Chapter 34 Neuropsychology
Goals of Neuropsychology
When neural damage is present or cognitive changes are observed or reported during clinical evaluation, an extended neuropsychological evaluation is appropriate. The prominent neuropsychologist, Arthur Benton (1975), best described neuropsychology as “a refinement of clinical neurological observation [that] serves the function of enhancing clinical observation [and] is closely allied to clinical neurological evaluation and in fact can be considered to be a special form of it.” (p. 68) In clinical settings, neuropsychological assessment aims to extend the clinical neurological exam by: (1) providing important diagnostic information and predictions, even in conditions not detected via other procedures (e.g., anoxia), (2) attributing cognitive strengths and weaknesses to their appropriate factors (e.g., psychiatric symptoms, neurological disease, demographic factors), (3) predicting functional ability to enhance treatment planning, and (4) monitoring cognitive changes and treatment effectiveness across time (Lezak et al., 2004). Neuropsychological assessment is also used in forensic settings and for research in neuroscience, but discussion on these topics is beyond the clinical focus of this chapter.
Before the advent of neuroimaging in the 1970s and 1980s, one of the main goals of neuropsychology was lesion localization. Since current structural imaging techniques are capable of localizing lesions with remarkable accuracy, the focus of neuropsychology has shifted toward characterizing patients’ cognitive and behavioral profiles. Such profiles can be used to make differential diagnosis decisions, especially when lesions may not be evident. For example, some types of dementias present with a cortical profile (i.e., impairments of memory and language), whereas others present with a characteristic subcortical profile (i.e., impairments of processing speed, executive functioning, and mood) (Table 34.1). Repeated neuropsychological evaluations can also be a sensitive method for monitoring the progression of neurodegenerative diseases over time (e.g., Huntington disease [HD], Alzheimer disease [AD]), recovery after an acute injury (e.g., stroke or traumatic brain injury [TBI]), or postoperative recovery following neurosurgery (e.g., temporal lobectomy). Evaluating the effectiveness of medical procedures also entails repeated assessment of cognitive abilities with pre- and post-treatment testing.
Table 34.1 Neuropsychological Characteristics of Cortical versus Subcortical Dementia Using Alzheimer Disease and Huntington Disease as Examples
| Alzheimer Disease (Cortical Dementia) |
Huntington Disease (Subcortical Dementia) |
|
|---|---|---|
| LEARNING AND MEMORY | ||
| Episodic memory | Impaired encoding/consolidation | Impaired information retrieval |
| Poor delayed recall and recognition memory | Recognition memory is better than delayed recall | |
| Retrograde amnesia | Severe, temporally graded, retrograde amnesia | Mild, non-graded, retrograde amnesia |
| Priming | Impaired | Preserved |
| Implicit procedural/motor learning | Preserved | Impaired |
| Implicit cognitive skill learning | Preserved | Impaired |
| ATTENTION/CONCENTRATION | Relatively preserved | Poor auditory and visual attention |
| PROCESSING SPEED | Relatively intact | Very slow |
| EXECUTIVE FUNCTIONING | ||
| Set shifting | Better able to shift focus | Difficulty with perseveration |
| Working memory | Mild deficits in ability to manipulate information, but preserved phonological loop and visuospatial sketchpad | Early notable deficits in phonological loop, visuospatial sketchpad, and ability to manipulate information |
| LANGUAGE AND SEMANTIC KNOWLEDGE | ||
| Speech | Preserved | Dysarthric and slow |
| Fluency | More impaired semantic fluency than phonemic fluency | Severe and equal impairment in phonemic and semantic fluency |
| Naming | Impaired; more semantic errors (e.g., calling a lion “an animal”) | Relatively preserved; more perceptual errors (e.g., calling a bucket “a cup”) |
| Structure of semantic knowledge | Tend to focus on concrete perceptual information | Able to focus on abstract conceptual knowledge |
Adapted from Salmon, D.P., Filoteo, J.V., 2007. Neuropsychology of cortical versus subcortical dementia syndromes. Semin Neurol 27, 7-21.
Neuropsychological Evaluation
Test Administration
Two major approaches to neuropsychological evaluation currently dominate the field: the fixed battery approach and the flexible battery approach (Barr, 2008). The fixed battery approach requires that the same tests are administered to every patient in a standardized manner. One example of a fixed battery is the Halstead-Reitan battery (Box 34.1), for which comprehensive norms have been published by Heaton and colleagues (Heaton et al., 1991). An advantage to the fixed battery approach is that the information gathered is comprehensive and systematically assesses multiple domains of cognitive functioning. Additionally, if repeated assessments are available, test scores can be directly compared with baseline information, and tests are well validated and normed. Drawbacks of the fixed battery approach include its length (up to 8 hours), because it may be too long for some patients to tolerate and is difficult to afford with the limited reimbursement schedules in managed care. Furthermore, an extended assessment may not be necessary to address the referral question.
Box 34.1
Heaton Adaptation of Halstead-Reitan Neuropsychological Test Battery
Adapted from Heaton, R.K., Grant, I., Matthews, C.G., 1991. Comprehensive Norms for Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources, Odessa, Florida.
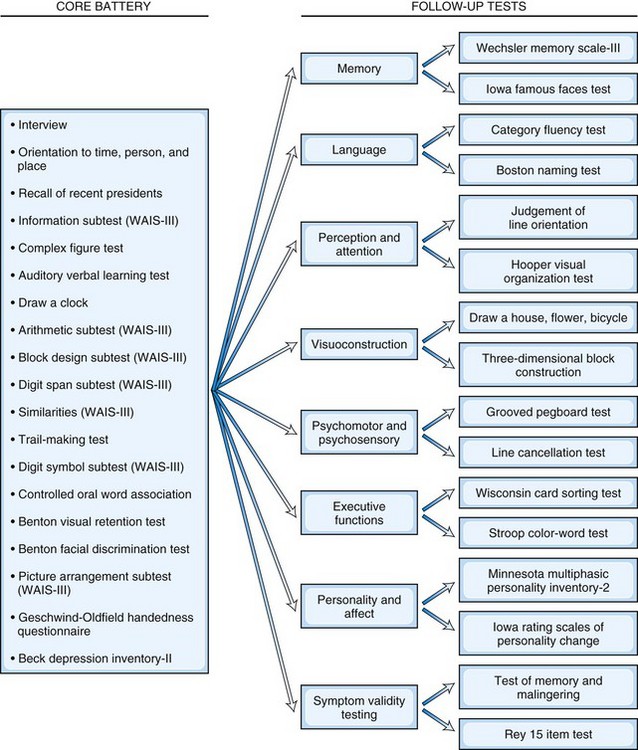
In contrast to the fixed battery approach, the flexible battery (or hypothesis-driven) approach allows neuropsychologists to develop a test battery based on the referral question, the patient’s history, and the clinical interview. In the flexible battery approach, a brief set of basic tests is initially administered, and additional tests of more specific abilities are used to conduct in-depth follow-up assessments based on each particular patient’s needs. For example, clinicians using the Iowa-Benton method (Tranel, 2008) specifically tailor testing to each patient based on their presenting concern by administering the appropriate portions of a core battery, which are then followed up with tests that assess suspected impairments in more detail (Fig. 34.1). Considerations when selecting tests include age, primary language, level of education, ethnicity/cultural factors, reading level, expected level of global cognitive impairment (to avoid ceiling or floor effects in testing), and physical disabilities (Smith et al., 2008). Although this approach is more tailored to the individual needs of the patient (and is therefore briefer), it can be less comprehensive than the fixed battery approach. Most neuropsychologists’ approaches fall somewhere between the use of a set battery and a completely individualized examination.
Test Interpretation
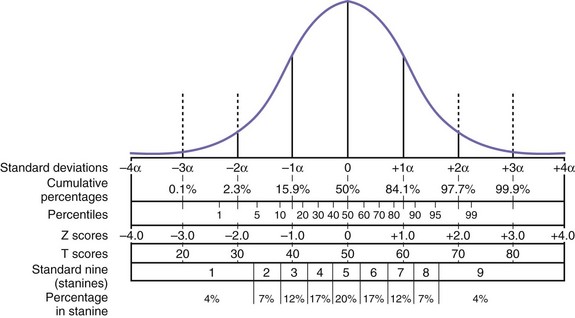
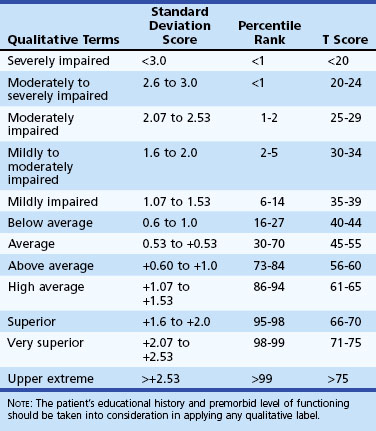
Neuropsychological test scores are interpreted most often by using group statistics to make inferences about individual patients through the use of normative data, which are typically collected by test developers as a standardization sample. Normative data aid in test interpretation by accounting for variables that are likely to influence test performance (e.g., demographic factors) so that accurate and appropriate conclusions are drawn. Confounding variables are accounted for by stratifying test scores according to gender, age, and/or level of education. An individual’s raw score is compared with the distribution of scores from his or her peer group to determine where it falls within the range of expected performances. Fig. 34.2 and Table 34.2 show a normal distribution and interpretive guidelines for use in neuropsychological interpretation. The usefulness of normative data depends strongly on the size and representativeness of the standardization sample. Clinical interpretation can also be greatly affected by the goodness-of- fit between the individual patient and the standardization sample. For example, it would not be appropriate to make determinations about the test performance of an 82-year-old man with 8 years of education by comparing his test score with those of a group of 40-year-olds with an average of 12 years of education. Furthermore, it is important to use the most recent norms available, because cohort effects may lead to differences between current patients and those from whom data were collected years ago. When appropriate norms are not available, there is a danger of overdiagnosis or underdiagnosis of cognitive impairment. Accurate interpretation of neuropsychological test performance necessarily incorporates information about the sample from which the test norms were developed.
Table 34.2 Descriptive Terms Associated with Performance within Various Ranges of the Normal Distribution
Another approach to test interpretation is through the use of cut scores. Tests that rely on cut scores often measure performances with low base rates or deficits very few healthy people demonstrate. Some tests are fairly straightforward in their capability to measure abilities that are largely intact in normal subjects but impaired in disordered patients. For example, most people are able to bisect a line without difficulty, but patients with left-sided visuospatial neglect typically identify the midpoint of the line to be to the right of center. Other tests, however, are more complex and require more sophisticated analyses to develop valid cut scores. Smith et al. (2008) provide an excellent explanation for how cut scores are useful individual statistics (i.e., test scores) that allow inferences about which diagnostic group a patient is likely to belong to (e.g., AD versus mild cognitive impairment versus healthy). Test validation studies commonly use sensitivity and specificity data and base rate information to calculate likelihood ratios and positive predictive values for individual tests. Likelihood ratios and positive predictive values are differing expressions of the probability that a patient has a particular condition given his or her test score. Smith et al. (2008) put these concepts another way by saying, “the positive predictive value allows for statements such as: ‘Based on the patient having earned a score of y on test z, the probability that this patient has the condition of interest is x.’ ” (p. 47). A common example of the application of cut scores can be found in the use of screening instruments to quickly identify potential impairments. For example, a cut score of 23 on the Mini-Mental State Examination (MMSE) has frequently been used as an indicator of cognitive impairment for dementia. Although cut scores can save time and provide useful heuristics, these scores may miss “true hits” and identify “false positives.”
The comparison of current performance with past test scores is another important component of test interpretation, especially if cognitive decline is suspected. Rarely, however, do individuals have previous test data available for these comparisons. When no previous test scores are available, evidence of the patient’s premorbid intellectual functioning is estimated. Several techniques are available for estimating premorbid intellect. Measures known as hold tests (i.e., cognitive tests that are resistant to neurological insult), such as reading ability measures, are frequently used because they are more resistant to many processes that cause decline in cognitive functioning (Smith-Seemiller et al., 1997). Some common measures of reading ability are the National Adult Reading Test (Nelson, 1982), Wide Range Achievement Test Revision 4 (Wilkinson and Robertson, 2006), and Wechsler Test of Adult Reading (Psychological Corporation, 2001), all of which assess the ability to read irregularly spelled words aloud. In some cases, reading ability measures may not be appropriate estimates, because they are affected by the progression of disease (e.g., dementia) (Cockburn et al., 2000; Taylor, 1999). In such conditions, estimates that use demographic variables (e.g., age, education level, primary occupation) in regression-based formulas to predict premorbid IQ may be more useful (e.g., the Barona formula; see Box 34.2). Similarly, academic records (e.g., college grade point average, achievement tests from middle school) can provide estimates of functioning prior to illness or injury. Most contemporary neuropsychologists use a combination of these strategies, either formally (e.g., Oklahoma Premorbid Intelligence Estimate-3) (Schoenberg et al., 2002) or informally, to arrive at the best estimate of premorbid ability.
Box 34.2
Barona Regression-Based Premorbid IQ Estimation Formula
Barona premorbid IQ estimation formula: VIQ = 54.23 + 0.49 (age) + 1.92 (sex) + 4.24 (race) + 5.25 (education) + 1.89 (occupation) + 1.24 (region)
Age: 16 to 17 years = 1; 18 to 19 years = 2; 20 to 24 years = 3; 25 to 34 years = 4; 35 to 44 years = 5; 45 to 54 years = 6; 55 to 64 years = 7; 65 to 69 years = 8; and 70 to 74 years = 9
Race: black = 1; other ethnicity = 2; white = 3
Education in years: 0 to 7 years of school = 1; 8 years = 2; 9 to 11 years = 3; 12 years = 4; 13 to 15 years = 5; and 16 or more years of school = 6
Occupation: farm laborers, farm foremen, and laborers (unskilled workers) = 1; operatives, service workers, farmers, and farm managers (semiskilled) = 2; not in the labor force = 3; craftsmen and foremen (skilled workers) = 4; managers, officials, proprietors, clerical, and sales workers = 5; professional and technical = 6
Region: Southern region = 1; North Central region = 2; Western region = 3; Northeast region = 4
Adapted from Barona, A., Reynolds, C., Chastain, R., 1984. A demographically based index of premorbid intelligence for the WAIS-R. J Clin Consult Psychol 52, 885-887.
Brief Mental Status Examination
Mini-Mental State Examination
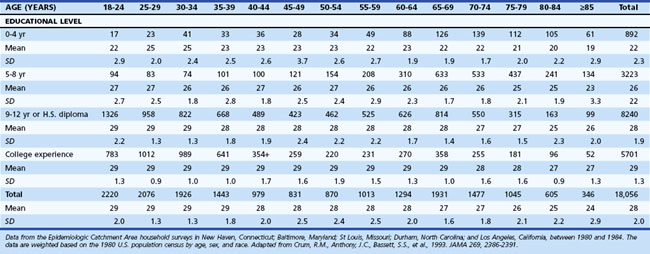
One of the most widely used mental status examinations is the MMSE (Folstein et al., 1975), a 30-point standardized screening tool for assessing orientation, attention, short-term recall, naming, repetition, simple verbal and written commands, writing, and construction (Fig. 34.3). The MMSE has been used in a variety of settings (e.g., community, institutions, general hospital, specialty clinics), with many different neurological and psychiatric conditions (e.g., dementia, stroke, depression), across age ranges, and with different cultural and ethnic subgroups. Demographic variables such as age and education have been shown to systematically influence MMSE scores, so normative data or cut scores should account for these variables. One example of appropriate norms comes from the Epidemiologic Catchment Area study (Crum et al., 1993); these are presented in Table 34.3. Whereas many intact individuals achieve total scores near 30, a cut score of 23 on the MMSE has been shown to have adequate sensitivity and specificity (86% and 91%, respectively) for detecting dementia in community samples (Cullen et al., 2005). However, when working with highly educated patients (i.e., ≥16 years of formal education) a cut score of 27 is recommended. A cut score of 27 has correct classification rate of 90.1% and a likelihood ratio of 9.6, which means that highly educated individuals with cognitive complaints and MMSE scores below 27 are almost 10 times more likely to have dementia than those with scores greater than 27 (O’Bryant et al., 2008). MMSE scores can also track changes across time. Longitudinal studies have found patients with AD show an average annual rate of change of 2.81 points, although change rates are not uniform across illness stages or gender (Chatfield et al., 2007).
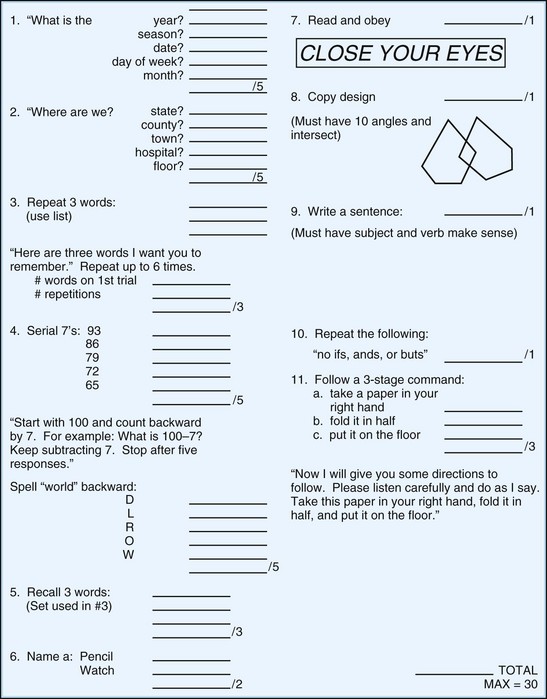
Fig. 34.3 Mini-Mental State Examination.
(Reprinted with permission from Folstein, M.F., Folstein, S.E., McHugh, P.R., 1975. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189-198.)
Table 34.3 Mini-Mental State Examination Score by Age and Educational Level, Number of Participants, Mean, Standard Deviation, and Selected Percentiles
Despite its widespread use, the MMSE has some drawbacks. One potential threat to the test’s internal validity is the non-standardized administration of some of the items. Examples of these frequent adaptations of the MMSE include the use of nonorthogonal (i.e., semantically related) word stimuli for registration and recall, nonstandard scoring of serial 7s, and nonstandard inclusion of spelling world backwards. Standard scoring instructions can alleviate some of these problems. Another drawback of the MMSE is that it has “ceiling effects” that can miss cognitive impairments in high-functioning individuals. The MMSE also has difficulty differentiating individuals with mild cognitive impairment (MCI) from controls and those with dementia (Mitchell, 2009). Finally, because this test relies on a single total score, partial administration of the measure (e.g., due to sensory impairments of the patient) provides no information about cognitive status.
Modified Mini-Mental State Examination
Some of the criticisms of the MMSE led to the development of the Modified Mini-Mental State Examination (3MS) (Teng and Chui, 1987), a 15-item extension of the MMSE that assesses orientation (self, time, place), attention (simple and complex), memory (recall and recognition), language (naming, verbal fluency, repetition, following commands, writing), construction, and executive functioning (similarities). It remains relatively brief to administer (10 minutes), and age- and education-corrected normative data are available (Tschanz et al., 2002). Regression-based prediction formulas for the 3MS allow for more accurate assessments of change across time (Tombaugh, 2005). The broader scoring range (0 to 100) has been shown to be more sensitive than that of the MMSE in identifying dementia (McDowell et al., 1997; Tschanz et al., 2002) and other cognitive disorders (Bland and Newman, 2001) in large community samples. A cut score for cognitive impairment is typically 77 (Bland and Newman, 2001; McDowell et al., 1997), and a change of 5 points over the course of 5 to 10 years indicates the presence of clinically meaningful decline (Andrew and Rockwood, 2008).
Montreal Cognitive Assessment
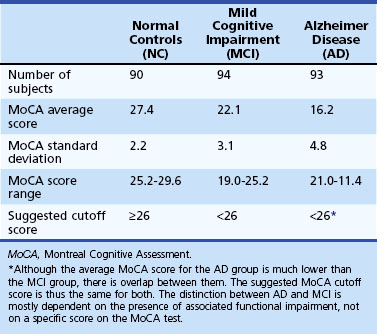
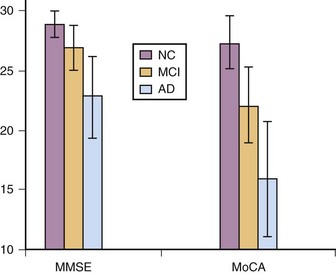
The Montreal Cognitive Assessment (MoCA) was originally developed as a screening tool to correct the MMSE’s insensitivity to MCI (Nasreddine et al., 2005). Executive functioning, immediate and delayed memory, visuospatial abilities, attention, working memory, language, and orientation to time and place are all assessed in this one-page measure (Fig. 34.4). The total score ranges from 0 to 30 points, and a cut score of 26 has demonstrated very good specificity (by correctly identifying 87% of healthy participants) and excellent sensitivity when differentiating MCI (90%) and AD (100%) from healthy comparisons (Table 34.4). More importantly, the positive predictive value of the MoCA was 89% for both MCI and AD. The psychometric properties of the MoCA contrast with the MMSE’s sensitivity of 18% for MCI and 78% for AD (although the MMSE had a specificity of 100% in the same study, meaning it correctly ruled out dementia in all cases) (Fig. 34.5). Studies in Parkinson disease (PD) (Hoops et al., 2009; Nazem et al., 2009) and HD (Videnovic et al., 2010) populations have also shown that the MoCA has promise as a measure sensitive to early stages of different types of dementia. The MoCA has the advantage of assessing more cognitive domains, therefore reducing the likelihood that impairments or disorders would be overlooked (e.g., executive dysfunction, a hallmark symptoms of vascular dementia). Another advantage is that the test is free to clinicians (http://www.mocatest.org) and has been translated into 31 different languages and dialects.

Fig. 34.4 Montreal Cognitive Assessment.
(Reprinted with permission from Nasreddine, Z.S., Phillips, N.A., Bedirian, V., et al., 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695-699.)
Telephone Interview for Cognitive Status—Modified
The Telephone Interview for Cognitive Status—Modified (mTICS) is a relatively brief screening instrument that was designed to quickly and accurately assess cognition over the telephone, although it can also be used in face-to-face settings. This 13-item measure is heavily weighted toward immediate and delayed free recall, which might make it particularly useful in detecting mild impairments (Duff et al., 2009; Lines et al., 2003) such as amnestic MCI (Fig. 34.6). Age-, education-, gender-, and race-corrected normative data are available (Hogervorst et al., 2004).
Neuropsychological Characteristics of Neurological Disease
In this section we briefly address the neurocognitive sequelae of some of the major neurological disorders. Although many of these disorders have psychiatric characteristics as well, these will only be briefly discussed. Please see Chapter 8, Behavior and Personality Disturbances, for a more comprehensive discussion on the psychiatric aspects of neurological disorders.
Mild Cognitive Impairment
Research into aging and dementia is increasingly focused on characterizing the earliest period of cognitive impairment before the development of dementia, and evidence points to a long prodromal period. This transitional stage between normal aging and dementia has been referred to as mild cognitive impairment (Petersen, 2004). The initial diagnostic criteria for MCI, defined by Petersen and colleagues at the Mayo Clinic, are subjective memory complaints, an objective memory deficit approximately 1.5 standard deviations below peers, otherwise intact cognition, no functional impairments, and the absence of dementia. By definition, objective cognitive testing is required to diagnose MCI. Objective deficits must be observed across multiple memory tests to accurately classify persons with amnestic MCI (Brooks et al., 2008).
Initially, MCI was defined as a purely amnestic condition, with a focus on deficits in immediate and delayed recall of verbal and nonverbal information. Isolated difficulties in other cognitive abilities were not fully considered in diagnosis. More recently, however, the criteria have been broadened to include other cognitive domains beyond memory. Several different subtypes of MCI have been proposed, including single-domain impairment (either memory or non-memory) and multiple-domain impairments (with or without memory impairment) (Petersen et al., 2009). To illustrate, Duff et al. (2010c) applied criteria for amnestic and non-amnestic MCI to individuals in the prodromal phase of Huntington disease and found that nearly 40% of these individuals met criteria for some type of MCI. Unlike single-domain amnestic MCI, which tends to progress into Alzheimer dementia, the most common deficit in prodromal HD was slow processing speed, not poor memory. Furthermore, the prevalence of MCI increased as patients’ motor symptoms worsened and they approached clinical diagnosis. Other MCI subtypes are hypothesized to progress to their own dementia outcomes. For example, single, non-memory, executive dysfunction domain subtype might reflect a prodromal stage of frontotemporal dementia or, if either executive dysfunction or visuospatial deficits are present, Lewy body disease (Molano et al., 2010). Multiple domain MCI (e.g., deficits in memory and processing speed) might be indicative of eventual vascular dementia. Neuropsychological testing is essential for obtaining the necessary information to differentiate MCI subtypes and track their progression into the various forms of dementia.
MCI has attracted so much attention because of its prognostic value, with a 10-fold increase in the rate of dementia for individuals with MCI (Petersen et al., 1999). Patients of memory disorders clinics with MCI progress to dementia at a rate of 10% to 15% per year (Farias et al., 2009) and community dwelling adults at an annual rate of 6% to 10% per year (Petersen et al., 2009). Individuals with MCI also experience global cognitive decline at over twice the rate of what is observed in nonimpaired individuals (Wilson et al., 2010). Since not everyone with MCI progresses to dementia, several predictors of the progression from MCI have been identified. On neuropsychological testing, more severe memory deficits have been associated with transition from MCI to dementia, as has mild executive dysfunction (Griffith et al., 2006), visuospatial dysfunction (Molano et al., 2010), and processing speed (Duff et al., 2010c). An absence of expected practice effects has also been linked to continued cognitive decline (Duff et al., 2007). Despite this growing research, no standard has yet been established for predicting the MCI-to-dementia conversion.
Alzheimer Disease
Alzheimer disease-related dementia is the most common type, with prevalence rates as high as 10% in community-based samples (Fitzpatrick et al., 2004) and 68% in memory disorder clinics (Paulino Ramirez Diaz et al., 2005). Definitive diagnosis requires postmortem neuropathological examination of brain tissue for the hallmark signs of plaques and neurofibrillary tangles in the hippocampal and entorhinal regions (Braak and Braak, 1991); however, premortem diagnostic criteria are widely employed in clinical and research settings. Currently, the diagnosis of dementia in AD can be made with evidence of a gradually progressive episodic memory impairment plus impairment in at least one other cognitive ability (aphasia, apraxia, agnosia, or executive dysfunction) alongside functional decline (American Psychiatric Association, 2000). For research purposes, the National Institute of Neurological and Communicative Disorders and Stroke Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD are the most commonly used. Recently, workgroups convened by the National Institutes of Aging and the Alzheimer’s Association have proposed revised criteria for MCI, preclinical AD, and AD dementia to improve upon the 25-year-old NINCDS-ADRDA standards (Alzheimer’s Association, 2010). The aim of the revisions is to differentiate the various stages of AD pathology and to differentiate AD dementia from other acknowledged dementia etiologies (e.g., FTD, vascular dementia [VaD]). Advantages of the new revisions include the incorporation of biomarkers, neuroimaging, and genetic findings into the diagnostic criteria. The proposed criteria particularly emphasize the importance of measurable cognitive decline, especially for MCI and preclinical AD. Formal neuropsychological assessment is needed to measure AD related declines, using the most sensitive and accurate tools available. The bedside exams mentioned earlier in this chapter also have some degree of utility in detecting cognitive changes, but their comparatively limited scope and sensitivity and their susceptibility to ceiling effects make them better suited for screening purposes.
Complaints about “memory problems” are what often lead to a clinical evaluation of possible AD (Godbolt et al., 2005; Lehrner et al., 2005). Consistent with patient report and behavioral observations, performances across a comprehensive battery of neuropsychological assessment measures is likely to identify stark memory deficits for both verbally (e.g., lists, stories, paired associates) and visually (e.g., concrete or abstract figures) mediated information (Harciarek and Jodzio, 2005). An inability to recall more than 50% of previously learned information is not unusual (Au et al., 2003). Deficits in delayed recall are also notable in AD (Moulin et al., 2004), and intrusion errors are common (e.g., adding extra words to delayed recall trials on word-list memory tasks). Furthermore, performance on recognition trials with memory cues is as impaired as free recall trials (Delis et al., 2005), with few correct “hits” and relatively high numbers of false-positive errors. Other cognitive deficits are seen in a number of other domains including language functions (e.g., paraphasias, naming), semantic knowledge, visuospatial abilities, executive functioning, and motor planning (Smith and Bondi, 2008). Indeed, according to the newly proposed AD diagnostic criteria, a non-memory cognitive deficit may be the primary cognitive presentation of AD if it is present alongside a known AD biomarker or genetic risk factor. Global cognitive functioning in AD declines at a rate nearly four times faster than what is observed in cognitively intact individuals of the same age (Wilson et al., 2010). Given the pattern of deficits in AD, it is not surprising that measures of semantic fluency, delayed free recall, and global cognitive status demonstrate the highest levels of sensitivity and specificity for detecting patients with early AD (Salmon et al., 2002), even over other measures commonly used in the diagnosis of AD (e.g., confrontation naming) (Testa, et al., 2004). Many patients with AD are also anosognosic, which means they are unaware of the extent of their cognitive deficits, if at all (Smith and Bondi, 2008).
Although memory deficits are usually glaring compared to other deficits in AD, some studies suggest that older AD patients (i.e., older than 80) do not have the same disproportionate performances as their younger counterparts (Bondi et al., 2003). As dementia progresses to moderate stages, learning and memory performances are likely to produce floor effects on many standardized neuropsychological measures, and more profound deficits are apparent in other cognitive domains. Deficits in praxis also begin to develop. These pervasive declines in late AD often make formal neuropsychological testing unnecessary or impossible.
Vascular Dementia
Cerebrovascular disease frequently leads to cognitive impairment, which is to be expected given the high prevalence of vascular pathology in both demented and non-demented individuals (Matthews et al., 2009). Extracerebellar lacunar, large vessel, or strategically placed infarcts and hemorrhage lead to varying types and degrees of cognitive impairment. Small vessel disease is the most frequently observed vascular pathology that leads to cognitive declines. Cognitive changes can occur abruptly and in a stepwise pattern with coinciding cerebrovascular accidents (CVAs), or they may fluctuate or remain static. Neuroimaging is likely to detect lesions that are a result of a CVA and enhance diagnostic certainty, but imaging is not required to accurately identify dementia with a vascular etiology. Neurological evidence (e.g., history of strokes, sensorimotor changes consistent with stroke) in combination with cognitive changes can also signify vascular dementia (VaD) (American Psychiatric Association, http://www.dsm5.org).
The clinical presentation of VaD varies depending on the number of infarcts, severity of neural damage following stroke, and location of the CVA. Although many VaD patients may acknowledge “memory problems,” which may lead to suspicions of AD, further questioning reveals that these complaints are quite different from those typically seen in AD. Since many patients do not experience a notable stroke preceding the onset of VaD, they may not present to memory disorder clinics because their primary symptoms are an inability to solve simple problems, slowed cognitive processing, apathy, and/or depression (Roman, 2005). Classic dementia symptoms might be reported (e.g., difficulty remembering names, appointments, medications) but changes in instrumental activities of daily living that require complex organizational and problem-solving skills (e.g., managing finances, following directions, “figuring things out”) are likely more prominent in a patient with VaD compared to one with AD. Depression, emotional lability, and delusions are also reported, and apathy is a hallmark symptom (McKeith and Cummings, 2005). Upon first inspection, VaD may initially be cognitively indistinguishable from other dementias in many ways; VaD and AD patients have similarly poor orientation to time, person, and place, for example (Mathias and Burke, 2009). Yet, during a detailed neuropsychological examination, behavioral observations and formal tests are likely to reveal a unique pattern of deficits in VaD. VaD patients tend to have better long-term verbal memory than their AD counterparts but worse frontal-executive functioning (Looi and Sachdev, 1999). Declines in speeded processing and complex attention are also prominent in VaD, and they tend to have a rapid initial onset (Boyle et al., 2004). Free recall might occasionally appear similar in VaD and AD, but patients with VaD significantly outperform their AD counterparts on tests of recognition memory (Tierney et al., 2001). Better recognition memory suggests that encoding processes are relatively intact in VaD compared to AD patients, but information retrieval is deficient. This retrieval deficit appears to be quite common in subcortical dementias, with which VaD appears to have several components in common. Patients with VaD also perform more poorly on phonemic fluency tasks relative to semantic fluency tasks, which suggests greater executive dysfunction due to disruption of the frontal subcortical circuitry rather than degeneration in the temporal lobes. Lastly, VaD patients often perform worse than AD patients on emotion recognition tasks (Mathias and Burke, 2009).
Mixed Dementia
More common than AD and VaD in their pure forms is mixed dementia, which involves the coexistence of AD and VaD pathology or a combination of AD and VaD with another pathological process (e.g., Lewy bodies). Across several studies, from 25% to 50% of patients with AD also have sufficient vascular pathology to be deemed VaD (Langa et al., 2004; Zekry et al., 2002), and in autopsy studies the prevalence of mixed dementia ranges from 2% to 56% (Jellinger and Attems, 2007). Persons with multiple pathologies are three times more likely to develop dementia than others with only one identifiable diagnosis (Schneider and Arvanitakis et al., 2007). Neuropsychological and neuroimaging comparisons between AD, VaD, and mixed dementia tend to show that the latter two conditions are most similar (Zekry et al., 2002). Multiple types of dementia pathology can co-occur, with each leading to its own pattern of deficits and impairments.
Frontotemporal Dementia
Frontotemporal dementia is another common type of dementia and is in fact the second most common in those younger than age 65 (Arvanitakis, 2010). Current estimates suggest that 4% of dementia patients will be diagnosed with FTD (Brunnstrom et al., 2009), and it is frequently misdiagnosed as AD. The term frontotemporal lobar degeneration (FTLD) is also frequently used in connection with FTD, but usually when referring to the pathological aspects of FTD, not the clinical entity (McKhann et al., 2001). As the newly proposed criteria for FTD in the DSM-V suggests (American Psychiatric Association, http://www.dsm5.org), FTD has multiple clinical manifestations that include behavioral and language variants. Behavioral observations, neuropsychological testing, and neuroimaging (e.g., frontal or temporal lobe atrophy on computed tomography [CT] or magnetic resonance imaging [MRI], hypoperfusion or hypometabolism on single-photon emission tomography [SPECT] or positron emission tomography [PET]) are used in combination to establish a clinically probable diagnosis of FTD. A definitive diagnosis can be made with histopathological evidence through biopsy or postmortem examination.
Although cognitive deficits are clearly evident on formal testing, the earliest and most common complaints for the behavioral variant of FTD are related to changes in personality and behavior (Arvanitakis, 2010). Increases in impulsive actions and disinhibition, poor judgment, compulsive and stereotypic behaviors, lack of hygiene, hyperorality, and loss of social graces are all prominent. A formerly mild-mannered and conscientious patient who develops the behavioral variant of FTD may present as overly frank, crass, and uncaring. The patient is usually indifferent to their behavior, but spouses and adult children are usually embarrassed by his or her conduct. Many of these behavioral issues may have underlying cognitive components. Patients’ indifference to their behavior ultimately reflect an inability to cognitively recognize emotions or perspectives in others (Gregory et al., 2002). Deficits on cognitive testing typically involve impaired mental flexibility, planning deficits, slowed word/design generation, multiple response errors, and impairments in reversal learning. The Wisconsin Card Sorting Test, Trail Making Test-Part B, and the Stroop Color-Word Test are all common tests of executive functioning that capture decline in the behavioral variant of FTD. Phonemic fluency also tends to be more impaired than semantic fluency. As the disease progresses, even simple go/no go tasks are difficult. Despite the array of cognitive deficits in FTD, visuospatial functioning tends to be well preserved, and some suggest that a lack of deficits in this domain might be one of the best ways to differentiate FTD from other diseases such as PD (Arvanitakis, 2010).
The language variants of FTD are different clinical entities than the behavioral variant, and they develop if there is disproportionate atrophy, hypometabolism, or hypoperfusion in the temporal lobes (Arvanitakis, 2010). Confrontation naming, word finding, speech production, comprehension, and/or syntax all gradually worsen over the course of disease. Memory functioning and visuospatial skills are relatively spared. The proposed DSM-V diagnostic criteria suggest that the language variant of FTD be divided into four subtypes: primary progressive aphasia (PPA), semantic variant, nonfluent/agrammatic variant, and logopenic/phonologic variant (American Psychiatric Association, http://www.dsm5.org). All of these variants involve impaired confrontation naming (e.g., poor Boston Naming Test scores) but have otherwise unique neuropsychological profiles. Primary progressive aphasia is characterized by the insidious and gradual decomposition of nearly all language functions such as word finding, object naming, grammar, speech production, and comprehension. The semantic variant of FTD involves impaired single-word comprehension, object and/or person knowledge, and dyslexia in the presence of spared single-word repetition, motor speech production, and syntax. Non-fluent/agrammatic FTD results in impaired motor speech production, grammatical errors, and comprehension of complex sentences, with intact single-word comprehension and object knowledge. Lastly, the logopenic/phonologic variant presents with deficits in spontaneous word retrieval and repetition of sentences and phrases, while single-word comprehension, object knowledge, and motor speech and grammar production are spared. Needless to say, neuropsychological testing may be the ideal exam for parsing out the complexity of FTD variants, given the details of their cognitive profiles.
Parkinson Disease with Dementia and Dementia with Lewy Bodies
Parkinson disease with dementia (PDD) and dementia with Lewy bodies (DLB) have been collectively classified as Lewy body dementias given their shared α-synuclein pathology (Lippa et al., 2007). The neuropsychological findings in the two disorders are largely similar, so they are often distinguished by differences in the onset of signs and symptoms (Metzler-Baddeley, 2007). The cognitive symptoms of PDD often develop after the onset of motor signs (e.g., bradykinesia, gait instability, tremor, rigidity), while the cognitive symptoms in DLB tend to precede motor features or occur within 1 year of the manifestation of motor signs. Furthermore, cognition fluctuates more drastically, and motor signs develop more quickly in DLB when compared to the insidious onset of PDD signs and symptoms (McKeith et al., 2005). Although the time course of cognitive symptoms and motor signs in DLB and PDD may serve as a helpful heuristic, caution should be used when differentiating these dementias from one another based on onset alone, since subtle cognitive declines can be detected early in the course of PDD (Tröster, 2008).
The neuropsychological profiles of PDD and DLB are similar (Tröster and Fields, 2008). Up to 40% of PD patients progress to dementia at some point during their illness (Padovani et al., 2006). There is considerable heterogeneity in the presentation and progression of cognitive deficits, with some patients displaying only minor cognitive slowing. Overall, the neuropsychological profile of PDD includes bradyphrenia, memory impairment, visuospatial deficits, and executive dysfunction (Troster and Fields, 2008). Of these cognitive difficulties, executive dysfunction—including impairments in decision making, planning, set shifting, and monitoring of goal-directed behaviors—may be among the earliest signs of cognitive decline in PDD. Language problems (e.g., decreased verbal fluency, poor comprehension, difficulty producing syntactically complex sentences) can occur frequently. Memory problems in PDD reflect the subcortical dementia profile, with poor initial learning and delayed recall but relatively intact recognition (Beatty et al., 2003). Psychiatric manifestations are also common in PD, including mood disturbances, anxiety, apathy, and psychosis (Richard, 2005; Weintraub and Stern, 2005; Williams-Gray et al., 2006). Similar psychiatric problems are also observed in patients with DLB; however, the most striking symptom in these patients is the development of well-formed visual hallucinations (Metzler-Baddeley, 2007). These hallucinations are typically not frightening to the patient, and they can be any visual object; however, they often take human form.
In addition to characterizing the cognitive sequelae of PD and the effects of pharmacological treatment, neuropsychologists are often called upon to evaluate candidates for deep brain stimulation (DBS) of the subthalamic nucleus. If patients with PD are severely cognitively impaired, DBS may be contraindicated, since significant declines in executive function, learning and memory, and verbal fluency can occur following surgery (Parsons et al., 2006). Furthermore, older PD patients with MCI prior to DBS may be at greater risk for postoperative declines (Halpern et al., 2009).
Huntington Disease
Huntington disease is a genetically transmitted neurodegenerative condition that primarily affects the basal ganglia and is characterized by a triad of clinical symptoms: choreoathetosis, cognitive decline, and psychiatric dysfunction. The diagnosis of HD is based on a neurological evaluation (e.g., Unified Huntington’s Disease Rating Scale [UHDRS]) (Huntington Study Group, 1996) with the manifestation of an unequivocal extrapyramidal movement disorder, although the cognitive and behavioral alterations can be the most debilitating aspect of the disease and place the greatest burden on HD families (Nehl and Paulsen, 2004).
Slowed processing speed, followed by declines in episodic memory and visuospatial processing, may be some of the earliest cognitive changes in people with the HD gene expansion (Duff et al., 2010c; Solomon et al., 2007). The perception of time and timing precision are also affected in HD (Paulsen et al., 2004), and differences in this ability are particularly sensitive to approaching diagnosis in the prodromal phase of the disease (Paulsen et al., 2004; Rowe et al., 2010). Executive dysfunction (e.g., strategies in planning and problem solving, self-monitoring, cognitive flexibility) and “frontal” neurobehavioral symptoms such as apathy and disinhibition are also prominent, even in prodromal HD (Duff et al., 2010a, 2010b). Patients with HD are impaired on cognitive tests that require executive functions, such as the Wisconsin Card Sorting Test, the Stroop Color-Word Test (Beglinger et al., 2005; Montoya et al., 2006), and clinical rating scales of executive control (Paulsen et al., 1996). Even extremely face-valid tests of judgment and decision making have detected impairments in HD (Stout et al., 2001). Another cognitive deficit observed in HD and prodromal HD is poor attention and concentration, affecting such processes as resource allocation, response flexibility, and vigilance (Montoya et al., 2006; Nehl et al., 2001). Recent research has suggested that poor attention in HD may be due to an inability to automatize task performance, which results in the diversion of cognitive resources to tasks that are normally automatic in healthy people (Thompson et al., 2010). Memory problems are also frequently reported by patients with HD and their families. Objective deficits in learning and memory have been widely reported, with these patients displaying a typical subcortical profile (Duff et al., 2010a; Paulsen et al., 1995). Additionally, emotion recognition declines regardless of modality (i.e., facial expression, verbal intonation), especially for fear, anger, and disgust (Johnson et al., 2007; Snowden et al., 2008).
Language deficits may initially appear present in HD; however, communication deficits are likely due to dysarthria—one of the more prominent motor features of HD—and not cognitive decline per se. Dysarthria-related changes include insufficient breath support, varying prosody, increased response latencies, and mild misarticulations (Rohrer et al., 1999). As HD progresses, phrase length decreases, and pauses in speech output are extended. Dysarthria likely accounts for the deficits observed on tasks of letter and category fluency early in the disease (Henry et al., 2005). These deficits are best described as a “paucity of speed” as opposed to dysfluency. Regardless of impairments in speech production, other language functions remain intact, including syntactic structure, content, and the integrity of word associations. Speech output continues to worsen as the disease progresses, typically resulting in a profound communication deficit.
Multiple Sclerosis
Estimates of the prevalence of cognitive disability in multiple sclerosis (MS) range between 40% and 50% in community-based samples (Amato et al., 2006; Jonsson et al., 2006) to more than 55% in clinical settings (Feinstein, 2004). Slowed processing speed is perhaps the most prominent neuropsychological deficit in MS (Parmenter et al., 2007; Rao et al., 1991). A variety of other cognitive declines observed in MS include working memory, cognitive flexibility, inhibition, problem solving, attention, verbal fluency, and spatial reasoning. Confrontation naming and verbal comprehension are generally not affected in MS, and dementia is uncommon (Rao et al., 1991). Consistent correlations between brain lesion magnitude and severity of cognitive dysfunction have also been reported (Benedict et al., 2002).
A distinct cognitive profile is difficult to establish in MS, since individual differences in the microscopic and macroscopic pathology of the disease lead to tremendous variability depending on the location of sclerotic plaques (e.g., subcortical white matter versus spinal cord). Furthermore, the cognitive sequelae of MS are complicated by the fact that deficits vary across the different subtypes of MS, with progressive subtypes performing worse over time (Huijbregts et al., 2006). Although deficits vary, the typical cognitive profile of MS has been described as a subcortical one, similar to PD or HD, given the deficits outlined above. Depression and other disturbances of emotional functioning occur in nearly half of these patients (Siegert and Abernethy, 2005). Psychiatric disturbances have also been linked with additional cognitive disability (Feinstein, 2004). Depression in MS has also been found to be related to cognitive deficits, particularly speeded attention and executive functioning (Arnett et al., 2002).
Epilepsy
The cognitive effects of epilepsy are unique for each patient, because there is no single cognitive profile associated with any epilepsy subtype (e.g., partial seizures, complex partial, primary generalized). Cognitive deficits also vary according to the location of the seizure foci, frequency of seizures, duration of seizure disorder, age of onset, and antiepileptic drug effects (Jokeit and Schacher, 2004; Lee and Clason, 2008). Regardless of whether a seizure is part of a single idiopathic event or a chronic epileptic disorder, the abnormal electrical activity produced during the ictal period is usually associated with cognitive and psychiatric changes. In chronic conditions, residual interictal alterations in cognition and mood also occur. Given the heterogeneity of cognitive deficits in epilepsy, neuropsychological testing is particularly indicated to provide an accurate characterization of patients’ unique cognitive deficits.
Memory deficits are one of the most common clinical manifestations of cognitive impairment in epilepsy, likely due to the high incidence of seizures originating in the medial temporal lobes (MTL) (Bornstein et al., 1988). Research has suggested that patients with focal seizure onset in the right MTL may experience greater nonverbal and visual memory loss, whereas those with onset in the left MTL have a reduced capacity for verbal information (Davis et al., 2006; Glosser and Donofrio, 2001; Kim et al., 2003). However, these findings must be interpreted with caution, since other studies have had contrasting results (Giovagnoli et al., 2005; Raspall et al., 2005). Although visual memory deficits have been observed in right MTL epilepsy, general visuospatial functioning and perception are not usually impaired in these patients (Lee and Clason, 2008). In addition to memory, Lee and Clason (2008) note in their review of the literature that general intellectual functioning can be impaired. In general, those with left MTL seizures and less cognitive reserve (i.e., protective factors, such as education, that generate resilience against the behavioral effects of neuropathology) tend to demonstrate broader cognitive deficits (Oyegbile et al., 2004). Narrative discourse (i.e., the ability to formulate and tell a story) is also affected in epilepsy. Bell and colleagues (2003) found that temporal lobe epilepsy (TLE) patients used non-communicative language, produced less story content, and spoke slower than healthy controls when asked to tell a story. Interestingly, there were no differences between controls and epilepsy patients in procedural discourse (i.e., explaining how to do a task). Confrontation naming is also impaired in some epilepsy patients (Raspall et al., 2005). Language deficits are usually more pronounced if the focus of seizure activity is in the language dominant hemisphere. Postictal language recovery is also affected by seizure origin and generalization in frontal lobe epilepsy patients. Patients with seizures originating in the left frontal lobe and spreading to the language dominant temporal lobe have a slower recovery when compared to their counterparts with either right frontal lobe seizures or seizures confined to the frontal lobe only (Goldberg-Stern et al., 2004). Executive functioning is primarily affected in frontal lobe epilepsy, especially if seizures originate in left frontal lobe, but less so in TLE. Dysfunction is usually characterized by poor cognitive flexibility, response inhibition, and perseveration (McDonald et al., 2005; Milner, 1963).
Neuropsychological evaluation is an integral part of neurosurgical treatments for pharmaco-resistant epilepsy (Helmstaedter, 2004). Preoperative evaluations and Wada testing provide useful information about the localization and lateralization of seizure-induced cognitive impairment. Assessment of patients’ cognitive reserve can be useful for predicting postoperative outcomes (e.g., better memory performance at baseline usually means greater memory loss after resection). Postoperative testing is also useful for treatment planning and quality control following resection, and ultimately refines the effectiveness of surgical procedures. Neuropsychological test batteries for surgery candidates are usually very extensive. Given the frequency of temporal lobectomy surgery relative to other procedures, most batteries are heavily focused on assessing memory and language functioning; however, executive functioning, attention, cognitive efficiency, and visuoperception are also examined thoroughly. Although fMRI has potential as a noninvasive method for lateralizing function, Wada testing is still well suited for determining how the nonepileptic hemisphere will function in isolation. Helmstaedter (2004) suggests that the Wada procedure is indicated when neuropsychological testing suggests that seizures are originating from cortex directly involved in language functioning, or if there is an atypical lateralization of language functioning. Other indications include early seizure onset (due to the increased potential for cortical reorganization) and left-handedness. Ultimately, neuropsychological assessment in Wada testing can help reduce the potential for postsurgical disconnection syndromes, aphasia, and functional loss due to unexpectedly poor contralateral compensation.
In addition to the cognitive aspects of epilepsy, psychiatric and behavioral disorders are prevalent (McCagh et al., 2009). Depression is the most common mood disorder, with 40% to 60% of epilepsy patients reporting symptoms, particularly persons with TLE in the dominant hemisphere (Grabowska-Grzyb et al., 2006). Symptoms of depression also appear to be associated with the future onset of focal seizures in some patients. One study found that depression can increase the likelihood of unprovoked seizures by six times (Hesdorffer et al., 2000). Increased anxiety is also present in about 1 in 5 epilepsy patients and does not appear to be directly associated with the neuropathology of epilepsy, but rather the unpredictability of seizures, social consequences of epilepsy, and societal stigmatization (Mensah et al., 2007).
A complicating issue in identifying cognitive and behavioral deficits associated with epilepsy is the independent effect antiepileptic drugs (AEDs) commonly have on neuropsychological functioning (Carreno et al., 2008). Episodic memory, concentration, and psychomotor functioning are the most frequently affected by both classic and new AEDs, although only modestly in most cases (Meador, 2002). The varying effects these medications have on cognition must be weighed against the benefits of minimizing the frequency and severity of seizures.
Traumatic Brain Injury
Traumatic brain injury is one of the most common forms of neurological damage in the United States, particularly in young adults (National Center for Injury Prevention and Control, 2003). In cases of moderate to severe injury, impairments in several cognitive domains can occur, depending on what caused the injury (i.e., blunt force versus projectile), the severity of the injury, site of the lesion, premorbid cognitive and personality factors, and treatment received after the injury. Advances in knowledge regarding the various cognitive sequelae of TBI has allowed for better diagnostic clarity. Beyond the established methods for staging the severity of TBI (e.g., length of loss of consciousness or posttraumatic amnesia, Glasgow Coma Scale [GCS]), neuropsychological testing can be used to increase diagnostic accuracy by measuring the classic cognitive deficits that result from head injury.
Neuropsychological testing is frequently indicated in TBI, because the range of deficits following parenchymal damage can vary significantly. For example, a circumscribed TBI involving the ventromedial prefrontal cortex can produce changes in moral reasoning and social judgment that result in impulse control problems, utilitarian moral reasoning, marked misinterpretation of social cues, or lowered frustration tolerance (Koenigs et al., 2007; Young et al., 2010). Yet, a milder TBI elsewhere might only lead to subtle deficits in attention and processing speed. Despite individual clinical variability, several common neurobehavioral sequelae of moderate to severe TBI can be identified (Table 34.5). Cognitive recovery is protracted for moderate to severe TBIs, with improvements still occurring 2 years after the injury; however, even after this length of time, individuals do not necessarily return to preinjury levels.
Table 34.5 Common Neurocognitive Sequelae of Moderate to Severe Traumatic Brain Injury
| Cognitive Domain | Clinical Manifestation of Impairment |
|---|---|
| Attention | Difficulty with sustained attention |
| Poor concentration | |
| Psychomotor impersistence | |
| Memory | Problems with acquiring and retaining new verbal or nonverbal information |
| Problems in retrieving verbal and nonverbal memories | |
| Speed of information processing | Slowed sensorimotor skills and information processing |
| Executive functioning | Problems in convergent and divergent reasoning |
| Poor judgment | |
| Difficulty planning | |
| Problems in self-monitoring and self-correcting behavior | |
| Awareness of symptoms | Difficulty recognizing deficits |
| Unrealistic expectations concerning the recovery of functions | |
| Problems related to poor treatment compliance | |
| Language and communication | Problems in word comprehension |
| Impaired reading, spelling, and writing ability | |
| Tendency to become fragmented in free speech | |
| Integrative functions | Problems in adequate or time efficient execution of various perceptual-motor-spatial-sequential tasks |
In addition to moderate and severe TBI, the cognitive sequelae of mild TBI (mTBI) have become an increasing concern, since estimates suggest that 80% of all 2 million TBIs in the United States fall under this classification (National Center for Injury Prevention and Control, 2003; Kraus et al., 1996). In their review of the literature, Mittenberg and Roberts (2008) identified reduced memory, processing speed, and attention span as the most common deficits in mTBI. Global cognitive functioning is also affected in mTBI, but much less so than what is observed in moderate to severe TBI. Furthermore, cognition appears to recover for 95% of patients within 1 to 3 months following the injury (Schretlen and Shapiro, 2003). Other symptoms following mTBI may linger. For instance, a potential concern following mTBI is postconcussion syndrome (PCS), which is characterized by headaches, fatigue, irritability, depression, and poor concentration. Approximately 28% of mTBI patients exhibit symptoms of PCS 6 months following their injury, well past the point of neuropsychological recovery, indicating that PCS may be more related to anxiety surrounding the injury and not underlying neuropsychological impairment, although this is controversial (Mittenberg and Strauman, 2000; Panayiotou et al., 2010; Ryan and Warden, 2003). Treatment for patients with persisting PCS frequently involves education about what they can reasonably expect from their injury and prognosis.
Alzheimer’s Association. New diagnostic criteria and guidelines for Alzheimer’s disease. Accessed June 1, 2010 at http://www.alz.org/research/diagnostic_criteria/, 2010.
Amato M.P., Zipoli V., Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245:41-46.
American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, fourth ed. Washington, DC.
American Psychiatric Association. DSM-5 development. Available at http://www.dsm5.org
Andrew M.K., Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2008;61:827-831.
Arnett P.A., Higginson C.I., Voss W.D., et al. Relationship between coping, cognitive dysfunction, and depression in multiple sclerosis. Clin Neuropsychol. 2002;16:341-355.
Arvanitakis Z. Update on frontotemporal dementia. Neurologist. 2010;16:16-22.
Au A., Chan A.S., Chiu H. Verbal learning in Alzheimer’s dementia. J Int Neuropsychol Soc. 2003;9:363-375.
Barr W.B. Historical development of the neuropsychological test battery. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:3-17.
Beatty W.W., Ryder K.A., Gontkovsky S.T., et al. Analyzing the subcortical dementia syndrome of Parkinson’s disease using the RBANS. Arch Clin Neuropsychol. 2003;18:509-520.
Beglinger L.J., Nopoulos P.C., Jorge R.E., et al. White matter volume and cognitive dysfunction in early Huntington’s disease. Cogn Behav Neurol. 2005;18:102-107.
Bell B., Dow C., Watson E.R., et al. Narrative and procedural discourse in temporal lobe epilepsy. J Int Neuropsychol Soc. 2003;9:733-739.
Benedict R.H.B., Bakshi R., Simon J., et al. Frontal cortex atrophy predicts cognitive impairment in multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2002;14:44-51.
Benton A.L. Neuropsychological assessment. In: Tower D.B., editor. The nervous system, vol. 2. New York: Raven Press; 1975. The clinical neurosciences
Bland R.C., Newman S.C. Mild dementia or cognitive impairment: the Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506-510.
Bondi M.W., Houston W.S., Salmon D.P., et al. Neuropsychological deficits associated with Alzheimer’s disease in the very-old: discrepancies in raw vs standardized scores. J Int Neuropsychol Soc. 2003;9:783-795.
Bornstein R.A., Pakalnis A., Drake M.E.Jr., et al. Effects of seizure type and waveform abnormality on memory and attention. Arch Neurol. 1988;45:884-887.
Boyle P.A., Paul R.H., Moser D.J., et al. Executive impairments predict functional declines in vascular dementia. Clin Neuropsychol. 2004;18:75-82.
Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239-259.
Brooks B.L., Iverson G.L., Holdnack J.A., et al. Potential for misclassification of mild cognitive impairment: a study of memory scores on the Wechsler Memory Scale-III in healthy older adults. J Int Neuropsychol Soc. 2008;14:463-478.
Brunnstrom H., Gustafson L., Passant U., et al. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch Gerontol Geriatr. 2009;49:146-149.
Carreno M., Donaire A., Sanchez-Carpintero R. Cognitive disorders associated with epilepsy: diagnosis and treatment. Neurologist. 2008;14(6 Suppl 1):S26-S34.
Chatfield M., Matthews F.E., Brayne C. Using the Mini-Mental State Examination for tracking cognition in the older population based on longitudinal data. J Am Geriatr Soc. 2007;55:1066-1071.
Cockburn J., Keene J., Hope T., et al. Progressive decline in NART score with increasing dementia severity. J Clin Exp Neuropsychol. 2000;22:508-517.
Crum R.M., Anthony J.C., Bassett S.S., et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;296:2386-2391.
Cullen B., Fahy S., Cunningham C.J., et al. Screening for dementia in an Irish community sample using MMSE: a comparison of norm-adjusted versus fixed cut-points. Int J Geriatr Psychiatry. 2005;20:371-376.
Davis R.N., Andresen E.N., Witgert M.E., et al. Is basic memory structure invariant across epilepsy patient subgroups? J Clin Exp Neuropsychol. 2006;28:987-997.
Delis D.C., Wetter S.R., Jacobson M.W., et al. Recall discriminability: utility of a new CVLT-II measure in the differential diagnosis of dementia. J Int Neuropsychol Soc. 2005;11:708-715.
Duff K., Beglinger L.J., Theriault D., et al. Cognitive deficits in Huntington’s disease on the Repeatable Battery for the Assessment of Neuropsychological Status. J Clin Exp Neuropsychol. 2010;32:231-238.
Duff K., Paulsen J.S., Beglinger L.J., et al. “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci. 2010;22:196-207.
Duff K., Palusen J.S., Mills J., et al. Mild cognitive impairment in prediagnosed Huntington disease. Neurology. 2010;75:500-507.
Duff K., Beglinger L.J., Adams W.H. Validation of the modified telephone interview for cognitive status in amnestic mild cognitive impairment and intact elders. Alzheimer Dis Assoc Disord. 2009;23:38-43.
Duff K., Beglinger L.J., Schultz S.K., et al. Practice effects in the prediction of long-term cognitive outcome in three patient samples: a novel prognostic index. Arch Clin Neuropsychol. 2007;22:15-24.
Farias S.T., Mungas D., Reed B.R., et al. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151-1157.
Feinstein A. The neuropsychiatry of multiple sclerosis. Can J Psychiatry. 2004;49:157-163.
Fitzpatrick A.L., Kuller L.H., Ives D.G., et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195-204.
Folstein M.F., Folstein S.E., McHugh P.R. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
Giovagnoli A.R., Erbetta A., Villani F., et al. Semantic memory in partial epilepsy: verbal and non-verbal deficits and neuroanatomical relationships. Neuropsychologia. 2005;43:1482-1492.
Glosser G., Donofrio N. Differences between nouns and verbs after anterior temporal lobectomy. Neuropsychology. 2001;15:39-47.
Godbolt A.K., Cipolotti L., Anderson V.M., et al. A decade of pre-diagnostic assessment in a case of familial Alzheimer’s disease: tracking progression from asymptomatic to MCI and dementia. Neurocase. 2005;11:56-64.
Goldberg-Stern H., Gadoth N., Cahill W., et al. Language dysfunction after frontal lobe partial seizures. Neurology. 2004;62:1637-1638.
Grabowska-Grzyb A., Jedrzejczak J., Naganska E., et al. Risk factors for depression in patients with epilepsy. Epilepsy Behav. 2006;8:411-417.
Gregory C., Lough S., Stone V., et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125:752-764.
Griffith H.R., Netson K.L., Harrell L.E., et al. Amnestic mild cognitive impairment: diagnostic outcomes and clinical prediction over a two-year time period. J Int Neuropsychol Soc. 2006;12:166-175.
Halpern C.H., Rick J.H., Danish S.F., et al. Cognition following bilateral deep brain stimulation surgery of the subthalamic nucleus for Parkinson’s disease. Int J Geriatr Psychiatry. 2009;24:443-451.
Harciarek M., Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer’s disease: a review. Neuropsychol Rev. 2005;15:131-145.
Heaton R.K., Grant I., Matthews C.G. Comprehensive Norms for Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, FL: Psychological Assessment Resources; 1991.
Helmstaedter C. Neuropsychological aspects of epilepsy surgery. Epilepsy Behav. 2004;5(Suppl. 1):S45-S55.
Henry J.D., Crawford J.R., Phillips L.H. A meta-analytic review of verbal fluency deficits in Huntington’s disease. Neuropsychology. 2005;19:243-252.
Hesdorffer D.C., Hauser W.A., Annegers J.F., et al. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246-249.
Hogervorst E., Bandelow S., Hart J.Jr., et al. Telephone word-list recall tested in the rural aging and memory study: two parallel versions for the TICS-M. Int J Geriatr Psychiatry. 2004;19:875-880.
Hoops S., Nazem S., Siderowf A.D., et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738-1745.
Huijbregts S.C., Kalkers N.F., de Sonneville L.M., et al. Cognitive impairment and decline in different MS subtypes. J Neurol Sci. 2006;245:187-194.
Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136-142.
Jellinger K.A., Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80-87.
Johnson S.A., Stout J.C., Solomon A.C., et al. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington’s disease. Brain. 2007;130(Pt 7):1732-1744.
Jokeit H., Schacher M. Neuropsychological aspects of type of epilepsy and etiological factors in adults. Epilepsy Behav. 2004;5(Suppl. 1):S14-S20.
Jonsson A., Andresen J., Storr L., et al. Cognitive impairment in newly diagnosed multiple sclerosis patients: a 4-year follow-up study. J Neurol Sci. 2006;245:77-85.
Kim H., Yi S., Son E.I., et al. Material-specific memory in temporal lobe epilepsy: effects of seizure laterality and language dominance. Neuropsychology. 2003;17:59-68.
Koenigs M., Young L., Adolphs R., et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908-911.
Kraus J.F., McArthur D.L., Silverman T.A., et al. Epidemiology of brain injury. In: Naravan R.K., Wilberger J.E., Povlishock J.T. Neurotrauma. New York: McGraw-Hill, 1996.
Langa K.M., Foster N.L., Larson E.B. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901-2908.
Lee G.P., Clason C.L. Classification of seizure disorder and syndromes, and neuropsychological impairment in adults with epilepsy. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:437-465.
Lehrner J, Gufler R., Guttmann G., et al. Annual conversion to Alzheimer disease among patients with memory complaints attending an outpatient memory clinic: the influence of amnestic mild cognitive impairment and the predictive value of neuropsychological testing. Wien Klin Wochenschr. 2005;117:629-635.
Lezak M.D., Howieson D.B., Loring D.W. The practice of neuropsychological assessment. In: Lezak M.D., Howieson D.B., Loring D.W. Neuropsychological Assessment. fourth ed. Oxford, UK: Oxford University Press; 2004:3-14.
Lines C.R., McCarroll K.A., Lipton R.B., et al. Telephone screening for amnestic mild cognitive impairment. Neurology. 2003;60:261-266.
Lippa C.F., Duda J.E., Grossman M., et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology and biomarkers. Neurology. 2007;68:812-819.
Looi J.C., Sachdev P.S. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology. 1999;53:670-678.
Mathias J.L., Burke J. Cognitive functioning in Alzheimer’s and vascular dementia: a meta-analysis. Neuropsychology. 2009;23:411-423.
Matthews F.E., Brayne C., Lowe J., et al. Epidemiological pathology of dementia: attributable-risks at death in the medical research council cognitive function and aging study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180
McCagh J., Fisk J.E., Baker G.A. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009;86:1-14.
McDonald C.R., Delis D.C., Norman M.A., et al. Response inhibition and set shifting in patients with frontal lobe epilepsy or temporal lobe epilepsy. Epilepsy Behav. 2005;7:438-446.
McDowell I., Kristjansson B., Hill G.B., et al. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377-383.
McKeith I., Cummings J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. 2005;4:735-742.
McKeith I.G., Dickson D.W., Lowe J., et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863-1872.
McKhann G.M., Albert M.S., Grossman M., et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803-1809.
Meador K.J. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002;58(8 Suppl 5):S21-S26.
Mensah S.A., Beavis J.M., Thapar A.K., et al. A community study of the presence of anxiety disorder in people with epilepsy. Epilepsy Behav. 2007;11:118-124.
Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex. 2007;43:583-600.
Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:90-100.
Mitchell A.J. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411-431.
Mittenberg W., Roberts D.M. Mild traumatic brain injury and postconcussion syndrome. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:430-436.
Mittenberg W., Strauman S. Diagnosis of mild head injury and the postconcussion syndrome. J Head Trauma Rehabil. 2000;15:783-791.
Molano J., Boeve B., Ferman T., et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133(Pt 2):540-556.
Montoya A., Price B.H., Menear M., et al. Brain imaging and cognitive dysfunctions in Huntington’s disease. J Psychiatry Neurosci. 2006;31:21-29.
Moulin C.J., James N., Freeman J.E., et al. Deficient acquisition and consolidation: intertrial free recall performance in Alzheimer’s disease and mild cognitive impairment. J Clin Exp Neuropsychol. 2004;26:1-10.
Nasreddine Z.S., Phillips N.A., Bedirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: Steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003.
Nazem S., Siderowf A.D., Duda J.E., et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57:304-308.
Nehl C., Paulsen J.S. Cognitive and psychiatric aspects of Huntington disease contribute to functional capacity. J Nerv Ment Dis. 2004;192:72-74.
Nehl C., Ready R.E., Hamilton J., et al. Effects of depression on working memory in presymptomatic Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:342-346.
Nelson, H., 1982. The National Adult Reading Test. NFER-Nelson, Windsor.
O’Bryant S.E., Humphreys J.D., Smith G.E., et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963-967.
Oyegbile T.O., Dow C., Jones J., et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736-1742.
Padovani A., Costanzi C., Gilberti N., et al. Parkinson’s disease and dementia. Neurol Sci. 2006;27(Suppl. 1):S40-S43.
Panayiotou A., Jackson M., Crowe S.F. A meta-analytic review of the emotional symptoms associated with mild traumatic brain injury. J Clin Exp Neuropsychol. 2010;32:463-473.
Parmenter B.A., Shucard J.L., Shucard D.W. Information processing deficits in multiple sclerosis: a matter of complexity. J Int Neuropsychol Soc. 2007;13:417-423.
Parsons T.D., Rogers S.A., Braaten A.J., et al. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurol. 2006;5:578-588.
Paulino Ramirez Diaz S., Gil Gregorio P., Manuel Ribera Casado J., et al. The need for a consensus in the use of assessment tools for Alzheimer’s disease: the Feasibility Study (assessment tools for dementia in Alzheimer Centres across Europe), a European Alzheimer’s Disease Consortium’s (EADC) survey. Int J Geriatr Psychiatry. 2005;20:744-748.
Paulsen J.S., Salmon D.P., Monsch A.U., et al. Discrimination of cortical from subcortical dementias on the basis of memory and problem-solving tests. J Clin Psychol. 1995;51:48-58.
Paulsen J.S., Stout J.C., DeLaPena J., et al. Frontal behavioral syndromes in cortical and subcortical dementia. Assessment. 1996;3:327-337.
Paulsen J.S., Zimbelman J.L., Hinton S.C., et al. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington’s disease. AJNR Am J Neuroradiol. 2004;25:1715-1721.
Petersen R., Smith G., Waring S., et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
Petersen R.C., Roberts R.O., Knopman D.S., et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
Psychological Corporation. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001.
Rao S.M., Leo G.J., Bernardin L., et al. Cognitive dysfunction in multiple sclerosis I Frequency, patterns, and prediction. Neurology. 1991;41:685-691.
Raspall T., Donate M., Boget T., et al. Neuropsychological tests with lateralizing value in patients with temporal lobe epilepsy: reconsidering material-specific theory. Seizure. 2005;14:569-576.
Richard I.H. Anxiety disorders in Parkinson’s disease. Adv Neurol. 2005;96:42-55.
Rohrer D., Salmon D.P., Wixted J.T., et al. The disparate effects of Alzheimer’s disease and Huntington’s disease on semantic memory. Neuropsychology. 13, 1999. 381–338
Roman G.C. Clinical forms of vascular dementia. In: Paul R.H., Cohen R., Ott B.R., et al. Vascular Dementia: Cerebrovascular Mechanisms and Clinical Management. Totowa, NJ: Humana Press; 2005:7-21.
Rowe K.C., Paulsen J.S., Langbehn D.R., et al. Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology. 2010;24:435-442.
Ryan L.M., Warden D.L. Post concussion syndrome. Int Rev Psychiatry. 2003;15:310-316.
Salmon D.P., Thomas R.G., Pay M.M., et al. Alzheimer’s disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59:1022-1028.
Schneider J.A., Arvanitakis Z., Bang W., et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197-2204.
Schoenberg M.R., Scott J.G., Duff K., et al. Estimation of WAIS-III intelligence from combined performance and demographic variables: development of the OPIE-3. Clin Neuropsychol. 2002;16:426-437.
Schretlen D.J., Shapiro A.M. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15:341-349.
Siegert R.J., Abernethy D.A. Depression in multiple sclerosis: a review Neurol Neurosurg. Psychiatry. 2005;76:469-475.
Smith G.E., Bondi M.W. Normal aging, mild cognitive impairment, and Alzheimer’s disease. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:762-780.
Smith G.E., Ivnik R.J., Lucas J. Assessment techniques: tests, test batteries, norms, and methodological approaches. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:38-57.
Smith-Seemiller L., Franzen M.D., Burgess E.J., et al. Neuropsychologists’ practice patterns in assessing premorbid intelligence. Arch Clin Neuropsychol. 1997;12:739-744.
Snowden J.S., Austin N.A., Sembi S., et al. Emotion recognition in Huntington’s disease and frontotemporal dementia. Neuropsychologia. 2008;46:2638-2649.
Solomon A.C., Stout J.C., Johnson S.A., et al. Verbal episodic memory declines prior to diagnosis in Huntington’s disease. Neuropsychologia. 2007;45:1767-1776.
Stout J.C., Rodawalt W.C., Siemers E.R. Risky decision making in Huntington’s disease. J Int Neuropsychol Soc. 2001;7:92-101.
Taylor R. National Adult Reading Test performance in established dementia. Arch Gerontol Geriatr. 1999;29:291-296.
Teng E.L., Chui H.C. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314-318.
Testa J.A., Ivnik R.J., Boeve B., et al. Confrontation naming does not add incremental diagnostic utility in MCI and Alzheimer’s disease. J Int Neuropsychol Soc. 2004;10:504-512.
Thompson J.C., Poliakoff E., Sollom A.C., et al. Automaticity and attention in Huntington’s disease: when two hands are not better than one. Neuropsychologia. 2010;48:171-178.
Tierney M.C., Black S.E., Szalai J.P., et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol. 2001;58:1654-1659.
Tombaugh T.N. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch Clin Neuropsychol. 2005;20:485-503.
Tranel D. Theories of clinical neuropsychology and brain-behavior relationships. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:25-37.
Tröster A.I. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentiation, early detection, and implications for mild cognitive impairment” and biomarkers. Neuropsychol Rev. 2008;18:103-119.
Tröster A.I., Fields J.A. Parkinson’s disease, progressive supranuclear palsy, corticobasal degeneration, and related disorders of the frontostriatal system. In: Morgan J.E., Ricker J.H. Textbook of Clinical Neuropsychology. New York: Taylor & Francis; 2008:536-577.
Tschanz J.T., Welsh-Bohmer K.A., Plassman B.L., et al. An adaptation of the Modified Mini-Mental State Examination: analysis of demographic influences and normative data: the Cache County study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28-38.
Videnovic A., Bernard B., Fan W., et al. The Montreal Cognitive Assessment as a screening tool for cognitive dysfunction in Huntington’s disease. Mov Disord. 2010;25:401-404.
Weintraub D., Stern M.B. Psychiatric complications in Parkinson disease. Am J Geriatr Psychiatry. 2005;13:844-851.
Wilkinson G.S., Robertson G. WRAT4: Wide Range Achievement Test professional manual, fourth ed. Lutz, FL: Psychological Assessment Resources; 2006.
Williams-Gray C.H., Foltynie T., Lewis S.J., et al. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20:477-505.
Wilson R.S., Aggarwal N.T., Barnes L.L., et al. Cognitive decline in incident Alzheimer disease in a community population. Neurology. 2010;74:951-955.
Young L., Bechara A., Tranel D., et al. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65:845-851.
Zekry D., Hauw J.J., Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50:1431-1438.