, ed 3, Philadelphia: WB Saunders; 1976:1384.
47 Jatene A.D. Left ventricular aneurysmectomy: Resection or reconstruction. J Thorac Cardiovasc Surg. 1985;89:321-331.
48 Dor V., Kreitmann P., Jourdan J., et al. Interest of physiological closure (circumferential plasty on contractile areas) of left ventricle after resection and endocardiectomy for aneurysm of akinetic zone: Comparison with classical technique about a series of 209 left ventricular resections [abstract]. J Cardiovasc Surg. 1985;26:73.
49 Dor V., Saab M., Coste P., et al. Left ventricular aneurysm: a new surgical approach. Thorac Cardiovasc Surg. 1989;37:11-19.
50 Batista R.J., Santos J.L., Takeshita N., et al. Partial left ventriculectomy to improve left ventricular function in end-stage heart disease. J Cardiac Surg. 1996;11:96.
51 Batista R.J.V., Verde J., Nery P., et al. Partial left ventriculectomy to treat end-stage heart disease. Ann Thorac Surg. 1997;64:634.
52 Franco-Cereceda A., McCarthy P.M., Blackstone E.H., et al. Partial left ventriculectomy for dilated cardiomyopathy: Is this an alternative to transplantation? J Thorac Cardiovasc Surg. 2001;121:879.
53 McCarthy J.F., McCarthy P.M., Starling R.C., et al. Partial left ventriculectomy and mitral valve repair for end-stage congestive heart failure. Eur J Cardiothorac Surg. 1998;13:337.
54 Etoch S.W., Koenig S.C., Laureano M.A., et al. Results after partial left ventriculectomy versus heart transplantation for idiopathic cardiomyopathy. J Thorac Cardiovasc Surg. 1999;117:952.
55 Suma H., RESTORE Group. Left ventriculoplasty for nonischemic dilated cardiomyopathy. Semin Thorac Cardiovasc Surg. 2001;13:514.
56 Dor V., Sabatier M., DiDonato M., et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: Comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg. 1998;116:47.
57 DiDonato M., Sabatier M., Dor V., et al. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J Thorac Cardiovasc Surg. 2001;121:91.
58 Athanasuleas C.L., Stanley A.W.Jr, Buckberg G.D., et al. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. RESTORE Group. Reconstructive Endoventricular Surgery, Returning Torsion Original Radius Elliptical Shape to the LV. J Am Coll Cardiol. 2001;37:1199.
59 Jones R.H., Velazquez E.J., Michler R.M., et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705-1717.
60 Buckberg G.D., Athanasuleas C.L. The STICH trial: Misguided conclusions. J Thorac Cardiovasc Surg. 2009;138:1060-1064.
61 Cirillo M. A new surgical ventricular restoration technique to reset residual myocardium’s fiber orientation: The “KISS” procedure. Ann Surg Innov Res. 2009;3:6-14.
62 Kerwin W.F., Botvinick E.H., O’Connell J.W., et al. Ventricular contraction abnormalities in dilated cardiomyopathy: Effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol. 2000;35:1221.
63 Jarcho J. Resynchronizing ventricular contraction in heart failure. N Engl J Med. 2005;352:1594.
64 Shamin W., Francis D.P., Yousufuddin M., et al. Intraventricular conduction delay: A prognostic marker in chronic heart failure. Int J Cardiol. 1999;70:171.
65 Auricchio A., Stellbrink C., et al. The pacing therapies for congestive heart failure study: Rationale, design, and endpoint of a prospective randomized multicenter study. Am J Cardiol. 1999;83:130D.
66 Cazeau S., Leclereq C., Lavergne T., et al. The Multisite Stimulation in Cardiomyopathies Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;334:873.
67 Abraham W.T., Fisher W.G., for the multicenter Insync Randomized Clinical Evaluation Investigators and Coordinators. Double-blind, randomized, controlled trial of cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845.
68 Cleland J., Daubert J., Erdman E., et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539.
69 Kass D.A., Chen C.H., Curry C., et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567.
70 Auricchio A., Stellbrink C., Block M., et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993.
71 Gras D., Mabo P., Tang T., et al. Multisite pacing as a supplemental treatment of congestive heart failure: Preliminary results of the Medtronic Inc. InSync Study. Pacing Clin Electrophysiol. 1998;21:2249.
72 Cazeau S., Leclercq C., Lavergne T., et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873.
73 Goldman S., Johnson G., for the V-HeFT VA Cooperative Studies Group. Mechanism of death in heart failure. The Vasodilator-Heart Failure Trials. Circulation. 1993;87(Suppl VI):VI-V24.
74 Abraham W.T., Fisher W.G., Smith A.L., for the MIRACLE Investigators and Coordinators: Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Results of a randomized, double-blind, controlled trial to assess cardiac resynchronization therapy in heart failure patients [abstract]. Circulation. 2001;104:II.
75 Hohnloser S., Kuck K., Dorlan P., et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481.
76 Bardy E., Lee K., Mark D., et al. Amiodarone or an implanted cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225.
77 Buxton A.E., Lee K.L., et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882.
78 Moss A.J., Zareba W., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877.
79 Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140.
80 Epstein A.E., DiMarco J.P., et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Circulation. 2008;117:e350.
81 Linde C., Abraham W.T., et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834-1843.
82 Moss A.J., Hall W.J., et al. Cardiac-resynchronization therapy for the prevention of heart failure events. N Engl J Med. 2009;361:1329.
83 el-Banayosy A., Arusoglu L., Kizner L., et al. Predictors of survival in patients bridged to transplantation with the Thoratec VAD device: A single-center retrospective study on more than 100 patients. J Heart Lung Transplant. 2000;19:964-968.
84 Fukamachi K., McCarthy P.M., Smedira N.G., et al. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181-2184.
85 Ochiai Y., McCarthy P.M., Smedira N.G., et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation. 2002;106(Suppl 1):I-198–I-202.
86 Rao V., Oz M.C., Flannery M.A., et al. Revised screening scale to predict survival after insertion of a left ventricular assist device. J Thorac Cardiovasc Surg. 2003;125:855-862.
87 Schenk S., McCarthy P.M., Blackstone E.H., et al. Duration of inotropic support after left ventricular assist device implantation: Risk factors and impact on outcome. J Thorac Cardiovasc Surg. 2006;131:447-454.
88 Farrar D.J. Preoperative predictors of survival in patients with Thoratec ventricular assist devices as a bridge to heart transplantation. J Heart Lung Transplant. 1994;13:93-100.
89 Deng M.C., Loebe M., el-Banayosy A., et al. Mechanical circulatory support for advanced heart failure: Effect of patient selection on outcome. Circulation. 2001;103:231-237.
90 Frazier O.H., Rose E.A., Oz M.C., et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186-1195.
91 Miller L.W., Pagani F.D., Russell R.D., et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885-896.
92 Miller L.W., Lietz K. Candidate selection for long-term left ventricular assist device therapy for refractory heart failure. J Heart Lung Transplant. 2006;25:756-764.
93 Fitzpatrick J.R., Frederick J.R., Hsu V. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant. 2008;27:1286-1292.
94 Holman W.L., Kormos R.L., Naftel D.C., et al. Predictors of death and transplant in patients with a mechanical circulatory support device: A multi-institutional study. J Heart Lung Transplant. 2009;28:44-50.
95 Zimpfer D., Zrunek P., Roethy W., et al. Left ventricular assist devices decrease fixed pulmonary hypertension in cardiac transplant candidates. J Thorac Cardiovasc Surg. 2007;133:689-695.
96 Radovancevic B., Vrtovec B., de Kort E., et al. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J Heart Lung Transplant. 2007;26:815-818.
97 Kamdar F., Boyle A., Liao K., et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352-359.
98 Miller L.W., Pagani F.D., Russell S.D., et al. Use of a continuous flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885-896.
99 Feller E.D., Sorensen E.N., Haddad M., et al. Clinical outcomes are similar in pulsatile and nonpulsatile left ventricular assist device recipients. Ann Thorac Surg. 2007;83:1082-1088.
100 Frazier O.H., Gemmato C., Myers T.J., et al. Initial clinical experience with the HeartMate II axial-flow left ventricular assist device. Tex Heart Inst J. 2007;34:275-281.
101 John R., Kamdar F., Liao K., et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-1235.
102 Mueller H.S. Role of intra-aortic counterpulsation in cardiogenic shock and acute myocardial infarction. Cardiology. 1994;84:168.
103 Braunwald E., Treatment of heart failure-assisted circulation, Heart disease: A textbook of cardiovascular medicine, ed 6, 2001; WB Saunders Company; Philadelphia
104 Allen B.S., Rosenkrantz F., Buckberg G.D., et al. Studies on prolonged acute regional ischemia: VI. Myocardial infarction with LV power failure: A medical/surgical emergency requiring urgent revascularization with maximal protection of remote muscle. J Thorac Cardiovasc Surg. 1989;98:691.
105 Maccioli G., Lucas W., Norfleet E. The intra-aortic balloon pump: A review. J Cardiothorac Anesth. 1988;2:365-373.
106 Dietl C.A., Berkheimer M.D., Woods E.L., et al. Efficacy and cost effectiveness of pre-operative IABP in patients with ejection fraction of 0.25 or less. Ann Thorac Surg. 1996;62:401-408.
107 Noon G.P., Ball J.W., Short H.D. Bio-medicus centrifugal ventricular support for postcardiotomy cardiac failure: A review of 129 cases. Ann Thorac Surg. 1996;61:291-295.
108 Aragon J., Lee M.S., Kar B., et al. Percutaneous left ventricular assist device: “TandemHeart” for high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:346-352.
109 Vranckx P., Foley D.P., de Feijter P.J., et al. Clinical introduction of the TandemHeart as a percutaneous left ventricular assist device for circulatory support during high-risk percutaneous coronary intervention. Int J Cardiovasc Intervent. 2003;5:35-39.
110 Kar B., Butkevich A., Civitello A.B., et al. Hemodynamic support with a percutaneous left ventricular assist device during stenting of an unprotected left main coronary artery. Tex Heart Inst J. 2004;31:84-86.
111 Neuzil P., Kmonicek P., Skoda J., et al. Temporary (short-term) percutaneous left ventricular assist device (Tandem Heart™) in a patient with STEMI, multivessel coronary artery disease, cardiogenic shock and severe peripheral artery disease. Acute Card Care. 2009;11:146-150.
112 Pitsis A.A., Visouli A.N., Burkhoff D., et al. Feasibility study of a temporary percutaneous left ventricular assist device in cardiac surgery. Ann Thor Surg. 2007;84:1993-1999.
113 Khalife W.I., Kar B. The TandemHeart® pVAD™ in the treatment of acute fulminant myocarditis. Tex Heart Inst J. 2007;34:209-213.
114 Thiele H., Sick P., Boudriot E., et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276-1283.
115 Gregoric I.D., Poglajen G., Span M., et al. Percutaneous ventricular assist device support during off-pump surgical coronary revascularization. Ann Thorac Surg. 2008;86:637-639.
116 Bruckner B.A., Jacob J.P., Gregoric I.D. Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Tex Heart Inst J. 2008;35:447-450.
117 Meyns B., Dens J., Sergeant P., et al. Initial experiences with the Impella device in patients with cardiogenic shock. Thorac Cardiovasc Surg. 2003;51:312-317.
118 Seyfarth M., Sibbing D., Bauer I. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52:1584-1588.
119 Dixon S.R., Henriques J.P.S., Mauri L. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): Initial U.S. experience. JACC: Cardiovasc Interv. 2009;2:91-96.
120 Atoui R., Samoukovic G., Al-Tuwaijri F. The use of the Impella LP 2.5 percutaneous microaxial ventricular assist device as hemodynamic support during high-risk abdominal surgery. J Card Surg. 2010;25:238-240.
121 Ferrari M., Poerner T.C., Brehm B.R. First use of a novel plug-and-play percutaneous circulatory assist device for high-risk coronary angioplasty. Acute Card Care. 2008;10:111-115.
122 Abiomed, BVS 5000 clinical update, Available at: www.abiomed.com/clinical_information/BVS5000_Update.cfm Accessed December 27, 2009
123 Abiomed, AB5000 clinical update, Available at: www.abiomed.com/clinical_information/AB5000_Update.cfm Accessed December 27, 2009
124 Abiomed, Data presented in video lecture from Dr. Ralph de la Torre: AMI Patients and Mechanical Support, Available at: www.abiomed.com/clinical_information/physician_videos.cfm Accessed December 27, 2009.
125 BVS5000 Bi-ventricular Support Training Manual. Danvers, MA: Abiomed, July 1997.
126 Fukumachi K., McCarthy P.M., Smedira N.G., et al. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181-2184.
127 Elbeery J.R., Owen C.H., Savitt M.A., et al. Effects of the left ventricular assist device on right ventricular function. J Thorac Cardiovasc Surg. 1990;99:809-816.
128 Santamore W.P., Gray L.A.Jr. Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg. 1996;61:350-356.
129 Marasco S.F., Lukas G., McDonald M., et al. Review of ECMO (Extra Corporeal Membrane Oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008;17(Suppl 4):S41-S47.
130 Hoshi H., Shinshi T., Takatani S. Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs. 2006;30:324-333.
131 Asama J., Shinshi T., Hoshi H., et al. A compact highly efficient and low hemolytic centrifugal blood pump with a magnetically levitated impeller. Artif Organs. 2006;30:160-167.
132 Santise G., Petrou M., Pepper J.R., et al. Levitronix CentriMag as a short-term salvage treatment for primary graft failure after heart transplantation. J Heart Lung Transplant. 2006;25:495-498.
133 John R., Liao K., Lietz K., et al. Experience with the Levitronix CentriMag circulatory support system as a bridge to decision in patients with refractory acute cardiogenic shock and multisystem organ failure. J Thorac Cardiovasc Surg. 2007;134:351-358.
134 Shuhaiber J.H., Jenkins D., Berman M., et al. The Papworth experience with the Levitronix CentriMag ventricular assist device. J Heart Lung Transplant. 2008;27:158-164.
135 Frazier O.H., Rose E.A., Oz M.C., et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186-1195.
136 www.thoratec.com, 2009; Accessed December 27
137 Slaughter M., Tsui S., El-Banayosy A., et al. Results of a multicenter clinical trial with the Thoratec implantable ventricular assist device. J Thorac Cardiovasc Surg. 2007;133:1573-1580.
138 Copeland J.G., Smith R.G., Arabia F.A., et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351:859-867.
139 Minami K., El-Banayosy A., Sezai A., et al. Morbidity and outcome after mechanical support using Thoratec, Novacor, and HeartMate for bridging to heart transplantation. Artif Organs. 2000;24:421-426.
140 Miller L.W., Pagani F.D., Russel S.D., et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885-896.
141 Frazier O.H., Rose E.A., Oz M.C., et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186-1195.
142 Lee S., Kamdar F., Madlon-Kay R., et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J Heart Lung Transplant. 2010;29:209-215.
143 Patel N.D., Weiss E.S., Schaffer J., et al. Right heart dysfunction after left ventricular assist device implantation: A comparison of the pulsatile HeartMate I and axial-flow HeartMate II devices. Ann Thorac Surg. 2008;86:832-840.
144 Saleh S., Liakopoulos O.J., Buckberg G.D. The septal motor of biventricular function. Eur J Cardiothorac Surg. 2006;29S:S126-S138.
145 Lee S., Kamdar F., Madlon-Kay R., et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J Heart Lung Transplant. 2010;29:209-215.
146 Rose E.A., Gelijns A.C., Moskowitz A.J., et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435-1443.
147 Long J.W., Kfoury A.G., Slaughter M.S., et al. Long term destination therapy with the HeartMate XVE left ventricular assist device: Improved outcomes since the REMATCH study. Congest Heart Fail. 2005;11:133-138.
148 Slaughter M.S., Rogers J.G., Milano C.A., et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2282-2285.
149 Lietz K., Long J.W., Kfoury A.G., et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: Implications for patient selection. Circulation. 2007;116:497-505.
150 Kirklin J.K., Naftel D.C., Stevenson L.W., et al. INTERMACS database for durable devices for circulatory support: First annual report. J Heart Lung Transplant. 2008;27:1065-1072.
151 Stevenson L.W., Pagani F.D., Young J.B., et al. INTERMACS profiles of advanced heart failure: The current picture. J Heart Lung Transplant. 2009;28:535-541.
152 Holman W.L., Kormos R.L., Naftel D.C., et al. Predictors of death and transplant in patients with a mechanical circulatory support device: A multi-institutional study. J Heart Lung Transplant. 2009;28:44-50.
153 Levy W.C., Mozaffarian D., Linker D.T., et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation. 2006;113:1424-1433.
154 Levy W.C., Mozaffarian D., Linker D.T., et al. Can the Seattle Heart Failure Model be used to risk-stratify heart failure patients for potential left ventricular assist device therapy? J Heart Lung Transplant. 2009;28:231-236.
155 Schaffer J.M., Allen J.G., Weiss E.S., et al. Evaluation of risk indices in continuous-flow left ventricular assist device patients. Ann Thorac Surg. 2009;88:1889-1896.
156 Patten R.D., Denofrio D., El-Zaru M, et al. Ventricular assist device therapy normalizes inducible nitric oxide synthate expression and reduces cardiomyocyte apoptosis in the failing human heart. J Am Coll Cardiol. 2005;45(9):1425-1427.
157 Zafeirides A., Jeevanandam V., Houser S.R., et al. Regression of cellular hypertrophy after left ventricular assist device support. Circulation. 1998;98:656-662.
158 Madigan J.D., Barbone A., Choudhri A.F., et al. Time course of reverse remodeling of the left ventricle during support with a left ventricular assist device. J Thorac Cardiovasc Surg. 2001;121:902-908.
159 Terracciano C.M.N., Hardy J., Birks E.J., et al. Clinical recovery from end-stage heart failure using left ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation. 2004;109:2263-2265.
160 Cooley D.A., Liotta D., Hallman G.L., et al. Orthotopic cardiac prosthesis for two staged cardiac replacement. Am J Cardiol. 1969;24:723.
161 Cooley D.A., Akutsu T., Norman J.C., et al. Total artificial heart in two-stage cardiac transplantation. Cardiovasc Dis. 1981;8:305.
162 DeVries W.L., Anderson J.L., Joyce L.D., et al. Clinical use of the total artificial heart. N Engl J Med. 1984;310:273.
163 Organ Procurement and Transplant Network (OPTN) Web site; www.OPTN.org/data; Accessed March 8, 2010
164 Menasche P. Cell transplantation in myocardium. Ann Thorac Surg. 2003;75:20.
165 Siminiak T., Fiszer D., Jerzykowska O., et al, Percutaneous transvenous transplantation of autologous myoblasts in the treatment of postinfarction heart failure: The POZNAN trial; March 7, 2004; Presented at the American College of Cardiology, 53rd Annual Scientific Session, New Orleans
166 Len N. Mobilizing cells to the injured myocardium. J Am Coll Cardiol. 2004;44:1521.
167 Rosenstrauch D., Poglajen G., Zidar N., et al. Stem cell therapy for ischemic heart failure. Tex Heart Inst J. 2005;32:339.
168 Snakumar B., Harry L., Paleolog E. Modulating angiogenesis. JAMA. 2004;292:972.
169 Chaudhri B.B., del Monte F., Harding S.E., et al. Gene transfer in cardiac myocytes. Surg Clin N Am. 2004;84:141.
170 Askuri A., Penn M. Targeted gene therapy for the treatment of cardiac dysfunction. Semin Thorac Cardiovasc Surg. 2002;14:167.
171 Sandham J.D., Hull R.D., Brant R.F., et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5.
172 Mangano D.T. Monitoring pulmonary artery pressures in coronary artery disease. Anesthesiology. 1980;53:364.
173 Carpentier A., Chauvaud S., Fabiani J.N., et al. Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J Thorac Cardiovasc Surg. 1980;79:338.
174 Horton S., Khodaverdian R., Chatelain P., et al. Left ventricular assist device malfunction: An approach to diagnosis by echocardiography. J Am Coll Cardiol. 2005;45:1435.
175 Scalia G.M., McCarthy P.M., Savage R.M., et al. Clinical utility of echocardiography in the management of implantable ventricular assist devices. J Am Soc Echocardiogr. 2000;13:754.
176 Stone M. Transesophageal echocardiography and surgical devices: Cannulas, catheters, intraaortic balloon pumps, ventricular assist devices, and occluders. In Konstadt S., Shernan S., Oka Y., editors: Clinical Transesophageal Echocardiography: A Problem Oriented Approach, ed 2, Philadelphia: Lippincott Williams & Wilkins, 2003.
177 Mets B. Anesthesia for left ventricular assist device placement. J Cardiothorac Vasc Anesth. 2000;14:316.
178 Castillo J.G., Anyanwu A.C., Adams D.H., et al. Real-time 3-D echocardiographic assessment of current continuous flow rotary left ventricular assist devices. J Cardiothorac Vasc Anesth. 2009;23:702-710.
179 Kuhl H.P., Schreckenberg M., Rulands D., et al. High-resolution transthoracic real-time three-dimensional echocardiography: Quantitation of cardiac volumes and function using semi-automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;43:2083-2090.
180 Gopal A.S., Schnellbaecher M.J., Shen Z., et al. Freehand three-dimensional echocardiography for determination of left ventricular volume and mass in patients with abnormal ventricles: Comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 1997;10:853-861.
181 Arai K., Hozumi T., Matsumura Y., et al. Accuracy of measurement of left ventricular volume and ejection fraction by new real-time three-dimensional echocardiography in patients with wall motion abnormalities secondary to myocardial infarction. Am J Cardiol. 2004;94:552-558.
182 Ochiai Y., McCarthy P.M., Smedira N.G., et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: Analysis of 245 patients. Circulation. 2002;106:I198-I202.
183 Maeder M.T., Leet A., Ross A., et al. Changes in right ventricular function during continuous-low left ventricular assist device support. J Heart Lung Transplant. 2009;28:360-366.
184 Farrar D.J., Hill J.D., Pennington D.G., et al. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the Thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113:202-209.
185 Adams D.H., Anyanwu A.C., Chikwe J., et al. The year in cardiovascular surgery. J Am Coll Cardiol. 2009;53:239-240.
186 Ramakrishna H., Fassl J., Sinha A., et al. The Year in cardiothoracic and vascular anesthesia: selected highlights from 2009. J Cardiothorac Vasc Anesth. 2010;24:7-17.
187 Thunberg C., Gaitan B., Arable F., et al. Ventricular assist devices today and tomorrow. J Cardiothorac Vasc Anesth. 2010;24:656-680.