4 Juvéderm® family
Summary and Key Features
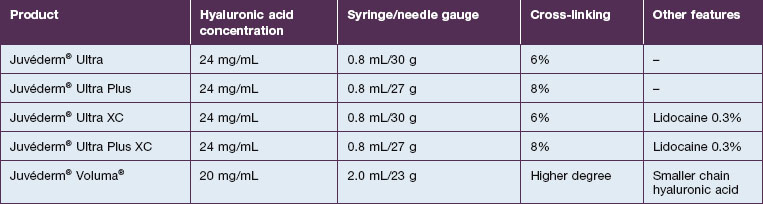
• Juvéderm® features a proprietary cross-linking process that results in a concentration of 24 mg/mL of hyaluronic acid; this unique process results in a soft, viscous, non-beaded gel and is designed to improve the durability of this filler
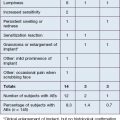
• Juvéderm® is available in the USA as Juvéderm® Ultra, Juvéderm® Ultra Plus, Juvéderm® Ultra XC, and Juvéderm® Ultra Plus XC
• Juvéderm® Voluma® is also available in many countries. Voluma® is a 20 mg/mL smooth hyaluronic acid preparation with high cohesivity and viscosity. These features provide extra ‘lift’ when injected into tissue
• Like all hyaluronic acid fillers Juvéderm® can be dissolved using hyaluronidase
• The most common areas for treatment are the lips, nasolabial furrows, commissures, chin, and nasojugal depressions
• Many dermatologists prefer Juvéderm® for the perioral areas, especially the lips, over other hyaluronic acid fillers because it is a ‘soft’ filler that feels more natural to the patient in sites where a hard texture would be more discernable
• Some dermatologists prefer the pre-mixed lidocaine-loaded XC Juvéderm® gels, while others like the flexibility of diluting the gels with lidocaine in the clinic
• Juvéderm® Voluma® has been developed specifically for volumizing by treating the three-dimensional aspects of facial aging
Juvéderm formulations
Juvéderm® is available in a variety of formulations. In the USA, it is distributed as Juvéderm® Ultra, Juvéderm® Ultra Plus, Juvéderm® Ultra XC, and Juvéderm® Ultra Plus XC (Table 4.1). Juvéderm® Ultra Plus contains a cross-linked composition of 8% versus 6% in Juvéderm® Ultra, making it a more robust filler. The two XC Juvéderm® products contain 0.3% lidocaine but are otherwise identical to the Ultra formulations. In some countries Juvéderm® Voluma® is also available. Voluma® is a 20 mg/mL smooth HA preparation with high cohesivity and viscosity. These features provide extra ‘lift’ when injected into tissue.
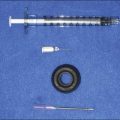
Juvéderm® injectable gel is a sterile, biodegradable, non-pyrogenic, viscoelastic, clear, colorless, homogenized gel implant (Fig. 4.1). It consists of cross-linked HA produced by bacterial fermentation by Streptococcus equus. The gel is formulated to a concentration of 20–24 mg/mL and suspended in a physiological buffer. The package insert states that the product is approved for ‘injection into the mid- to deep dermis for correction of moderate to severe facial wrinkles and folds (such as nasolabial folds)’ but is commonly used by physicians for a variety of soft tissue augmentation indications.
Using Juvéderm®
The most common areas for treatment with Juvéderm® products are the lips, nasolabial furrows, commissures, chin, and nasojugal depressions (Box 4.1). Prior to treatments the patient should undergo detailed informed consent with the physician, reviewing all the risks and benefits of using this filler. After answering all questions and obtaining written permission, the physician can begin treatment. A topical anesthetic cream applied to the skin for at least 10 minutes (longer if possible) makes the process more comfortable. For the perioral area, injectable local anesthesia such as a submucosal field block is also helpful in reducing discomfort. However, if too much volume of anesthetic is injected, it may distort the contours enough to confuse the injector. Niamtu states that, after application of a topical dental anesthetic, small aliquots of plain lidocaine can be injected at several locations at the sulcus produced by the attached gingival and the unattached mucosa in the upper and lower lips. Additional injection points on the undersurface of the nasolabial fold between the mucosa and the skin extend the field of anesthesia. This normally gives excellent anesthesia for infiltration of Juvéderm® into the nasolabial furrows, commissures, and lips.
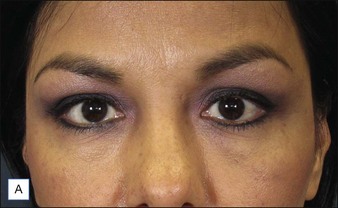
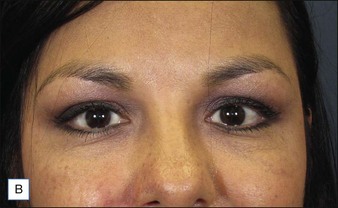
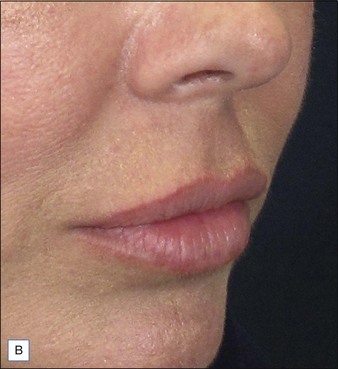
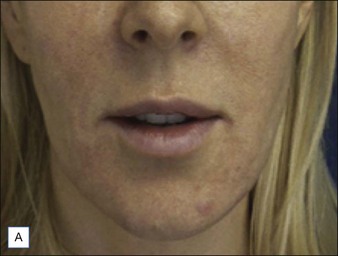
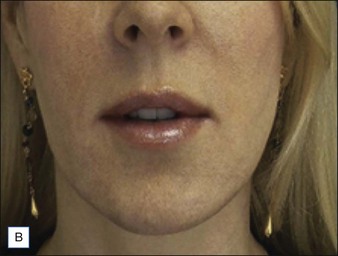
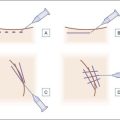
Before approaching the patient, the physician should confirm that the needle is firmly locked into the syringe; then the plunger should be depressed (while pointing it away from the patient) until a small amount of gel flows out of the needle to prime the needle and ensure adequate flow. Typically, a combination of serial puncture and linear-threading injection techniques is used. The Juvéderm® gel should be placed deep enough in the dermis that surface lumps are not produced. If the material flows too superficially, the lumps can usually be massaged into the proper contour. Juvéderm® gel is quite soft and can be molded quite easily immediately after injection, using fingers and/or cotton-tipped applicators. Determining the end point of the filling injection takes experience, but overcorrection is discouraged. Generally, it is better for the physician to undercorrect and then have the patient return for a secondary injection than to overcorrect. This is especially true of treatment of the periocular area where overfilling can result in visibility of the injected product. Injections should create a more youthful appearance to the treated area (Fig. 4.2).
Immediately afterward, the patient should apply ice to the treated areas and should be instructed to avoid making extensive facial expressions, which may make the gel move from its intended location, for at least 2 hours. This includes excessive chewing or talking. Heavy exercise or exposure to excessive heat may increase swelling and bruising. It is wise to have patients avoid alcoholic beverages and anticoagulant medications such as aspirin, non-steroidal inflammatory agents, fish oil, or warfarin for 1 day after treatment. Most patients can return to social occasions the following day (see Case study 1).
Clinical choices
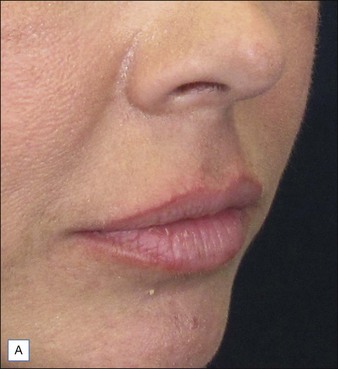
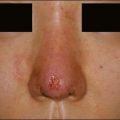
Many dermatologists prefer Juvéderm® for the perioral areas, especially the lips, over other HA fillers. Juvéderm® has a well-deserved reputation as a ‘soft’ filler that feels more natural to the patient in sites where a hard texture would be more discernable. More often, patients are looking for subtle improvements that do not appear ‘overdone’ (Fig. 4.3). The choice to use the Ultra or the Ultra Plus gel is more personal since there is no good clinical evidence that one performs significantly better than the other in specific sites. One might theorize that the more robust Ultra Plus would last longer and feel firmer in the tissues; however, there is no compelling evidence to support this.
Volumizing with Juvéderm® gels
Although Juvéderm® Ultra, Juvéderm® Ultra Plus, Juvéderm® Ultra XC, and Juvéderm® Ultra Plus XC are used by some dermatologists for replacing volume loss of soft tissue, the most common applications are to treat wrinkles and folds. Juvéderm® Voluma® has been developed specifically for treating the three-dimensional aspects of facial aging (see Case study 2; Fig. 4.4). Available in Canada and other countries, the 20 mg/mL is positioned as a ‘smooth, cohesive HA filler’, according to Carruthers et al. Allergan uses a unique manufacturing process, mixing both low and high molecular weight HA polymer chains, to increase cross-linking and produce a product of both high viscosity and high cohesivity. This process theoretically reduces migration from deep injection sites. Because of its increased viscosity, Juvéderm® Voluma® is packaged with two 23-gauge 1-inch (25 mm) ultra-thin wall needles and two 18-gauge 70 mm cannulas. There is a known incompatibility between sodium hyaluronate and quaternary ammonium salts such as benzalkonium chloride. The package insert therefore advises the user never to put Juvéderm® Voluma® in contact with these products, or with substances treated with these products.
Baumann LS, Shamban AT, Lupo MP, et al. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: a multicenter, double-masked, randomized, within-subject study. Dermatologic Surgery. 2007;33:S128–S135.
Brody HJ. The use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatologic Surgery. 2005;31:893–897.
Carruthers J, Carrauthers A, Tezel A, et al. Volumizing with a 20-mg/mL smooth, highly cohesive, viscous hyaluronic acid filler and its role in facial rejuvenation therapy. Dermatologic Surgery. 2010;36:1886–1892.
Grunebaum LD, Allemann IB, Dayan S, et al. The risk of alar necrosis associated with dermal filler injection. Dermatologic Surgery. 2009;35:1635–1640.
Hoffmann K. Volumizing effects of a smooth, highly cohesive, viscous 20-mg/mL hyaluronic acid volumizing filler: prospective European study. BMC Dermatology. 2009;9:9.
Juvéderm 2011 [package insert]
Levy PM, De Boulle K, Raspaldo H. A split-face comparison of a new hyaluronic acid facial filler containing pre-incorporated lidocaine versus a standard hyaluronic acid facial filler in the treatment of naso-labial folds. Journal of Cosmetic and Laser Therapy. 2009;11(3):169–173.
Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatologic Therapy. 2006;19(3):141–150.
Niamtu J, III. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatologic Surgery. 2005;31:10–11.
Pinsky MA, Thomas JA, Murphy DK, et al. Juvéderm vs. Zyplast Nasolabial Fold Study Group. Juvéderm injectable gel: a multicenter, double-blind, randomized study of safety and effectiveness. Aesthetic Surgery Journal. 2008;28:17–23.
Raspaldo H. Volumizing effect of a new hyaluronic acid sub-dermal facial filler: a retrospective analysis based on 102 cases. Journal of Cosmetic and Laser Therapy. 2008;10:134–142.
Raspaldo H, De Boulle K, Levy PM. Longevity of effects of hyaluronic acid plus lidocaine facial filler. Journal of Cosmetic Dermatology. 2010;9(1):11–15.
Rohrich RJ, Monheit G, Nguyen AT, et al. Soft-tissue filler complications: the important role of biofilms. Plastic and Reconstructive Surgery. 2010;125:1250–1256. Online. Available http://www.ncbi.nlm.nih.gov/pubmed/19935452
Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatologic Surgery. 2009;35:1653–1660.
Wahl G. European evaluation of a new hyaluronic acid filler incorporating lidocaine. Journal of Cosmetic Dermatology. 2008;7:298–303.