Chapter 121 Neutrophils
The Phagocytic Inflammatory Response
The phagocyte system includes both granulocytes (neutrophils, eosinophils, and basophils) and mononuclear phagocytes (monocytes and tissue macrophages). Neutrophils and mononuclear phagocytes share primary functions, including the defining properties of large particle ingestion and microbial killing. Phagocytes participate primarily in the innate immune response but also help initiate acquired immunity. Mononuclear phagocytes, including tissue macrophages and circulating monocytes, are discussed in Chapter 122.
Neutrophils provide the rapid effector arm of the innate immune system. They circulate in the bloodstream for only about 6 hr (Table 121-1), but upon encountering specific chemotactic signals, they adhere to the vascular endothelium and transmigrate into tissues, where they ingest and kill microbes and release chemotactic signals to recruit more neutrophils and to attract dendritic cells and other initiators of the acquired immune response.
| NEUTROPHILS | |
| Average time in mitosis (myeloblast to myelocyte) | 7-9 day |
| Average time in postmitosis and storage (metamyelocyte to neutrophil) | 3-7 day |
| Average half-life in the circulation | 6 hr |
| Average total body pool | 6.5 × 108 cells/kg |
| Average circulating pool | 3.2 × 108 cells/kg |
| Average marginating pool | 3.3 × 108 cells/kg |
| Average daily turnover rate | 1.8 × 108 cells/kg |
| MONONUCLEAR PHAGOCYTES | |
| Average time in mitosis | 30-48 hr |
| Average half-life in the circulation | 36-104 hr |
| Average circulating pool (monocytes) | 1.8 × 107 cells/kg |
| Average daily turnover rate | 1.8 × 109 cells/kg |
| Average survival in tissues (macrophages) | Months |
From Boxer LA: Function of neutrophils and mononuclear phagocytes. In Bennett JC, Plum F, editors: Cecil textbook of internal medicine, ed 20, Philadelphia, 1996, WB Saunders.
Hematopoiesis
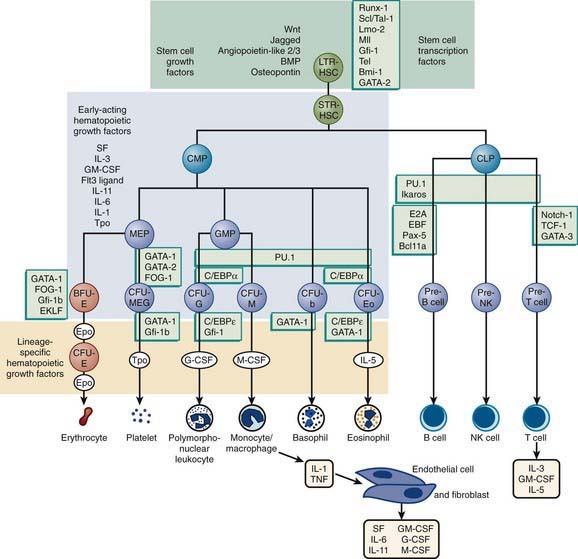
The hematopoietic progenitor system can be envisioned as a continuum of functional compartments with the most primitive compartment composed of very rare pluripotential stem cells, which have high self-renewal capacity and give rise to more mature stem cells, including cells that are committed to either lymphoid or myeloid development (Fig. 121-1). Common lymphoid progenitor cells give rise to T- and B-cell precursors and their mature progeny (Chapter 117). Common myeloid progenitor cells eventually give rise to committed single-lineage progenitors of the recognizable precursors through a random process of lineage restriction in a stepwise process (Chapter 440). The capacity of lineage-specific committed progenitors to proliferate and differentiate in response to demand provides the hematopoietic system with a remarkable range of response to changing requirements for mature blood cell production.
The proliferation, differentiation, and survival of immature hematopoietic progenitor cells are governed by hematopoietic growth factors (HGFs), a family of glycoproteins (Chapter 440). Besides regulating proliferation and differentiation of progenitors, these factors influence the survival and function of mature blood cells. During granulopoiesis and monopoiesis, multiple cytokines regulate the cells at each stage of differentiation from pluripotent stem cells to nondividing terminally differentiated cells (monocytes, neutrophils, eosinophils, and basophils). As cells mature, they lose receptors for most cytokines, especially those that influence early cell development; however, they retain receptors for cytokines that affect their mobilization and function, such as granulocyte colony–stimulating factor (G-CSF) and macrophage colony–stimulating factor (M-CSF). Mature phagocytes also express receptors for chemokines, which help direct the cells to sites of inflammation. Chemokine receptors such as CXCR4 and its ligand SDF-1 play a key role in retention of developing myeloid cells within bone marrow.
Neutrophil Maturation and Kinetics
Granulocytes survive for only 6-12 hr in the circulation, and therefore daily production of 2 × 104 granulocytes/µL of blood is required to maintain a level of circulating granulocytes of 5 × 103/µL (see Table 121-1). The relatively small peripheral blood pool includes the rapidly interchanging circulating and marginating pools; the latter provides entrance into the tissue phase, where neutrophils may survive for hours or days. The circulating pool is fed and buffered by a much larger marrow population of mature neutrophils and myeloid precursors, representing the marrow reserve and proliferating pools, respectively. Proliferation of myeloid cells, encompassing approximately 5 mitotic divisions, takes place only during the 1st 3 stages of neutrophil development, in myeloblasts, promyelocytes, and myelocytes. After the myelocyte stage, the cells terminally differentiate into nondividing, maturing metamyelocytes, bands, and neutrophils.
Neutrophil Function
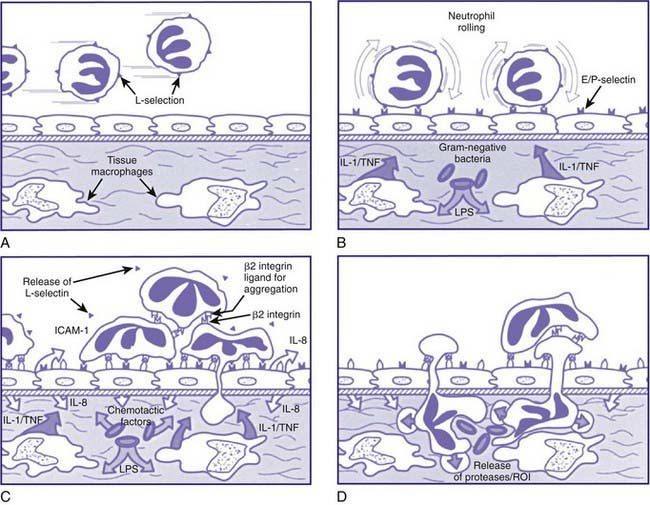
Neutrophil responses are initiated as circulating neutrophils flowing through the postcapillary venules detect low levels of chemokines and other chemotactic substances released from a site of infection. These soluble effectors of inflammation trigger subtle changes in the array and activity of surface molecules on both endothelial cells and neutrophils. The initial associations are low affinity, reversible, and mediated primarily by selectin-carbohydrate interactions. This leads to leukocyte rolling, in which loose adhesions are made and broken, causing neutrophils to move reversibly along the endothelial surface. The rolling of neutrophils allows more intense exposure of neutrophils to activating factors such as tumor necrosis factor (TNF) or IL-1 (Fig. 121-2). Neutrophil activation leads to induction of qualitative and quantitative changes in the family of β2 integrin adhesion receptors (the CD11/CD18 group of surface molecules), leading to tight heterotypic adhesion between neutrophils and endothelial cells and homotypic adhesion of neutrophils with each other. The net result of these interdependent intercellular interactions is that neutrophils flatten onto the endothelial cells, while neutrophil/neutrophil and neutrophil/platelet aggregates partially occlude the venule and reduce blood flow.
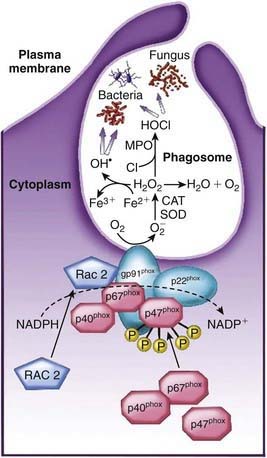
The neutrophil ingests microbes that are opsonized (prepared for ingestion) by opsonins, serum proteins such as immunoglobulin and complement component C3. The opsonins facilitate phagocytosis, in which the pathogens are engulfed into a closed vacuole, the phagosome (Fig. 121-3). As phagocytosis proceeds, two cellular responses essential for optimal microbicidal activity occur concomitantly: degranulation and activation of nicotinamide-adenine dinucleotide phosphate (NADPH)–dependent oxidase. Fusion of neutrophil granule membranes with the phagosome membrane occurs, resulting in the delivery of potent antimicrobial proteins and small peptides into the phagosome. In coordinated succession, the contents of the specific granules and then contents of the azurophil granules are secreted into the phagosome.
Occurring concomitantly are assembly and activation of NADPH oxidase at the phagosome membrane (see Fig. 121-3). This enzyme generates large amounts of superoxide (O2−) from molecular oxygen that in turn decomposes to produce hydrogen peroxide (H2O2) and singlet oxygen. H2O2 can react with O2− to form hydroxyl radicals. Myeloperoxidase, a major azurophil granule component, catalyzes the reaction of H2O2 with ubiquitously present chloride ions to create hypochlorous acid (HOCl) in the phagosome. H2O2 and HOCl are potent microbicidal agents that can directly denature proteins and nucleic acids as well as activate some of the neutrophil proteases. These events, together with the acidification of the lysosomal vacuole, break down and clear pathogens from sites of infection.
Dinauer MC, Newburger PE. The phagocyte system and disorders of granulopoiesis and granulocyte function. In: Orkin SH, Ginsburg D, Nathan DG, et al, editors. Nathan and Oski’s hematology of infancy and childhood. Philadelphia: Saunders Elsevier; 2009:1109-1217.
Eyles JL, Roberts AW, Metcalf D, et al. Granulocyte colony-stimulating factor and neutrophils—forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol. 2006;2:500-510.
Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678-689.
Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88-102.
Scapini P, Lapinet-Vera JA, Gasperini S, et al. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195-203.