Chapter 21 New Approaches to the Surgical Treatment of End-Stage Heart Failure
According to the American Heart Association, there are approximately 6–7 million people in the United States with congestive heart failure (HF). Available statistics indicate that the incidence of HF in the population approaches 10 per 1000 after age 65, with 550,000 new cases each year. Heart failure (HF) is the leading cause of hospitalization in patients older than age 65, with a reported associated cost of $24 to $50 billion annually. On a global scale, HF reportedly affects 0.4% to 2.0% of the adult population.1
While many patients successfully achieve temporary relief of HF symptoms with medical management, the underlying pathophysiology inevitably progresses and pharmacologic interventions alone will eventually become inadequate in the vast majority. A variety of surgical procedures can be performed to improve cardiac function and potentially arrest (or even reverse) the progression to severe dysfunction, but until very recently, surgical intervention (short of transplantation or placement of a ventricular assist device) was considered contraindicated in patients with advanced HF. Surprisingly good outcomes with “corrective” interventions, however, have now resulted in patients presenting for surgical treatment of their HF on a regular basis.2,3
SURGICAL OPTIONS FOR HEART FAILURE
A growing number of surgical procedures exist (or have been developed) to relieve HF symptoms and arrest the progression of the disease through correction of abnormal myocardial depolarization, enhancement of myocardial blood supply, improvement in ventricular loading conditions, and restoration of more normal ventricular geometry. Box 21-1 provides a list of current surgical interventions for HF.
Cumulative worldwide experience with such interventions thus far suggests that these procedures not only relieve symptoms but may also attenuate or possibly arrest the progressive myocardial remodeling that accompanies chronic HF. In some cases, partial reversal of the adverse myocardial remodeling has been demonstrated and combination therapy (surgical intervention with targeted pharmacologic treatment) intended to enhance reverse remodeling is actively being investigated.4
REVASCULARIZATION
Despite an increased perioperative risk of morbidity and mortality in this population, the world’s literature reports current survival between 57% and 75% at 5 years with in-hospital mortality between 1.7% and 11%. A recent review reported an 83.5% survival at 2 years after revascularization compared with only 57.2% survival in patients with congestive heart failure (CHF) who were not revascularized.5 In general, morbidity and mortality tend to correlate inversely with EF and directly with NYHA functional class. Additional factors predisposing patients to higher morbidity and mortality include advanced age, female sex, hypertension, diabetes, and emergent operations. The decreases in morbidity and mortality after revascularization in this high-risk population in recent years are at least partially attributable to improvements in surgical technique and myocardial protection, but the concurrent performance of mitral valve repair and ventricular reshaping address the adverse ventricular loading conditions present and may also contribute to improved outcomes. The results of ongoing clinical trials evaluating combinations of surgical procedures (e.g., revascularization plus ventricular reshaping versus revascularization alone) are eagerly awaited.
CORRECTION OF MITRAL REGURGITATION
Despite the slightly increased operative risk, the current literature supports mitral valve repair or replacement as beneficial to patients with severely depressed LV function, HF, and MR. In a recent series, Romano and Bolling reported operative mortality of 5% with 1- and 2-year survival rates of 80% and 70%, respectively.6 Not only was long-term mortality reduced, but the increase in LV systolic function (on average by 10%) enabled a downgrading of NYHA class and resulted in an improved quality of life. It has been shown that 1-, 2-, and 5-year survival rates of 91%, 84%, and 77%, respectively, can be obtained in patients with LVEF less than 30%. In addition, the rate of re-hospitalization for HF was decreased during the period of follow-up compared with a cohort that did not receive a mitral repair. Thus, both medical and economic benefits may result from mitral valve repair in this population.
While the majority of end-stage HF patients will exhibit functional MR, there may be additional concurrent valvular pathology present in a given patient. An intraoperative transesophageal echocardiography (TEE) evaluation of the valvular anatomy, the mechanism of the MR, and direct surgical inspection will determine the feasibility of repair. It is generally believed that valve repair is preferable to valve replacement, because there are demonstrated hemodynamic advantages associated with preservation of the subvalvular apparatus7 and long-term anticoagulation is not required.
LEFT VENTRICULAR RESHAPING
The modified Dor procedure (endoventricular circular patch plasty) is successfully used to reshape the dilated, spherical LV of patients who have had an anterior wall myocardial infarction with resulting aneurysm and akinesis/dyskinesis. Essentially, a Dacron patch is placed within the LV cavity so as to exclude the large akinetic/dyskinetic area of the anterior wall. This restores LV geometry to a more normal elliptical shape and improves systolic function. When performed concurrently with coronary artery bypass grafting (CABG), significant early and late improvements in both NYHA functional class and EF have been demonstrated with an in-hospital mortality rate of 12%. A trial of 439 patients undergoing this procedure found an improved in-hospital mortality of 6.6% and an 18-month survival of 89.2%. In this series, CABG was performed concurrently in 89%, mitral valve repair in 22%, and mitral valve replacement in 4%.8
PROCEDURES TO ARREST THE DILATION OF THE FAILING VENTRICLE
Acorn CorCap Cardiac Support Device
The CorCap Cardiac Support Device (Acorn Cardiovascular, Inc., Minneapolis, MN) is an investigational mesh fabric that is surgically wrapped around the heart in an attempt to prevent further dilation. This device has been shown in animal models to reduce wall stress, myocyte hypertrophy, and myocardial fibrosis. A global multicenter study by Oz and coworkers9 showed promising results. There were significant reductions in LV end-diastolic dimensions, MR grade, and NYHA classification in patients 12 months after CorCap implantation. At the same time, a significant improvement was seen in LVEF and quality of life. Ongoing randomized clinical trials in Europe and the United States will further address the safety of implantation of the CorCap device and ultimate outcomes.
CARDIAC RESYNCHRONIZATION THERAPY AND IMPLANTABLE CARDIOVERTER-DEFIBRILLATORS
The progression of disease resulting in advanced cardiac failure is typically accompanied by conduction defects and arrhythmias, and pacemakers and implantable cardioverter-defibrillators (ICDs) are commonly used in this population. In addition to the well-known defects in sinus or atrioventricular node function, intraventricular conduction defects delay the onset of RV or LV systole in 30% to 50% of patients with advanced HF.10 This lack of coordination of LV and RV contractions further impairs CO and has been reported to increase the risk of death in this population.
Studies have shown that atrial-synchronized biventricular pacing (pacing the LV and RV in a carefully timed manner) can “resynchronize” RV and LV contraction, improving CO and overall hemodynamics. This enhances these patients’ ability to exercise (which improves their NYHA functional class) and decreases the length and frequency of their hospitalizations, which improves their quality of life.11
Sudden death from ventricular fibrillation (VF) accounts for approximately 300,000 deaths annually in the United States. Patients with advanced HF experience VF with a frequency 6 to 9 times that of the general population, and VF causes 40% of all deaths in this population even in the absence of apparent disease progression based on symptoms. Thus, ICDs are commonly indicated for patients with advanced cardiac failure. An ICD is a device capable of arrhythmia detection and automatic defibrillation. ICDs successfully terminate VF in greater than 98% of episodes, and studies have demonstrated that an ICD increases survival and decreases the risk of sudden death in patients with ischemic cardiomyopathy and decreased LV function.12
VENTRICULAR ASSIST DEVICES
Currently Available Ventricular Assist Devices: Indications and the Basic Strategy of Their Use
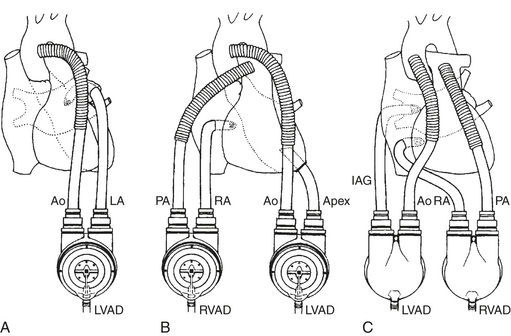
While the VAD is acting to maintain the circulation, decompression of the failing ventricle will decrease ventricular wall tension and therefore myocardial oxygen demand. To decompress the failing ventricle, cannulas must be placed in the heart to divert blood to the pump, and all currently approved devices use essentially the same cannulation strategies within the heart and great vessels. For LV support, blood is drained from the LA or, more commonly, the LV apex and returned to the ascending aorta. For RV support, blood is drained from the RA or RV and returned to the main pulmonary artery. These cannulation strategies are common to all VADs currently approved for clinical use and are diagrammed in Figure 21-1.
Temporary Ventricular Assist Device Use: Bridge to Recovery
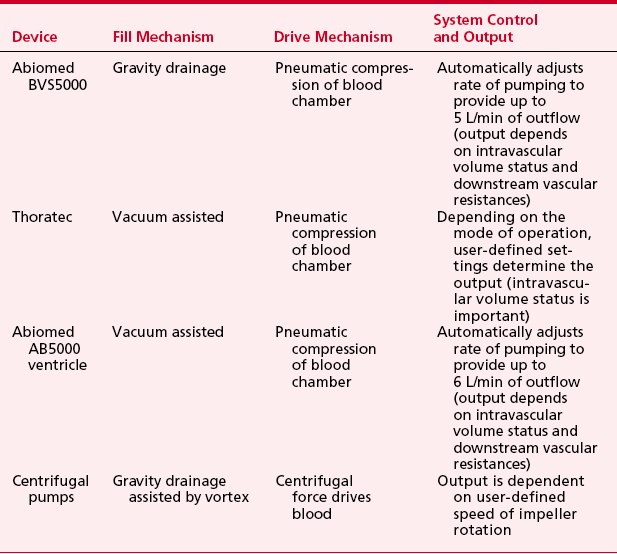
In patients in whom there has been an acute myocardial insult resulting in refractory cardiogenic shock but myocardial recovery is expected, a short-term VAD (e.g., the Abiomed BVS5000; Abiomed, Danvers, MA), an intermediate-term VAD (e.g., the Thoratec VAD system; Thoratec Laboratories, Pleasanton, CA, or the AB5000 ventricle; Abiomed, Danvers, MA), or standard centrifugal devices can support the circulation as a “bridge to recovery.” The Abiomed BVS5000, the Thoratec, and the AB5000 ventricle are paracorporeal, pneumatically driven devices capable of providing pulsatile support for the LV (LVAD), the RV (RVAD), or both ventricles (BiVAD) as needed. With all these devices, the paracorporeal pump heads are connected to inflow and outflow cannulas anastomosed to the heart and great vessels. Table 21-1 summarizes the basic characteristics of these devices. The artificial blood contacting surfaces, unidirectional valves, and pulsatile nature of the pumping mechanism in these devices require that the patient be anticoagulated during support.
Intermediate- to Long-Term Ventricular Assist Device Use: Bridge to Transplantation
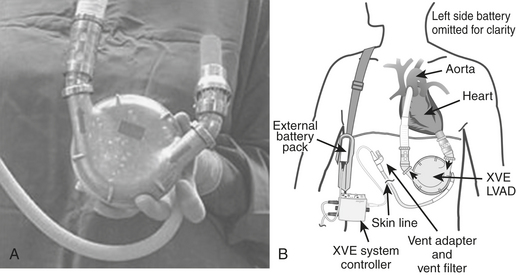
The Novacor (Fig. 21-2) and the HeartMate (World Heart, Inc., Oakland, CA) (Fig. 21-3) are the most commonly used bridge-to-transplantation devices for patients with advanced LV failure. Patients with biventricular failure are typically bridged with the Thoratec devices. While there are new and promising devices currently in clinical trials, the Thoratec is presently the only widely available mechanical circulatory support device capable of providing intermediate to long-term support of the native heart as a bridge-to-transplantation for patients who require biventricular support. According to the most recent voluntary Novacor, HeartMate, and Thoratec registries, more than 1700 patients have been implanted with the Novacor, more than 5600 with the HeartMate, and more than 2500 with the Thoratec worldwide. Of those implanted with the Thoratec for bridge to transplantation, 59% required biventricular assistance. Rates of successful bridging to transplantation with these devices are reportedly on the order of 51% to 78%.13
An implanted Novacor is shown in Figure 21-2, and a HeartMate is seen in Figure 21-3. As depicted, the pump heads of these devices are implanted in a surgically created pocket in the preperitoneal space. Inflow and outflow conduits are connected to the heart and great vessels as shown. Blood is drained to the implanted pump head from the LV apex and is ejected in a pulsatile fashion into the ascending aorta. Adequate intravascular volume status is a critical factor in optimizing pump outputs.
Permanent Ventricular Assist Device Use: Destination Therapy
Based on the encouraging results of the REMATCH trial,14 the HeartMate LVAS was FDA-approved in 2002 for use as “destination therapy” in the United States. Briefly, REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) was conducted to determine if the HeartMate could be effective as an alternative therapy for patients with end-stage cardiac failure who are not eligible to receive a transplant. In addition to patient survival, the study collected data on the implanted patient’s quality of life, the cost of the patient’s care, and any adverse events that occurred during the treatment. In essence, the REMATCH study demonstrated that the use of a left-sided VAD was not only an effective tool to treat patients with advanced HF but also resulted in more than twice the survival rate and an improved quality of life in comparison to optimal medical management. This was especially the case if the patient was younger than 60 years. Summarized results of the REMATCH trial appear in Table 21-2.
| HeartMate | Medical Treatment | |
|---|---|---|
| 1-Year survival (patients < 60 yr) | 52% (74%) | 25% (33%) |
| 2-Year survival | 23% | 8% |
| Median survival | 408 days | 150 days |
Complications of Currently Available Ventricular Assist Devices
Regardless of which short- or long-term device is in use, the major complications of VAD use include coagulopathic bleeding in the perioperative period, potential for thromboembolism during support, and infection. Sepsis is the leading cause of death. Additional complications of VAD use include RV failure while on isolated LV assistance (on the order of 30% due to the induced changes in RV geometry), potential device malfunction, and progressive multisystem organ failure despite VAD support.15
TOTAL ARTIFICIAL HEART
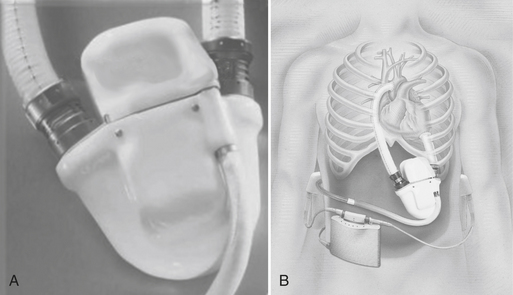
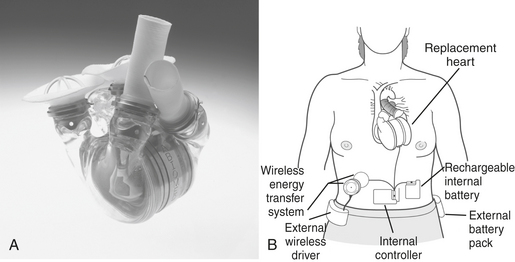
The Abiocor Implantable Replacement Heart (Fig. 21-4) represents a major advance in artificial heart technology because it is truly totally implantable; there are no percutaneous cables, conduits, or wires. The device is motor driven, so a source of compressed air to drive the pumping action is not required, allowing patients complete mobility. The device itself weighs approximately 2 pounds and is orthotopically implanted.
NEW THERAPIES
Cellular Transplantation into the Myocardium
A frequently encountered occurrence in the phase I studies was the fact that many patients had episodes of ventricular tachycardia after the procedure. They were successfully treated with amiodarone or electric cardioversion. Phase II studies are now in progress in the United States and Europe. Many other cell types are now being experimentally injected into the myocardium or given intravenously in an attempt to regrow cardiac myocytes. These cells include adult bone marrow stem cells, embryonic stem cells, and cardiac progenitor cells found mainly in the atrium. They have been injected alone or with multiple growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF), vasoactive endothelial growth factor (VEGF), and angiopoietin-1, which mobilize progenitor cells and induce new cell growth. The transplanted cells may morph into new cardiac muscle cells or they may improve cardiac function by boosting the growth of new blood vessels or releasing other growth factors that encourage cell proliferation and survival. Any of these effects could explain some of the early positive results seen to date.16
Gene Transfer in Cardiac Myocytes
Gene therapy has received much interest by the media during the past decade. Multiple research groups are trying to cure or at least alleviate symptoms brought on by HF through gene therapy. To change the genetic programming of a cell it is necessary to inoculate the cell with DNA. To achieve this goal, vectors are being used. Vectors commonly used range from plasmid DNA to different virus types (e.g., adenovirus, herpesvirus). The two cellular pathways that are being targeted are the sarcoplasmic reticulum and the β-adrenergic pathway. The targets of gene therapy are to increase β-adrenergic function, adenylyl cyclase, the V2 vasopressin receptor, and sarcoplasmic reticulum Ca2+ ATPase (SERCA2a), while also decreasing phospholambam and β-adrenoreceptor kinase (BARK 1). All of these new therapies hold great promise and many trials are being done.17
ANESTHETIC CONSIDERATIONS IN THE PATIENT WITH SEVERELY IMPAIRED CARDIAC FUNCTION
Transesophageal Echocardiography and Ventricular Assist Devices
The role of TEE where mechanical ventricular assist devices are concerned begins before placement of the device, with a TEE evaluation focused on detecting or ruling out specific anatomic pathologic processes that may prevent the device from functioning as intended, or lead to preventable complications, and often must be surgically addressed before starting support by the device.18
Pre-LVAD placement, TEE is used to detect specific anatomic pathologies that will19:
During LVAD placement, TEE is used to:
SUMMARY
1. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140-1151.
2. Vitali E., Colombo T., Fratto P., et al. Surgical therapy in advanced heart failure. Am J Cardiol. 2003;91(suppl):88F.
3. Westaby S., Narula J. Preface: Surgical options in heart failure. Surg Clin North Am. 2004;84:15.
4. Birks E., Tansley P., Hardy J., et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N ENF J MED. 2006;355:1873.
5. Liao L., Cabell C.H., Jollis J.G., et al. Usefulness of myocardial viability or ischemia in predicting long-term survival for patients with severe left ventricular dysfunction undergoing revascularization. Am J Cardiol. 2004;93:1275.
6. Romano M.A., Bolling S.F. Mitral valve repair as an alternative treatment for heart failure patients. Heart Fail Monit. 2003;4:7.
7. Reese T.B., Tribble C.G., Ellman P.I., et al. Mitral repair is superior to replacement when associated with coronary artery disease. Ann Surg. 2004;239:6715.
8. Athanasuleas C.L., Stanley A.W.Jr., Buckberg G.D., et al. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. RESTORE Group. Reconstructive Endoventricular Surgery, Returning Torsion Original Radius Elliptical Shape to the LV. J Am Coll Cardiol. 2001;37:1199.
9. Oz M.C., Konerzt W.F., Kleber F.X., et al. Global surgical experience with the acorn cardiac support device. J Thorac Cardiovasc Surg. 2003;126:983.
10. Jarcho J. Resynchronizing ventricular contraction in heart failure. N Engl J Med. 2005;352:1594.
11. Cleland J., Daubert J., Erdman E., et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539.
12. Bardy E., Lee K., Mark D., et al. Amiodarone or an implanted cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225.
13. Minami K., El-Banayosy A., Sezai A., et al. Morbidity and outcome after mechanical support using Thoratec, Novacor, and HeartMate for bridging to heart transplantation. Artif Organs. 2000;24:421.
14. Rose E.A., Gelijns A.C., Moskowitz A.J., et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435.
15. Horton S., Khodaverdian R., Powers A., et al. Left ventricular assist device malfunction: A systemic approach to diagnosis. J Am Coll Cardiol. 2004;43:1574.
16. Rosenstrauch D., Poglajen G., Zidar N., Gregoric I. Stem cell therapy for ischemic heart failure. Tex Heart Inst J. 2005;32:339.
17. Chaudhri B.B., del Monte F., Harding S.E., et al. Gene transfer in cardiac myocytes. Surg Clin North Am. 2004;84:141.
18. Horton S., Khodaverdian R., Chatelain P., et al. Left ventricular assist device malfunction: An approach to diagnosis by echocardiography. J Am Coll Cardiol. 2005;45:1435.
19. Mets B. Anesthesia for left ventricular assist device placement. J Cardiothorac Vasc Anesth. 2000;14:316.