9 New and novel fillers
highlighting elastin and soft tissue augmentation, platelet-rich plasma and a combination of carboxymethyl cellulose (CMC), and polyethylene oxide (PEO)
Summary and Key Features
• Dermal elastin confers on tissue the ability to stretch and recoil and is important in cutaneous support
• Elastin may be a useful tissue filler and attempts to regenerate elastin are currently underway using recombinant tropoelastin
• Hyaluronic acid may be used as the crosslinking agent for tropoelastin and is being trialled in this form as a cutaneous filling agent
• Platelet rich plasma is a platelet rich concentrate in a small volume of plasma
• Platelets are a rich source of at least 7 growth factors including transforming vasoactive and epithelial factors
• Simplified methods have allowed harvesting of platelets and separation from other blood cells without lysing of the platelets
• Platelet rich plasma may have roles both in topical wound healing and in injected approaches as an augmentation and tissue regenerative agent.
• Platelet rich plasma has been shown to induce neocollagenesis, neovascularization and neolipogenesis after injection
• A novel filler comprising carboxymethyl cellulose and polyetheleneoxide (Laresse) has been used as a tissue filler
• Laresse has been compared favourably to Restylane in a short term study
• Laresse has been injected into upper lips, nasolabial and melomental folds in a small cohort of patients with few adverse reactions
Elastin and soft tissue augmentation
The raisons d’être of elastin regeneration and injecting elastin products
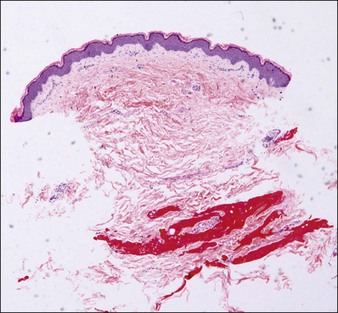
Damaged elastic fibers are responsible for an aged and wrinkled appearance (Fig. 9.1). Given the importance of elastin to the skin and its loss in the aging process, it is not surprising that various attempts have been made to maintain or replenish elastin levels. Treatments aiming to repair or regenerate elastin in elastic tissues should consider all the molecules implicated in elastin fiber formation. However, as elastin fibers develop, they ultimately consist of over 90% elastin and so the integration of sufficient tropoelastin into elastin fibers is clearly the major target. Effective treatment approaches are restricted owing to the obvious physical challenge of transferring materials and / or treatments across the epidermis and into the dermis, resulting in a preference for small molecules and physical treatments. Tretinoin or all-trans retinoic acid is a small molecule utilized for many years in topical formulations to increase elastin production through increased tropoelastin and fibrillin expression and secretion. Molecules such as aldosterone and mineralocorticoid receptor antagonists may impact on elastin fiber deposition in skin. Soy and rice (USPTO 19469891) extracts may also increase elastin formation, as can a combination of zinc and copper. More recently, a dill extract has also been shown by Cenizo et al to have the potential to promote elastin formation by promoting LOXL synthesis and secretion into the dermis.
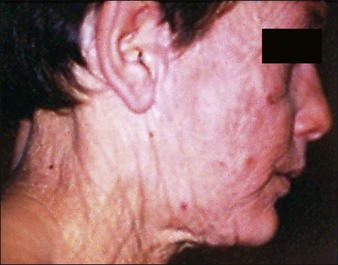
Courtesy of Hu Q, Reymond JL, Pinel N, et al 2006 Inflammatory destruction of elastic fibers in acquired cutis laxa is associated with missense alleles in the elastin and fibulin-5 genes. Journal of Investigative Dermatology 126(2):283-290.
A new approach to treat aged and damaged skin using elastin is currently in clinical development in Australia by Elastagen Pty Ltd. The approach is unique to skin augmentation treatments in that it is based on a recombinant human tropoelastin protein, which is identical to the one naturally occurring in healthy human skin. As seen in early clinical studies, this high degree of similarity to the natural elastin protein promises a significantly higher level of tissue compatibility than achieved with animal-derived products or synthetic polymer materials (Fig. 9.2). The treatment process, trademarked as elastatherapy®, also benefits from a new formulation chemistry that enables the tropoelastin protein to be cross-linked with a low-concentration HA component. There is no requirement for the toxic cross-linking agents often used in other products and the use of hyaluronidase to correct poor treatment outcomes is still an option – a significant advantage over alternative dermal filler materials. Elastin treatments benefit from the cell-supporting properties of elastin and the potential to stimulate skin cells, leading to the formation of new collagen at the treatment site. This latter property coupled with the product’s smooth, cohesive structure may present significant advantages over particulate products, which target collagen regeneration but carry a significant risk of lumps and nodules. The product range also includes the potential to bulk the skin with a long-lasting, highly biocompatible elastin material and the unique prospect of restoring elastin to improve the skin’s physical properties and suppleness.
Platelet-rich plasma (PRP)
The raisons d’être for the use of platelet-rich plasma in cosmetic and reconstructive medicine
Method
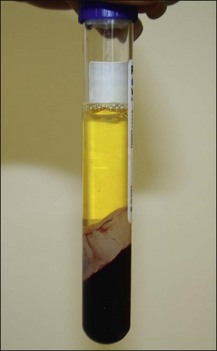
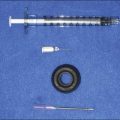
PRP production has been greatly simplified so that it can be used in the office and operating room. A sterile centrifugation process allows platelet separation from red blood cells and their sequestration in high concentrations without lysing the platelets or damaging them so that they no longer can actively secrete their growth factors. Many ‘kits’ are available and the resultant separation is illustrated in Figure 9.3, in which the plasma utilized from the separated elements is the yellow supernatant.
Carboxymethyl cellulose plus polyethylene oxide dermal filler (Laresse®)
Introduction
Pilot clinical study
Case Study 3 Clinical results
Laresse® study
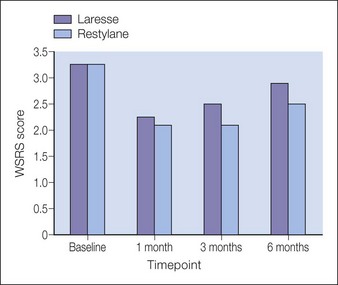
The WSRS scores for all 12 patients are plotted in Figure 9.5. The number of patients was too small to determine the level of statistical significance for a comparison of the two products. The GAIS scores were also determined from the responses of both the patient and the treating physician and results from the patients and the treating investigator indicate that the correction is still noticeable by the patient and the investigator at 3 and 6 months for both treatments. The mean baseline score for both groups was 3.4 and both fillers demonstrated measurable clinical correction at 6 months.
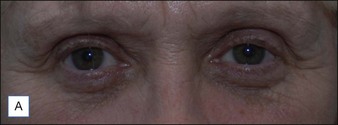
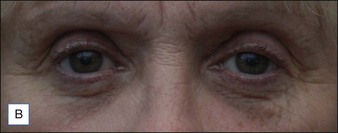
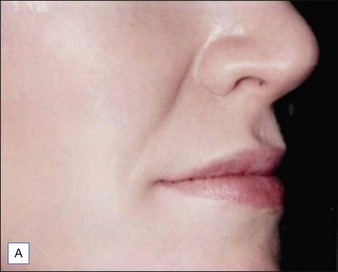
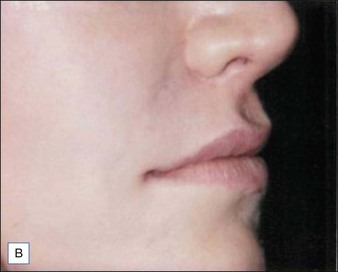
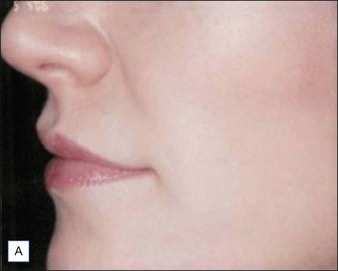
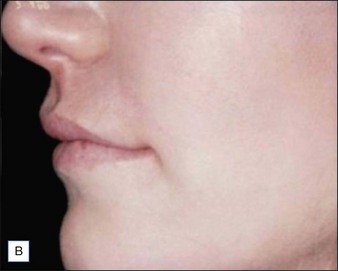
Figures 9.6 and 9.7 illustrate a patient treated with 1.8 mL Laresse® to the nasolabial fold and upper lip.
Further clinical experience
There were no side effects noted other than occasional bruising and expected transient edema.
Andre P, Lowe NJ, Parc A, et al. Adverse reactions to dermal fillers: a review of European experiences. Journal of Cosmetic Laser Therapy. 2005;7:171–176.
Bergstrom KG. Beyond tretinoin: cosmeceuticals for aging skin. Journal of Drugs in Dermatology. 2009;8:674–677.
Cenizo V, Andre V, Reymermier C, et al. LOXL as a target to increase the elastin content in adult skin: a dill extract induces the LOXL gene expression. Experimental Dermatology. 2006;15:574–581.
Chapman SL, Sicot FX, Davis EC, et al. Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:68–74.
Cleary EG, Sandberg LB, Jackson DS. The changes in chemical composition during development of the bovine nuchal ligament. Journal Cell Biology. 1967;33:469–479.
Day DJ, Littler CM, Swift RW, et al. The wrinkle severity rating scale: a validation study. American Journal of Clinical Dermatology. 2004;5:49–52.
Falcone SJ, Berg RA. Temporary polysaccharide dermal fillers: a model for persistence based on physical properties. Dermatol Surg Aug;. 2009;35(8):1238–1243.
Gogoleski S, Jovanovic M, Perren SM, et al. Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3 hydroxybutyrate) (PHB), and poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). Journal of Biomedical Materials Research. 1993;27:1135–1148.
Goodman G. Post acne scarring: a review. Journal of Cosmetic and Laser Therapy. 2003;5:77–95.
Harrison S, Vavken P, Kevy S, et al. Platelet activation by collagen provides sustained release of anabolic cytokines. American Journal of Sports Medicine. 2011;39:729–734.
Hu Q, Reymond JL, Pinel N, et al. Inflammatory destruction of elastic fibers in acquired cutis laxa is associated with missense alleles in the elastin and fibulin-5 genes. Journal of Investigative Dermatology. 2006;126(2):283–290.
Indik Z, Yeh H, Ornstein-Goldstein N, et al. Structure of the elastin gene and alternative splicing of elastin mRNA: implications for human disease. American Journal of Medical Genetics. 1989;34:81–90.
Jacobson M, Fufa D, Abreu EL, et al. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair and Regeneration. 2008;16(3):370–378.
Kielty CM, Shuttleworth CA. Microfibrillar elements of the dermal matrix. Microscopy Research and Technique. 1997;38:413–427.
Kim KD, Wang JC, Robertson DP, et al. Reduction of radiculopathy and pain with Oxiplex/SP gel after laminectomy, laminotomy, and discectomy: a pilot clinical study. Spine. 2003;28:1080–1087. discussion 1087-1088
Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatologic Surgery. 2011;237:931–938.
Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatologic Surgery. 2005;31:1616–1625.
Lundorff P, Donnez J, Korell M, et al. Clinical evaluation of a viscoelastic gel for reduction of adhesions following gynaecological surgery by laparoscopy in Europe. Human Reproduction. 2005;20:514–520.
Maki JM, Sormunen R, Lippo S, et al. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. American Journal of Pathology. 2005;167:927–936.
Mei-Dan O, Laver L, Nyska M, et al. [Platelet rich plasma – a new biotechnology for treatment of sports injuries]. Harefuah. 2011;150:453–457. 490
Nakatomi Y, Tsuruga E, Nakashima K, et al. EMILIN-1 regulates the amount of oxytalan fiber formation in periodontal ligaments in vitro. Connective Tissue Research. 2011;52:30–35.
Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multi-center comparison of the efficacy and tolerability of Restylane(r) versus Zyplast for the correction of nasolabial folds. Dermatologic Surgery. 2003;29:588–595.
Narins R, Jewell M, Rubin M, et al. Clinical conference: management of rare events following dermal fillers – focal necrosis and angry red bumps. Dermatologic Surgery. 2006;32:426–434.
Noblesse E, Cenizo V, Bouez C, et al. Lysyl oxidase-like and lysyl oxidase are present in the dermis and epidermis of a skin equivalent and in human skin and are associated to elastic fibers. Journal of Investigative Dermatology. 2004;122:621–630.
Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatologic Surgery. 1995;21:543–549.
Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. Journal of Craniofacial Surgery. 2005;16:1043–1054.
Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. Journal of Drugs in Dermatology. 2010;9:466–472.
Schmelzer CE, Getie M, Neubert RH. Mass spectrometric characterization of human skin elastin peptides produced by proteolytic digestion with pepsin and thermitase. Journal of Chromatography A. 2005;1083:120–126.
Schwartz HE, Blackmore JM, Cortese SM, et al. Compositions of polyacids and polyethers and methods for their use in reducing adhesions (US patent no: 6 869 938). San Luis Obispo, CA: FzioMed, Inc.; 2005.
Sclafani AP. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. Journal of Cosmetic Dermatology. 2010;9:66–71.
Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch Facial Plast Surg Mar;. 2012;14(2):132–136.
Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Archives of Physical Medicine and Rehabilitation. 2012;14(2):132–136.
Sephel GC, Buckley A, Davidson JM. Developmental initiation of elastin gene expression by human fetal skin fibroblasts. Journal of Investigative Dermatology. 1987;88:732–735.
Watson RE, Long SP, Bowden JJ, et al. Repair of photoaged dermal matrix by topical application of a cosmetic ‘antiageing’ product. British Journal of Dermatology. 2008;158:472–477.
Yamauchi Y, Tsuruga E, Nakashima K, et al. Fibulin-4 and -5, but not fibulin-2, are associated with tropoelastin deposition in elastin-producing cell culture. Acta Histochemica et Cytochemica. 2010;43:131–138.