Neurosurgical Anaesthesia
APPLIED ANATOMY AND PHYSIOLOGY
Spinal Cord
Cerebrospinal Fluid
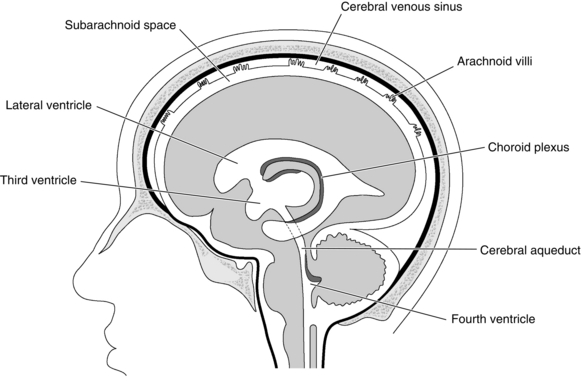
Cerebrospinal fluid (CSF) fills the cerebral ventricles and the subarachnoid space around the brain and the spinal cord. The CSF acts as a buffer, separating the brain and spinal cord from the hard bony projections inside the skull and the vertebral canal. It is produced by the choroid plexus in the lateral, third and fourth ventricles by a combination of filtration and secretion (Fig. 32.1). The total volume of CSF is 150–200 mL. CSF passes back into the venous blood through arachnoid villi. Blockages which obstruct the normal flow of CSF through the ventricular system or prevent its reabsorption lead to a build-up in CSF pressure, dilation of the ventricles and hydrocephalus.
Intracranial Pressure
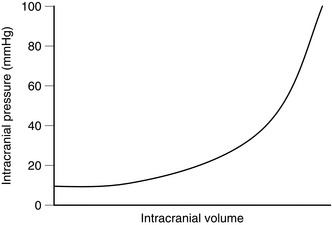
With normal cerebral compliance (the correct physiological parameter is elastance, which is the reciprocal of compliance), the intracranial pressure (ICP) is 7–15 cmH2O (5–11 mmHg) in the horizontal position. When moving to the erect position, the ICP decreases initially, but then, because of a decrease in reabsorption of CSF, the pressure returns to normal. ICP is related directly to intrathoracic pressure and has a normal respiratory swing. It is increased by coughing, straining and positive end-expiratory pressure. In the presence of reduced cerebral compliance, small changes in cerebral volume produce large changes in ICP. Such critical changes may be induced by drugs used during anaesthesia (e.g. volatile anaesthetic agents and vasodilators), elevations in PaCO2 and posture, as well as by surgery and trauma (Fig. 32.2).
Cerebral Blood Flow
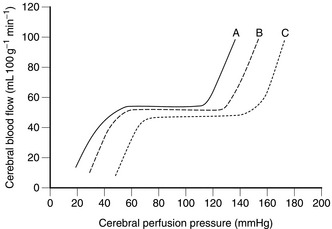
Under normal conditions, the brain receives about 15% of the cardiac output, which corresponds to a cerebral blood flow (CBF) of approximately 50 mL 100 g– 1 tissue min– 1 or 600–700 mL min– 1. The cerebral circulation is able to maintain an almost constant blood flow between a mean arterial pressure of 60 and 140 mmHg by the process of autoregulation. This is mediated by a primary myogenic response involving local alteration in the diameter of small arterioles in response to changes in transmural pressure. Above and below these limits, or in the traumatized brain, autoregulation is impaired or absent, so that cerebral blood flow is closely related to cerebral perfusion pressure (CPP) (Fig. 32.3). This effect is also seen in association with cerebral hypoxia and hypercapnia, in addition to acute intracranial disease and trauma. Cerebral perfusion pressure may be reduced as a result of systemic hypotension or an increase in ICP; CBF is maintained until the ICP exceeds 30–40 mmHg. The Cushing reflex increases CPP in response to an increase in ICP by producing, first, reflex systemic hypertension and tachycardia and then bradycardia, despite these compensatory mechanisms also contributing to an increase in ICP. In the treatment of closed head injuries, if both ICP and mean arterial pressure are being monitored, it is essential to maintain the resultant CPP with vasopressor therapy if cerebral perfusion is borderline because even transient absence of flow to the brain may produce focal or global ischaemia with infarction. Figure 32.3 also demonstrates that haemorrhagic hypotension associated with excess sympathetic nervous activity results in a loss of autoregulation at a higher CPP than normal, while the use of vasodilators to induce hypotension shifts the curve to the left, maintaining flow at lower levels of perfusion pressure. Vasodilators also differ in their effects; autoregulation is preserved at a lower CPP with sodium nitroprusside than with autonomic ganglionic blockade (however, vasodilators are rarely used during neuroanaesthesia). Cerebral blood flow is closely coupled to cerebral metabolic rate. Local increases in cerebral metabolic rate are associated with very prompt increases in CBF. The increased electrical activity associated with convulsions produces an increase in lactic acid and other vasodilator metabolites. This, together with an increase in CO2 production, produces an increase in CBF. Conversely, cerebral metabolic depression, in association with either deliberate or accidental hypothermia or induced by drugs, reduces CBF.
Effects of Oxygen and Carbon Dioxide on Cerebral Blood Flow
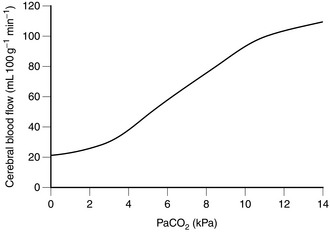
Physiologically, carbon dioxide is the most important cerebral vasodilator. Even small increases in PaCO2 produce significant increases in CBF and, therefore, ICP. There is an almost linear relationship between PaCO2 and CBF (Fig. 32.4). Over the normal range, an increase of PaCO2 by 1 kPa increases CBF by 30%. Conversely, hyperventilation to produce a PaCO2 of 4 kPa produces cerebral vasoconstriction and a decrease in ICP, although this is compensated for by an increase in CSF production over a more prolonged period of hyperventilation, such as that used in the treatment of head injuries. This is why there is no advantage in aggressive hyperventilation regimens in head injury management. Hypocapnia below a PaCO2 of 4 kPa has little acute effect on ICP, and hyperventilation beyond this point to lower ICP should be avoided except as a last resort because the vasoconstriction induced may be associated with a reduction in jugular bulb oxygen saturation, suggesting hypoperfusion and ischaemia. At a PaCO2 above 10 kPa, the vessels are maximally dilated and there is little, if any, further increase in CBF.
GENERAL PRINCIPLES OF NEUROSURGICAL ANAESTHESIA
Recovery from Anaesthesia
The majority of patients are allowed to wake up as usual at the end of operation, preferably in a dedicated neurosurgical recovery room. The Glasgow Coma Scale (Table 32.1) or an equivalent for children is recorded. Patients should return rapidly to at least their preoperative level of consciousness. A failure to achieve this, or a deterioration after an initial awakening, should alert carers to possible ischaemia or raised ICP. Re-imaging or immediate wound exploration is then required. Seizures after elective intracranial neurosurgery are surprisingly rare and, if they occur, should be treated immediately and the cause identified.
TABLE 32.1
| Clinical Sign | Response | Score |
| Eyes open | Spontaneously | 4 |
| To verbal command | 3 | |
| To pain | 2 | |
| No response | 1 | |
| Best motor response to verbal command or to painful stimulus | Obeys Localizes pain Flexion withdrawal Abnormal flexion (decorticate rigidity) |
6 5 4 3 |
| Extension (decerebrate rigidity)No response | 21 | |
| Best verbal response | Orientated, converses | 5 |
| Disorientated, converses | 4 | |
| Inappropriate words | 3 | |
| Incomprehensible sounds | 2 | |
| No response | 1 | |
| Total (minimum 3, | ||
| maximum 15) |
ANAESTHESIA FOR ELECTIVE INTRACRANIAL SURGERY
Cerebrovascular Lesions
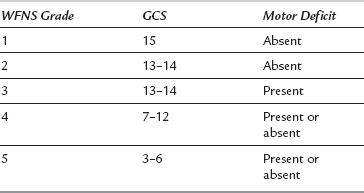
Patients with a vascular lesion such as an intracranial aneurysm or arteriovenous malformation (AVM) may present acutely with a subarachnoid or intracerebral haemorrhage. Congenital lesions are seen frequently in young and previously healthy patients. Intracranial aneurysms occur in the older age group and may be associated with other, more widespread cardiovascular disease. Subarachnoid haemorrhage is now graded using the World Federation of Neurosurgeons’ (WFNS) scale (Table 32.2). Although application of clips (‘clipping’) at craniotomy should prevent the risk of further bleeding, significant perioperative morbidity and mortality can result from vasospasm, which may occur pre- or postoperatively The current trend is to undertake emergency cerebral angiography and clipping of an aneurysm in good-grade patients, but to delay surgery in the poor-grade patients until their condition improves. The calcium channel blocker nimodipine is used to reduce or prevent vasospasm. By preference, it is given orally because the hypotensive effects are less.
ANAESTHESIA FOR SURGERY OF THE SPINE AND SPINAL CORD
Anaesthesia for Cervical Spine Surgery
MANAGEMENT OF THE HEAD-INJURED PATIENT
1. Initial airway maintenance, remembering that patients with craniofacial injuries often have associated damage to the cervical spine. Tracheal intubation is usually necessary, must be accomplished without excessive neck manipulation and should be performed by an experienced person. It is important to make intubation as atraumatic as possible; consequently, sedation and neuromuscular blockade should be used irrespective of the level of consciousness, except in the most severe situation. The benefits of succinylcholine usually outweigh the potential risks. Nasotracheal intubation is contraindicated because of the possibility of a basal skull fracture.
2. Maintenance of adequate ventilation with oxygen-enriched air. Avoidance of hypoxaemia and hypercapnia is essential.
3. Maintenance of an adequate circulating volume and arterial pressure. Hypotension after head injury greatly worsens outcome. Other injuries which may affect the circulatory state must be identified while resuscitation is being performed.
4. Sedation and analgesia, and neuromuscular blockade, are usually continued to allow management of other injuries, CT scanning and possible inter-hospital transfer.
5. Detailed assessment of thoracic, abdominal and limb injuries and appropriate therapy to stabilize the patient’s cardiovascular and respiratory systems are required before transfer to the CT scanner and X-ray room. Other life-threatening injuries must be dealt with to prevent secondary brain injury caused by hypoxaemia or hypotension.
6. Invasive arterial pressure monitoring, together with ECG, capnography and pulse oximetry are all important in the early detection of deterioration in ICP, cardiovascular stability or respiratory function. A contused, oedematous and non-compliant brain tolerates only minimal changes in oxygen supply or carbon dioxide tension before ICP increases still further.
7. After the CT scan, many patients are transferred directly to the neurosurgical operating theatre for evacuation of haematoma or insertion of an intraventricular catheter or pressure transducer. Patients who are scanned in peripheral hospitals have their scans relayed to the main neurosurgical centre. The patient is then transferred directly by ambulance to the neurosurgical operating theatre, but both cardiovascular and neurological stability must be achieved before the journey. Realistically, this involves the transfer of a sedated, intubated and ventilated patient, often pretreated with mannitol to minimize acute increases in ICP.
INTENSIVE CARE MANAGEMENT OF HEAD-INJURED PATIENTS
Detailed Neurological Assessment
The Glasgow Coma Scale (Table 32.1), which is based upon eye opening, and verbal and motor responses, is used in non-sedated patients. Brain function may also be assessed by use of the electroencephalogram (or a processed EEG monitor such as the cerebral function analyzing monitor [CFAM]), transcranial Doppler and near-infrared spectroscopy.