Chapter 38 Neurourology
Lower Urinary Tract and Its Neurological Control
Bowel and Its Neurological Control
Sexual Function and Its Neurological Control
Neurogenic Bladder Dysfunction
Complications Arising from Neurogenic Bladder Dysfunction
Management of Neurogenic Bladder Dysfunction
Lower Urinary Tract and Its Neurological Control
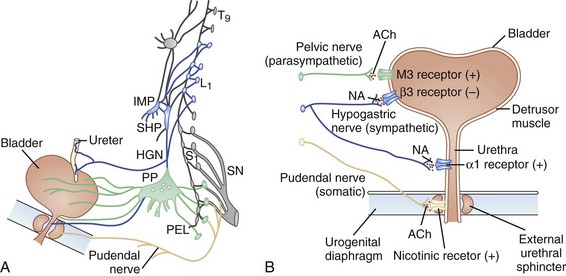
The lower urinary tract consists of the bladder and urethra and has two roles: storage of urine and voiding at appropriate times. Control of the detrusor and urethral sphincter muscles in these two mutually exclusive states is dependent upon both local spinal reflexes and central cerebral control. The pontine micturition center, which receives input from higher centers (including the periaqueductal gray of the midbrain, hypothalamus, and cortical areas such as the medial prefrontal cortex) is responsible for switching between the two states. The frequency of micturition in a person with a bladder capacity of 400 to 600 mL is once every 3 to 4 hours. Voiding takes 2 to 3 minutes, so this means that for more than 98% of life, the bladder is in a storage phase. Switching to a voiding phase is initiated by a conscious decision triggered by the perceived state of bladder fullness and an assessment of the social appropriateness of doing so. To effect both storage and voiding, connections between the pons and the sacral spinal cord must be intact, as must the peripheral innervation that originates from the most caudal segments of the cord. During the storage phase, sympathetic- and pudendal-mediated contraction of the internal and external urethral sphincters, respectively, maintains continence. Inhibition of the parasympathetic outflow prevents detrusor contractions (Fowler et al., 2008). When it is deemed appropriate to void, the pontine micturition center is no longer tonically inhibited, and reciprocal activation-inhibition of the sphincter-detrusor reverses. Relaxation of the pelvic floor and external and internal urethral sphincters, accompanied by parasympathetic-mediated detrusor contraction, results in effective bladder emptying. Intact neural circuitry between the pontine micturition center and bladder ensures coordinated activity between the detrusor and sphincter muscles. Fig. 38.1 reviews the innervation of the bladder.
Functional brain imaging studies have demonstrated that neurological control of the bladder in humans is essentially similar to that demonstrated in experimental animals. A number of positron emission tomography (PET) and, more recently, functional magnetic resonance imaging (fMRI) studies have investigated human control of urinary storage and voiding. The initial PET experiments of Blok and colleagues identified the brain centers activated during attempted micturition (Blok et al., 1997, 1998). In those able to void during the scanning, activity was shown in a region of the medioposterior pons called the M-region. In those subjects unable to void, a distinct region in the ventrolateral pontine tegmentum was activated, the L-region. Although it had been demonstrated in cats that separate pontine nuclei exist for the storage and voiding phases of bladder activity, subsequent experiments have failed to consistently demonstrate activity in this distinct L-region. In the cortex, the PET scans showed significant activity in the right inferior frontal gyrus and the right anterior cingulate gyrus during voiding that was not present during the withholding phase. Nour and associates then corroborated these findings with their own PET study of 12 healthy male volunteers, showing activity in a number of brain areas including the cerebellum (Nour et al., 2000). Other areas that show activation in fMRI during bladder filling include the anterior cingulate gyrus and right insula; fMRI has shown that the medial prefrontal cortex, responsible for complex cognitive and socially appropriate behavior, plays an important role in voiding. A recent article reviews the functional imaging studies evaluating bladder functions and their contributions to our understanding of bladder control (Fowler and Griffiths, 2010).
Bowel and Its Neurological Control
Similar to the bladder, the lower bowel also exists mostly in the storage mode. Continence is maintained by a combination of the acute anorectal angle, maintained by puborectalis contraction, and internal anal sphincter tone, determined by sympathetic activity. In health, defecation can be delayed if necessary by contraction of the external anal sphincter and pelvic floor musculature, which requires sensory feedback from the anorectum. The process of defecation involves a series of neurologically controlled actions that begin in response to the conscious sensation of a full rectum. When this is perceived and if judged to be appropriate, defecation is initiated by maneuvers to raise the intraabdominal pressure and by straining down, causing descent of the pelvic floor. The internal anal sphincter pressure falls as a result of the rectoanal inhibitory reflex, and the pubococcygeus and striated external sphincter muscles relax. Functional imaging has been applied to evaluate central processing of different types of gastrointestinal (GI) stimulation. Hobday and associates used fMRI to identify the brain centers involved in the processing of anal (somatic) and rectal (visceral) sensation in healthy adults (Hobday et al., 2001). Rectal stimulation produced activation of somatosensory cortex, insula, anterior cingulate, and prefrontal cortex (PFC); anal canal stimulation produced similar regions of activity, although anterior cingulate activity was absent, and the primary somatosensory activation was slightly more superior in location. The activation of cingulate cortex with rectal stimulation may signify the function of the limbic system in the processing of visceral stimuli.
The processing of rectal sensation is relevant in bladder function because unlike other gut organs, it has an important sensory role, and the rectum is a visceral organ that contains both unmyelinated C fibers and thinly myelinated Aδ afferents. In contrast, the anal canal has somatic innervation from the pudendal nerve. The study by Hobday and associates has highlighted the differences in its cortical representation from that of the rectum. The various brain imaging studies of visceral stimulation, including the foregoing report, have been reviewed (Derbyshire, 2003). When different visceral stimuli—esophageal distension, esophageal pain, rectal distension, or the mechanisms operative in irritable bowel syndrome—were analyzed, esophageal stimulation was found to activate the insula most consistently, with other commonly involved areas including somatosensory and motor cortices. Considerable variation was observed in whether the periaqueductal gray was activated or not. Lower GI stimuli predominantly activated the prefrontal and orbitofrontal cortices as well as the insula, with variability in cingulate activation. Overall, esophageal stimulation involved a more central sensory and motor neural circuit, whereas lower GI stimulation activated areas with projections to autonomic and affective control centers such as the brainstem and amygdala.
Sexual Function and Its Neurological Control
Much remains to be discovered about cortical control of sexual function. Although it is thought that cerebral processing determines libido and desire, the ability to effect a sexual response is determined by spinal autonomic reflexes. Libido is hormone dependent with a major hypothalamic component, and loss of libido may be the earliest symptom of a pituitary tumor. In experimental animals, the deep anterior midline structures that form the limbic system have been shown to be important for sexual responses, and the medial preoptic–anterior hypothalamic area has an integrating function (Andersson, 2001). Functional brain imaging experiments including five PET studies and seven fMRI studies have highlighted the key areas of brain activity associated with sexual functioning—for example, the role of the hypothalamus in reproductive function, regulation of human sexuality, and regulation of erection through medial preoptic area, or the roles of the insula and claustrum in autonomic regulation and visceral sensory processing. The aspects of sexual function covered include penile sexual stimulation (Arnow et al., 2002; Georgiadis and Holstege, 2005), male ejaculation (Holstege et al., 2003), and visual sexual stimuli (Karama et al., 2002).
Neurogenic Bladder Dysfunction
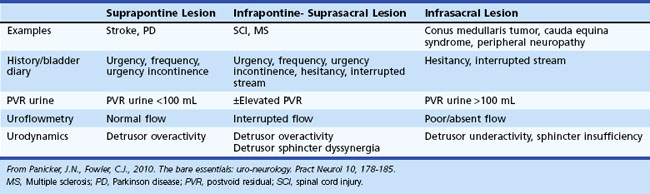
Lesions of the nervous system, central or peripheral, can result in characteristic patterns of bladder dysfunction depending upon the level of the lesions in the neurological axis (Panicker et al., 2010). The storage function of the bladder is affected following suprapontine or infrapontine/suprasacral lesions. This results in involuntary spontaneous or induced contractions of the detrusor muscle (detrusor overactivity), which can be identified during the filling phase of urodynamics. The voiding function of the bladder can be affected by infrapontine lesions. Following spinal cord damage, there is simultaneous contraction of the external urethral sphincter and detrusor muscle, detrusor-sphincter dyssynergia, which results in incomplete bladder emptying and abnormally high bladder pressures. Following lesions of the conus medullaris or cauda equina, voiding dysfunction can be due to poorly sustained detrusor contractions and possibly non-relaxing urethral sphincters (Table 38.1).
Cortical Lesions
Bladder Dysfunction
It has been known since the 1960s that anterior regions of the frontal lobes are critical for bladder control. Among patients with disturbed bladder control, various frontal lobe disturbances have been reported: intracranial tumors, damage after rupture of an aneurysm, penetrating brain wounds, and prefrontal lobotomy (leukotomy). The typical clinical picture of frontal lobe incontinence is a patient with severe urgency and frequency of micturition and urge incontinence but without dementia; the patient is socially aware and embarrassed by the incontinence. Micturition is normally coordinated, indicating that the disturbance is in the higher control of these processes. Urinary retention also has been described in patients with brain lesions. A small number of case histories have described patients with right frontal lobe disorders who had urinary retention and in whom voiding was restored when the frontal lobe disorder was treated successfully (Fowler, 1999).
Urinary incontinence develops in some patients after stroke. Urodynamic studies in incontinent patients have been carried out, and the general conclusion drawn from studying patients with disparate cortical lesions is that voiding mostly is normally coordinated. The most common cystometric finding is that of detrusor overactivity. It has not been possible to demonstrate a correlation between any particular lesion site and urodynamic findings. Urinary incontinence at 7 days following stroke predicts poor survival, disability, and institutionalization independent of level of consciousness. It has been suggested that incontinence in such cases is the result of severe general loss of function, or that persons who became incontinent may be less motivated to recover both continence and general function. Patients with hemorrhagic stroke are more likely to have detrusor underactivity in urodynamics compared to patients with ischemic stroke, who more often have detrusor overactivity (Han et al., 2010). Small-vessel disease of the white matter (leukoaraiosis) is associated with urgency incontinence, and it is increasingly becoming apparent that this is an important cause for incontinence in the functionally independent elderly (Tadic et al., 2010).
The cause of urinary incontinence in dementia is probably multifactorial. Not all incontinent older adults are cognitively impaired, and not all cognitively impaired older adults are incontinent. In a study of patients with progressive cognitive decline, incontinence was observed to occur in more advanced stages of Alzheimer disease (AD), whereas it could occur earlier on in the course of patients with dementia with Lewy bodies (DLB) (Ransmayr et al., 2008).
Sexual Dysfunction
The role of hormonal dysfunction has yet to be fully determined. On the basis of measurements of sex hormones and pituitary function, it has been suggested that the hyposexuality of TLE results from a subclinical hypogonadotropic hypogonadism, and that dysfunction of medial temporal lobe structures may dysmodulate hypothalamopituitary secretion (Murialdo et al., 1995). Erectile dysfunction (ED) with preservation of libido can occur in men with temporal lobe damage with or without epilepsy and may be characterized by loss of nocturnal penile tumescence. Surgery for epilepsy rarely restores erectile function, although a survey of operated patients showed a higher level of satisfaction with sexual function among those who were free of seizures. Sexual dysfunction is common after head injury, particularly in patients who demonstrate cognitive damage. Hypersexual behavior may occur after frontal lobe damage. Lesions of the frontal lobes, the basal-medial part in particular, may lead to loss of social control, which also may affect sexual behavior.
Basal Ganglia Lesions
Bladder Dysfunction
Bladder symptoms in Parkinson disease (PD) correlate with neurological disability (Araki and Kuno, 2000) and stage of disease; both findings appear to support a link between dopaminergic degeneration and symptoms of urinary dysfunction. In line with current thinking about staging of PD in terms of underlying neuropathology (Braak et al., 2004), it appears that bladder dysfunction does not occur until some years after the onset of motor symptoms, and the dysfunction is correlated with the extent of dopamine depletion (Sakakibara et al., 2001b). This means that the underlying pathological process is likely to have extended into the neocortex and explains why the clinical context in which bladder dysfunction is seen in PD is common in patients with cerebral symptoms, as well as with adverse effects of long-standing treatment with dopaminergic agents.
The most frequent complaints are of nighttime frequency, urgency, and difficulty voiding (Sakakibara et al., 2001a), and the most common abnormality in urodynamic studies is detrusor overactivity (Araki et al., 2000). Of the several possible explanations for this finding, the hypothesis most widely accepted is that in healthy persons, the basal ganglia has an inhibitory effect on the micturition reflex, and with neuronal loss in the substantia nigra, detrusor overactivity develops. Studies on anesthetized cats demonstrated that rhythmic bladder contractions were inhibited by intracerebroventricular administration of a dopamine D1 receptor agonist but were not affected by a D2 receptor agonist. From this evidence, it was concluded that the D1 receptor provides the main inhibitory influence on the micturition reflex (Yoshimura et al., 2003). Clinical studies that have looked at the effect of l-dopa or apomorphine on bladder behavior in patients with PD have, however, produced conflicting results. In patients demonstrating the on/off phenomenon, cystometry done after taking l-dopa showed improvement of overactivity in some and worsening in others. The unpredictable effect of antiparkinsonian medications on urinary symptoms has not been definitively shown to correlate with age or stage of disease (Winge and Fowler, 2006). Many patients with PD have nocturnal polyuria as well, which contributes to bladder symptoms and would not be expected to improve with antimuscarinics. In addition to neurogenic bladder dysfunction, benign prostatic obstruction may occur concomitantly in some men with PD and contribute to bladder dysfunction. A recent study suggests that contrary to previous teaching, transurethral prostate resection may be successful in carefully selected PD men and is associated with minimal risk of incontinence (Roth et al., 2009).
In a patient with severe urinary symptoms but mild parkinsonism, a diagnosis of multiple system atrophy (MSA) should be considered. The onset of urogenital symptoms in MSA may precede overt neurological involvement; ED and bladder symptoms begin on average 4 to 5 years before diagnosis and 2 years before more specific neurological symptoms appear. The neuronal degeneration of MSA affects the central nervous system (CNS) at several locations that are important for bladder control, which probably explains why urinary complaints occur so early and are so severe in this condition. It is thought that detrusor overactivity is caused by neuronal loss in the pontine region, whereas incomplete bladder emptying is caused by loss of parasympathetic innervation of the detrusor after neuronal degeneration in the intermediolateral cell columns of the spinal cord. In addition, anterior horn cell loss in the Onuf nucleus results in denervation of the urethral sphincter so that the patient has a combination of detrusor overactivity, incomplete bladder emptying, and a weak sphincter. Bladder dysfunction may change during the progression of MSA, and serial studies have shown that the mean postmicturition residual volume increases as the condition progresses (Ito et al., 2006). Bladder symptoms in other parkinsonian syndromes are less prominent than in MSA, and although they may occur as part of the patient’s general disability, they rarely are as severe as in MSA and do not occur at a stage of the disease when a neurological cause is not evident.
Bowel Dysfunction
Constipation is now thought to be a preclinical manifestation of PD, and a symptom questionnaire showed that this was considered to be the most bothersome nonmotor symptom for PD patients (Sakakibara et al., 2001a). Several possible causes for constipation are recognized: a slow colonic transit time has been demonstrated in a number of studies; this finding may be secondary to a reduction in dopaminergic myenteric neurons. An abnormality of the defecation process has also been demonstrated in some patients with PD, with paradoxical contraction of the external anal sphincter and pubococcygeus causing outlet obstruction. This phenomenon can result in anismus and is thought to be a form of focal dystonia. Bowel dysfunction appears earlier and progresses faster in patients with MSA than in those with PD (Stocchi et al., 2000).
Sexual Dysfunction
Experimental evidence from animals and humans shows that dopaminergic mechanisms are involved in determining libido and inducing penile erection. In animal studies, the medial preoptic area of the hypothalamus has been shown to regulate sexual drive, and selective stimulation of dopamine D2 receptors in this region increases sexual activity in rats (Andersson, 2001). An increase in libido in some patients with PD treated with dopamine agonists and l-dopa as part of the “hedonistic homeostatic dysregulation” syndrome (Giovannoni et al., 2000) is a well-recognized phenomenon, although its incidence is uncertain. The cause of ED in PD is unclear, but it is a significant problem and in one study was shown to affect 60% of a group of men with PD, compared with 37.5% of age-matched healthy men. ED usually affects men later in the course of PD, with onset years after the diagnosis of neurological disease has been established. A survey of relatively young patients with PD (mean age, 49.6 years) and their partners revealed a high level of sexual dysfunction, with the most severely affected couples being those in which the patient was male.
ED may be the first symptom in men with MSA, predating the onset of any other neurological symptoms by several years. The disorder appears to be chronologically distinct from the development of postural hypotension. The reason for the apparently early selective involvement of neural mechanisms for erection is not known. Preserved erectile function in a man with parkinsonism strongly contradicts the diagnosis of MSA. The available literature on female sexual problems in movement disorders is limited (Jacobs et al., 2000; Oertel et al., 2003).
Brainstem Lesions
Voiding difficulty is a rare but recognized symptom of a posterior fossa tumor and has been reported in series of patients with brainstem disorders (Fowler, 1999). In an analysis of urinary symptoms of 39 patients who had had brainstem strokes, lesions that resulted in micturition disturbance usually were dorsally situated (Sakakibara et al., 1996)—a finding consistent with the known location of the brainstem centers involved in bladder control. The proximity in the dorsal pons between the pontine micturition center and medial longitudinal fasciculus means that a disorder of eye movements, such as an internuclear ophthalmoplegia (INO), is highly likely in patients with a pontine disorder causing a voiding difficulty.
Multiple Sclerosis
The pathophysiological consequences of progressive MS affecting the spinal cord for the bladder are similar to those of SCI, but the medical context of increasing disability is such that management must be quite different. Estimates of the proportion of patients with MS who have lower urinary tract symptoms vary according to the severity of the neurological disability in the group under study, but a figure of about 75% is frequently cited (Marrie et al., 2007). Several studies have shown that urinary incontinence is considered to be one of the worst aspects of the disease; 70% of a self-selected group of patients with MS responding to a questionnaire classified the impact bladder symptoms had on their life as “high” or “moderate” (Hemmett et al., 2004). A strong association between bladder symptoms and the presence of clinical spinal cord involvement, including paraparesis and UMN signs, has been recognized on examination of the lower limbs in patients with MS. This observation has also been made in patients with a similar condition, acute disseminated encephalomyelitis (ADEM) (Panicker et al., 2009).
As the neurological condition progresses, bladder dysfunction may become more difficult to treat. However, unlike with the bladder dysfunction that follows SCI, progressive neurological diseases such as MS rarely result in upper urinary tract involvement. This is the case even when long-standing MS has resulted in severe disability and spasticity. The reason for this sparing of the upper urinary tract is not known, but it means that in such patients, management should emphasize symptomatic relief (Fowler, 1999).
A particular problem in MS is that neurological symptoms may deteriorate acutely when the patient has an infection and pyrexia, including urinary tract infection (UTI). As MS progresses, recurrent infections are likely to result in deficits that accumulate and lead to progressive neurological deterioration (Buljevac et al., 2002).
Bowel Dysfunction
Half of all patients need help with bowel management. A questionnaire survey of patients with SCI found that bowel dysfunction was a major problem, rated as only slightly less serious than loss of mobility (Glickman and Kamm, 1996). Bowel management may be equally problematic for patients with progressive spinal cord disease such as MS, with prevalence rates for bowel dysfunction reported at 30% to 50% (DasGupta and Fowler, 2003). The loss of rectal sensation and the normal urge to defecate means that bowel emptying must be induced at a convenient time by digital anal stimulation, use of suppositories or enemas, or manual evacuation. The consequence of losing the ability to postpone bowel emptying because of impaired sensation of impending defecation and inability to voluntarily contract the anal sphincter is that fecal incontinence is common.
Sexual Dysfunction
Male Sexual Dysfunction
The level and completeness of a spinal cord lesion determine erectile and ejaculatory capability after SCI (Bors and Comarr, 1960). With a complete cervical lesion, psychogenic erections are lost, but the capacity for spontaneous or reflex erections may be intact. With low spinal cord lesions, particularly if the cauda equina is involved, little or no erectile capacity may be retained. Theoretically, a lesion below spinal level L2 leaves psychogenic erections intact, but in practice it is uncommon for men with such a lesion to have erections adequate for intercourse. Psychogenic erections are more likely to be preserved in incomplete lesions. Preservation of ejaculation function after a spinal cord lesion is unusual (Sipski, Alexander, and Gómez-Marín, 2006). Although earlier studies indicated a much lower figure, it is now known that 60% to 65% of men with MS have ED, often coexisting with urinary symptoms, with urodynamically demonstrable overactivity in a majority of those affected. Typically, in the early stages of MS, the chief complaint is difficulty sustaining an erection for intercourse. With advancing neurological disability, erectile function may cease, and difficulty with ejaculation may develop. A study of pudendal evoked potentials in men with MS found that those with severely delayed latencies (i.e., with more severe spinal cord disease) were more likely to be unable to ejaculate (Betts et al., 1994). Though it has been said that a diagnosis of MS should be considered in a young man presenting with impotence, this possibility seems unlikely in the absence of clinical spinal cord disease. In one series, only a single patient had erectile difficulties at the time of the first symptoms of MS, and neurological disease did not develop subsequently in any of the men who presented with ED.
Female Sexual Dysfunction
Studies of women with SCI at different levels, both complete and incomplete, have advanced current understanding of the neural pathways involved in female sexual response. It has been hypothesized that the sensory experience of orgasm may have an autonomic basis because orgasmic capacity is preserved in a proportion of affected women, particularly with higher cord lesions (Sipski et al., 2001); fMRI studies in SCI patients suggest preservation of vagal pathways. Sexual dysfunction in women with MS is common, affecting 50% to 60%, although probably underdiagnosed, with the incidence increasing with worsening disability. Neurogenic problems during intercourse include decreased lubrication and reduced orgasmic capacity. A double-blind, randomized, placebo-controlled crossover study of sildenafil citrate in treating females with sexual dysfunction due to MS showed no overall benefit with the drug, shown to be far more effective in men with MS (DasGupta et al., 2004). In women with advanced disease, additional problems may include lower limb spasticity, loss of pelvic sensation, genital dysesthesia, and fear of incontinence (Hulter and Lundberg, 1995).
Conus and Cauda Equina Lesions
The cauda equina contains the sacral parasympathetic outflow together with the somatic efferent and afferent fibers. Damage to the cauda equina leaves the detrusor decentralized rather than denervated because the postganglionic parasympathetic innervation is unaffected. This distinction may explain why bladder dysfunction after a cauda equina lesion is unpredictable and why even detrusor overactivity has been described (Podnar and Fowler, 2010).
Inability to evacuate the bowel may be a severe problem, and manual evacuation may be necessary for the long term. Additional denervation of the anal sphincter can result in incontinence of flatus and feces. Damage to the cauda equina results in sensory loss, and both men and women complain of perineal sensory loss and loss of erotic genital sensation, for which no effective treatment is available. In men, ED is also a complaint (Podnar et al., 2006).
For most patients, contact with other persons similarly affected may prove supportive.
Disturbances of Peripheral Innervation
Diabetic Neuropathy
Bladder involvement once was considered an uncommon complication of diabetes, but the increase in use of techniques for studying bladder function has shown that such involvement is common, although often asymptomatic. Bladder dysfunction does not occur in isolation, and other symptoms and signs of generalized neuropathy are necessarily present in affected patients. The onset of the bladder dysfunction is insidious, with progressive loss of bladder sensation and impairment of bladder emptying over years, eventually culminating in chronic low-pressure urinary retention (Hill et al., 2008). Urodynamic studies demonstrate impaired detrusor contractility, reduced urine flow, increased postmicturition residual volume, and reduced bladder sensation. It seems likely that vesical afferent and efferent fibers are involved, causing reduced awareness of bladder filling and decreased bladder contractility.
Amyloid Neuropathy
Autonomic manifestations are common in amyloid neuropathy and include ED, orthostatic hypotension, bladder dysfunction, distal anhidrosis and abnormal pupils. Lower urinary tract symptoms generally appear early on and are present in 50% of patients within the first 3 years of the disease. Patients most often complain of difficulty in bladder emptying and incontinence (Andrade, 2009). Often, however, bladder dysfunction may be asymptomatic and uncovered only during investigations. Urodynamic studies have demonstrated reduced bladder sensations, underactive detrusor, poor urinary flow, and opening of the bladder neck. Bladder wall thickening may be seen on ultrasound scan. Some 10% of patients with familial amyloidotic polyneuropathy (FAP) type I may proceed to end-stage renal disease (Lobato, 2003) and may complain of polyuria. Urinary incontinence has been shown to be associated with higher post–liver transplant mortality (Adams et al., 2000).
Autoimmune Autonomic Ganglionopathy
Patients with autoimmune autonomic ganglionopathy present with rapid onset of severe autonomic failure, with orthostatic hypotension, GI dysmotility, anhidrosis, bladder dysfunction, ED, and sicca symptoms and may have ganglionic acetylcholine receptor (AChR) antibodies. Bladder dysfunction generally manifests with voiding difficulty and incomplete emptying. Severity and distribution of autonomic dysfunction appear to depend upon the level of antibody titers (Gibbons and Freeman, 2009).
Pure Autonomic Failure
Pure autonomic failure (PAF) is a degenerative postganglionic autonomic disorder. There is now evidence to suggest that the underlying basis is a synucleinopathy, with Lewy bodies confined primarily to the autonomic ganglia neurons. Nocturia and voiding dysfunction are common, and bladder emptying is often affected. Bladder dysfunction in PAF appears to be as common but less severe than in MSA, and this could possibly reflect slower progression of the disease (Sakakibara et al., 2000). ED and constipation are common.
Diagnostic Evaluation
History
History forms the cornerstone for evaluation and should address both storage and voiding dysfunction. Patients with storage dysfunction complain of frequency for micturition, nocturia, urgency, and urgency incontinence. Urgency, frequency, and nocturia, with or without incontinence, is called overactive bladder syndrome, urge syndrome, or urgency-frequency syndrome (Abrams et al., 2002). Patients experiencing voiding dysfunction report hesitancy for micturition, a slow and interrupted urinary stream, the need to strain to pass urine, and double voiding. Patients may be in complete urinary retention. The history of voiding dysfunction is often unreliable, and patients may be unaware of incomplete bladder emptying. Therefore, the history should be supplemented by a bladder scan (see later discussion).
Bladder Diary
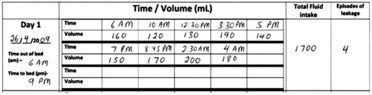
The bladder diary supplements history taking and records the frequency for micturition, volumes voided, episodes of incontinence, and fluid intake over the course of a few days (Fig. 38.2).
Investigations
Screening for Urinary Tract Infections
It is advisable for patients presenting with bladder symptoms to be screened for UTIs. Combined rapid tests of urine using reagent strips (“dipstick” test) have a negative productive value of 98% for excluding UTI. However, the positive predictive value for confirming infection is only 50% (Fowlis et al., 1994), and hence if abnormal, a urine sample should be sent off to the lab for culture.
Bladder Scan
Because the extent of incomplete bladder emptying cannot be predicted from history or clinical examination, it is pertinent to estimate the postvoid residual urine by ultrasonography. This is most commonly carried out using a portable bladder scanner (Fig. 38.3), or by “in-out” catheterization, especially in patients who perform intermittent self-catheterization. It is recognized that a single measurement of a postvoid residual is often not representative; if possible, a series of measurements should be made over the course of 1 or 2 weeks.
Urodynamic Studies
Noninvasive Bladder Investigations
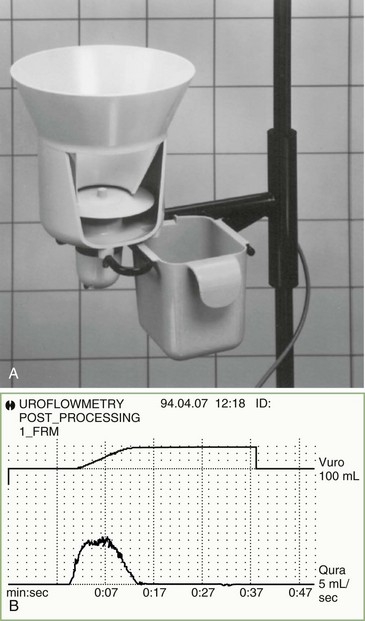
Uroflowmetry is a valuable noninvasive investigation, particularly when combined with an ultrasound measurement of the postvoid residual volume. A commonly used design for a flow meter consists of a commode or urinal into which the patient passes urine as naturally as possible. In the base of the collecting system is a spinning disc, and flow of urine onto this disc tends to slow its speed of rotation, which a servomotor holds constant. The urinary flow is calculated based upon the power necessary to maintain the rotation speed. A graphic printout of the urinary flow is obtained, and time taken to reach maximum flow, maximum and average flow rates, and voided volume are analyzed (Fig. 38.4). It is important that the patient performs the test with a comfortably full bladder containing, if possible, a volume of at least 150 mL; privacy is essential insofar as a spurious result may be obtained if the individual is not fully relaxed.
Investigations Requiring Catheterization
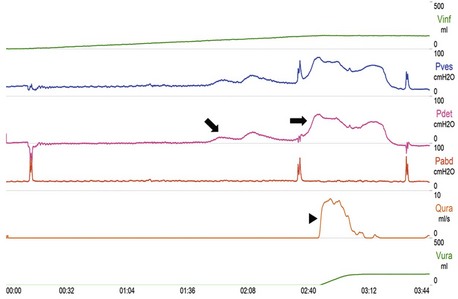
Cystometry evaluates the pressure/volume relationship during nonphysiological filling of the bladder and during voiding. The detrusor pressure is derived by subtraction of the abdominal pressure (measured using a catheter in the rectum) from the intravesical pressure (measured using a catheter in the bladder). The rate of filling is recorded by the machine, which pumps sterile water or saline through the catheter in the bladder. For speed and convenience, most laboratories use filling rates of between 50 and 100 mL/min. This nonphysiological rapid filling does mean that the full bladder capacity can be reached usually within 7 or 8 minutes. The first sensation of bladder filling may be reported at around 100 mL, and full capacity is reached between 400 and 600 mL. In healthy persons, the bladder expands to contain this amount of fluid without an increase of pressure more than 15 cm H2O. A bladder that behaves in this way is said to be “stable.” The main abnormality sought during filling cystometry in patients with a neurological disease is the presence of detrusor overactivity (Fig. 38.5). This is a urodynamic observation characterized by involuntary detrusor contractions that may be spontaneous or provoked. It should be emphasized that on urodynamics, detrusor overactivity of neurogenic origin is indistinguishable from other causes for detrusor overactivity. When bladder filling has been completed, the patient voids into the flow meter, with the bladder and rectal lines still in place. Valuable information can be obtained by measuring detrusor pressure and urine flow simultaneously.
Whether it is necessary to perform a complete urodynamic study in all patients with a suspected neurogenic bladder is a subject of debate. Patients with spinal cord injury, spina bifida, and possibly advanced MS should undergo urodynamic studies because of the higher risk for upper tract involvement and renal impairment, although ultrasound is a less invasive method for monitoring. Guidelines underlying the key role of urodynamics for baseline evaluation, management, and follow-up of a neurogenic bladder in these patient groups have been published. However, in other conditions such as early MS, stroke, and PD, some authors have recommended restricting the initial evaluation to noninvasive tests, on the basis that the risk for upper urinary tract damage is less (Fowler et al., 2009). In the absence of evidence-based medical data comparing these two models of management, the decision for performing complete baseline urodynamics would depend upon local resources and recommendations.
Electromyography
Sphincter Electromyography in the Evaluation of Suspected Cauda Equina Lesions
Lesions of the cauda equina are an important cause for pelvic floor dysfunction. Most often, EMG of the external anal sphincter demonstrating changes of chronic reinnervation with a reduced interference pattern and enlarged polyphasic motor units (>1 mV amplitude) can be found in patients with long-standing cauda equina syndrome (Podnar et al., 2006). Though EMG may demonstrate pathological spontaneous activity 3 weeks or more after injury, these changes of moderate to severe partial denervation or complete denervation often become lost in the tonically firing motor units of the sphincter.
Sphincter Electromyography in the Diagnosis of Multiple System Atrophy
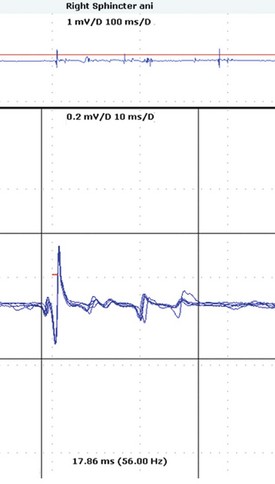
Neuropathological studies have shown that anterior horn cells in the Onuf nucleus are selectively lost in MSA, and this results in changes in the sphincter muscles that can be identified by EMG. The anal sphincter is once again most often studied, and compared to the changes of chronic reinnervation in patients with cauda equina syndrome described earlier, the changes of reinnervation in MSA tend to result in prolonged-duration motor units, presumably because the progressive nature of that disease precludes motor unit “compaction.” These changes can be detected easily, but it is important to include measurement of the late components of the potentials (Fig. 38.6).
Although the value of sphincter EMG in the differential diagnosis of parkinsonism has been widely debated, a body of opinion exists that maintains that a highly abnormal result in a patient with mild parkinsonism is of value in establishing a diagnosis of probable MSA (Vodusek, 2001). This correlation is important not only for the neurologist but also for the urologist because inappropriate surgery for a suspected prostate enlargement as the cause for bladder troubles can then be avoided.
Management of Neurogenic Bladder Dysfunction
The pattern of lower urinary tract symptoms and findings from relevant bedside investigations such as uroflowmetry and bladder scan are useful in the localization of neurological lesions (see Table 38.1). Variations from these expected patterns of symptoms and findings should warrant a search for additional urological conditions that may be occurring concomitantly and alter the pattern of lower tract dysfunction.
The goals one would wish to achieve when managing neurogenic bladder dysfunction are urinary continence, preventing UTIs, and preserving upper urinary tract function (Stohrer et al., 2009). Attaining these goals would help improve quality of life of patients with neurological disease. The management of neurogenic bladder dysfunction must address both voiding and storage dysfunction.
General Measures
Nonpharmacological measures are generally effective in the early stages when symptoms are mild. A fluid intake of around 1 to 2 L a day is suggested, although this should be individualized; it is often helpful to assess fluid balance by means of a bladder diary (Hashim and Abrams, 2008). Caffeine reduction may reduce urgency and frequency, especially for patients who drink coffee or tea in excess. Bladder retraining, whereby patients void by the clock and voluntarily “hold on” for increasingly longer periods, aims to restore the normal pattern of micturition. If voiding dysfunction has been excluded, pelvic floor exercises and neuromuscular stimulation may play a role in ameliorating overactive bladder symptoms.
Voiding Dysfunction
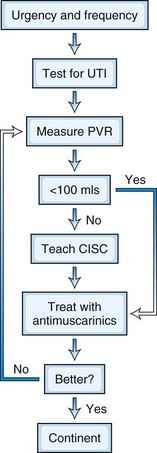
Knowledge of a patient’s postvoid residual volume is critical in planning treatment of bladder symptoms. There is no consensus regarding the figure of residual volume at which intermittent self-catheterization should be initiated. However, in general, because patients with a neurogenic bladder have reduced bladder capacity, a volume of more than 100 mL or more than one-third of bladder capacity is taken as the amount of residual urine that contributes to bladder dysfunction (Fowler et al., 2009). The widespread use of intermittent self-catheterization has greatly improved management of neurogenic bladder dysfunction. Incomplete emptying can exacerbate detrusor overactivity, and an overactive bladder constantly stimulated by a residual volume responds by contracting and producing symptoms of urgency and frequency, thus making antimuscarinic medications less effective. Sterile intermittent catheterization was first introduced in the 1960s, but subsequently a clean rather than sterile technique was found to be adequate. Intermittent catheterization is best performed by patients themselves, who should be taught by someone experienced with this method, such as nurse continence advisors. Neurological lesions affecting manual dexterity, weakness, tremor, rigidity, spasticity, impaired visual acuity, and cognitive impairment may make it impossible for the patient to self-catheterize, in which case it may be performed by the partner or care assistant. The incidence of symptomatic UTIs is low when performed regularly.
Reflex voiding using trigger techniques and the Credé maneuver (nonforceful, smooth, even pressure applied from the umbilicus toward the pubis) are usually not recommended, as they may result in high detrusor pressure and incomplete bladder emptying during voiding (Fowler et al., 2009). Suprapubic vibration using a mechanical “buzzer” has been demonstrated to be effective in patients with MS with incomplete bladder emptying and detrusor overactivity, but its effect is limited (Prasad et al., 2003). Alpha-blockers relax the internal urethral sphincter in men, and there is evidence that they improve bladder emptying and reduce postvoid residual volumes (O’Riordan et al., 1995). However, this is not consistently seen in clinical practice unless there is concomitant bladder outlet obstruction. Botulinum toxin injections into the external urethral sphincter may improve bladder emptying in patients with SCI who have significant voiding dysfunction (Naumann et al., 2008).
Storage Dysfunction
Antimuscarinic Medications
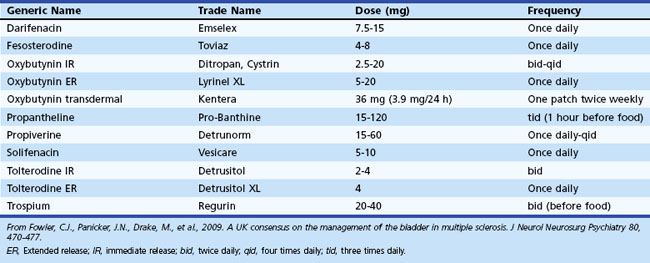
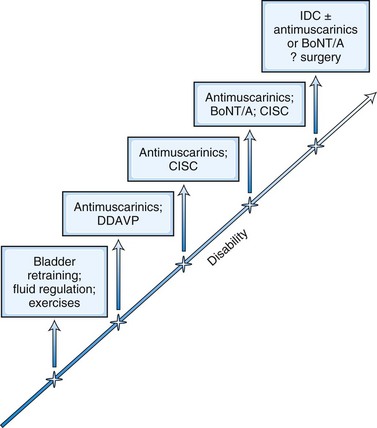
Detrusor overactivity is a major cause for incontinence in patients with neurogenic bladder disorders. The sensation of urgency is experienced as the detrusor muscle begins to contract, and if the pressure continues to rise, the patient senses impending micturition. Antimuscarinic medications are the mainstay of treatment for detrusor overactivity. Table 38.2 lists the medications available in the United Kingdom. Oxybutynin was one of the earlier drugs introduced, and subsequently several newer agents have been marketed. Meta-analyses suggest that efficacy is similar between these medications. Adverse events arise owing to their nonspecific anticholinergic action and include dry mouth, blurred vision for near objects, tachycardia, and constipation. These drugs can also block central muscarinic M1 receptors and cause impairment of cognition and consciousness in susceptible individuals. This may be mitigated by medications that have low selectivity for the M1 receptor, such as darifenacin, or restricted permeability across the blood-brain barrier (BBB), such as trospium. The postvoid residual urine may increase following treatment, and it should be monitored by repeat bladder scans, especially if initial beneficial effects are short lasting. In many patients, there may also be underlying voiding dysfunction, and the judicious use of antimuscarinic medication coupled with clean intermittent self-catheterization (CISC) often proves most effective for managing neurogenic bladder dysfunction (Fowler et al., 2009) (Fig. 38.7).
Desmopressin
Desmopressin, a synthetic analog of arginine vasopressin, temporarily reduces urine production and volume-determined detrusor overactivity by promoting water reabsorption at the distal and collecting tubules of the kidney. It is useful for treating urinary frequency or nocturia in patients with MS, providing symptom relief for up to 6 hours (Bosma et al., 2005). It is also helpful in managing nocturnal polyuria, characterized by increased production of urine in the night. This may be seen in patients with PD and also various neurological conditions associated with dysautonomia and orthostatic hypotension. However, it should be prescribed with caution in patients over the age of 65 or with dependent leg edema, and it should not be used more than once in 24 hours for fear of hyponatremia or congestive heart failure.
Cannabinoids
Positive anecdotal reports on the effect of cannabis in controlling bladder symptoms in MS patients and identification of the cannabinoid receptor in animal and human studies led to studies of cannabis-based medicinal extracts in this group. An open-label study in patients with advanced MS produced promising results, with diminished frequency, nocturia, and incontinence (Freeman et al., 2006). A license was recently granted in the United Kingdom for the use of a cannabis-based medicine to treat spasticity in MS, but this does not cover bladder dysfunction.
Botulinum Toxin
Botulinum toxin type A injected into the detrusor muscle under cystoscopic guidance appears to be a highly promising, although at the time of writing an unlicensed treatment, for intractable detrusor overactivity. The effect lasts 9 to 13 months and significantly improves storage symptoms and quality of life (Kalsi et al., 2007), though patients often have to perform CISC afterwards. Studies also suggest that patients receiving botulinum toxin injections have fewer UTIs and reduced catheter bypassing (urethral leakage when using an indwelling catheter). Botulinum toxin was introduced on the theoretical basis that it blocks synaptic release of acetylcholine from the parasympathetic nerve endings and produces a paralysis of detrusor muscle (and indeed a demonstrable increase in bladder capacity has been reported in the various studies). However, accumulating evidence indicates that the mechanism of action is more complex and may actually involve the afferent innervation as well.
Surgery
Various urological surgeries can be carried out to treat incontinence and are summarized in Table 38.3. A surgical procedure to rectify a disorder causing incontinence in an otherwise fit and healthy person often is highly successful. Even after SCI, a surgical option might be the best solution for long-term bladder management. This, however, does not apply to patients with progressive neurological disease causing incontinence. For example, at a time when the bladder is becoming unmanageable by intermittent catheterization and an antimuscarinic medication, the patient with MS may only just be managing to remain independent. This is not the moment to suggest major urological surgery, and in practice few patients with progressive neurological disease affecting bladder control opt for surgery. With the advent of botulinum toxin for the bladder, some of these debilitating symptoms can now be better controlled.
Table 38.3 Urological Procedures That May Be Performed to Treat Various Causes for Incontinence
| Stress incontinence: pelvic floor weakness |
TOT, Transobturator tape; TVT, tension-free vaginal tape.
Stepwise Approach to Neurogenic Bladder Dysfunction
The treatment options offered to a patient should reflect the severity of bladder dysfunction, which generally parallels the extent of neurological disease (Fig. 38.8). However, beyond a certain point, incontinence may become refractory to all treatment options, and it is at this stage that a long-term indwelling catheter should be offered. Although most patients can be managed along these lines, there are specific situations where specialist urology services should be involved earlier on in the disease course (Box 38.1).
Box 38.1 Red Flags That Would Suggest Early Referral to a Specialist Urology Service
 Suspicion of concomitant urological pathologies such as bladder outlet obstruction due to prostate enlargement in men or stress incontinence in women
Suspicion of concomitant urological pathologies such as bladder outlet obstruction due to prostate enlargement in men or stress incontinence in women
 Symptoms refractory to treatment
Symptoms refractory to treatment
 Consideration of suprapubic catheterization
Consideration of suprapubic catheterization
 Consideration of urological procedures such as intradetrusor injections of botulinum toxin A or surgeries such as augmentation cystoplasty or urinary diversion
Consideration of urological procedures such as intradetrusor injections of botulinum toxin A or surgeries such as augmentation cystoplasty or urinary diversion
Urinary Tract Infections
The presence of asymptomatic bacteria alone in a patient performing intermittent self-catheterization should not be an indication for antibiotics (Fowler et al., 2009). The usual indication would be presence of associated symptoms (local or systemic) and certainly if involvement of the upper urinary tract (pyelonephritis) occurs. In an individual with recurrent UTIs (more than 2 in 6 months or more than 3 in a year), the catheterization technique should be reviewed and if optimal, it is worthwhile to exclude a urological cause such as a bladder stone. In individuals with proven recurrent UTIs and when no urological structural abnormality has been identified, it is reasonable to start prophylactic low-dose antibiotics for a finite duration. Rotation of antibiotics is one approach to minimize antibiotic resistance developing. It is also important to distinguish relapsing from remitting infections and ensure that undertreatment of a causative organism is not the underlying cause of the symptoms. There is debate about the pros/cons of introducing CISC as a preventive measure against UTIs, as well as the threshold above which CISC should be recommended (e.g., a residual of 150 mL). The input of a specialist nurse in lower urinary tract dysfunction who teaches CISC is particularly helpful here.
The value of cranberry juice in preventing UTIs in neurogenic patients is debatable.
Management of Neurogenic Sexual Dysfunction
Sexual dysfunction in neurological disease can be due to several different causes (Foley and Werner, 2000) (Box 38.2). Primary sexual dysfunction results from the actual neurological lesions directly affecting the neural pathways for sexual functions. For example, lesions in the spinal cord may cause loss of tactile sensations from the genitalia. Physical disabilities such as spasticity or pain resulting from the neurological disease can interfere with sexual functions as well. This is known as secondary sexual dysfunction. Sexual dysfunction arising from the psychological, emotional, or cultural impact of living with a neurological disease is known as tertiary sexual dysfunction. A holistic approach to managing sexual dysfunction involves identifying all these contributory factors.
Box 38.2 Potential Factors Predisposing to Sexual Dysfunction in Neurological Disease
Management of Erectile Dysfunction
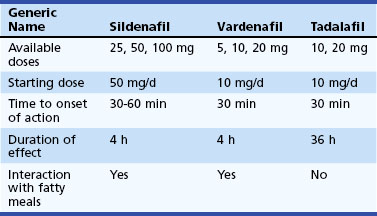
After the introduction of sildenafil, two other medications with a similar mechanism of action have been introduced. All three medications are generally well tolerated, but there are some differences to be considered. Sildenafil and vardenafil are effective after 30 to 60 minutes from administration, respectively, and last for up to 4 hours. By contrast, tadalafil is effective from 30 minutes after administration, buts its peak efficacy is expected after approximately 2 hours. Efficacy is maintained for up to 36 hours and may, therefore, mean less planning and pressure to have sexual intercourse to a schedule. A fatty meal may affect the absorption of sildenafil and vardenafil, with potential bearing on efficacy. Specific characteristics of the three currently available phosphodiesterase 5 inhibitors are shown in Table 38.4.
Management of Ejaculation Dysfunction
Impaired ejaculation is a problem for many men with spinal cord lesions. There is as yet no medical intervention that restores ejaculatory function, although a small proportion of patients report some improvement of “erotic sensitivity” with yohimbine. This is an alkaloid derived from the bark of the African tree, Pausinystalia yohimbe, and the South American herb, quebracho, Aspidosperma quebracho-blanco. Available as an oral preparation, it is principally a monoamine oxidase inhibitor (MAOI) that stimulates release of norepinephrine. Yohimbine can be used 1 to 2 hours before intercourse. Its main side effects include rise in blood pressure and increased frequency of micturition. It may also have an anxiogenic effect. Midodrine, an α1-agonist, has recently been shown to improve ejaculation in men with SCI (Soler et al., 2007).
Sexual Dysfunction in Women
Until recently, sexual dysfunction in women has been generally neglected by mainstream clinical practice. Sexual dysfunction has significance on both the affected woman and her partner and often is an underlying strain in a relationship. The success of sildenafil in treating ED in men led to a placebo-controlled randomized study of its effect in women (Dasgupta et al., 2004). The only benefit seemed to be a slight but significant improvement in vaginal lubrication, explained by the vasodilatory action of sildenafil. However, this was not associated with an improvement in orgasmic function or on quality of life. Anesthetic gels or pain modulation may be useful for women with dyspareunia.
Abrams P., Cardozo L., Fall M., et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167-178.
Adams D., Samuel D., Goulon-Goeau C., et al. The course and prognostic factors of familial amyloid polyneuropathy after liver transplantation. Brain. 2000;123(Pt 7):1495-1504.
Andersson K.E. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417-450.
Andrade M.J. Lower urinary tract dysfunction in familial amyloidotic polyneuropathy, Portuguese type. Neurourol Urodyn. 2009;28:26-32.
Araki I., Kitahara M., Oida T., et al. Voiding dysfunction and Parkinson’s disease: urodynamic abnormalities and urinary symptoms. J Urol. 2000;164:1640-1643.
Araki I., Kuno S. Assessment of voiding dysfunction in Parkinson’s disease by the international prostate symptom score. J Neurol Neurosurg Psychiatry. 2000;68:429-433.
Arnow B.A., Desmond J.E., Banner L.L., et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014-1023.
Betts C.D., Jones S.J., Fowler C.G., et al. Erectile dysfunction in multiple sclerosis. Associated neurological and neurophysiological deficits, and treatment of the condition. Brain. 1994;117(Pt 6):1303-1310.
Blok B.F., Sturms L.M., Holstege G. Brain activation during micturition in women. Brain. 1998;121(Pt 11):2033-2042.
Blok B.F., Willemsen A.T., Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120(Pt 1):111-121.
Bors E., Comarr A. Neurological disturbances of sexual function with special references to 529 patients with spinal cord injury. Urological Survey. 1960;10:191-222.
Bosma R., Wynia K., Havlikova E., et al. Efficacy of desmopressin in patients with multiple sclerosis suffering from bladder dysfunction: a meta-analysis. Acta Neurol Scand. 2005;112:1-5.
Braak H., Ghebremedhin E., Rub U., et al. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121-134.
Buljevac D., Flach H.Z., Hop W.C., et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952-960.
DasGupta R., Fowler C.J. Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies. Drugs. 2003;63:153-166.
Dasgupta R., Wiseman O.J., Kanabar G., et al. Efficacy of sildenafil in the treatment of female sexual dysfunction due to multiple sclerosis. J Urol. 2004;171:1189-1193. discussion 1193
Derbyshire S.W. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol. 2003;98:12-20.
Foley F., Werner M. Sexuality and Intimacy. In: Kalb R., editor. Multiple sclerosis: the questions you ask, the answers you need. New York: Demos Vermonde Press, 2000.
Fowler C.J. Neurological disorders of micturition and their treatment. Brain. 1999;122(Pt 7):1213-1231.
Fowler C.J., Griffiths D., de Groat W.C. The neural control of micturition. Nat Rev Neurosci. 2008;9:453-466.
Fowler C.J., Griffiths D.J. A decade of functional brain imaging applied to bladder control. Neurourol Urodyn. 2010;29:49-55.
Fowler C.J., Panicker J.N., Drake M., et al. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:470-477.
Fowlis G.A., Waters J., Williams G. The cost effectiveness of combined rapid tests (Multistix) in screening for urinary tract infections. J R Soc Med. 1994;87:681-682.
Freeman R.M., Adekanmi O., Waterfield M.R., et al. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:636-641.
Georgiadis J.R., Holstege G. Human brain activation during sexual stimulation of the penis. J Comp Neurol. 2005;493:33-38.
Gibbons C.H., Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146:8-12.
Giovannoni G., O’Sullivan J.D., Turner K., et al. Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry. 2000;68:423-428.
Glickman S., Kamm M.A. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996;347:1651-1653.
Han K.S., Heo S.H., Lee S.J., et al. Comparison of urodynamics between ischemic and hemorrhagic stroke patients; can we suggest the category of urinary dysfunction in patients with cerebrovascular accident according to type of stroke? Neurourol Urodyn. 2010;29:387-390.
Hashim H., Abrams P. How should patients with an overactive bladder manipulate their fluid intake? BJU Int. 2008.
Hemmett L., Holmes J., Barnes M., et al. What drives quality of life in multiple sclerosis? QJM. 2004;97:671-676.
Hill S.R., Fayyad A.M., Jones G.R. Diabetes mellitus and female lower urinary tract symptoms: a review. Neurourol Urodyn. 2008;27:362-367.
Hobday D.I., Aziz Q., Thacker N., et al. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361-368.
Holstege G., Georgiadis J.R., Paans A.M., et al. Brain activation during human male ejaculation. J Neurosci. 2003;23:9185-9193.
Hulter B.M., Lundberg P.O. Sexual function in women with advanced multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995;59:83-86.
Ito T., Sakakibara R., Yasuda K., et al. Incomplete emptying and urinary retention in multiple-system atrophy: when does it occur and how do we manage it? Mov Disord. 2006;21:816-823.
Jacobs H., Vieregge A., Vieregge P. Sexuality in young patients with Parkinson’s disease: a population based comparison with healthy controls. J Neurol Neurosurg Psychiatry. 2000;69:550-552.
Kalsi V., Gonzales G., Popat R., et al. Botulinum injections for the treatment of bladder symptoms of multiple sclerosis. Ann Neurol. 2007;62:452-457.
Karama S., Lecours A.R., Leroux J.M., et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1-13.
Lobato L. Portuguese-type amyloidosis (transthyretin amyloidosis, ATTR V30M). J Nephrol. 2003;16:438-442.
Marrie R.A., Cutter G., Tyry T., et al. Disparities in the management of multiple sclerosis–related bladder symptoms. Neurology. 2007;68:1971-1978.
Murialdo G., Galimberti C.A., Fonzi S., et al. Sex hormones and pituitary function in male epileptic patients with altered or normal sexuality. Epilepsia. 1995;36:360-365.
Naumann M., So Y., Argoff C.E., et al. Assessment: botulinum neurotoxin in the treatment of autonomic disorders and pain (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1707-1714.
Nour S., Svarer C., Kristensen J.K., et al. Cerebral activation during micturition in normal men. Brain. 2000;123(Pt 4):781-789.
O’Riordan J.I., Doherty C., Javed M., et al. Do alpha-blockers have a role in lower urinary tract dysfunction in multiple sclerosis? J Urol. 1995;153:1114-1116.
Oertel W.H., Wachter T., Quinn N.P., et al. Reduced genital sensitivity in female patients with multiple system atrophy of parkinsonian type. Mov Disord. 2003;18:430-432.
Panicker J.N., Kalsi V., de Seze M. Approach and evaluation of neurogenic bladder dysfunction. In: Fowler C.J., Panicker J.N., Emmanuel A. Pelvic Organ Dysfunction in Neurological Disease: Clinical Management and Rehabilitation. Cambridge University Press, 2010.
Panicker J.N., Nagaraja D., Kovoor J.M., et al. Lower urinary tract dysfunction in acute disseminated encephalomyelitis. Mult Scler. 2009;15:1118-1122.
Podnar S., Fowler C.J. Pelvic organ dysfunction following cauda equina damage. In: Fowler C.J., Panicker J.N., Emmanuel A. Pelvic Organ Dysfunction in Neurological Disease: Clinical Management and Rehabilitation. Cambridge University Press, 2010.
Podnar S., Trsinar B., Vodusek D.B. Bladder dysfunction in patients with cauda equina lesions. Neurourol Urodyn. 2006;25:23-31.
Prasad R.S., Smith S.J., Wright H. Lower abdominal pressure versus external bladder stimulation to aid bladder emptying in multiple sclerosis: a randomized controlled study. Clin Rehabil. 2003;17:42-47.
Ransmayr G.N., Holliger S., Schletterer K., et al. Lower urinary tract symptoms in dementia with Lewy bodies, Parkinson disease, and Alzheimer disease. Neurology. 2008;70:299-303.
Roth B., Studer U.E., Fowler C.J., et al. Benign prostatic obstruction and Parkinson’s disease–should transurethral resection of the prostate be avoided? J Urol. 2009;181:2209-2213.
Sakakibara R., Hattori T., Uchiyama T., et al. Micturitional disturbance in pure autonomic failure. Neurology. 2000;54:499-501.
Sakakibara R., Hattori T., Yasuda K., et al. Micturitional disturbance and the pontine tegmental lesion: urodynamic and MRI analyses of vascular cases. J Neurol Sci. 1996;141:105-110.
Sakakibara R., Shinotoh H., Uchiyama T., et al. Questionnaire-based assessment of pelvic organ dysfunction in Parkinson’s disease. Auton Neurosci. 2001;92:76-85.
Sakakibara R., Shinotoh H., Uchiyama T., et al. SPECT imaging of the dopamine transporter with [(123)I]-beta-CIT reveals marked decline of nigrostriatal dopaminergic function in Parkinson’s disease with urinary dysfunction. J Neurol Sci. 2001;187:55-59.
Sipski M.L., Alexander C.J., Rosen R. Sexual arousal and orgasm in women: effects of spinal cord injury. Ann Neurol. 2001;49:35-44.
Sipski M., Alexander C.J., Gómez-Marín D. Effects of level and degree of spinal cord injury on male orgasm. Spinal Cord. 2006;44:798-804.
Soler J.M., Previnaire J.G., Plante P., et al. Midodrine improves ejaculation in spinal cord injured men. J Urol. 2007;178:2082-2086.
Stocchi F., Badiali D., Vacca L., et al. Anorectal function in multiple system atrophy and Parkinson’s disease. Mov Disord. 2000;15:71-76.
Stohrer, M., Castro-Diaz, D., Chartier-Kastler, E., et al., 2009. Guidelines on Neurogenic Lower Urinary Tract Dysfunction. European Association of Urology Guidelines: European Association of Urology.
Tadic S.D., Griffiths D., Murrin A., et al. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage. 2010;51:1294-1302.
Vodusek D.B. Sphincter EMG and differential diagnosis of multiple system atrophy. Mov Disord. 2001;16:600-607.
Winge K., Fowler C.J. Bladder dysfunction in Parkinsonism: mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21:737-745.
Yoshimura N., Kuno S., Chancellor M.B., et al. Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br J Pharmacol. 2003;139:1425-1432.