CHAPTER 41 New advances in glaucoma surgery
Introduction
Trabeculectomy, since its introduction in 1968 by Cairns1, has been the most widely performed glaucoma incisional procedure and continues today to be considered by many the gold standard by which all other surgical glaucoma procedures are measured. Because of the low attainable intraocular pressure (IOP) levels, and the relative simplicity of performing the actual procedure, trabeculectomy rapidly gained acceptance. However, despite its advantages, a large number of potentially visually devastating complications may result, in both the short and long term. These have been well documented in the literature and include, but are not limited to, infections (blebitis, endophthalmitis), bleb-related complications (encapsulation, fibrosis, leakage, dysesthesia, overhang, corneal dellen), hypotony and its associated complications (shallow/flat anterior chamber, maculopathy, overfiltration), as well as others such as aqueous misdirection syndrome, accelerated cataract formation, and endothelial cell loss2.
Another procedure which has continued to gain acceptance, especially in complex eyes with previous surgical failure and increasingly also as a primary procedure, are the glaucoma drainage devices (GDDs). The first such device was introduced by Molteno in the 1960s3. Other similar models followed including the Ahmed valve4, the Krupin eye disc5, and the Baerveldt drainage device6. These long tube shunt devices were designed to carry aqueous from the anterior chamber to a reservoir 10–12 millimeters (mm) posterior to the limbus, taking advantage of the larger posterior potential subconjunctival space, as well as theoretically avoiding the less metabolically active limbal zone where it may be more likely for a bleb to fibrose and fail. However, like trabeculectomy, these procedures too have an extensive complication profile. Most significantly, the risk of hypotony and suprachoroidal hemorrhage have led to the design or performance of modern day GDD surgery to emphasize flow control with either a valved plate mechanism such as the Ahmed, or partial and sometimes complete ligature of the tube with suture material. Despite these measures, hypotony and its associated complications remain a risk for the patient undergoing tube shunt surgery. In addition to these risks, GDD surgery has its own unique set of postoperative risks, including tube or plate exposure, tube occlusion, tube-related corneal endothelial cell loss even with properly positioned tubes, ptosis, and diplopia.
The Ex-PRESS shunt
The Ex-PRESS mini glaucoma shunt, like trabeculectomy, is designed to provide aqueous humor with an outlet into the subconjunctival space resulting in a bleb. The stainless steel device has a rounded tip in the anterior chamber to drain aqueous humor through the shaft trans-sclerally to end in a footplate which rests under a scleral flap and prevents migration of the shunt anteriorly. A spur on the anterior chamber side of the device prevents device extrusion. Several designs of the device offer slight modifications of the footplate or tip. While initially the device was implanted subconjunctivally without a scleral flap, complication rates were unacceptably high, with hypotony, shunt migration, and conjunctival erosion7. Later, the shunt was placed under a trabeculectomy-style scleral flap, which improved the safety profile of the device.
Technique
As in standard trabeculectomy, a conjunctival peritomy is followed by a scleral flap dissected forward so as to provide a clear view of the scleral spur. The dimensions of this flap should be slightly larger than that in standard trabeculectomy so as to cover fully the footplate of the device. Once the glistening white fibers of the scleral spur are visible, the anterior chamber should be pressurized with an ophthalmic viscosurgical device (OVD) via a paracentesis incision, or a cataract incision, if part of a combined procedure. An entry is made into the anterior chamber at the level of the scleral spur using either a sapphire blade, manufactured by Optonol, or a 25-gauge hypodermic needle (Fig. 41.1). The angle of entry into the anterior chamber must be parallel to the iris plane. An entry angled perpendicular to the spur will result in an excessively posterior shunt impinging on iris, perhaps with tip occlusion, corectopia, and photophobia. An entry angled excessively anteriorly will rest the tip near the corneal endothelium. The ideal position for the shunt is to be parallel to the iris surface (Fig. 41.2). Once the shunt is in a satisfactory position, the OVD may be aspirated with a dry technique and the anterior chamber pressurized with balanced saline solution (BSS). The scleral flap is sutured at its corners similar to a standard trabeculectomy; flow can be assessed with a surgical sponge. With suture tension adjusted to attain satisfactory flow, the conjunctiva is closed in a watertight fashion.
Discussion
Several studies have demonstrated effective IOP lowering and decreased medication usage with the Ex-PRESS shunt8,9 and more recently, prospective and retrospective studies have found the device to be comparable with conventional trabeculectomy10,11. In traditional trabeculectomy surgery, aqueous egress from the anterior chamber is regulated by the size of the ostium and of the scleral flap as well as suture tension. Although the Ex-PRESS shunt depends also on subconjunctival flow to lower IOP, it provides a consistent and reproducible ostium size, which may be challenging with a standard trabeculectomy punch. As well, without a large ostium, the Ex-PRESS shunt is inserted through a small entry into the anterior chamber improving anterior chamber stability during entry, as well as obviating the need for a surgical iridectomy. While in trabeculectomy, a surgical iridectomy may also be avoided, the creation of the large punch opening, combined with a shallowing of the anterior chamber, commonly results in iris presenting at the trabeculectomy site requiring a surgical iridectomy. This is notable in patients with narrow angles or shallow anterior chambers where enhanced anterior chamber stability may be more important. In our experience the Ex-PRESS shunt is well suited for narrow angle patients who require filtration surgery.
Non-penetrating Schlemm’s canaloplasty
Technique
The dissection is continued forward into peripheral clear cornea for approximately 2 mm exposing the trabeculo-Descemet window (TDW) (Fig. 41.3). Extreme care must be taken with the TDW as it easily ruptures, even with a wet surgical sponge. Once the TDW has been exposed, the deep flap is excised. The microcatheter is introduced into one of the cut ends of the canal and under fiberoptic illumination guidance is advanced carefully around the entire circumference until the tip emerges from the opposite cut end (Fig. 41.4). Once this has been achieved a suture, typically 10-0 Prolene, which the authors prefer, is tied to the distal end with two loose ends tied to the loop. The microcatheter is retracted 360° in the opposite direction, while an assistant injects a metered dose of OVD into the canal. Once fully retracted, the suture is cut from the microcatheter and the result is two loose suture ends emerging from each cut end of Schlemm’s canal. These ends are carefully corresponded and tied down in a slipknot fashion over the TDW. As the suture is tensioned, an inward dimpling of the TDW should be visualized, occasionally accompanied by an increase in aqueous humor percolation seen to emerge through the TDW (Fig. 41.5). The second suture is then tied down in a similar fashion. The superficial scleral flap is replaced and sutured down in a watertight fashion with typically five interrupted sutures, and finally followed by watertight conjunctival closure.
Discussion
The goal of canaloplasty is to restore or augment the outflow of aqueous via the conventional pathway by suture mediated distension of Schlemm’s canal. The tensioned suture attempts to reverse the process of canal collapse in glaucomatous eyes and thus to lower IOP. In the longest follow-up studies to date, at 2 years postoperatively, the mean IOP in patients undergoing canaloplasty alone was reduced from 23.3 mmHg to 16.3 mmHg and the mean number of medications used was reduced from 2.0 to 0.6. In patients undergoing combined cataract surgery with canaloplasty, the mean IOP was reduced from 23.1 mmHg to 13.4 mmHg and medication numbers decreased from 1.7 to 0.212. Suture tension has been shown in studies to be critical to obtain IOP lowering, with greater distension correlating with greater lowering of IOP13. Aqueous egress through the TDW and into the scleral lake, the potential space underneath the superficial scleral flap, also occurs and is potentially an important mechanism of IOP reduction. From the scleral lake, aqueous may exit into Schlemm’s canal, into the suprachoroidal space, directly into scleral, episcleral, and choroidal vascular channels, and/or emerge subconjunctivally to form a bleb, despite watertight closure. In some cases, fibrosis of the scleral lake, or at the level of the TDW, increases IOP necessitating an yttrium–aluminum–garnet (YAG) laser goniopuncture to restore aqueous flow into this space. To date, clinical studies available show excellent efficacy in IOP lowering with a low complication profile, reduced medication usage, and minimal postoperative management. However, the primary challenges are the technical difficulties involved in performing canaloplasty. This procedure is covered in more detail elsewhere in this book.
The Glaukos trabecular microbypass iStent
While canaloplasty approaches Schlemm’s canal ab externo, the iStent is designed is implanted ab interno, leaving conjunctiva and sclera undisturbed. This heparin-coated 1 mm titanium L-shaped stent has a half-pipe design with a snorkel that rests in the anterior chamber. It bypasses what is thought to be the main point of resistance in open angle glaucoma eyes: the juxta-canalicular trabecular meshwork (JTM). The half pipe has a pointed tip, designed to facilitate engaging the meshwork and inner wall for stent delivery, and retention barbs on the surface, designed to abut the inner wall to prevent stent migration (Fig. 41.6).
Technique
Once visualization is satisfactory, the tip of the device engages the trabecular meshwork and is advanced tangentially to the elbow of the device, being careful not to advance excessively, as this could result in tearing of the trabecular meshwork and inner wall of the canal (Fig. 41.7). Once a satisfactory position is attained, the index finger of the surgeon’s implantation hand is depressed, releasing the snorkel and device from the four-pronged grasping mechanism. A small amount of blood reflux may be encountered and should be expected, confirming the placement of the device in the canal. OVD can be used to displace the blood, clearing the view of the snorkel and device, to confirm its acceptable position (Fig. 41.8). Occasionally, the snorkel requires a gentle tap from the OVD cannula to ensure that it is fully seated in the canal. Irrigation and aspiration are then used to remove OVD from the eye, and the incisions are checked to be watertight.
Discussion
The ab interno approach of the iStent allows surgeons to bypass the JTM resistance point facilitating access of aqueous to Schlemm’s canal, thereby augmenting outflow via the conventional pathway. In a mathematical model of trabecular meshwork bypass, unidirectional and bidirectional flow would improve aqueous outflow facility by 13% and 26% respectively; also, the higher the initial IOP, the greater is the resultant reduction14,15. However, this relationship has been less clear in in vitro studies. While the insertion of a single stent resulted in an IOP reduction from 21.4 mmHg to 12.4 mmHg, insertion of additional stents did not result in as clear a benefit in IOP reduction16. Despite the theoretical mathematical models of JTM bypass via the iStent, the clinical efficacy in IOP lowering depends on circumferential flow in Schlemm’s canal, which may not be present in glaucomatous eyes with collapse of the canal. This raises further questions as to whether multiple stents provide an increased likelihood of circumferential flow, as well as whether strategic placement of the stents adjacent to or overlying collector channels increases IOP lowering. These issues remain insufficiently studied and require further investigation. Despite these unanswered questions, two studies have been published showing effective IOP lowering as well as decrease in topical medication usage in patients undergoing trabecular microbypass stent implantation combined with cataract surgery. One study reported a mean reduction of IOP from 21.7 mmHg to 17.4 mmHg at 12 months follow-up and a decrease in number of medications from 1.6 to 0.417. The other demonstrated IOP reduction from 19.6 mmHg to 15.8 mmHg and a reduction in medication numbers from 2.7 to 1.7 at 12 months follow-up18.
Trabectome microelectrocautery
The hand piece is composed of a 25-gauge aspiration port, a 19-gauge infusion sleeve, and a bipolar electrocautery unit 150 µm away from an insulated footplate. The footplate measures 800 µm from the heel of the hand piece to the tip and the maximum width measures 230 µm and the maximum thickness 110 µm (Fig. 41.9). The triangular shape and pointed tip of the footplate, which is angled 90° to the shaft of the hand piece, along with its smooth surface, are designed to facilitate penetration into Schlemm’s canal as well as to insulate the outer wall of the canal from electrosurgical injury and trauma.
Technique
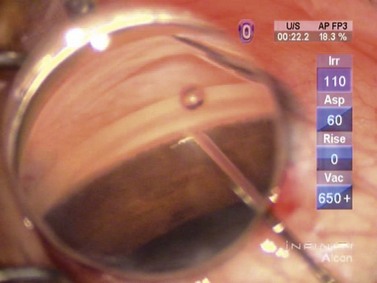
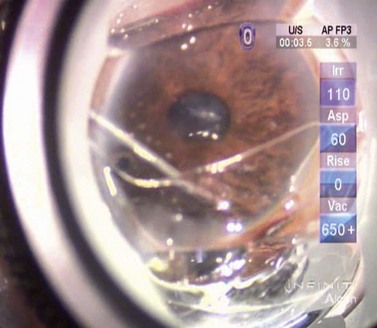
After the cataract has been removed, the patient’s head is turned approximately 15–30° and the operating microscope tilted away from the surgeon. A gonioprism, such as the Swan–Jacob gonioprism, permits a direct view of the anterior chamber angle, which is inflated with a heavy OVD. The Trabectome hand piece is inserted into the eye and, under direct visualization, the pointed tip is used to incise the trabecular meshwork until the footplate of the device rests in the canal. The foot pedal is depressed to initiate ablation and the hand piece advanced in the direction that the tip is pointing until further progress is prevented by either the angle of approach or visualization (Fig. 41.10). The hand piece tip may be turned to face the opposite direction and a similar ablation performed until no further progress can be made. Blood reflux is common, but is usually managed adequately by irrigation and aspiration provided by the hand piece as well as the heavy OVD, which tamponades the blood. Once the ablation is completed, the OVD is removed and the wounds checked to be watertight. A clear corneal suture and intracameral air at the conclusion of surgery are associated with smaller amounts of postoperative hyphema19.
Discussion
Similar to the trabecular microbypass stent, the Trabectome creates a direct communication for aqueous between the anterior chamber and the Schlemm’s canal collector channels through the length of the ablated arc. This bypasses the JTM resistance point. Several studies have been published, both as a stand-alone procedure, as well as combined with phacoemulsification. In the stand-alone group, the mean IOP fell from 25.7 mmHg to 16.6 mmHg at 24 months of follow-up with the mean medication numbers decreasing from 2.93 to 1.24. However, there was a significant drop-off in the number of patients who reached 24 months follow-up (from n = 738 to n = 46). In patients undergoing combined surgery, the mean IOP decreased from 20.0 to 15.5 mmHg with the mean medication usage decreasing from 2.65 to 1.44; again at the 24 month follow-up there was a significant drop in numbers (n = 366 to n = 45)20. The most common complications reported with the Trabectome include transient postoperative hyphema, peripheral anterior or goniosynechiae, transient corneal injury, Descemet’s detachment and hemorrhage, persistent Descemet’s injury, inadvertent iris injury, and transient early hypotony19,20. Patients with narrow angle configurations may not be suitable for the Trabectome treatment.
The advantages of the Trabectome are similar to those for the trabecular microbypass stent, including avoidance of a bleb and its associated risks, the ability to use the same incisions as those created for cataract surgery, the relative simplicity of the procedure, the theoretical protection against hypotony with downstream episcleral venous resistance, and finally the preservation of conjunctiva should traditional filtration surgery be required later. Although the device shows promise, questions remain. Histologic studies have shown that the footplate of the hand piece effectively protects the outer wall of the canal and collector channel ostia from injury or trauma21, but the long-term effect of electrosurgical energy in the angle has yet to be studied effectively from a biochemical or inflammatory mediator standpoint.
The gold suprachoroidal microshunt
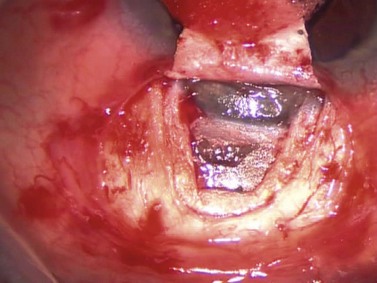
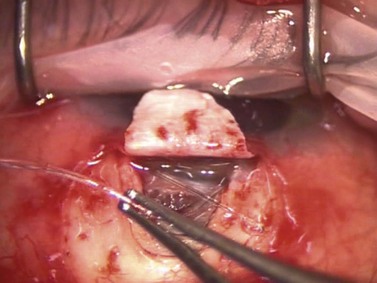
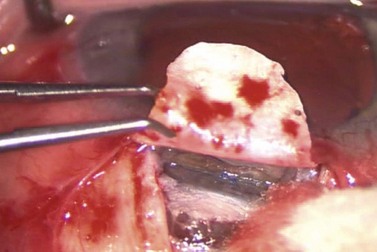
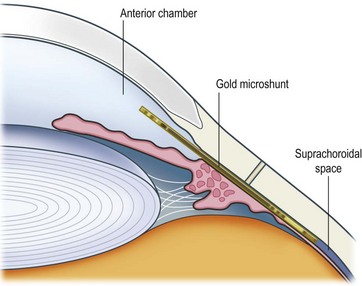
Another outflow route which may be augmented to reduce IOP is the uveoscleral pathway, which accounts for from 20–55% of aqueous outflow normally. The suprachoroidal gold microshunt is designed to augment aqueous outflow from the anterior chamber to the suprachoroidal space. The device consists of two 24-carat gold leaflets, fused together vertically to contain channels that drain aqueous. Some aqueous also flows around the device into the suprachoroidal space. The head of the device rests in the anterior chamber with its tail in the suprachoroidal space (Fig. 41.11). Versions of the device incorporate variations in lumen size. The most recent model consists of 24 µm wide by 50 µm high lumens of which nine are open and ten closed; these latter may be opened by laser. The device is 5.2 mm long, with widths varying from 2.5 mm anteriorly to 3.2 mm posteriorly.
Technique
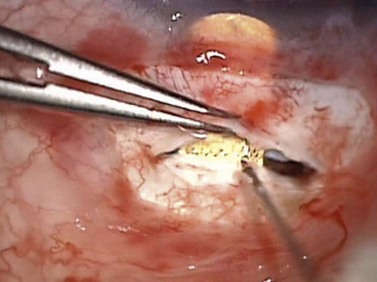
The gold shunt is gently slid into the scleral pocket until its head is seen in the anterior chamber. Because the channels within the device may be crushed with any blunt force, handling the body of the device is inadvisable. With a 27-gauge hypodermic needle on the surface of the device, it is manipulated into position, or alternatively a Sinskey hook may be used to manipulate the posterior aspect of the shunt via one of its posterior wing holes (Fig. 41.12). Proper positioning of the device requires all of its anterior openings to be visible in the anterior chamber, and with its tail and all of the posterior openings fully in the suprachoroidal space. Once satisfactorily achieved, the sclera is closed in a water-tight fashion with four to five interrupted 10-0 nylon sutures. This is followed by watertight conjunctival closure. OVD is removed from the anterior chamber with a dry technique using a blunt cannula on a syringe half-filled with balanced saline solution.
Discussion
The cyclodialysis cleft has been used to lower IOP by augmenting uveoscleral outflow, but has been abandoned due to the unpredictability of results, persistent hypotony as a significant complication, as well as often sudden and dramatic elevations in IOP late postoperatively due to cleft closure. Despite the unpredictability in the creation of a cyclodialysis cleft, several devices including hyaluronic acid implants, Teflon tube implants, hydroxyethyl methacrylate capillary strips, and scleral strips have been attempted as suprachoroidal implants to act as spacers to maintain a cyclodialysis communication22–25. A Phase III multicenter trial is in progress comparing patients who have failed filtering surgery randomized to receive either the Ahmed GDD or the gold microshunt.
Recently, data on 38 patients with uncontrolled open angle glaucoma patients, with or without prior filtering surgery, have reported a mean IOP decrease from 27.6 mmHg to 18.2 mmHg at a mean follow-up time of 11.7 months, with topical medication numbers falling from 2.0 to 1.5. Complications reported related to the device include synechia formation to the shunt, hyphema, and exudative retinal detachment26. Other shunt-related complications which may occur include shunt–cornea touch, cataract formation, shunt migration, exposure, prolonged anterior chamber inflammation, hypotony, vitreous hemorrhage, and chronic pain.
Endoscopic cycloplasty and goniosynechialysis for angle closure glaucoma
Technique
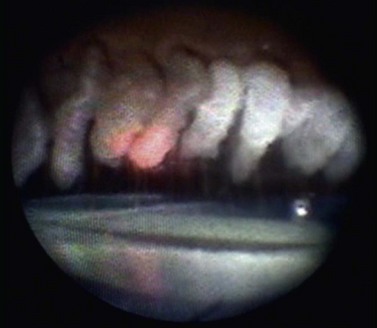
After standard phacoemulsification and intraocular lens (IOL) implantation, the ciliary sulcus is inflated with OVD. The endoscopic probe is inserted through the clear corneal cataract incision and the surgeon watches the external monitor. Once the ciliary processes are visualized, the foot pedal is depressed with the aiming beam focused on the posterior tails of the ciliary processes. This shrinks the ciliary processes posteriorly, curling them away from the posterior iris surface (Fig. 41.13). If additional IOP lowering is desired, the anterior aspect of the process may be treated as well, but only after the posterior tail has been ablated and posterior curling of the individual process observed. This is performed for approximately 270°, the approximate maximum permissible arc through a single incision.
An intraoperative gonioscopic mirror can be used to determine whether peripheral anterior synechiae (PAS) are present. If these are present, anterior segment microforceps (MST Surgical, Redmond, WA) may be used to grasp peripheral iris and release the PAS under gonioscopic visualization (Fig. 41.14). Once completed, automated irrigation and aspiration remove all residual OVD. The irrigation and aspiration hand piece may be used to grasp peripheral iris and exert gentle centripetal tension to release PAS. Care must be taken not to apply excessive centripetal force as this may result in iridodialysis, tearing of the iris root, and/or hyphema. Depending on the amount of PAS present, this may or may not be necessary. Although not well defined, if the cumulative arc degrees of open angle are greater than 180°, goniosynechialysis is unlikely to be necessary.
Discussion
While ECP has been reserved for eyes refractory to all other glaucomatous medical, laser, or surgical therapy, this technique, which we have termed ‘endoscopic cycloplasty’ (ECPL), combined with cataract extraction and IOL implantation appears to be effective in opening the angle in angle closure or angle closure glaucoma patients and may have a unique role in the treatment of these sometimes difficult to manage cases. Additionally, this technique has been observed to be successful in the treatment of angle closure secondary to plateau iris, occasionally with dramatic results (Fig. 41.15). As has been previously demonstrated, cataract extraction alone does not relieve a plateau iris configuration and these patients continue to be at risk for angle closure and angle closure glaucoma from this mechanism even when pseudophakic27.
Potential complications associated with ECP include prolonged or persistent anterior chamber inflammation, inadvertent zonular or capsular injury, inadvertent iris ablation, and hypotony. Potential complications associated with goniosynechialysis include hyphema, iridodialysis, iris tears, cyclodialysis, and Descemet’s injury and detachment. Although a significant concern with cycloablation procedures is persistent hypotony, with the performance of endoscopic cycloplasty for 270° of arc length in conjunction with cataract extraction in a series of 101 consecutive patients, data presented revealed no cases of hypotony at 6 months follow-up28.
1 Cairns J. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66(4):673-679.
2 Borisuth N, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10(2):112-116.
3 Molteno A. New implant for drainage in glaucoma: clinical trial. Br J Ophthalmol. 1969;53:606-615.
4 Coleman A, Hill R, Wilson MR, et al. Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1995;120(1):23-31.
5 Krupin T, Podos SM, Becker B, et al. Valve implants in filtering surgery. Am J Ophthalmol. 1976;81(2):232-235.
6 Lloyd M, Baerveldt G, Heuer DK, et al. Initial clinical experience with the Baerveldt implant in complicated glaucomas. Ophthalmology. 1994;101(4):640-650.
7 Wamsley S, Moster MR, Rai S, et al. Results of the use of the Ex-PRESS miniature glaucoma implant in technically challenging, advanced glaucoma cases: a clinical pilot study. Am J Ophthalmol. 2004;138(6):1049-1051.
8 Dahan E, Carmichael TR. Implantation of a miniature glaucoma device under a scleral flap. J Glaucoma. 2005;14(2):98-102.
9 Coupin A, Li Q, Riss I. Ex-PRESS miniature glaucoma implant inserted under a scleral flap in open-angle glaucoma surgery: a retrospective study. Fr J Glaucoma. 2007;30(1):18-23.
10 Maris P, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma. 2007;16:14-19.
11 de Jong L. The Ex-PRESS glaucoma shunt versus trabeculectomy in open-angle glaucoma: a prospective randomized study. Adv Ther. 2009;26(3):336-345.
12 Lewis R, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009;35(5):814-824.
13 Lewis R, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg. 2007;33(7):1217-1226.
14 Zhou J, Smedley GT. A trabecular bypass flow hypothesis. J Glaucoma. 2005;14(1):74-83.
15 Zhou J, Smedley GT. Trabecular bypass: effect of Schlemm canal and collector channel dilation. J Glaucoma.. 2006;15(5):446-455.
16 Bahler C, Smedley GT, Zhou J, et al. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthalmol. 2004;138(6):988-994.
17 Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular microbypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19(3):393-399.
18 Vandewalle E, Zeyen T, Stalmans I. The iStent trabecular microbypass stent: a case series. Bull Soc Belge Ophtalmol. 2009;311:23-29.
19 Minckler D, Baerveldt G, Alfaro MR, et al. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112:962-967.
20 Minckler D, Mosaed S, Dustin L, et alThe Trabectome Study Group. Trabectome (Trabeculotomy – internal approach): Additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:149-160.
21 Minckler D, Baerveldt G, Ramirez MA, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc. 2006;104:40-50.
22 Klemm M, Balazs A, Draeger J, et al. Experimental use of space-retaining substances with extended duration: functional and morphological results. Graefes Arch Clin Exp Ophthalmol. 1995;233(9):592-597.
23 Nesterov A, Batmanov YE, Cherkasova IN, et al. Surgical stimulation of the uveoscleral outflow: experimental studies on enucleated human eyes. Acta Ophthalmol. 1979;57(3):3.
24 Pinnas G, Boniuk M. Cyclodialysis with Teflon tube implants. Am J Ophthalmol. 1969;68(5):879-883.
25 Krejci L. Cyclodialysis with hydroxyethyl methacrylate capillary strip. Ophthalmologica. 1972;164:113-121.
26 Melamed S, Ben Simon GJ, Goldenfeld M, et al. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: a pilot study. Arch Ophthalmol. 2009;127(3):264-269.
27 Tran H, Liebmann JM, Ritch R. Iridociliary apposition in plateau iris syndrome persists after cataract extraction. Am J Ophthalmol. 2003;135(1):40-43.
28 Tam DY, Vold SD, Ahmed IK. ASCRS 2008, Endocyclophotocoagulation. In: Various Mechanisms of Glaucoma, Paper #416949.

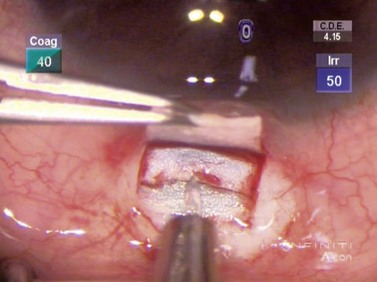
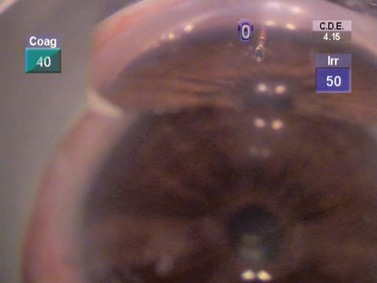
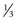 scleral thickness. A deep scleral flap is created, approximately 1 mm within the borders of the superficial flap and the depth such that approximately 100 µm of sclera remain in the bed of the dissection. As this dissection is carried forward, the glistening white fibers of the scleral spur should appear in the bed of the dissection and, immediately anterior to this, the canal of Schlemm is seen to split open. The dissection must be carried forward at a consistent depth: dissecting excessively deeply will penetrate the eye; conversely superficial dissection will pass over the canal without accessing it.
scleral thickness. A deep scleral flap is created, approximately 1 mm within the borders of the superficial flap and the depth such that approximately 100 µm of sclera remain in the bed of the dissection. As this dissection is carried forward, the glistening white fibers of the scleral spur should appear in the bed of the dissection and, immediately anterior to this, the canal of Schlemm is seen to split open. The dissection must be carried forward at a consistent depth: dissecting excessively deeply will penetrate the eye; conversely superficial dissection will pass over the canal without accessing it.