CHAPTER 325 Neuropathology of Traumatic Brain Injury
It is conceptually useful to divide traumatic brain injury (TBI) into primary and secondary types of damage provided that it is understood that the primary types of brain damage are not static but dynamic lesions that evolve over time and thus may be potentially amenable to treatment.1–3
Primary traumatic brain damage is the result of mechanical forces producing tissue deformation at the moment of injury. These deformations may directly damage the blood vessels, neurons and their processes, glia, and microglia in a focal, multifocal, or diffuse pattern and initiate dynamic and evolving processes that differ for each cellular component (Table 325-1). At the least severe end of the spectrum, the changes may be only molecular, and with increasing damage, microscopic and macroscopic lesions become increasingly apparent. This temporal evolution implies that at any given point in time, the pathologic picture is a summative complex of the evolving cascades of damage involving the blood vessels, neurons and their processes, glia, and microglia.4
TABLE 325-1 Features of Primary Traumatic Brain Injury
Note: Most fatal cases of traumatic brain injury are mixtures of focal, multifocal, and diffuse injuries.
Secondary traumatic brain damage (Table 325-2) occurs as a complication of the different types of primary brain damage and includes ischemic and hypoxic damage, expansion of hemorrhagic lesions, cerebral swelling, and consequences of raised intracranial pressure (ICP). Secondary insults such as hypotension, hypoxemia from respiratory complications, electrolyte abnormalities, and pyrexia may further add to the total injury burden.
TABLE 325-2 Features of Secondary Traumatic Brain Injury
|
2 Focal, multifocal, or diffuse ischemic injury from perfusion failure in microcirculation or macrocirculation
|
CPP, cerebral perfusion pressure; ICP, intracranial pressure.
Biomarkers of this structural damage, such as S-100, tau, neuron-specific enolase (NSE), and myelin basic protein (MBP), may have potential utility as diagnostic, prognostic, and therapeutic adjuncts.5
Consciousness is mediated by parallel distributed neuronal networks involving thalamic (cholinergic) and extrathalamic (serotoninergic, noradrenergic, and histaminergic) ascending arousal systems, responsible for wakefulness, and ascending sensory cortical and thalamocorticothalamic loops responsible for awareness of self. These neuronal networks may all be damaged to varying degrees by direct mechanical injury or raised ICP, leading to the various grades of coma, or may be differentially damaged, as in the vegetative state in which the circuits subserving wakefulness are intact but the awareness circuits are not functioning.6
In any given patient, there may be a complex and dynamic interplay of the different primary and secondary types of brain damage and secondary insults to produce a constellation of lesions that is unique both in anatomical site and number. For example, the consequences of primary vascular damage may be bleeding into brain tissue to produce an intracerebral hematoma or interference in the perfusion of the brain tissue with resultant ischemic damage (secondary brain damage), or a combination of the two resulting in increased ICP leading to its sequelae. Thus, head injury is not a single entity but consists of many different types of lesion that may occur rarely in isolation, or more commonly, in varied combinations. This heterogeneity of lesions in TBI makes it unlikely that there is any single pharmacologic agent that will be effective in treating all these intersecting cascades of damage.7,8
Age, genetic predisposition, preexisting disease, drugs, alcohol, and nutritional status are all factors that may influence traumatic injury. The delayed consequences of traumatic injury may continue to evolve for years after the event and include processes such as atrophy, gliosis, neural deafferentation and reinnervation, synaptic plasticity, trans-synaptic degeneration, immune reactions, wallerian degeneration, and neurogenesis.9
Traumatic Axonal Injury
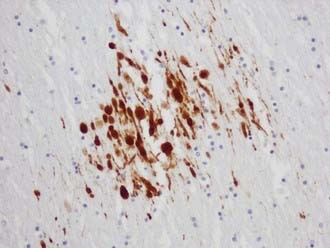
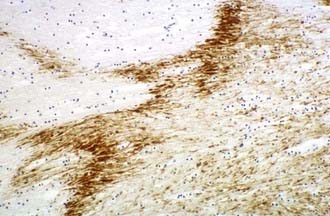
The visualization of damaged axons by traditional silver stains has been greatly improved by a battery of immunocytochemical methods targeting molecules such as amyloid precursor protein-β (APP-β) carried by fast axoplasmic transport and cytoskeletal proteins such as the various neurofilament proteins (NFPs) and tubulins carried by slow axoplasmic transport. Impairment of fast and slow axoplasmic flow leads to progressive axonal swelling and eventual disconnection and formation of axonal retraction bulbs (ARBs) (Fig. 325-1), a process termed secondary axotomy. Current techniques are unable to distinguish traumatic axonal injury (TAI) due to mechanical deformation from axonal injury (AI) due to nontraumatic pathologic processes such as infarcts, hemorrhages, abscesses, neoplasms, and demyelination. The term AI is thus nonspecific and refers to axonal damage of any etiology. TAI may be focal, multifocal, or diffuse. There is increasing recognition of different types of TAI (Table 325-3) and that the anatomic distribution in a detailed neuropathologic work-up including large brain sections may give a clue to the putative mechanism of injury.10
| Primary axotomy | Tissue tears or lacerations at the severe end of mechanical deformation |
| Secondary axotomy | Progressive impairment of axonal transport resulting in axonal swelling and eventual disconnection with the formation of axonal retraction bulbs |
| Neurofilament compaction | Neurofilament side-arm cleavage |
| Impaired axonal transport without swelling | Impaired axoplasmic transport |
APP-immunopositive axonal damage is an almost universal finding in cases of fatal TBI,11 whereas traditional silver stains only showed damage in about 30% of fatal head injuries.12
Axonal Amyloid Precursor Protein in Traumatic Axonal Injury
APP can be demonstrated immunohistochemically in damaged axons within 35 minutes of the insult.13 APP is normally anterogradely transported along the axon by fast axoplasmic transport as a membrane-bound vesicular protein that accumulates rapidly proximal to the site of injury.14 Reversible APP-β immunoreactive axonal changes have been shown in some experimental animal studies, but whether this also occurs in humans is unknown.
Axonal Amyloid Precursor Protein Patterns in Traumatic Axonal Injury
Multifocal (diffuse) traumatic axonal injury is defined as axonal swellings and bulbs scattered throughout the white matter of cerebral hemispheres, brainstem, and cerebellum as individually affected axons. A spectrum of change is seen that is usually multifocal rather than truly diffuse. Vascular axonal injury (VAI) is defined as axonal swellings and bulbs that cluster around infarct or in ischemic brain in a distribution of vascular compromise associated with raised ICP. The affected axons are often arranged in clusters that have a zigzag, irregular, or geographic pattern15 (Fig. 325-2).
Recent studies in humans have confirmed that APP-immunopositive axons may persist for years after the injury and that this may be associated with the formation of intra-axonal amyloid-β but without evidence of extracellular amyloid-β plaque deposition.16,17 This is in contrast to previous studies that have shown extracellular deposition of amyloid-βcontaining plaque-like structures close to damaged axons just hours after trauma18,19 linking TBI to the development of Alzheimer’s disease.20
Pathogenesis of Traumatic Axonal Injury
Detailed experimental investigation of the pathobiology of TAI has revealed a spectrum of axonal damage.21 Most traumatically injured axons are not mechanically severed at the time of impact as originally believed; instead, they progress gradually to axonal disconnection over several hours.22–24
Axonal deformation at the moment of injury results in a focal impairment of axoplasmic transport and subsequent focal swelling of the axon due to abnormal accumulation of neurofilaments and membranous organelles, and over the next 6 to 12 hours, there is disconnection of the proximal axonal segment from the distal segment. The separated distal segment undergoes wallerian degeneration over time. The transient focal disruption of the axonal membrane allows an influx of Ca2+, which activates multiple Ca2+-dependent deleterious cascades that involve calpains, caspases, and calcineurin and cause disruption of the cytoskeleton. Calcium-linked dephosphorylation of neurofilament side arms results in focal neurofilament compaction (NFC) type of axonal injury.25,26
Diffuse Axonal Injury
Diffuse axonal injury (DAI) was first described as a clinicopathologic syndrome in patients unconscious from the time of trauma, with widespread traumatic axonal damage throughout the brain in the absence of intracranial mass lesions. Similar less severe axonal changes were also found in mild and moderate TBI resulting in the concept that these axonal changes were also the substrate for the transient disorders of consciousness associated with mild and moderate TBI.27 Thus, it was conceptualized that there was a spectrum of DAI, with the severe end of the spectrum correlating with post-traumatic dementia and the mild end of the spectrum correlating with the concussive syndromes.28
The application of more sensitive immunocytochemical techniques such as APP has expanded the spectrum of axonal damage demonstrable in mild, moderate, and severe TBI.29
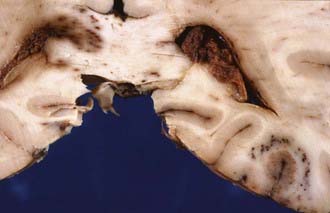
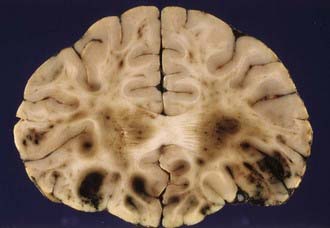
The severity of DAI has been graded on the basis of the combination of macroscopic and microscopic lesions using silver impregnation techniques to identify axonal swellings and bulbs.12 In grade 1 DAI, widespread axonal damage is present in the corpus callosum, white matter of the cerebral hemispheres, brainstem, and cerebellum. In grade 2 DAI, there are additional focal abnormalities (usually small hemorrhages) in the corpus callosum (Fig. 325-3). In grade 3 DAI, there are, in addition to the findings of grade 2, small focal lesions in the rostral brainstem (Fig. 325-4). Focal lesions in the corpus callosum and dorsolateral rostral brainstem in grades 2 and 3 DAI may be visible on neuroimaging, but in grade 1 DAI without macroscopic focal marker lesions, conventional imaging techniques may not reveal any abnormalities. Neuroimaging of the small focal hemorrhagic lesions in the deep white matter, corpus callosum, and rostral brainstem have been used as surrogate markers of DAI.
The principal mechanical loading associated with the induction of DAI is rotational acceleration of the unrestricted head, resulting in shear, tensile, and compressive strains that produce dynamic deformation of brain tissue.30 The large size of the human brain plays an important role in the generation of relatively high shear strains between different regions of tissue. The concentration of AI in midline structures may be due to dural barriers such as falx cerebri and tentorium cerebelli, which act as partial barriers to motion of the brain in a given direction.
Although DAI may occur in the absence of impact (contact) forces, most fatal human head injuries are the result of head impacts.31 The contact forces when the head is struck by or strikes a hard object characteristically produce focal lesions such as contusions, but they may also induce rapid acceleration-deceleration, potentially damaging axons. Nonimpact rotational acceleration of the head during car crashes may be followed by single or multiple head impacts against the interior of the motor car or road in the case of pedestrians, motor cyclists, and pedal cyclists.
Traumatic Vascular Injury
There is a large potential spectrum of traumatic vascular injuries that may occur in isolation or in different combinations (Table 325-4). Injury to the intraparenchymal blood vessels may be (1) focal vascular injury, such as contusion, intracerebral hemorrhage, or subarachnoid hemorrhage; (2) multifocal vascular injury, which includes a combination of those injuries; or (3) diffuse vascular injury, such as petechial hemorrhage or microhemorrhage. Injury to the extraparenchymal blood vessels may include (1) injury that bridges veins and arteries, such as acute subdural hematoma (ASDH) or chronic subdural hematoma (CSDH); (2) injury to meningeal arteries and veins, such as extradural (epidural) hematoma (EDH); (3) injury to the venous sinuses, such as EDH; (4) injury to the large arteries in neck; (5) injury to the internal carotid and vertebral arteries, including thrombosis, dissection, subintimal hemorrhage, laceration, and arteriovenous fistula; (6) injury to the blood vessels of the circle of Willis; and (7) injury to the middle, anterior, and posterior cerebral arteries, basilar artery, intracranial internal carotid artery, and vertebral artery, including thrombosis, dissection, subintimal hemorrhage, laceration, and arteriovenous fistula.
| Intraparenchymal Blood Vessels | |
| Focal vascular injury | Contusions or intracerebral hemorrhage |
| Multifocal vascular injury | Contusions and intracerebral hemorrhage |
| Diffuse vascular injury | Numerous petechial hemorrhages and microhemorrhages |
| Extraparenchymal Blood Vessels | |
| Bridging veins and arteries | Acute subdural hematoma, chronic subdural hematoma, subarachnoid hemorrhage |
| Meningeal arteries and veins | Extradural (epidural) hematoma, subarachnoid hemorrhage |
| Venous sinuses | Extradural (epidural) hematoma, subarachnoid hemorrhage |
| Large Arteries in the Neck | |
| Internal carotid and vertebral arteries | Thrombosis, dissection, subintimal hemorrhage, laceration, A-V fistula |
| Blood Vessels of the Circle of Willis | |
| Middle, anterior, and posterior cerebral arteries; basilar artery; intracranial, internal carotid, and vertebral arteries | Thrombosis, dissection, subintimal hemorrhage, laceration, A-V fistula, subarachnoid hemorrhage |
A-V, arteriovenous.
Cerebral Contusions
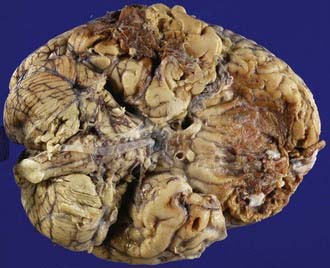
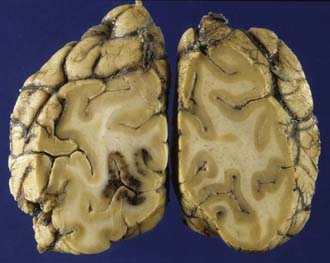
Cerebral contusions (bruises) are focal injuries that result when mechanical forces damage the small blood vessels (capillaries, veins, or arteries) and other tissue components (nerve and glial cells and their processes) of the neural parenchyma. The bleeding from damaged blood vessels is usually the most obvious feature on macroscopic and microscopic examination, the manifestations ranging from microhemorrhages to confluent hemorrhage disrupting the tissue (Fig. 325-5). Contusions are typically surface lesions of the brain, but some also include similar hemorrhagic lesions in the deeper structures of the brain.
Contusions are dynamic lesions that evolve with time. The progressive expansion or “blossoming” of contusions is demonstrated well by computed tomography (CT) and magnetic resonance (MRI) imaging.32 The damage to the blood vessels sets in train an intertwined cascade of events leading to hemorrhage, breakdown of the blood-brain barrier, and infarction secondary to compromise of the microcirculation (including compromise resulting from thrombotic occlusion of blood vessels).33 This produces a spectrum of macroscopic changes varying from focally dilated blood vessels to burst brain lobes.
Acute surface contusions are characterized by focal vascular damage leading to punctate hemorrhages or small linear hemorrhages aligned at right angles to the cortical surface due to extension of hemorrhage along the perivascular plane. Occasionally, local subarachnoid hemorrhage adjacent to a contusion accumulates within the sulcus to form a sulcal hematoma. This may lead to an erroneous diagnosis of intracerebral hemorrhage on head CT scan. The radiating streak-like cortical hemorrhages on microscopy consist of perivascular accumulations of red cells, and serial sectioning may show evidence of focal traumatic rupture or tearing of the affected blood vessel with bleeding into the perivascular space or neural parenchyma. Damaged blood vessels may thrombose, leading to additional ischemic complications. Contusions often increase in size over hours to days owing to the evolving events related to the interplay of hemorrhage, vasogenic edema, and ischemic necrosis. In the first 24 hours after trauma, contused brain tissue biopsies show an inflammatory response, which is predominantly intravascular and consists of vascular margination of polymorphonuclear leukocytes. Extravascular polymorphonuclear leukocytes can be demonstrated in injured brain tissue only a few minutes after TBI. Three to 5 days later, the inflammation is predominantly parenchymal and consists of monocyte-macrophages, reactive microglia, polymorphonuclear cells, and CD4 and CD8 T-lymphocytes, correlating with delayed postcontusional brain swelling. Inflammatory cells produce free radicals and cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α, which mediate blood-brain barrier injury that leads to brain swelling and induce DNA fragmentation in neurons and oligodendrocytes.34
Surgical and autopsy studies have also provided evidence that apoptosis (an active process requiring energy and protein synthesis) also occurs in human cerebral contusions in addition to necrosis.35 Although necrosis and apoptosis have been considered as distinct separate mechanisms, it is possible that in TBI they represent poles in a continuum of cell injury.36 TUNEL-positive neurons and oligodendrocytes have been identified in human contusions.37 Increased expression of the antiapoptotic protein Bcl-2 has been observed in surviving neurons after human TBI.38 Bcl-2 proteins may participate in the control of cell death and survival by regulating the release of mitochondrial cytochrome c, which is involved in the activation of caspases, especially caspase-3, which cleaves substrates associated with DNA damage and repair, including DNA-fragmentation factor (DFF45/40), poly (ADP-ribose) polymerase (PARP), and the cytoskeletal proteins actin and laminin. Caspase-3 is activated in the injured cerebral cortex of human TBI.38
The next phase is that of resorption of damaged tissue and progressive reactive gliosis. Very small hemorrhages may be completely resorbed within 2 to 3 weeks, whereas larger hemorrhages may take many weeks or months to resorb. The extravasated red blood cells are sequentially broken down to various blood pigments, including hemosiderin. Necrotic brain tissue is phagocytosed by macrophages derived from monocytes at sites where there has been disruption of the blood-brain barrier and lipid macrophages appear 2 to 5 days after the insult. The end result of these processes of resorption is a shrunken brown cystic lesion involving the crests of gyri (plaque jaunt), often with fibrous scarring of adjacent meninges with the formation of a meningocerebral cicatrix (Fig. 325-6).
Contusions most frequently involve the inferior frontal lobes and the inferolateral temporal lobes and poles where brain tissue comes in contact with the irregular bony surfaces of the anterior and middle cranial fossae due to the relative motion of the brain and skull at these sites.39 The occipital lobes and cerebellum are rarely contused in the absence of skull fractures.
Types of Contusion
Coup contusions are contusions that occur beneath the site of impact. Coup contusions are a type of contact injury and are produced by compressive forces operating beneath an area of skull in-bending or tensile forces generated by the negative pressure produced beneath an area of skull in-bending suddenly snapping back into place. Contrecoup contusions occur opposite the impact site. Intermediate coup contusions are intracerebral lesions that occur deeply within the neural parenchyma between the impact site and the opposite side of the brain. Fracture contusions occur beneath the site of a fracture. Gliding contusions occur in the parasagittal regions and are believed to be due to the rostrocaudal movement of the brain resulting from impact or impulsive loading forces. The hemorrhages involve the deeper layers of the cortex and the convolutional white matter and spare the surface of the gyrus. Gliding contusions are often associated with DAI.40 Herniation contusions involve the medial temporal lobes and the cerebellar tonsils and are produced by movement of the brain impacting on the rigid tentorium cerebelli or the bony margins of the foramen magnum.
Contusion Patterns and Head Impacts
Previous studies have suggested that the contusion pattern depends on the direction and magnitude of the impacting force and whether the head is accelerated by the impact (e.g., blow to the movable head), is not accelerated by the impact (e.g., blow to the supported head), or is in a state of acceleration at the moment of impact (e.g., fall on head). Thus, a lateral impact in the frontotemporal area may produce a surface contusion of the contralateral temporal lobe and contusions of both uncinate gyri; a lateral temporoparietal impact may result in a contrecoup contusion of the temporal lobe; a midline occipital impact may produce bilateral frontal and temporal lobe contusions; an occipital impact lateral to the midline may cause contrecoup contusions of the frontal and temporal lobes; frontal impacts may result in bilateral or unilateral contusions of the frontal and temporal lobes; and vertex impacts may produce contusions of the brainstem and tears of the corpus callosum and pituitary stalk.41 The surface contusions will be most severe in the frontal and temporal lobes irrespective of the cranial impact site provided the forces acting on the head are sufficient to impart movement of the brain over the irregular bony surfaces of the anterior and middle cranial fossae. Both frontal and occipital impacts result in contusions that are most severe in the frontal lobes, and it therefore cannot be extrapolated that the site of head impact necessarily is diametrically opposite the area of most severe contusion.40
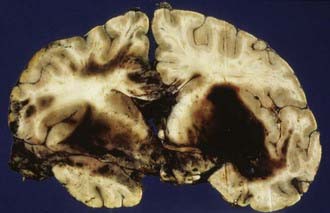
Patients with contusions may show progressive or sudden deterioration. Sudden deterioration is a feature especially of patients with severe bifrontal contusions, temporal pole pulping, and “burst” lobes (Fig. 325-7). Contusions are also one of the causes of neurological deterioration after a lucid interval (“talk-and-die” patients), mimicking extracerebral hematomas.42 However, contusions may be totally absent in patients who have sustained severe or lethal head injury.
Traumatic Subarachnoid Hemorrhage
Traumatic SAH may be a marker of adverse outcome owing to its association with other important cerebral lesions (subdural hematomas [SDHs] and contusions), which directly influence outcome, or to vasospasm and ischemia produced by mechanisms similar to those of aneurysmal SAH.43
Fatal, massive, nonaneurysmal basal subarachnoid hemorrhage may follow assaults in the context of minor neck trauma and alcoholic intoxication.44 The victims die rapidly, although a few cases have been described in which the patient has remained conscious for a few hours. Rupture of the vertebral artery is the most common cause of this type of injury, and the ruptures may be extracranial (28.8%), intracranial (50%), or within the junction zone (21.2%).45
Sequelae of traumatic SAH include the following:
Traumatic Intracerebral Hemorrhage
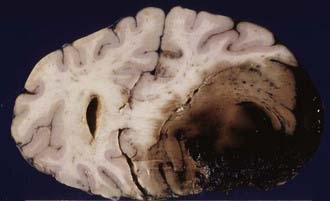
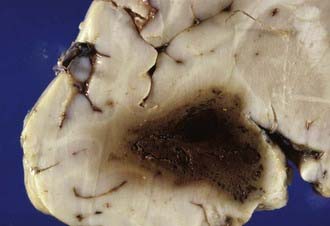
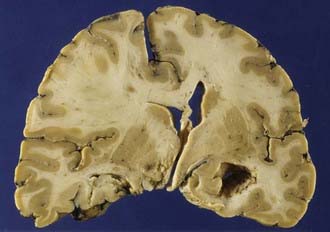
Traumatic intracerebral hemorrhages are defined as hematomas 2 cm or larger not in contact with the surface of the brain and are present in 15% of autopsy cases of severe head injury.46 Lobar intracerebral hemorrhages are those that involve a lobe of the brain and occur usually in the temporal (Fig. 325-8) or frontal lobes. The pathogenesis of intracranial hemorrhage (ICH) is likely due to deformation and rupture of the intrinsic blood vessels (single or multiple) at the time of injury. Damage to multiple small blood vessels may result in the coalescence of many smaller hemorrhages. Traumatic ICHs are often multiple (Fig. 325-9), and 28% are associated with subdural and 10% with epidural hematoma, and they can arise in areas that appear normal on CT scans obtained soon after injury.47 One third to one half of patients with ICH are unconscious on admission, and up to 20% demonstrate a classic lucid interval before the onset of coma.47 Patients who are deeply comatose with large hematomas have a high mortality rate. Large hematomas act as space-occupying lesions and result in intracranial hypertension and then transtentorial herniation. Animal studies have shown marked hypoperfusion around hematomas as well as ipsilateral hemisphere ischemia.
Neuroimaging has shown that many traumatic hematomas develop hours to days after the injury and may not be visible on scanning soon after the traumatic event. Delayed traumatic intracerebral hematoma (DTICH) is a common cause of secondary neurological deterioration after head injury, and progressive increase in size of the ICH has been reported in up 51% of patients on repeated CT scans in the first 24 hours.48
Traumatic Intraventricular Hemorrhage
A small amount of intraventricular hemorrhage (IVH) is frequently found in head-injured patients who do not survive long enough to reach the hospital, but bleeding may be significant (at the severe end of the spectrum) (Fig. 325-10). It is often impossible to determine the source of hemorrhage, but bleeding may be due to small tears in the veins of the ventricle walls; tears in the corpus callosum, septum pellucidum, and fornices; or tears in the choroid plexus. It has been suggested that the sudden dilation of the ventricular system at the time of impact leads to deformation and rupture of subependymal veins.
Delayed Deterioration after Traumatic Brain Injury
Patients who “talk and die” after TBI constitute an important group that would be expected to survive because the brain has not sustained a diffuse injury sufficient to produce an initial coma. In 75% of cases, the deterioration is due to an expanding intracranial hematoma.42 Between 12% and 32% of patients speak at some time between trauma and subsequent deterioration (“talk and deteriorate”), and 30% to 40% of these die or are left with severe disability.49
Extradural (Epidural) Hematoma
EDH occurs in about 2% of all types of head injury and in up to 15% of fatal head injuries.50 The clots are most frequently found in the temporoparietal regions (73%), where the middle meningeal arteries and veins have been damaged usually by a fracture involving the squamous temporal bone. Eleven percent of clots occur in the anterior cranial fossa (anterior meningeal artery), 9% in the parasagittal regions (sagittal sinus), and 7% in the posterior fossa (occipital meningeal artery and transverse and sigmoid sinuses).51 Bruising of the overlying scalp is usually a reliable guide to the site of the hematoma.
About 10% to 40% of EDHs are of venous origin as the result of tearing of venous dural sinuses, emissary veins, or venous lakes within the dura mater.52 Most traumatic venous EDHs occur in children, and most are not associated with a skull fracture.52
Often, the initial injury may be apparently trivial, and the patient may experience a lucid interval (only present in about 20% of EDHs). In about one third of cases, there are also other significant brain injuries such as ASDHs, contusions, and lacerations, and then the patient may experience no lucid interval and be unconscious from the time of injury.51 The systematic use of CT has resulted in the increased recognition of EDH from about 4% before to 9% after the introduction of head CT scanning.53
Subdural (Intradural) Hematoma
The outer membrane constitutes a source of inflammatory, angiogenic, fibrinolytic, and coagulation factors and is infiltrated by variable proportions of acute and chronic inflammatory cells associated with increased concentrations of the inflammatory cytokines IL-6 and IL-8.54 Striking eosinophilic infiltration of the neomembranes may occur and lead to a futile search for an associated infectious, inflammatory, or neoplastic condition.
Studies of the composition of the hematoma fluid have found marked imbalance of factors involved in regulation of coagulation and fibrinolysis, the effect of which is to prevent clotting of the hematoma fluid, and it has been suggested that the chronic SDH is a type of local coagulopathy.55
Acute Subdural Hematoma
There are two main types of traumatic ASDH. In traumatic ASDH related to contusions and lacerations, the subdural hematoma forms adjacent to damaged brain, often in association with severe diffuse primary brain damage, and these patients are unconscious from the time of injury. Often, the ASDH is continuous through contused, lacerated brain tissue with an intracerebral hemorrhage. This complex of SDH, cerebral contusion or laceration, and adjacent intracerebral hematoma is termed a burst lobe (the temporal or frontal lobes are most frequently involved) (see Fig. 325-7). Patients with burst lobes often show delayed neurological deterioration at about the third or fourth day after injury due to swelling of the damaged brain.
The second type of ASDH is related to rupture of bridging veins (those segments of the superficial cerebral veins that cross the subdural space to reach the venous sinuses) or occasionally to rupture of superficial cortical arteries or vessels within vascular stalks bridging the subdural space. Cortical bridging veins within the subarachnoid space are of uniform thickness, whereas in the potential subdural space, they are of irregular thickness, with the wall in some areas consisting only of endothelium, basement membrane, sparse collagen fibers, and a single layer of dural border cells.56 This predisposes to rupture into the potential subdural space rather than the subarachnoid space. In some cases of ruptured bridging vein ASDH, there is little or no associated brain damage, and these patients may experience a brief lucid interval before undergoing rapid neurological deterioration similar to that seen in typical cases of EDH. Unfortunately, the neurological deterioration is often so rapid that these patients fare no better than those in whom the hematoma is merely an extension of severe primary brain damage. Bridging vein ASDHs are found in about 13% of fatal TBI cases in the Glasgow series. It has been shown that bridging veins are most susceptible to angular acceleration forces and that 73% of traumatic ASDHs occur as the result of falls and assaults when short-duration, high-strain rate loading typically occurs.57 Only 11% of ASDHs occurred in the occupants of vehicle crashes, in which the angular acceleration is often of longer duration and less likely to rupture the bridging vessels.57
The reported mortality rate of traumatic ASDH varies from 30% to 90%, with the lower mortality rates occurring in patients who are operated on within 4 hours of injury.58 The severity of the underlying brain injury determines the outcome, even when the surgery has been prompt. This poor outcome has been correlated with neuropathologic studies showing ischemic brain damage in the hemisphere underlying the hematoma.59 An important factor leading to this ischemic damage is raised ICP producing impaired cerebral perfusion. Removal of the ASDH may result in the immediate reversal of global ischemia. However, mass effect and hemisphere compression are not the only factors of importance; hemisphere swelling beneath an ASDH often occurs even when the hematoma is thin. Excessive activation of excitatory neurotransmitter receptors, particularly the glutamatergic N-methyl-D-aspartate (NMDA) receptor, can cause neuronal damage indistinguishable from ischemic necrosis.
Chronic Subdural Hematoma
A subdural hematoma is chronic when it is discovered 2 to 3 weeks or longer after the initiating injury. Most patients are elderly people or chronic alcoholics, and cerebral atrophy appears to be an important predisposition.60,61 The lesions are bilateral in about 15% to 20% of cases. The head injury is often mild and, in up to half of cases, is denied altogether.60,61 The exact cause of the hemorrhage into the subdural space is usually unknown, although often attributed to rupture of a bridging vein. An atrophic brain permits the development of a subdural hematoma without the development of intracranial hypertension, although paradoxically, brain distortion is often so severe that even when the hematoma is evacuated, the brain remains depressed beneath the dura. Neuroimaging has shown that most smaller ASDHs normally resolve spontaneously and do not progress to a chronic SDH.
Brain Swelling
Massive swelling of all or part of the brain may be due to increased tissue water content of the brain (cerebral edema), increased intravascular blood volume (congestive brain swelling), or a combination of the two. Cerebral edema may be divided into cytotoxic, vasogenic, osmotic, hydrocephalic-interstitial, and hydrostatic subtypes.62
Cytotoxic and Vasogenic Edema
Cytotoxic (intracellular) edema is the result of an influx of ion particles (Na+) due to failure of energy-dependent ion pumps, leading to a shift of water from the extracellular to the intracellular space. Energy failure may be due to mitochondrial dysfunction as the result of ischemia or direct mechanical injury of the mitochondria. Cytotoxic edema affects all cell types, but astrocytic swelling is especially prominent because of localization of aquaporin-4 (AQP-4), a bidirectional transmembrane water channel, to the perivascular foot processes at the blood-brain barrier (BBB).63 AQP-4 plays an essential role in the formation and resorption of edema fluid from the brain parenchyma. AQP-4 deletion in models of cytotoxic edema reduces edema. Because the BBB remains intact, there is no enhancement on CT or MRI scans after intravenous contrast enhancement.
Impairment of the BBB leads to vasogenic edema due to a shift of water from the intravascular to the extravascular compartments and is characterized by protein-rich fluid in the extracellular space (hypodense white matter on CT). AQP-4 deletion in vasogenic edema results in decreased edema reabsorption.63
Diffusion-weighted imaging (DWI) and the calculated apparent diffusion coefficient (ADC) are able to differentiate vasogenic and cytotoxic edema.64 Cytotoxic and vasogenic edema often occur together or sequentially.
Brain Swelling in Head Injury
Diffuse Swelling of One Cerebral Hemisphere
Diffuse swelling of one cerebral hemisphere unrelated to contusion, intracerebral hemorrhage, or infarction may be associated with an ipsilateral ASDH (Fig. 325-11) but may also occur with larger epidural hematomas or as an isolated event.
Diffuse Cerebral Swelling of the Entire Brain
The reported incidence of diffuse brain swelling following head trauma on head CT scanning varies from 5% to 40%.65,66 Diffuse cerebral swelling occurs more frequently in children than in adults and may occur rapidly.67 A particularly severe catastrophic fatal form without obvious evidence of contusions or hemorrhages on neuroimaging occurs in young children. The pathogenesis of this progressive diffuse swelling unresponsive to treatment is poorly understood.68
Brain Damage Secondary to Raised Intracranial Pressure
Patterns of Raised Intracranial Pressure
Transtentorial Hernia from a Unilateral Supratentorial Mass Lesion
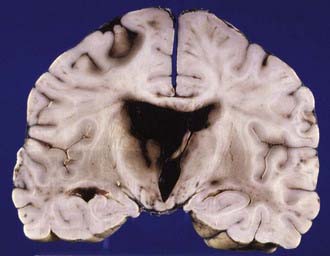
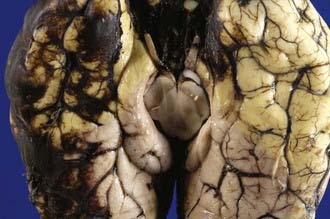
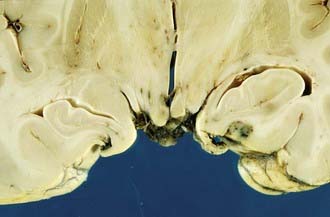
In raised ICP from supratentorial space-occupying lesions (e.g., intracerebral hematoma), the medial part of the temporal lobe on the side of the lesion is squashed against the midbrain (Fig.325-12) and squeezed through the tentorial hiatus (incisura), resulting in a swollen necrotic uncal hernia. Anteriorly placed mass lesions may produce uncal and anterior parahippocampal herniation, whereas more posteriorly placed mass lesions produce deep grooving and herniation of the posterior part of the parahippocampal gyrus.
The medial temporal hernia may also compress one or more branches of the posterior cerebral artery that supplies the inferior temporal and occipital lobes. If the hernia is of sufficient size and the vascular obstruction is intermittent, then variable hemorrhagic infarction in the posterior cerebral artery territories may occur, usually involving the posterior inferior temporal lobe and the medial occipital lobe (Fig. 325-13), including the calcarine cortex. Cortical blindness (Anton’s syndrome) results if the patient survives, and there may also be impairment of the ability to form new memories because of bilateral hippocampal involvement.
In transtentorial herniation, the parahippocampal gyri show medial wedge-shaped areas of hemorrhagic necrosis visible with the naked eye (Fig. 325-14) or nonhemorrhagic necrosis visible only on microscopy. These changes are invariably present when the ICP is greater than 5.3 kilopascal (kPa) (40 mm Hg) during life, in most patients with ICP between 2.7 and 5.3 kPa (22 and 40 mm Hg), and in no patients with ICP less than 2.7 kPa (20 mm Hg).69
Bilateral (central) transtentorial herniation occurs when there is diffuse swelling of both cerebral hemispheres (Fig. 325-15).
Tonsillar Herniation (Medullary Coning)
Medullary coning with rapid death can occur after lumbar puncture in a patient with raised ICP.
Transfalcine (Subfalcine) Herniation
Subfalcine herniation of the cingulate gyrus occurs when a mass lesion in a cerebral hemisphere enlarges sufficiently to squeeze the ipsilateral gyrus beneath the free margin of the falx cerebri (see Fig. 325-11). This may lead to compression of the pericallosal branches of the anterior cerebral artery, with a spectrum of ischemic and hemorrhagic infarction in the corpus callosum in the anterior cerebral artery territories of supply of the superior aspect of the cerebellar hemispheres. There may be compromise of branches of the superior cerebellar arteries, producing hemorrhagic and ischemic necrosis in the superior cerebellar artery vascular territories.
Ischemic Brain Damage in Traumatic Brain Injury
Neuropathologic evidence of ischemic brain damage is found in about 90% of patients who survive for several hours after injury and is significantly associated with a known episode of hypoxia, such as cardiac arrest, status epilepticus, or raised ICP.70
In experimental models, global ischemia and mechanical injury appear synergistic, producing more severe changes together than individually. When combined, mild global ischemic and fluid percussion mechanical insults that are insufficient to cause neuronal death by themselves cause massive hippocampal neuronal necrosis, even when the trauma and ischemia are separated by 24 hours.71
Microvascular Damageand Traumatic Brain Injury
Studies have revealed profound regional reductions in flow (18 mL/100 g per minute) around contusions and intracerebral hematomas, consistent with a “traumatic penumbra.” The tissue in the traumatic penumbra,72 similar to the ischemic penumbra, is at most risk for undergoing irreversible damage. This cerebral ischemia is most likely the result of microvascular compromise. In a study, intravascular microthrombosis correlated with selective neuronal necrosis.73
Focal Ischemia in Traumatic Brain Injury
Brainstem Lesions
CT and MRI have shown that the brainstem is frequently damaged in TBI.74 Brainstem lesions were found on MRI in 64% of patients with severe TBI.75 Before the advent of modern neuroimaging, primary focal brainstem lesions were considered to occur rarely in isolation from damage in the cerebral hemispheres.
Hemorrhage is one of the earliest recognizable signs of injury, and its presence in the brainstem (macroscopic or microscopic) may be the only evidence of a fatal injury. Numerous small hemorrhages in the brainstem, usually in association with similar lesions scattered throughout the cerebral hemispheres, are often seen in patients who die within minutes of a closed head injury. These are believed to be vascular markers of a type of diffuse brain damage incompatible with life.76 The bleeding may be periarterial, perivenous, pericapillary, or within the neuropil; microscopy reveals many more hemorrhages than can be seen with the naked eye.
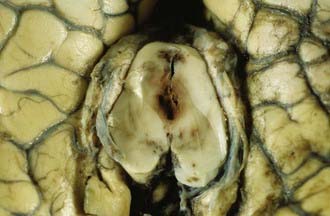
Pontomedullary Disruption
Traumatic damage of the pontomedullary junction is a well-recognized forensic entity. Tears at the pontomedullary junction may be complete or partial (Fig. 325-16), and differentiation from artifactual disruption may be aided by the presence of microscopic hemorrhages in the marginal surrounding tissue, subarachnoid or intraventricular hemorrhage, and adjacent axonal accumulation of APP. Complete disruptions are immediately fatal, but partial tears are compatible with several weeks’ survival.77
Blumbergs PC, Reilly PL, Vink R. Trauma. In: Love S, Louis DN, Ellison DW, editors. Greenfield’s Neuropathology. 8th ed. London: Hodder Arnold; 2008:733-832.
Blumbergs PC. Pathology. In: Reilly PL, Bullock R, editors. Head Injury. Pathophysiology and Management. 2nd ed. London: Hodder Education; 2005:41-72.
Chen XH, Johnson VE, Uryu K, et al. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214-223.
Farkas O, Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. In: Weber J, Maas A, editors. Progress in Brain Research. Philadelphia: Elsevier BV; 2007:43-59.
Gennarelli TA, Thibault LE, Graham DI. Diffuse axonal injury: an important form of traumatic brain damage. Neuroscientist. 1998;4:202-215.
Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993;11(suppl 1):5-11.
Kochanek PM, Berger RP, Bayir H, et al. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14:135-141.
Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537-546.
Mannion RJ, Cross J, Bradley P, et al. Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma. 2007;24:128-135.
Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of non-disruptive axonal injury: a review. J Neurotrauma. 1997;14:419-440.
Meaney DF, Margulies SS, Smith DH. Diffuse axonal injury. J Neurosurg. 2001;95:1108-1110.
Nortje J, Menon DK. Traumatic brain injury: physiology, mechanisms, and outcome. Curr Opin Neurol. 2004;17:711-718.
Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. 2002;16:220-242.
Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007;22:778-784.
Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1-12.
Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169-181.
Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719-738.
Sahuquillo J, Poca MA, Amoros S. Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr Pharm Des. 2001;7:1475-1503.
Smith DH, Meaney DF. Axonal damage in traumatic brain injury. Neuroscientist. 2000;6:483-495.
Unterberg AW, Stover J, Kress B, et al. Edema and brain trauma. Neuroscience. 2004;129:1021-1029.
Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185-192.
Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. In: Weber J, Maas A, editors. Progress in Brain Research. Philadelphia: Elsevier BV; 2007:303-316.
Zhang X, Chen Y, Jenkins LW, et al. Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Crit Care. 2005;9:66-75.
1 Sahuquillo J, Poca MA, Amoros S. Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr Pharm Des. 2001;7:1475-1503.
2 Blumbergs PC. Pathology. In: Reilly PL, Bullock R, editors. Head Injury. Pathophysiology and Management. 2nd ed. London: Hodder Education; 2005:41-72.
3 Blumbergs PC, Reilly PL, Vink R. Trauma. In: Love S, Louis DN, Ellison DW, editors. Greenfield’s Neuropathology. 8th ed. London: Hodder Arnold; 2008:733-832.
4 Farkas O, Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. In: Weber J, Maas A, editors. Progress in Brain Research. Philadelphia: Elsevier BV; 2007:43-59.
5 Kochanek PM, Berger RP, Bayir H, et al. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14:135-141.
6 Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537-546.
7 Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719-738.
8 Nortje J, Menon DK. Traumatic brain injury: physiology, mechanisms, and outcome. Curr Opin Neurol. 2004;17:711-718.
9 Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169-181.
10 Abou-Hamden A, Blumbergs PC, Scott G, et al. Axonal injury in falls. J Neurotrauma. 1997;14:699-713.
11 Gentleman SM, Roberts GW, Gennarelli TA, et al. Axonal injury: a universal consequence of fatal closed head injury. Acta Neuropathol. 1995;89:537-543.
12 Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49-59.
13 Hortobágyi T, Wise S, Hunt N, et al. Traumatic axonal damage in the brain can be detected using β-APP Immunohistochemistry within 35 min after head injury to human adults. Neuropathol Appl Neurobiol. 2007;33:226-237.
14 Sherriff FE, Bridges LR, Gentleman SM, et al. Markers of axonal injury in post mortem human brain. Acta Neuropathol (Berl). 1994;88:433-439.
15 Reichard RR, White CLIII, Hladik CL, et al. Beta-amyloid precursor protein staining of non-accidental central nervous system injury in paediatric autopsies. J Neurotrauma. 2003;20:347-355.
16 Chen XH, Johnson VE, Uryu K, et al. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214-223.
17 Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185-192.
18 Roberts GW, Gentleman SM, Lynch A, et al. A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422-1423.
19 Roberts GW, Gentleman SM, Lynch A, et al. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1994;57:419-425.
20 Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. In: Weber J, Maas A, editors. Progress in Brain Research. Philadelphia: Elsevier BV; 2007:303-316.
21 Smith DH, Meaney DF. Axonal damage in traumatic brain injury. Neuroscientist. 2000;6:483-495.
22 Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of non-disruptive axonal injury: a review. J Neurotrauma. 1997;14:419-440.
23 Meaney DF, Margulies SS, Smith DH. Diffuse axonal injury. J Neurosurg. 2001;95:1108-1110.
24 Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1-12.
25 Okonkwo DO, Pettus EH, Moroi J, et al. Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury. Brain Res. 1998;784:1-6.
26 Marmarou CR, Walker SA, Davis CL, et al. Quantitative analysis of the relationship between intra-axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J Neurotrauma. 2005;22:1066-1080.
27 Gennarelli TA, Thibault LE, Graham DI. Diffuse axonal injury: an important form of traumatic brain damage. Neuroscientist. 1998;4:202-215.
28 Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993;11(suppl 1):5-11.
29 Blumbergs PC, Scott G, Manavis J, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055-1056.
30 Ommaya AK, Goldsmith W, Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br J Neurosurg. 2002;16:220-242.
31 McLean AJ. Brain injury without head impact? J Neurotrauma. 1995;12:621-625.
32 Statham PF, Johnston RA, McPherson P. Delayed deterioration in patients with traumatic frontal contusions. J Neurol Neurosurg Psychiatry. 1989;52:351-354.
33 Lafuente JV, Cervos-Navarro J. Craniocerebral trauma induces hemorrheological disturbances. J Neurotrauma. 1999;16:425-430.
34 Holmin S, Soderlund J, Biberfeld P, et al. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291-298.
35 Zhang X, Chen Y, Jenkins LW, et al. Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Crit Care. 2005;9:66-75.
36 Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3-11.
37 Ng I, Yeo T, Tang W, et al. Apoptosis occurs after cerebral contusions in humans. Neurosurgery. 2000;46:949-956.
38 Clark R, Kochanek P, Chen M, et al. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 1999;13:813-821.
39 Adams JH, Scott G, Parker LS, et al. The contusion index: a quantitative approach to cerebral contusions in head injury. Neuropathol Appl Neurobiol. 1980;6:319-324.
40 Adams JH, Doyle D, Graham DI, et al. Gliding contusions in non-missile head injury in humans. Arch Pathol Lab Med. 1986;110:485-488.
41 Gurdjian ES. Cerebral contusions: re-evaluation of the mechanism of their development. J Trauma. 1976;16:35-51.
42 Reilly P, Graham D, Adams J, Jennett B. Patients with head injury who talk and die. Lancet. 1975;2:375-377.
43 Taneda M, Kataoka K, Akai F, et al. Traumatic subarachnoid hemorrhage as a predictable indicator of delayed ischemic symptoms. J Neurosurg. 1996;84:762-768.
44 Simonsen J. Traumatic subarachnoid hemorrhage in alcohol intoxication. J Forensic Sci. 1963;8:97-116.
45 Koszyca B, Gilbert JD, Blumbergs PC. Traumatic subarachnoid hemorrhage and extracranial vertebral artery injury: a case report and review of the literature. Am J Forensic Med Pathol. 2003;24:114-118.
46 Adams JH. Head injury. In: Adams JH, Duchen LM, editors. Greenfield’s Neuropathology. 5th ed. London: Edward Arnold; 1992:106-152.
47 Soloniuk D, Pitts LH, Lovely M, et al. Traumatic intracerebral hematomas: timing of appearance and indications for operative removal. J Trauma. 1986;26:787-794.
48 Oertel M, Kelly DF, McArthur D, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96:109-116.
49 Lobato RD, Rivas JJ, Gomez PA, et al. Head-injured patients who talk and deteriorate into coma: analysis of 211 cases studied with computerised tomography. J Neurosurg. 1991;75:256-261.
50 Freytag E. Autopsy findings in head injuries from blunt forces: statistical evaluation of 1,367 cases. Arch Pathol. 1963;75:402-413.
51 Jamieson KG, Yelland JD. Extradural hematoma: report of 167 cases. J Neurosurg. 1968;29:13-23.
52 Yilmazlar S, Kocaeli H, Dogan S, et al. Traumatic epidural hematomas of non-arterial origin: analysis of 30 consecutive cases. Acta Neurochir. 2005;147:1241-1248.
53 Guillermain P. Traumatic extradural hematomas. Adv Neurotraumatol. 1986;1:1-50.
54 Frati A, Salvati M, Mainiero F, et al. Inflammation markers and risk factors for recurrence in 35 patients with a post-traumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100:24-32.
55 Stoodley M, Weir B. Contents of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:425-434.
56 Yamashima T, Friede RL. Why do bridging veins rupture into the virtual subdural space? J Neurol Neurosurg Psychiatry. 1984;47:121-127.
57 Gennarelli TA, Thibault LE. Biomechanics of acute subdural hematoma. J Trauma. 1982;22:680-686.
58 Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74:212-218.
59 Graham D, Adams J, Doyle D. Ischemic brain damage in fatal non-missile head injuries. J Neurol Sci. 1978;39:213-234.
60 Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:399-406.
61 Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, et al. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107:223-229.
62 Unterberg AW, Stover J, Kress B, et al. Edema and brain trauma. Neuroscience. 2004;129:1021-1029.
63 Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007;22:778-784.
64 Hanstock CC, Faden AI, Bendall MR, et al. Diffusion-weighted imaging differentiates ischemic tissue from traumatised tissue. Stroke. 1994;25:843-848.
65 Eisenberg HM, Gary H, E Jr, Aldrich EF, et al. Initial CT findings in 753 patients with severe head injury: a report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688-698.
66 Marmarou A, Fatouros PP, Barzo P, et al. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J Neurosurg. 2000;93:183-193.
67 Zwienenberg M, Muizelaar JP. Severe pediatric head injury: the role of hyperemia revisited. J Neurotrauma. 1999;16:937-943.
68 Graham DI, Ford I, Adams JH, et al. Fatal head injury in children. J Clin Pathol. 1989;42:18-22.
69 Adams JH, Graham DI. The relationship between ventricular fluid pressure and the neuropathology of raised intracranial pressure. Neuropathol Appl Neurobiol. 1976;2:323-332.
70 Graham DI, Ford I, Adams JH, et al. Ischemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52:346-350.
71 Jenkins LW, Moszynski K, Lyeth BG, et al. Increased vulnerability of the mildly traumatised rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;477:211-244.
72 Menon DK. Procrustes, the traumatic penumbra and perfusion pressure targets in closed head injury. Anesthesiology. 2003;98:805-807.
73 Stein SC, Graham DI, Chen XH, et al. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. J Neurosurg. 2004;54:687-691.
74 Mannion RJ, Cross J, Bradley P, et al. Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma. 2007;24:128-135.
75 Firsching R, Woischneck D, Diedrich M, et al. Early magnetic resonance imaging of brain stem lesions after severe head injury. J Neurosurg. 1998;89:707-712.
76 Tomlinson BE. Brain-stem lesions after head injury. J Clin Pathol. 1970;4:154-165.
77 Pilz P, Strohecker J, Grobovschek M. Survival after traumatic pontomedullary tear. J Neurol Neurosurg Psychiatry. 1982;45:422-427.