CHAPTER 6 Neurons and Neuroglia
Neurons
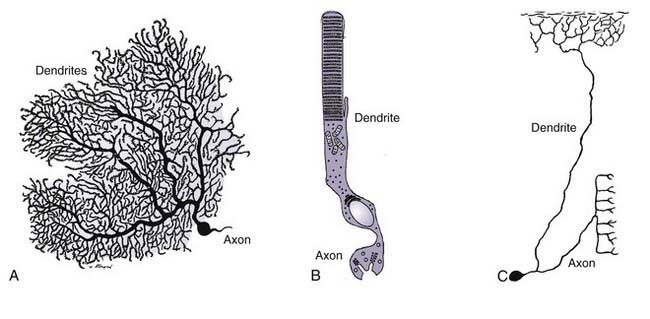
Webster’s dictionary defines a neuron as “the structural and functional unit of the nervous system, consisting of the nerve cell body and all its processes, as the dendrites and axon.”1 One may quibble with the attribution of structural properties to the neuron, but otherwise, this is a relatively concise and accurate definition. The problem of defining the neuron in any more detail is curiously difficult. This difficulty arises because more than any other cell type in the body, there is an enormous diversity of structural and functional characteristics possessed by the various cells that go by this name. Consider as three examples the Purkinje cell, the retinal photoreceptor cell, and the dorsal root ganglion neuron (Fig. 6-1). All three are considered neurons, yet the commonalities among them are difficult to pin down. The Purkinje cell is the most straightforward of the three (Fig. 6-1A). There is a clear cellular domain that is the neuronal dendrite, a prominent cell body, and an obvious single axon. The parts of the retinal photoreceptor (Fig. 6-1B) are less easily categorized. The apical portion serves as a receptor for light, yet it is hardly a normal dendrite. The cell body is apparent, but the axon is unconventional in its thickness and appearance. The dorsal root ganglion neuron (Fig. 6-1C) also diverges from the classic neuronal form. A cell body is clear, but the process that emanates from it bifurcates; one branch connects to a peripheral target, and the other branch connects to a target within the central nervous system (CNS). Functionally, the two branches are distinct, but morphologically, they are nearly identical; under most definitions, however, neither would qualify as a dendrite.
Neuronal Function
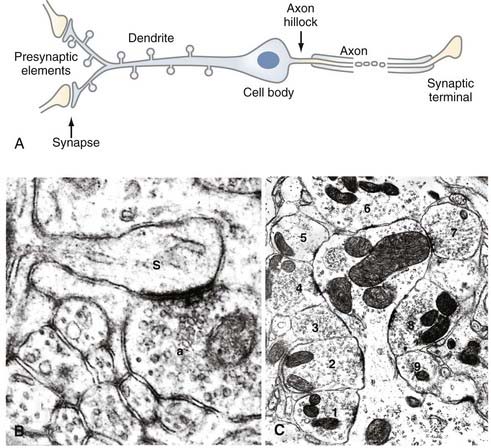
The emphasis in the previous section was on the diversity of nerve cell types. Discussion of the functioning of the adult neuron, however, requires a focus on commonalities. The goal of this section is not to describe the biochemical and biophysical mechanisms that the cell uses to create, store, and transmit electrical signals; this information is provided in Chapter 49. Rather, this section follows a packet of information as it moves through a typical nerve cell in the brain, with the goal of introducing the biology of the cell. Figure 6-2A is a diagram of one typical neuron. Synaptic input occurs on all synaptic spines, as shown in the enlarged spines at the far left. Synaptic spines can receive input from a single (Fig. 6-2B) or multiple (Fig. 6-2C) axons.
The information arrives at the nerve cell at a highly specialized structure known as a synapse. At this site, a process from the previous neuron in the circuit approaches to within a few hundred angstroms of the next cell but does not touch it. The gap between the cells remains continuous with the extracellular space. The presence of the gap requires a specialized mechanism for transferring an electrical signal from the presynaptic to the postsynaptic cell. This transfer is achieved by way of the presynaptic cell secreting a chemical transmitter substance into the gap. The transmitter is usually a small molecule such as acetylcholine or noradrenaline, but it can also be a peptide such as substance P or vasointestinal peptide. Diffusion carries this pulse of chemicals across the gap to the membrane of the postsynaptic cell, which is covered with receptor proteins. These receptors recognize the secreted chemicals and transform the information from the chemical pulse into an electrical event that can be propagated down the neuron. This mechanism is discussed in more detail later. A synapse can occur virtually anywhere on the postsynaptic cell, but the most common location is on the neuronal dendrite. The dendrite is usually the first part of the neuron to initiate an electrical response to a signal from the presynaptic neuron. On many neurons, the dendrites have specialized adornments known as spines, and it is with these structures that the presynaptic neuron forms a synapse. Spines can vary in size and shape, but they are generally no more than a few micrometers in length, with a bulb-like shape at the end of a tapered shaft (see Fig. 6-2). An electrical signal that initiates in a spine travels to the dendritic branch on which it occurs and moves down the dendrite toward the cell body. Although many neuritic processes look similar (especially in culture), a dendrite can usually be distinguished from an axon because it is tapered—decreasing in diameter as distance from the cell body increases. A nerve cell can have a single dendritic shaft emanating from its cell body (as in the Purkinje cell in Fig. 6-1A), or it can have several. A typical pyramidal cell in the cerebral cortex, for example, has a single apical dendrite but also several basal dendrites; a cerebellar granule cell has four to six short dendrites in a star-shaped configuration around the cell body.
The nerve cell body is the most prominent feature of the neuron in most basophilic stain preparations (e.g., hematoxylin-eosin). If one considers size alone, however, the cell soma is usually considerably smaller than the dendrite in both surface area and volume.2 This relatively small soma-to-process volume ratio represents a logistic problem for the cell body: the genetic machinery is located exclusively in the nucleus, yet the products of this machinery must be transported to sites on the cell that are up to a meter away.
The electrical signal reaching the cell body from the dendrite travels to the point on the cell where the axon emerges. The axon of a neuron is the structure that captures the summed electrical information in the dendrites and cell body and routes it to the next neuron (or target organ) in the circuit. Morphologically, the axon can be distinguished from the dendrite by the fact that it has a typically constant caliber over its entire length. Soon after the axon leaves the cell body, a specialized region known as the initial segment (or axon hillock) can be identified. This part of the cell is the biochemical boundary of the axon and the point of initiation of the action potential. Up to this point, the information packets from the dendrites and cell body travel primarily by electrotonic spread, with different packets of electrical activity coming together and summing in a graded (analog) manner. By contrast, the axon transmits information in a strictly digital fashion. If the combined electrogenic signal reaching the axon from the cell body is sufficiently strong, the axon will fire and pass the information along; otherwise, the signal stops and proceeds no farther in the circuit. If the decision is a “go,” the axon transforms the information into a self-propagating electrical wave known as an action potential that travels undiminished down the axon to its end. The action potential is an electrical signal that results from the coordinated functioning of sodium and potassium channels (see Chapter 49 for details), usually in collaboration with glial cells (see later).
Sensory Neurons
Mechanical Receptors
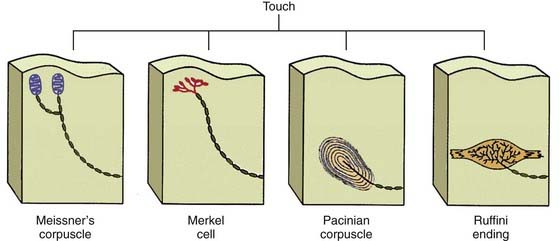
The simplest receptor cells of this type are used to receive information about touch and pain. As might be expected, most are located in the skin and other integuments. Receptors for light touch are illustrated in Figure 6-3. These receptor endings have different precisions and sensitivities and are specialized to receive different types of stimuli.3 Each receptor represents a specialized ending of a sensory ganglion axon. These include Ruffini’s endings or pacinian corpuscles (for deep receptors) and Merkel cells or Meissner’s corpuscles (for more superficial receptors). Sometimes the neurite of a mechanoreceptor is wrapped around the interfusal muscle fibers of one of the striated muscles. The cellular deformation associated with movement of the axonal membrane activates a series of stretch-sensitive ion channels. The ionic current through these channels initiates the electrical activity that signals a sensory stimulus to the organism.
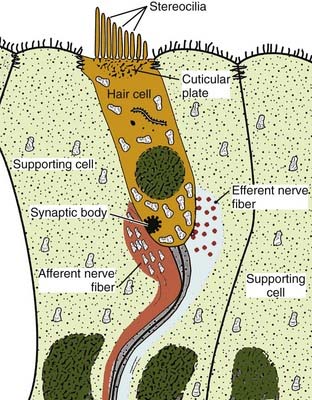
A subset of mechanical receptors has evolved to serve the auditory and vestibular systems. The principal function of these cells is the same as for light touch receptors: deformation of a hair opens a number of specialized ion channels, which results in the generation of an electrical signal. The difference in the acousticovestibular system is that the “hairs” are actually cilia on the basal surface of the cell and hence are part of the receptor cell itself. Indeed, the ion channels that are opened in response to movement of the hair are located at the tip of the cilium. A diagram of this cell type is shown in Figure 6-4. In the auditory system, the vibration of sound waves is transduced into the vibration of fluid in the cochlea. Receptor cells at different positions in the cochlear spiral respond to different auditory frequencies and transmit both pitch and volume information to the auditory system. In the vestibular system, a morphologically similar configuration of receptors is found in the semicircular canals. Movement of the head in any of the three orthogonal planes leads to movement of fluid in the canals. This movement displaces the cilia of the vestibular receptors and initiates an electrical signal that is transmitted through the eighth nerve to the brain, where the vestibular system interprets the information to determine the orientation and movement of the organism in space. In each of these systems, however, the common feature of the receptor is that deflection of a hair leads to deformation of the cellular membrane, which in turn results in opening of a specific set of ion channels. The resulting change in conductance leads to an electrical “packet” of information that moves along the neuron to the rest of the brain.
Chemical Receptors
A second class of receptors responds directly to specific chemicals by generating an electrical response that can be propagated to other parts of the nervous system. Receptors in this group are found in the papilla of the tongue, where they respond to the presence of salt, sweet, bitter, and sour and project to the gustatory centers of the brain by way of the seventh and ninth cranial nerves. A more sophisticated and chemically diverse set of sensors in this class is found in the lining of the nasal epithelium.4 These receptors are responsible for endowing the organism with a sense of smell. This more elaborate mechanism of chemical reception is based on a large family of G protein–linked receptor molecules. Family members of this receptor class number in the thousands, each apparently encoded by a different gene. Each receptor recognizes a different chemical structure and responds to binding of the chemical by stimulating release of the bound G protein, which activates adenylate cyclase. This, in turn, leads to an elevation in cyclic adenosine monophosphate, which then opens a cyclic nucleotide–gated channel and results in the generation of an electrical signal that is transmitted along the neuron to the olfactory portions of the brain.
Physical Receptors
Certain nerve cells are sensitive to the physical properties of their environment. The temperature receptors of the skin are one example of this group of receptor cells; the light-sensitive cells of the eye are another. These latter cells are known as photoreceptors, and they respond to electromagnetic radiation in the visible spectrum. They are further subdivided into rods and cones, depending on their wavelength specificity. Cones are narrowly tuned to transmit information about color, whereas rods have a broad frequency range and are most useful in low-light situations. Both classes of photoreceptors contain many flat membrane sacks that are stacked like pancakes at one end of the cell (see Fig. 6-1B). These sacks contain the photosensitive pigment rhodopsin, which allows light energy to be transduced into an electrical signal. Reception of light in the photoreceptor evolved through the use of the same class of G protein–linked receptor molecules as in the olfactory system. When light strikes the rhodopsin molecule, a chemical cascade occurs that is nearly identical to that described for the olfactory receptors.
Neuronal Structure
Visualization of Neurons
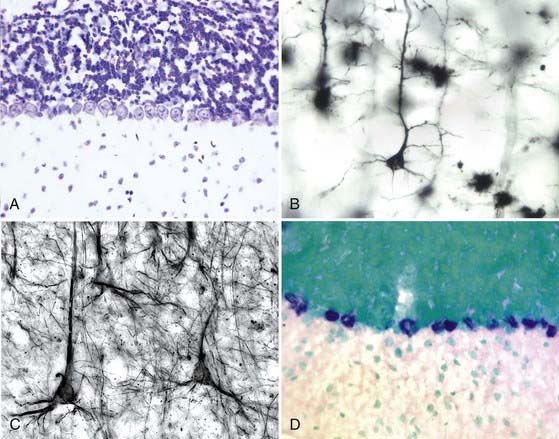
Neurons, like most other cells, are pale, clear, and difficult to see in living tissue. As a consequence, many of the significant advances in the study of the nervous system were made possible by improved methods of visualizing the nerve cell and its processes. The basophilic dyes are the oldest and still most widely used method of staining nervous tissue. Common stains of this class include hematoxylin and cresyl violet. These dyes bind avidly to RNA and DNA and thus highlight the heterochromatin of the nucleus and the rough endoplasmic reticulum of the cytoplasm (Fig. 6-5A). Basophilic stains are most commonly used on sections with a thickness ranging from 2 to 20 µm. Examination of nervous tissue stained with such reagents results in a clear picture of the cell body and proximal dendrites; the axons and distal dendrites are usually invisible, so white matter and neuropil are generally clear of stain.
During the 1800s, silver salts were found to have a special avidity for nervous tissue. Because of their binding to neuron-specific classes of intermediate filaments, a variety of protocols were developed that revealed the neuronal axon with great clarity. Among this class of stains, the Bodian and Bielschowsky stains are still commonly used. A special class of silver stain is the Golgi impregnation method. In this procedure, pieces of tissue are incubated for many weeks in heavy metal salts. During this time, a small number of cells take up the salts, with their entire intracellular spaces being filled. The tissue is then embedded and sectioned. When the sections are “developed” in reducing agents, an opaque black precipitate is formed that fills the impregnated cells completely. For unknown reasons, only 1% to 2% of the cells react in this fashion (seemingly at random). The details of an individual cell can be seen against a clear background (Fig. 6-5B). Although the technique reveals the finest details of dendritic structure, axons are more resistant to filling and are commonly invisible in Golgi preparations. The technique is used to best advantage in sections ranging from 80 to 120 µm thick.
In the second half of the 20th century, new technologies dramatically expanded our ability to visualize and analyze the nerve cells of the brain. Beginning in the 1950s and 1960s, the transmission electron microscope led to a quantum leap in the ability to resolve the details of nerve cellular structure. In this method, small pieces of tissue (typically 2 to 3 mm wide) are embedded in plastic and cut with a glass or diamond blade in sections ranging in thickness from tens to hundreds of nanometers. Before embedding, the tissues are usually stained with uranyl acetate, lead citrate, and osmium tetroxide, lipophilic dyes that reveal membrane structure with a high degree of clarity. Phosphotungstic acid is a frequently used stain that has a particular affinity for synapses. The resolution afforded by the electron microscope allows the fine structure of the cell to be revealed, and the organelles of the cell body can be analyzed. The unique advantages of using electron microscopy to view the nervous system include the ability to resolve synaptic structures (see Fig. 6-2B and C) such as synaptic vesicles, details of the presynaptic and postsynaptic membranes, and the material of the synaptic cleft. Axon and dendrite morphology, with their unique collections and arrangements of filaments, can also be seen.
In the 1970s and 1980s, serum antibodies were developed as highly specific stains by using the techniques of immunocytochemistry. Lightly fixed tissue is exposed to an antiserum or monoclonal antibody raised against a particular neuronal epitope. The antibody selectively binds to the neural structure that contains that epitope. Most frequently, this primary antibody is revealed through the application of a secondary antibody that is derivatized to carry a detection molecule—either a fluorescent compound, such as fluorescein or rhodamine, or an enzyme, such as horseradish peroxidase or alkaline phosphatase. In the former case, the location of the antibody is determined by examining the tissue with a fluorescence microscope. In the latter instance, the marker enzyme is localized through the use of a specific substrate whose action deposits an insoluble, chromagenic product. Immunocytochemistry is most commonly used to reveal proteins (e.g., tyrosine hydroxylase, microtubule-associated protein 2 [MAP2], the α6 receptor of γ-aminobutyric acid [GABAA]), but the location of carbohydrates (e.g., polysialic acid, gangliosides, chondroitin sulfate proteoglycan) can also be determined. Immunocytochemistry can be applied at the electron microscopic level as well, where peroxidase or gold particles are used to reveal the location of the secondary antibody. Figure 6-5C presents an example of immunocytochemistry using an antibody against a neurofilament protein, one of the major cytoskeletal components of neuronal cytoplasm.
In the 1980s and 1990s, advances in molecular biology enabled the detection of messenger RNA for specific proteins through a technique known as in situ hybridization (see Chapter 3). Medium- to high-abundance messages are localized to the cell body transcribing them by exposing lightly fixed tissue to labeled nucleotide probes that are complementary to the message sequence. The labeled probe is detected either because of its radioactivity (35S-labeled nucleotide precursors are commonly used) or because of the presence of other derivatives (such as biotin or digoxigenin attached to nucleotides). In either case, the tissue is treated with proteinase to remove the bound proteins and then hybridized at temperatures that ensure the specificity of the probe-message interaction. If radioactivity is used, the location of the hybridized message is determined by apposing the section to x-ray film or by dipping the section in liquid photographic emulsion, which forms a thin coating and is subsequently fogged wherever the labeled nucleotide is found. If nonradioactive methods are used in the detection protocol, their location is revealed with a secondary probe (derivatized avidin in the case of biotin-labeled probes or antibody to digoxigenin). A digoxigenin-labeled probe hybridized to the message for the Purkinje cell–specific nuclear receptor RORα is shown in Figure 6-5D. An important aspect of the interpretation of such images is that the location of the message marks the cell body where the message is synthesized, not the place where the protein is found. For example, the image shown in Figure 6-5D could represent messenger RNA encoding RORα (a nuclear protein), the δ2-glutamate receptor (found predominantly in dendritic spines), or synaptophysin (found principally in the presynaptic axon terminal).
Dendritic Structure
The dendrite represents a smooth, tapered extension of the neuronal cell body.5 The main tapered dendritic shafts have many of the same organelles as the cell body, including mitochondria, microtubules, neurofilaments, smooth endoplasmic reticulum, and ribosomes. The existence of ribosomes is correlated with clear evidence of local protein synthesis, and studies have illustrated that a unique subset of the total population of messenger RNA is transported into the dendrites6 based on specific sequences contained within the message. Despite this rough similarity to the cell body, the dendritic domain has several unique features that make it identifiable with both light and electron microscopy. Biochemically, the dendrite contains several proteins that are located nowhere else in the nerve cell. These proteins include MAP2, as well as several specific receptor species and channel proteins that differ from cell to cell.
As outlined earlier, the most typical site of contact between a postsynaptic cell and a presynaptic axon is a dendritic spine (see Fig. 6-2B and C). Spines can be found on the cell body (although this is rare in adults) or on the main dendritic branches, but they are most common on the fine terminal branches of the dendrites. The development of a dendritic spine is heavily influenced by the presence of a presynaptic afferent but does not require it. Studies of several pathologic conditions have shown that well-proportioned spines and their associated ultrastructure can be maintained (and possibly developed) in the absence of any presynaptic element. In this case, the structure is usually ensheathed by a glial cell.
Cell Body Structure
Neurons are synthetically active cells. Although the brain typically makes up only 2% of the weight of the human body, it consumes as much as 25% of the oxygen used by the organism. It has been estimated that more than 50% of human genes are either highly enriched or unique to the nervous system. Alternative splicing of transcripts is also more prevalent in the brain than in any other tissue.7 The ultrastructure of the nerve cell body reflects a high level of protein translation. The Golgi apparatus and rough endoplasmic reticulum are prominent features of the cell body. When there is a main apical dendrite, the Golgi apparatus and rough endoplasmic reticulum are often located between the nucleus and the emanation point of the dendrite. The concentration of rough endoplasmic reticulum is sufficiently great that the term Nissl substance is used to describe its prominent, dark floccular appearance on light microscopic images of basophilic dye preparations. Mitochondria are also in abundance, as might be expected given the high aerobic activity of the neuron. Primary and secondary lysosomes are present, and with aging, these organelles tend to accumulate large quantities of a waxy substance known as lipofuscin.8 The nucleus of most neurons is oval or spherical, with relatively clear nucleoplasm. In most large neurons there is a single prominent nucleolus, whereas in small neurons, such as the granule cells of the olfactory bulb, hippocampus, and cerebral or cerebellar cortex, there are scattered clumps of heterochromatic material with no nucleolus.
Axonal Structure
Although it is less prominent than in dendrites, recent work has shown that protein synthesis can also be detected in the axon.9 Even with this local synthetic source, the topology of the nerve cell presents a significant maintenance problem to neurons when the synaptic terminal is more than a meter away from the cell body. To achieve effective translocation between the protein synthetic machinery in the perikaryon and the axon terminal, the axon uses several mechanisms of transport. In the orthograde direction (cell body to axon terminal), bulk materials tend to flow at a pace of about 0.5 mm/day. Organelles and some proteins, however, are transported by rapid axonal transport, which can achieve rates of 400 mm/day. Material also moves from the axon terminal to the cell body, in the retrograde direction, at half the speed of fast orthograde transport.
Synaptic Structure
The physiology of synaptic function is covered in detail in Chapter 49. Underlying the details of ionic fluxes, however, are a number of crucial elements in the cell biologic structure of the neuron. On the presynaptic side, the axon terminates in a highly specialized presynaptic structure (see Fig. 6-2B and C). The shaft of the axon broadens in diameter and assumes a shallow cup-like form. Although microtubules are a prominent feature of the central axon domain, few extend into the terminal area. Instead, the major structural elements of the presynaptic terminal include neurofilaments and actin filaments. Mitochondria are more abundant than in the axon shaft, and a collection of small vesicles appears near the synaptic cleft itself. These vesicles contain the neurotransmitter substances that will be released on invasion of the terminal by an action potential. The vesicles are polymorphic in appearance. Those at excitatory synapses tend to be round, whereas those at inhibitory synapses are more ovoid or flattened. Synapses that release peptides contain larger vesicles with electron-dense material in their centers. These dense-core vesicles are typical of neurosecretory cells. Well in excess of a dozen proteins have been identified as being crucial to the process, which involves filling of the vesicle with transmitter, docking, fusion of the vesicle with cellular membranes, release, and finally, recycling.
The Cell Biology of Neuronal Death
Included among the important physiologic functions of a brain cell is its program of cell death. Cell death was once viewed as a passive process, but our understanding of its biology has advanced to the point where we now appreciate that most cell loss is an active process that involves a well-orchestrated program of gene expression, proteolysis, and rearrangement of cellular organelles.10–13 The programs of cell death are generally divided into the more active form of cell death known as apoptosis and the more passive form known as necrosis. This duality is oversimplified, however; a more nuanced and probably more accurate characterization is that there are many processes that contribute to cell death. It is the summation of these processes along with the internal protective mechanisms that usually come into play in a neuron under stress that ultimately determines whether a neuron will live or die.
Another emerging association with neuronal cell death is loss of cell cycle regulation. This represents an odd situation in that most adult neurons exit the cell cycle during embryogenesis, never to return (see Chapter 4). Nonetheless, in a variety of neurodegenerative conditions, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and ataxia-telangiectasia, as well as stroke and human immunodeficiency virus–associated dementia, neurons that are known to be at risk for death are found to re-express proteins that are normally found only in cells that are actively engaged in a cell cycle. These proteins include such cell cycle components as Ki-67, cdk4, cyclin D, p16, proliferating cell nuclear antigen, cyclin B, and cdc2.14–17 Curiously, the cycle is not a productive one; it is never completed. DNA replication is involved, as studies with DNA hybridization techniques have shown in both Alzheimer’s and Parkinson’s disease, and chromosome copy number increases, as would be expected during a normal S phase of the cell cycle. The actual mitotic process, however, does not occur; chromosomes do not condense and mitotic spindles are not observed, nor are any forms of cytokinesis. Studies in tissue culture suggest that cell cycle initiation is an early part of the death pathway inasmuch as blocking the cycle blocks the death. In the adult brain, however, the linkage between the two processes is not direct; it is estimated that it can be many months to a year from the time that a neuron intitates a cycle until it finally dies. Whether this is a new type of cell death or merely a precursor or trigger to apoptosis or necrosis is not known.
The idea that entrance into a cell cycle might be lethal for a neuron even while it is restorative for other cells in other tissues highlights the fact that neurons, because of their highly specialized form and function, are vulnerable to a variety of insults. Thus, because they are postmitotic and highly differentiated, they cannot tolerate reinitiation of the cell cycle.18 Similarly, because they are in a nonmitotic state, repair of DNA damage is a crucial neuroprotective activity. Syndromes that involve disruptions of genes that encode elements of the DNA repair pathways nearly always include neurodegeneration as part of their phenotype.19 Excitotoxicity also reflects unique neuronal vulnerability. Because its internal calcium concentration is exquisitely sensitive to neurotransmitters, it is highly susceptible to damage from overactive synaptic activity. Oxidative damage is also a common trigger for neurodegenerative disease, and failure of antioxidant strategies of the cell can result in death. In truth, both the triggers of neuronal cell death and the death pathways themselves probably have significant overlap and may represent a continuum rather than distinct and wholly separate events. For example, a regular target of oxidative damage is DNA, a lesion that would trigger both DNA repair and antioxidant responses. Similarly, the processes usually associated with apoptosis can be found in cells that have lost their ATP stores and thus would be considered to be undergoing necrosis.
Neurodegenerative Diseases
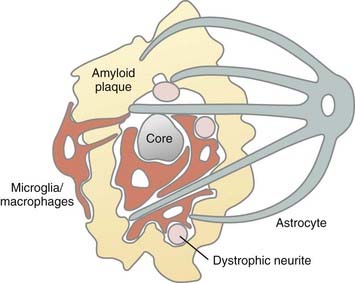
There are many types of neurodegenerative disorders that afflict humans in which these processes of neuronal cell death run amok. These disorders vary in their age at onset and the specificity of the affected cell populations. Alzheimer’s disease is an example of these conditions. Its disease process not only highlights several features of neuronal cell death but also emphasizes the importance of interaction among the various nervous system cell types. Alzheimer’s disease is a progressive dementia that affects millions of individuals in the United States alone. Clinically, the disease is manifested as loss of short-term memory, failure of executive function, and a variety of behavioral disorders such as depression and apathy. The disease is defined by the presence of two pathologic features: extracellular deposits of a peptide fragment known as β-amyloid in a largely insoluble aggregate known as a plaque and twisted configurations of fibrils (made up largely of neurofilaments and filament-associated proteins) known as a neuritic tangle. The course of the illness involves not only neurons but also several non-neuronal cells. In the vicinity of the plaque are reactive astrocytes, as well as activated microglial cells20,21 (discussed later in this chapter). The astrocytes appear to surround the plaque, as though trying to wall it off from the brain. The microglial cells appear to invade the plaque, as though trying to digest it. Associated with the dementia is loss of the majority of the neurons in several discrete neuronal populations: the hippocampus and entorhinal cortex, the basal nucleus of Meynert, the dorsal raphe, and the locus caeruleus. As the disease progresses, a more diffuse loss of neurons is observed that affects many regions of the frontal cortex and leads to significant atrophy of the brain in the later stages. As of this writing, the exact mechanism of neuronal death is controversial. Because the disease process itself can be many years in duration, only a tiny fraction of 1% of the cells in even the most affected population would be expected to be undergoing cell death at any one time. This slow loss of neurons makes it difficult to find the precise cells that are actively engaged in the process of dying. Perhaps as a consequence of this protracted disease course, no consistent evidence of either apoptosis or necrosis can be found in any of the affected populations. Like many other diseases, it is a condition that affects all the cells of the brain—neurons and non-neurons alike. For example, one probable scenario is that the aggregates of the β-amyloid peptide trigger a reaction in the astrocytes and microglial cells. This produces a complex inflammatory cascade that induces the release of numerous cytokines into the brain environment.22,23 Vascular deposits of amyloid are also found during the disease, and a role of vascular endothelial cells has additionally been proposed. Thus, this common neurodegenerative condition of the elderly highlights both the severe consequences for the individual of losing neurons in large numbers and emphasizes the integrated nature of the cellular functions of the brain. Neurons may be the long-distance carriers of information in the brain, but in both health and disease, the brain works as an ensemble of multiple different cell types.
Neuroglia
The term neuroglia, or “nerve glue,” was coined by Virchow in 1846 to describe an inactive connective tissue or cement that held neurons of the CNS in place.24 Although neuronal support is the major if not the only function of neuroglia, they are not passive caretakers of neurons. Neuroglia consist of morphologically and functionally distinct cell populations with irreplaceable structural and metabolic roles during brain development, neuronal function, and brain repair. In the human brain, neuroglia outnumber neurons by a factor of 10 and actively participate in information processing.25
How many neuroglial cell types are there? The classic metallic impregnation techniques developed by Cajal initially identified two neuroglial cell components: astrocytes and the “third element of the CNS.”26 Subsequently, del Rio-Hortega divided Cajal’s third element into the myelin-forming oligodendrocyte and the CNS resident immune cell, the microglia.27 Recently, a fourth population of glial cells has been identified in the adult human brain that appears to be as abundant as astrocytes, microglia, and oligodendrocytes.28 These cells can give rise to oligodendrocytes during brain development and are referred to here as oligodendrocyte progenitor cells (OPCs). As discussed later, however, all or some OPCs in the adult brain may have other functions. A fifth glial cell population in the CNS consists of ependymal cells, which line the ventricular system and central canal. In the peripheral nervous system (PNS), the Schwann cell is the major neuroglial component. With the exception of the ependymal cells, most CNS neuroglia extend multiple processes. The distribution and morphology of neuroglial cells in the adult brain are dictated by the functional requirements of these processes. Although many of these functions are understood, further characterization of the molecular properties of glial cells is needed to fully understand their functions.
Astrocytes
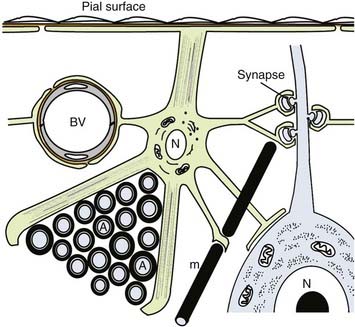
Astrocytes are the most abundant cell within the CNS. It has been estimated that astrocytes constitute 20% to 50% of the volume of many brain areas. Astrocytes mediate many functions through physical contact between their processes and other CNS components. Therefore, astrocyte distribution, shape, and function reflect in part the distribution of the CNS components that they contact. Many astrocyte functions occur ubiquitously throughout the CNS, whereas others are restricted in location. Astrocyte morphologies range from bipolar to stellate. The major functions of astrocytes are summarized in Figure 6-6 and are related to astrocyte distribution and morphology as presented in the following paragraphs.
How many types of astrocytes are there? Most textbooks identify three major astrocyte subtypes: radial glial, fibrous astrocytes of white matter, and protoplasmic astrocytes of gray matter. Astrocytes could be classified into dozens of subtypes (most found in gray matter) based on the differential expression of a variety of molecules, including ion channels, neurotransmitters, neurotrophin, and other cell surface receptors. Common to all astrocytes is a unique population of intermediate filaments enriched in glial fibrillary acidic protein. Bundles of 10-nm-thick intermediate filaments are a characteristic ultrastructural feature of astrocytes, as is an abundance of glycogen granules.29 The latter is a reflection of the important role that astrocytes play in brain energy metabolism.
Radial Glia
Transient populations of bipolar-shaped astrocytes are the first glial cells to appear in the developing mammalian brain.30 Their soma projects a short process to the ventricular surface and an elongated process to the pial surface. These radial glia transversely compartmentalize the developing neural tube and provide supportive scaffolding for the fragile embryonic neural tissue. This scaffolding plays an irreplaceable role in constructing the cytoarchitecture of the CNS by providing the physical substrate for many of the migrating postmitotic neurons that originate in the subventricular zone. These radial glia express a variety of extracellular matrix and adhesion molecules that provide the molecular cues for this neuronal migration. At the end of neuronal cell migration, most radial glia reenter the mitotic cycle and transform into astrocytes of white or gray matter. Radial glia, however, are not the only source of mature astrocytes; many originate from progenitor cells located in the subventricular zone. Bergmann’s glia in the cerebellum, cells of Müller in the retina, and tanycytes in the brain and spinal cord retain many characteristics of radial glia in the adult brain.31
White Matter Astrocytes
Curiously, no astrocyte functions are unique to white matter. Some functions, however, especially those related to structural support, appear to be more prominent in white matter. Furthermore, because of their high content of intermediate filaments, white matter astrocytes are easier to visualize than their gray matter counterparts. Astrocytes provide the cement or structural support to the adult CNS by extending processes to the ventricles and pial surface, where they form a continuous sheet called the glial limitans (see Fig. 6-6). In contrast to bipolar radial glia, most astrocytes that form the glial limitans in the adult brain are multipolar and extend relatively short processes to either the pial or the ventricular surface. Notable exceptions are the large astrocytic processes that form supportive scaffolding for the major white matter tracts and the pial limitans of the spinal cord. In all white matter tracts, smaller astrocytic processes serve as guides for axonal migration during development, secrete growth factors that regulate oligodendrogenesis and angiogenesis, and surround and support bundles of axons projecting to similar locations.
Neuronal communication occurs via chemical and ionic signals that cross the extracellular spaces of the CNS. The extracellular milieu of the CNS, therefore, must be tightly regulated. The astrocyte is the major homeostatic regulator of the CNS microenvironment. Most importantly, the astrocyte restricts the entry of serum factors into the CNS by extending processes that terminate in specialized “end-feet” that surround almost all blood vessels in the CNS. These astrocytic end-feet provide an extrinsic trophic effect that induces and maintains the tight junctions between neighboring endothelial cells, an essential element for formation and maintenance of the blood-brain barrier (see Chapter 8). Astrocytes also buffer the extracellular fluxes of ions and neurotransmitters associated with neuronal electrical activity. In white matter, astrocyte processes cover nodal regions of myelinated axons, where they buffer ionic fluxes associated with saltatory conduction. The “structural” astrocytes in white matter may represent a separate population from the astrocytes that send processes to nodes and vessels. This distinction reflects developmental differences in the timing of their initial appearance and progenitor cell origin. The structural astrocytes appear first, and many originate from radial glia. The structural astrocytes have been referred to as type I astrocytes and those associated with nodes and vessels as type II. Type II astrocytes are thought to originate from progenitor cells generated in the subventricular zone. In vitro, this progenitor cell may also give rise to oligodendrocytes.28
Gray Matter Astrocytes
When compared with white matter astrocytes, gray matter astrocytes are more abundant and project more and shorter processes. Gray matter astrocytes send processes to the pial surface, blood vessels, and nodes of Ranvier and therefore share many functions with white matter astrocytes. Gray matter contains less myelin and more vessels than white matter does, so gray matter astrocytes perform these functions at different proportions. The major difference between white and gray matter astrocytes is that the latter project processes to and ensheathe neuronal cell bodies, dendrites, and synapses (see Fig. 6-6). Ensheathment of synapses helps inactivate and recycle neurotransmitters, such as the excitatory amino acid glutamate. The glutamate and other neurotransmitter transporters are highly enriched in astrocyte processes that ensheathe synapses.32 Gray matter astrocytes are connected to each other by gap junctions and thereby form a syncytium that permits diffusion of ions and small molecules throughout the brain parenchyma.33 The potassium ions released from neurons during neurotransmission, for example, are taken up and diffused by astrocytes so that they do not interfere with future synaptic activity. Intercellular calcium waves are also generated from astrocyte to astrocyte in response to neuronal stimulation. Gap junctions have been detected between astrocytes and neurons and, along with astrocytic neurotransmitter receptors, may couple astrocyte and neuronal physiology.34
The human brain represents 2% of body weight yet consumes 25% of the body’s glucose.35 Glucose is the obligate energy substrate of the normal human brain and must be obtained from the circulation. The astrocyte is the major regulator of energy metabolism in the brain. One key to understanding this role is the physical connection between astrocytic processes and (1) capillaries, the external source of glucose, and (2) the synapse, a major energy consumer in the brain (see Fig. 6-6). The molecular events that couple synaptic activity, glucose uptake, neurotransmitter pools, and energy substrates can be stoichiometrically directed by synaptic activity. Best understood is the role of glutamate in the excitatory synapse.35 For each synaptically released glutamate molecule internalized by the astrocytic glutamate transporter, one glucose molecule enters the same astrocyte from the circulation via endothelial cells. From this glucose molecule, 2 ATP molecules are produced by glycolysis, and 2 lactate molecules are released and consumed by the synapse to yield 18 ATP molecules via oxidative phosphorylation (see Chapter 7). This activation also results in astrocytic release of glutamine, which enters the neuron and regenerates the neuronal glutamate pool. One can see from this description that much of brain energy metabolism related to neuronal function at the synapse occurs in astrocytes. Physiologic increases in brain activity visualized by proton emission tomography of 18F-2-deoxyglucose in vivo actually reflect increased blood flow and uptake of the tracer into astrocytes, not direct energy consumption by neurons.
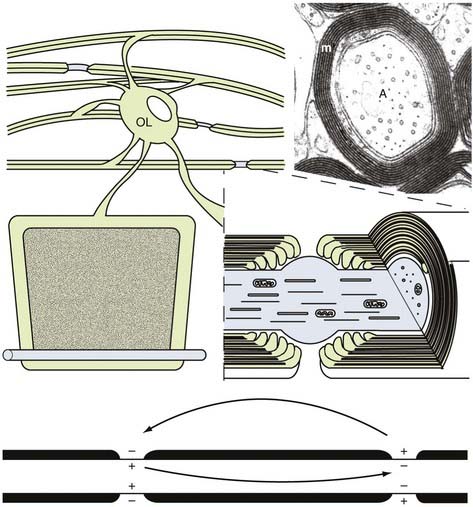
Oligodendrocytes
Rapid electrical communication between the 1011 neurons in the human brain controls and integrates the sophisticated mental and motor functions that set us apart from other species. Consider, for example, a complex task such as a 6-ft human dunking a basketball. The motor, sensory, and decision-making circuitry of the brain and peripheral nerves must integrate and coordinate jumping, manipulating the torso, handling the ball, avoiding defenders, and putting the ball in the basket. Millions of coordinated nerve impulses govern this action, and many must travel more than a meter in a fraction of a second. Without rapid nerve conduction, the 1011 neurons in the human brain would not be an advantage for function or survival. Two mechanisms have evolved to permit rapid conduction of nerve impulses. In invertebrates, axonal conduction velocity is related to the diameter of the axon.36 Large-diameter axons conduct at a much faster rate than do small-diameter axons. Although this situation is sufficient for regulating neural function in smaller, less sophisticated organisms, the CNS of humans cannot accommodate the increase in axonal volume required for rapid nerve conduction. For example, the large-diameter motor axons conduct at a velocity of approximately 40 m/sec. If this conduction velocity were regulated solely by axonal diameter, the diameter of this axon would be several millimeters. Multiplying this by the millions of axons in the spinal cord would result in a spinal cord as wide as a telephone pole. Therefore, an additional mechanism evolved to accommodate rapid nerve conduction in the vertebrate brain. Analogous to the conduction of electrical wire, the mammalian nervous system increases the resistance and decreases the capacitance of axonal membrane potentials by surrounding axons with a multilamellar, tightly compacted membrane insulation called myelin (Fig. 6-7). Myelin is a specialized extension of the plasma membrane of the oligodendrocyte in the CNS37 and of the Schwann cell in the PNS.38 The length of individual myelin segments or myelin internodes varies between several hundred micrometers and 2 mm, with larger diameter axons having longer and thicker (more spiral wraps) myelin internodes. Along each axon, individual myelin internodes are separated from their neighbors by a node of Ranvier, a specialized unmyelinated axonal segment (1 to 5 µm in length) enriched in sodium channels and analogous to the axon hillock or initial axonal segment. Enrichment of voltage-sensitive sodium channels at the node generates ionic impulses only at the node (see Fig. 6-7). The action potential “jumps” from node to node by a process called saltatory conduction (see Chapter 49). Propagation of the action potential is also an energy-dependent process. Saltatory conduction consumes less energy than nonsaltatory conduction does. Therefore, myelin has three general advantages for the function of the human CNS: fast axonal conduction, energy conservation, and space conservation. Myelin is essential for normal neurological function, and a variety of inherited, metabolic, and immune-mediated myelin diseases occur in humans. Axonal degeneration is a consistent and neurologically important phenotype in the brains of individuals with myelin disease,39 an observation indicating that myelin provides extrinsic trophic support for the survival of axons.40,41 Similarly, when myelin fails to form developmentally, axons fail to mature. Myelination is therefore essential for the maturation and survival of axons and should be considered an integral part of nerve regeneration paradigms.
The only known function of oligodendrocytes is myelination and axonal support. Consequently, the distribution of oligodendrocytes correlates with the density of axons requiring myelin. Oligodendrocyte-myelin ratios, however, are not directly proportional because different oligodendrocytes can myelinate different numbers of axons.42 Oligodendrocytes have small, round cell bodies, extend single processes to each myelin internode, and can be identified in tissue sections with a variety of myelin-specific antibodies. As expected, oligodendrocyte density is high in white matter tracts, where the cells often occur in rows oriented parallel to axons. Oligodendrocytes are also present at significant densities in gray matter because many axons terminating on and originating from neurons are myelinated. Oligodendrocyte cell bodies can be found close to neuronal cell bodies in gray matter or close to blood vessels in both gray and white matter. It is not known whether these cells perform functions other than myelination.
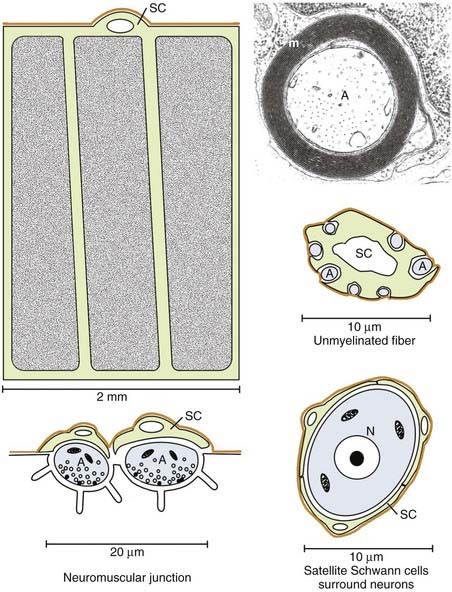
Schwann Cells
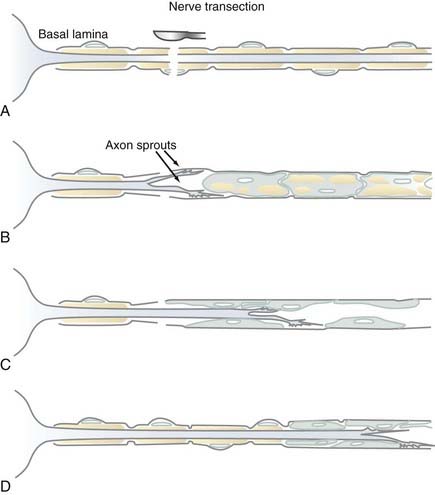
Myelin is formed by Schwann cells in the PNS, where it serves identical functions as CNS myelin. Schwann cells, however, differ from oligodendrocytes in that they can promote axonal regeneration. Schwann cells form single myelin internodes and surround these internodes with a specialized connective tissue or basal lamina (Fig. 6-8). The basal lamina forms a continuous tube around the entire length of each myelinated fiber. When a PNS axon is transected, the distal axonal segment rapidly degenerates, the myelin is removed, and Schwann cells multiply and form a continuum within each basal lamina tube (Fig. 6-9).43 The proximal end of the axon, still connected to the neuronal cell body, does not degenerate and can regrow into the Schwann cell tube, which provides a substrate and often direct continuity for regeneration and reinnervation of appropriate targets. Denervated peripheral nerve segments, as well as Schwann cells isolated and expanded in vitro, have been experimentally transplanted into the CNS or PNS to promote axonal regeneration.44,45 Although this is a common and often successful method of PNS regeneration in humans, Schwann cell transplantation as a therapy for human spinal cord injury is still under experimental development. Myelination is not the only function of Schwann cells. They also surround the perikaryon of PNS neurons, small-diameter axons, and portions of the neuromuscular junction (see Fig. 6-8). These Schwann cells have a molecular phenotype that differs significantly from myelinating Schwann cells.
Distribution of Microglia and Oligodendrocyte Progenitor Cells
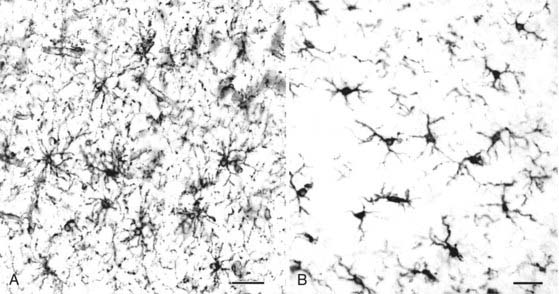
Although the functions of microglia and OPCs are not known to be intimately related, their distribution and morphology are compared here because of their striking similarity. Both cell types are ubiquitous in the normal adult CNS, they project multiple processes from relatively small cell bodies, and each cell occupies distinct domains with little overlap between the processes of neighboring cells (Fig. 6-10). The processes of neighboring microglial cells do not touch; the ends of OPC processes are often closely apposed, and some project to blood vessels. Microglia and OPCs can be distinguished molecularly by immunocytochemistry and to the experienced observer by morphology. Regional variation in the density and shape of each cell type (e.g., white versus gray matter) occurs, however, indicative of the fact that their distribution and shape are regulated by local environments. Based on these morphologic characteristics, both cell types form a lattice-like network that covers most of the brain parenchyma. They are therefore appropriately positioned to function as homeostatic regulators of normal brain function and as guards ready to respond to brain pathology or neural dysfunction. Although this hypothesis is clearly established for microglia, little is actually known about OPC function in the adult brain.
Function of Microglia
Microglia function as resident immune cells and phagocytes in the CNS. A lineal relationship between microglia and monocytes is supported by the following observations: all antibodies thus far raised against microglia also react with macrophages in peripheral tissues; microglia express activated monocyte markers, including major histocompatibility complex (MHC) class I and II; and microglia can process and present antigens to activated T cells. Microglia also display features not shared by peripheral macrophages and monocytes. Microglia proliferate spontaneously46 and express a unique ion channel pattern that directs large, inwardly rectifying currents dependent on potassium.47 Microglia can therefore be considered monocyte-derived cells that have evolved specialized features during their adaptation to the CNS. Although their functions in the normal adult brain are not fully understood, microglia have important roles in CNS development and disease. On the basis of current knowledge, it appears that most microglia originate from bone marrow–derived monocytes that enter the brain during early development. Initially ameboid in shape, these cells help phagocytose degenerating neurons and oligodendrocytes that undergo programmed cell death as part of normal development. They are mitotically active and secrete cytokines and growth factors that may help regulate gliogenesis and angiogenesis. After the early stages of brain development, ameboid microglial cells transform into ramified resident microglial cells that persist throughout adulthood.
Ramified microglia constitute 5% to 20% of the glial cells in the adult CNS.48 They are more abundant in gray than in white matter, and phylogenetically newer regions of the CNS (cerebral cortex, hippocampus) have more microglia than do older regions (cerebellum, brainstem). Regional variation in the number and shape of microglia suggests that microglial distribution and morphology are regulated by local environments and that microglia play a role in tissue homeostasis. Although the nature of this homeostasis remains to be elucidated, microglia respond quickly and dramatically to all forms of brain pathology.
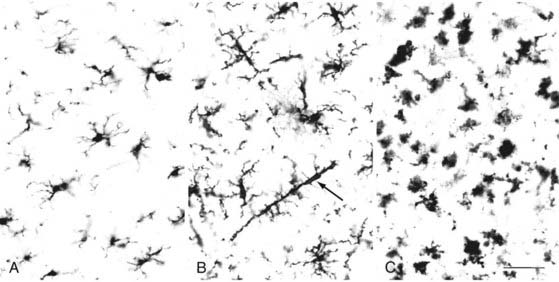
“Reactive” microglia can be distinguished from resting microglia by two criteria: change in morphology (Fig. 6-11) and upregulation of monocyte-macrophage molecules. Reactive microglia generally have larger cell bodies and shorter, thicker processes than do resting microglia. Reactive microglia also appear to be motile because they can surround or target structures such as dying cells, demyelinated axons, synapses, or as described later, amyloid plaque in Alzheimer’s disease. Reactive microglia express a variety of cell surface molecules that are not usually detectable in resting microglia, including MHC class II and CD68.46 In response to frank pathology, resident microglia follow a stereotyped pattern of first becoming “activated” and then phagocytic. Phagocytic CNS macrophages can also originate from circulating monocytes and from neighboring microglia that proliferate and migrate to the site of injury.
In addition to their role as phagocytic macrophages, resident microglia can respond to remote lesions and conditions lacking frank necrotic pathology or activation of the peripheral immune system. After transection of the PNS portion of the facial nerve, for example, resident microglia in the facial nerve nucleus become activated and displace afferent synaptic terminals from the injured facial motoneurons by a process called synaptic stripping.49 These cells become phagocytic only when the neurons die. As part of an early CNS immune defense mechanism, microglia play a primary role in CNS disease. Microglia release several potentially cytotoxic substances, including nitric oxide, TNF, proteases, cytokines, and glutamate.50 One of the more intriguing responses of microglia occurs in the brains of individuals with Alzheimer’s disease. Alzheimer’s disease is often referred to as an inflammatory disease of the CNS, but the inflammation in the brain does not consist of the classic perivascular accumulation and diffuse parenchymal distribution of blood-borne monocytes and lymphocytes in the cerebral cortex. It involves primary microglial activation. Most prominent among these responses is the migration to and infiltration of amyloid plaque by microglia (Fig. 6-12).20,21 It is likely that these microglia try, but fail to phagocytose amyloid, especially that associated with the center of the core plaque. As part of this activated microglial response to amyloid, neurites may become injured or dystrophic as a bystander effect of the secretion of microglial cytotoxic substance. Axons may also be transected by a similar mechanism in the lesions of multiple sclerosis (MS).40 Amyloid plaque is a good example of how microglia and astrocytes may coordinate reactions to brain pathology (see Fig. 6-12). Astrocytes near the core plaque reorient and send processes into the plaque.21 Although the functions of these astrocytic processes are unclear, they express high levels of proteoglycans51 and amyloid precursor protein, which is the source of amyloid. This is just one example of how microglia and astrocyte activation may affect brain function. Although glial cell reactivity is designed to be protective, it is possible (as proposed in Alzheimer’s disease) that attempts to remove amyloid may destroy axons and dendrites and release cytokines that drive susceptible neurons toward cell death. This and other examples have led to the concepts of good inflammation and bad inflammation. Understanding and regulating good and bad inflammation and other glial reactions is a major challenge to future neurosurgical interventions, including nerve regeneration, transplantation of stem or progenitor cells into the brains of patients with MS, and stroke therapies.
Turnover of Microglia
Because microglia originate from blood-borne monocytes, the possibility has been raised that genetically engineered monocytes or bone marrow transplants may be an effective mechanism of gene therapy for CNS disease. Based on bone marrow chimera experiments in normal adult rodents, ramified microglia have very low (<1%) turnover.52 In contrast, 20% to 70% of the monocytes and macrophages in the leptomeninges, choroid plexus, and perivascular spaces are replaced by cells from the bone marrow. Blood-borne cells appear to have a much higher CNS entry rate during brain development or in pathologic environments in the mature CNS. In this regard, bone marrow transplants have significantly increased the life span and quality of life of some children with leukodystrophies.53 Although the cellular mechanisms underlying the improved brain function are unknown, recent studies suggest that bone marrow also contains a stromal stem cell that can differentiate into microglia, astrocytes,54 and possibly oligodendrocytes and neurons. In the future, bone marrow transplants may prove to be a useful therapy for other CNS diseases.
Oligodendrocyte Progenitor Cells
Although little is known about OPC function in the adult brain, a significant literature describes the in vitro properties of OPCs isolated from neonatal rodent CNS. Because these cells give rise to oligodendrocytes in serum-free media and to type II astrocytes in serum-containing media, they were initially referred to as O2A cells.55 They can be identified in vitro by the expression of A2B5 gangliosides. Characterization of O2A cells in vivo was initially hampered by the ubiquitous expression of A2B5. This changed when O2A cells were demonstrated to express two polypeptide antigens: platelet-derived growth factor receptor α (PDGFαR) and the sulfated proteoglycan NG2. With the use of these markers, substantial evidence now exists that OPCs can give rise to oligodendrocytes in the developing brain.28 The potential for OPCs to give rise to astrocytes in vivo, however, remains to be established.
Oligodendrocyte Progenitor Cells in Development
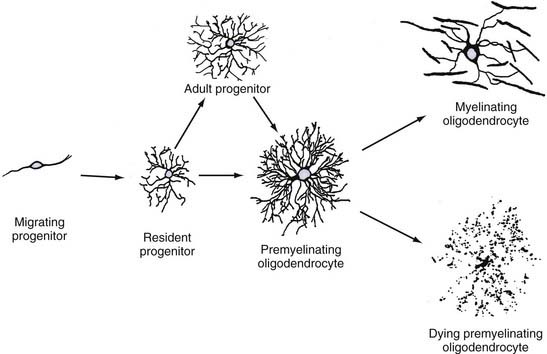
Because few studies have characterized OPCs during human brain development, the following description is based primarily on studies of rodent brain development. All available evidence suggests that gliogenesis is remarkably similar in human and rodent brains. OPCs originate from discrete regions of the subventricular zone during early brain development. In the spinal cord, OPCs originate from the ventral subventricular zone, dorsal to the floor plate, and their development depends on expression of sonic hedgehog56 and neuregulin.57 In the cerebrum, OPCs originate from the subventricular zone as PDGFαR-positive cells.28 These cells migrate throughout the brain and, shortly after leaving the subventricular zone, express detectable levels of NG2. These bipolar cells multiply as they migrate and eventually establish a network of stellate cells throughout the entire brain (Fig. 6-13; also see Fig. 6-10). The timing of oligodendrocyte production varies in different brain regions. Signals that regulate oligodendrocyte production are poorly understood, but PDGF-driven proliferation of OPCs appears to be one requirement.58 Stellate OPCs are highly mitotic (≈30% by short BrdU labeling) during early brain development,59 and these cells produce oligodendrocytes and new OPCs by symmetrical and asymmetric division. The oligodendrocyte initially displays a premyelinating phenotype by extending multiple myelin protein–positive processes that do not immediately ensheathe and myelinate axons (see Fig. 6-13).60 These cells have a limited life span and either go on to myelinate or die by programmed cell death.
Oligodendrocyte Progenitor Cells in Adult Brain
OPCs do not disappear as the brain matures, and they continue to express PDGFαR and NG2, but not markers specific for astrocytes, microglia, and oligodendrocytes.28 As analyzed in human cortical tissue slices, OPCs are abundant (see Fig. 6-10) and account for 10% to 15% of glial cells.61,62 OPCs are difficult to visualize in routinely processed surgical and autopsy tissues and thus are underrecognized by neuropathologists. When isolated from adult brain, they can give rise to oligodendrocytes.63 The stellate morphology and abundance of OPCs and the low turnover of oligodendrocytes in normal brain raise the possibility that they have functions unrelated to oligodendrocyte production. In adult rodent brain, OPC processes appose nodes of Ranvier,64 associate with synapses,65 and terminate on blood vessels. Cortical OPCs can also receive synaptic input from neurons.66–70 Collectively, these observations support the possibility that OPCs help astrocytes maintain CNS homeostasis, including regulation of neuronal electrical activity. It is possible that OPCs in the adult CNS consist of two populations of cells: those that can replenish oligodendrocytes and those that cannot.
Similar to astrocytes and microglia, OPCs respond to CNS injury by becoming activated: a more elongated shape, fewer processes, and upregulation of NG2.28 If the lesion includes demyelination and loss of oligodendrocytes, NG2-positive cells proliferate and new oligodendrocytes are produced.71 Definitive identification of proliferating OPCs as the source of new oligodendrocytes in the adult brain, however, remains to be established. OPCs have been identified in MS lesions,61,72 where they may also generate new oligodendrocytes that remyelinate some lesions.
Oligodendrocyte Progenitor Cells and Glial Neoplasms
Historically, glial tumors have been classified on the basis of their morphologic resemblance to the various cell types found in the normal CNS.73 Thus, more than 50% of intracranial tumors consist of astrocytomas, oligodendrogliomas, or ependymomas. Oligodendrogliomas, however, rarely express antigens that are normally found in mature myelin-forming oligodendrocytes. Because it is likely that a subpopulation of adult OPCs function as progenitor cells and retain the ability to divide, the possibility that adult OPCs contribute to glial neoplasms has been investigated. When various CNS tumors were examined, all the oligodendrogliomas and pilocytic astrocytomas abundantly expressed PDGFαR and NG2.74 These findings raise the possibility that OPCs might be the source of these glial neoplasms. It is tempting to speculate that cerebral oligodendrogliomas and infratentorial pilocytic astrocytomas originate from the same OPCs or O2A cell population that can give rise to both oligodendrocytes and astrocytes in vitro. Glioblastoma multiforme may be derived from a separate lineage of glial progenitor cells.
Ependymal Cells
Ependymal cells line the ventricular spaces of the adult brain. They have a simple ciliated cuboidal morphology.31 The cilia project into the ventricular space and spinal canal, oscillate approximately 200 times per minute, and are thought to assist in the rostrocaudal flow of cerebrospinal fluid. Ependymal cells are connected by apicolateral gap and zona occludens junctions. The lack of tight junctions between ependymal cells permits free exchange between the extracellular space of the brain and cerebrospinal fluid. Ependymal cells are mitotically active in the adult brain and thus are susceptible to genetic changes associated with tumorigenesis.
Transplantation Therapies
As described throughout this chapter, the nervous system consists of diverse cell populations that are essential for normal neurological function. The cells are also the target of inherited and acquired diseases of the nervous system, and therapies designed to replace diseased or destroyed cells are a major focus of translational neurosciences research. Two general cell replacement approaches—enhancement of endogenous repair and transplantation of exogenous cells—are being pursued. We focus here on transplantation therapies because they will be neurosurgery-based therapies. Proof of principle for effective transplantation of three neural cell populations—Schwann cells, neurons, and oligodendrocytes—has been established in animal models of CNS disease. Transplantation of Schwann cells or peripheral nerve segments is routinely used to enhance human peripheral nerve regeneration and will not be discussed further (see Chapter 239).
A more realistic strategy is replacement of dopaminergic neurons in individuals with Parkinson’s disease. Parkinson’s disease is characterized by progressive loss of dopaminergic neurons in the substantia nigra and reduced dopamine in the striatum. In animal models of Parkinson’s disease, fetal dopaminergic neurons survive when grafted into the striatum and improve motor function. Because these neurons are placed at the site of innervation, they need not extend long processes. Open-label transplantation trials of fetal dopaminergic neurons into the striata of a small number of patients with Parkinson’s disease showed efficacy.75–77 However, two placebo-controlled, double-blinded trials were disappointing as a whole but appeared to benefit a subpopulation of patients younger than 60 years.78,79 There has been much discussion regarding the possible reasons for the discrepancies between the open-label and double-blinded studies (for review, see Winkler and colleagues80). Parkinson’s disease is an attractive disease candidate for neuronal cell grafting, and transplantation of immature dopaminergic neurons remains a promising approach for treating advanced but not seriously affected patients.
The best example of endogenous cell replacement in the adult human brain is the generation of new oligodendrocytes in some but not all demyelinated lesions in individuals with MS.81,82 Because remyelination can occur in the adult human brain, transplantation therapies may be effective for the MS lesions that fail to repair. Much is known about the cellular aspects of oligodendrocyte production during myelination and remyelination. Transplanted OPCs can produce myelinating oligodendrocytes in the adult mammalian brain.83 Although MS would appear to be a logical target for cell replacement therapies, multiple obstacles need to be overcome before transplant trials for MS patients, including risk-benefit odds, source of donor cells, delivery of cells to multiple sites, age of recipients, lack of reliable surrogate markers for detecting effective treatment, and little proof of principle in relevant animal models. At present, individuals with inherited diseases of myelin appear to be more appropriate for human transplantation therapies. Their poor prognosis provides a positive risk-benefit assessment, and proof of principle in animal models has clearly been established inasmuch as transplantation of human OPCs into neonatal brains can rescue neurological phenotypes and extend the life spans of mice with inherited diseases of myelin.84 These OPCs colonized the developing brain and produced oligodendrocytes that successfully myelinated major white matter tracts. The neonatal brain appears to be a more receptive host for transplanted cells than the adult brain does. Cell replacement therapies will be a significant aspect of future research, and human clinical trials will follow. Some caution must be exercised, however, because one adverse event could substantially delay this promising therapeutic approach for treating diseases of the human brain.
Acknowledgment
We thank Drs. Grahame Kidd and Xinghua Yin for the preparation of Figures 6-6 to 6-9 and 6-12 and Dr. Chris Nelson for manuscript editing.
Bergles DE, Roberts JD, Somogyi P, et al. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187-191.
Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197-251.
Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267-310.
Deshmukh M, Johnson EM Jr. Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation–induced death of sympathetic neurons. Mol Pharmacol. 1997;51:897-906.
Dunnett SB, Bjorkland A, Lindvall O. Cell therapy in Parkinson’s disease—stop or go? Nat Rev Neurosci. 2001;2:365-369.
Gehrmann J, Kreutzberg GW. Microglia in experimental neuropathology. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:883-904.
Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368-378.
Hickey WF, Vass K, Lassmann H. Bone marrow–derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246-256.
Nave K-A, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535-561.
Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113-1121.
Peters A, Palay SL, Webster HF. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells, 3rd ed. New York: Oxford University Press; 1991.
Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22-31.
Spencer PS, Politis MJ, Pellegrino RG, et al. Control of Schwann cell behavior during nerve degeneration and regeneration. In: Gorio A, Millesi H, Mingrino S, editors. Posttraumatic Peripheral Nerve Regeneration: Experimental Basis and Clinical Implications. New York: Raven Press; 1981:411-426.
Stefanis L, Burke RE, Greene LA. Apoptosis in neurodegenerative disorders. Curr Opin Neurol. 1997;10:299-305.
Streit WJ. Microglial cells. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:85-96.
Trapp BD, Nave K-A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247-269.
Trapp BD, Nishiyama A, Cheng D, et al. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459-468.
Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in multiple sclerosis lesions. N Engl J Med. 1998;338:278-285.
Villegas-Perez M, Vidal-Sanz M, Bray GM, et al. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265-280.
Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977;34:585-589.
Winkler C, Kirik D, Bjorkland A. Cell transplantation in Parkinson’s disease: how can we make it work? Trends Neurosci. 2005;28:86-92.
1 Webster’s New Universal Unabridged Dictionary. Springfield, MA: Merriam-Webster, 1983.
2 Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267-310.
3 Darian-Smith I. The sense of touch: performance and peripheral neural processes. In: Smith D, editor. Handbook of Physiology. Bethesda, MD: American Physiological Society; 1984:739-788.
4 Axel R. The molecular logic of smell. Sci Am. 1995;273:154-159.
5 Palay S, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Berlin: Springer-Verlag; 1974.
6 Davis L, Banker GA, Steward O. Selective dendritic transport of RNA in hippocampal neurons in culture. Nature. 1987;330:477-479.
7 Licatalosi DD, Darnell RB. Splicing regulation in neurologic disease. Neuron. 2006;52:93-101.
8 Brizzee KR, Knox C. The aging process in the neuron. Adv Exp Med Biol. 1980;129:71-98.
9 Twiss JL, Fainzilber M. Ribosomes in axons—scrounging from the neighbors? Trends Cell Biol. 2009;19:236-243.
10 Deshmukh M, Johnson EMJr. Programmed cell death in neurons: focus on the pathway of nerve growth factor deprivation–induced death of sympathetic neurons. Mol Pharmacol. 1997;51:897-906.
11 Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245-267.
12 Mulligan G, Jacks T. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 1998;14:223-229.
13 Stefanis L, Burke RE, Greene LA. Apoptosis in neurodegenerative disorders. Curr Opin Neurol. 1997;10:299-305.
14 Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer’s disease brain. J Neurosci. 1998;18:2801-2807.
15 Copani A, Condorelli F, Caruso A, et al. Mitotic signaling by beta-amyloid causes neuronal death. FASEB J. 1999;13:2225-2234.
16 Nagy Z, Esiri MM, Cato AM, et al. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol (Berl). 1997;94:6-15.
17 Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996;132:413-425.
18 Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368-378.
19 McKinnon PJ. DNA repair deficiency and neurological disease. Nat Rev Neurosci. 2009;10:100-112.
20 Itagaki S, McGeer PL, Akiyama H, et al. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173-182.
21 Mochizuki A, Peterson JW, Mufson EJ, et al. Amyloid load and neural elements in Alzheimer’s disease and nondemented individuals with high amyloid plaque density. Exp Neurol. 1996;142:89-102.
22 Combs CK, Johnson DE, Karlo JC, et al. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid–stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. J Neurosci. 2000;20:558-567.
23 McDonald DR, Bamberger ME, Combs CK, et al. Beta-amyloid fibrils activate parallel mitogen-activated protein kinase pathways in microglia and THP1 monocytes. J Neurosci. 1998;18:4451-4460.
24 Virchow R. Uber das granulierte Ansehen der Wandungen der Gerhirnventrikel. Allg Z Psychiatr. 1846;3:242.
25 Coyle JT, Schwarcz R. Mind glue: implications of glial cell biology for psychiatry. Arch Gen Psychiatry. 2000;57:90-93.
26 Cajal SR. Contribución al conocimiento de la neuroglia del cerebro humano. Trab Lab Invest Biol. 1913;11:254.
27 del Rio-Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System, Vol 2. New York: Paul B. Hoeber; 1932:481-534.
28 Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol. 1999;58:1113-1121.
29 Peters A, Palay SL, Webster HF. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells, 3rd ed. New York: Oxford University Press; 1991.
30 Rakic P. Radial glial cells: scaffolding for brain. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:746-762.
31 Reichenbach A, Robinson SR. Ependymoglia and ependymoglialike cells. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:58-84.
32 Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713-725.
33 Ransom BR. Do glial gap junctions play a role in extracellular homeostasis? In: Spray DC, Dermietzel R, editors. Gap Junctions in the Nervous System. Austin, TX: RG Landes; 1996:161-173.
34 Spray DC, Scemes E, Rozental R. Cell-cell communication via gap junctions. In: Zigmond M, Bloom FE, Landis SC, et al, editors. Fundamental Neuroscience. San Diego, CA: Academic Press; 1999:317-343.
35 Magistretti P. Brain energy metabolism. In: Zigmond M, Bloom FE, Landis SC, et al, editors. Fundamental Neuroscience. San Diego, CA: Academic Press; 1999:389-413.
36 Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977;34:585-589.
37 Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197-251.
38 Webster HF. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J Cell Biol. 1971;48:348-367.
39 Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in multiple sclerosis lesions. N Engl J Med. 1998;338:278-285.
40 Trapp BD, Nave K-A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247-269.
41 Nave K-A, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535-561.
42 Blakemore WF. Observations on myelination and remyelination in the central nervous system. In: Garrod DR, Feldman JD, editors. Development in the Nervous System. Cambridge: Cambridge University Press; 1981:289-308.
43 Spencer PS, Politis MJ, Pellegrino RG, et al. Control of Schwann cell behavior during nerve degeneration and regeneration. In: Gorio A, Millesi H, Mingrino S, editors. Posttraumatic Peripheral Nerve Regeneration: Experimental Basis and Clinical Implications. New York: Raven Press; 1981:411-426.
44 Villegas-Perez M, Vidal-Sanz M, Bray GM, et al. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988;8:265-280.
45 Xu XM, Zhang SX, Li H, et al. Regrowth of axons into the distal spinal cord through a Schwann-cell–seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723-1740.
46 Giulian D. Microglia and neuronal dysfunction. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:671-684.
47 Kettenmann H, Banati R, Walz W. Electrophysiological behavior of microglia. Glia. 1993;7:93-101.
48 Streit WJ. Microglial cells. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:85-96.
49 Gehrmann J, Kreutzberg GW. Microglia in experimental neuropathology. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:883-904.
50 Benveniste EN. Cytokine production. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995:700-713.
51 DeWitt DA, Silver J, Canning DR, Perry G. Chondroitin sulfate proteoglycans are associated with the lesions of Alzheimer’s disease. Exp Neurol. 1993;121:149-152.
52 Hickey WF, Vass K, Lassmann H. Bone marrow–derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246-256.
53 Krivit W, Peters C, Shapiro EG. Bone marrow transplantation as effective treatment of central nervous system disease in globoid cell leukodystrophy, metachromatic leukodystrophy, adrenoleukodystrophy, mannosidosis, fucosidosis, aspartylglucosaminuria, Hurler, Maroteaux-Lamy, and Sly syndromes, and Gaucher disease type III. Curr Opin Neurol. 1999;12:167-176.
54 Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94:4080-4085.
55 Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390-396.
56 Orentas D, Hayes J, Dyer K, et al. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419-2429.
57 Vartanian T, Fischbach G, Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc Natl Acad Sci U S A. 1999;96:731-735.
58 Richardson WD, Pringle N, Mosley MJ, et al. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309-319.
59 Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307-321.
60 Trapp BD, Nishiyama A, Cheng D, et al. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459-468.
61 Chang A, Nishiyama A, Peterson J, et al. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404-6412.
62 Wilson HC, Scolding NJ, Raines CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176:162-173.
63 French-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in the adult rat optic nerve. Nature. 1986;319:499-502.
64 Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84-91.
65 Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan–positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83-95.
66 Bergles DE, Roberts JD, Somogyi P, et al. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187-191.
67 Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004;47:290-298.
68 Lin SC, Huck JH, Roberts JD, et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773-785.
69 Wigley R, Hamilton N, Nishiyama A, et al. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210:661-670.
70 Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): multifunctional cells with lineage and plasticity. Nat Rev Neurosci. 2009;10:9-22.
71 Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191-1201.
72 Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601-609.
73 Bailey P, Cushing H. A Classification of Tumors of the Glioma Group on a Histogenesis Basis with a Correlated Study of Prognosis. Philadelphia: JB Lippincott; 1926.
74 Shoshan Y, Nishiyama A, Chang A, et al. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci U S A. 1999;96:10361-10366.
75 Lindvall O, Hagell P. Clinical observations after neural transplantation in Parkinson’s disease. Prog Brain Res. 2000;127:299-320.
76 Dunnett SB, Bjorkland A, Lindvall O. Cell therapy in Parkinson’s disease—stop or go? Nat Rev Neurosci. 2001;2:365-369.
77 Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson’s disease. J Neuropathol Exp Neurol. 2001;60:741-752.
78 Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710-719.
79 Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403-414.
80 Winkler C, Kirik D, Bjorkland A. Cell transplantation in Parkinson’s disease: how can we make it work? Trends Neurosci. 2005;28:86-92.
81 Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22-31.
82 Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199-204.
83 Franklin RJ, Blakemore WF. Transplanting oligodendrocyte progenitors into the adult CNS. J Anat. 1997;190:23-33.
84 Windrem MS, Schanz SJ, Guo M, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553-565.