Chapter 14 Neuromuscular Retraining after Anterior Cruciate Ligament Reconstruction
INTRODUCTION
Rehabilitation after knee injuries, especially anterior cruciate ligament (ACL) ruptures, has significantly changed since the mid 1990s. In the past, rehabilitation focused on protection with a period of immobilization, guarded range of motion (ROM), strengthening exercises, and a delay in return to function. The return to functional activities and sports after ACL reconstruction is now more rapid, partly owing to the shift in rehabilitation philosophy and improved surgical techniques. Modern therapy focuses on immediate motion41,42 and weight-bearing, functional exercises, and proprioception and neuromuscular control,35,55 thus allowing an earlier regain of functional dynamic stability and return to functional activities.
TERMINOLOGY
Although often used interchangeably, proprioception, kinesthesia, and neuromuscular control have different definitions. Proprioception is the sense of awareness of the joint position, whereas kinesthesia is the sensation of joint movement.35 Wilk54 defined neuromuscular control as the afferent sensory recognition of joint position and the efferent response to that of awareness. Furthermore, neuromuscular control provides the functional component referred to as dynamic stabilization.
The joint and muscle receptors play a significant role in the mediation of extrinsic muscle stiffness32 and thus contribute to dynamic joint stability. The receptors in muscles contribute to muscle stiffness through two mechanisms: (1) force feedback, which is modulated by the GTO,29 and (2) length feedback, which is mediated by the muscle spindle pathway and facilitates motor activity.37,38
Two other possible mechanisms have been proposed for the joint receptors’ contribution to joint stability. The first mechanism is a direct ligamentous-muscular protective reflex. An example of this is the reflex between the ACL and the hamstring muscles. When tension is generated onto the ACL, the mechanoreceptors are stimulated, causing a reflex inhibition of the quadriceps and a facilitation of the hamstrings. This protects against increasing strain on the ACL. The second mechanism is the joint receptors’ indirect contribution to dynamic joint stability. In this mechanism, the joint receptors contribute to preparatory adjustments of muscles stiffness and dynamic joint stability. This is referred to as presetting.31 The authors believe this concept of presetting is critical in providing joint stability during functional activities such as preparation for deceleration or cutting.
Motor responses depend on the level of processing of afferent inputs within the CNS. The processing may occur at three different levels: the spinal cord, the brainstem, and the cerebral cortex.9 The site of processing affects the speed of motor responses. Spinal reflexes represent the shortest neuronal pathways and, consequently, the most rapid response to afferent stimuli.11,48,58 Theoretically, these spinal reflexes are faster than ligamentous failure.46,58 Conversely, sensory information mediated at the brainstem, cerebellum, and cortical levels include longer pathways and slower response times. Numerous studies11,21,48 have documented that sensory input processed at the CNS above the spinal cord level can be modified with training. Thus, owing to the adaptability of the responses at the brainstem and the cerebellum, these pathways are believed to be important in providing dynamic knee stability.18
After injury, the complex interactions within the neuromuscular system are disrupted, which can result in diminished proprioception and kinesthesia, abnormal patterns of muscle activity,15 and reduced joint dynamic stability.4,7,34 Furthermore, injury to one knee can affect the proprioception on the contralateral (uninvolved) extremity.28 Therefore, immediately after injury or surgery, the rehabilitation program must be directed toward creating an environment that promotes the restoration and development of motor responses and proprioception for both extremities.
Several other terms require classification. Open-loop control is defined as a movement that is brief, predictable, and produced in an unchanging environment that does not require sensory information for modification. Thus, these are movements that do not require feedback.48 Conversely, movements that rely on feedback from the sensory system, such as reflexive movements, are considered closed-loop control.
Chmielewski and coworkers12 defined motor skills in respect to the environment. Some motor skills, such as walking, stairclimbing, and extending the leg, although performed in a relatively stable environment, are referred to as closed skills. Conversely, open skills are performed in an environment that is changing and unpredictable. Examples of open skills include running in the woods, downhill skiing, or balancing on a wobble board. Other examples are playing sports such as basketball, soccer, or football.
EFFECTS OF INJURY ON PROPRIOCEPTION
Numerous authors4–7,28 have shown a decrease in proprioception and kinesthesia after ACL injury. After ACL injury, deafferentization of peripheral sensory receptors occurs.6,49 Changes in proprioception happen quickly. Lephart and associates35 reported that these changes occur within 24 hours from the injury. Alterations in proprioception may persist for as long as 6 years.17
After an injury, changes occur within the joint that affect normal recruitment and timing patterns of the surrounding musculature.20 Several theories regarding this deterioration of musculature activation have been proposed. One theory is that after an acute injury, an alteration occurs in the ratio of muscle spindle to GTO activity, leading to interference of the proprioception pathway. Another theory suggests that joint effusion after an acute injury alters the ability of the musculature to contract and, therefore, leads to decreased proprioception. A study by Palmieri-Smith and colleagues44 showed that effusion of the knee of just 30 ml significantly decreased the activation of the vastus medialis and lateralis muscles during a single-leg drop landing. Joint effusion of 30 ml is barely palpable by most clinicians.
Critical Points EFFECTS OF INJURY ON PROPRIOCEPTION, GAIT, AND DURATION OF INJURY
Injury to the ACL can lead to significant problems for the athlete. One study by Wojtys and Hutson59 showed there was a significant decrease in muscle activation timing and recruitment order in the medial and lateral quadriceps, medial and lateral hamstrings, and gastrocnemius in response to anterior tibial translation in individuals with ACL-deficient knees compared with an uninjured control group. This delay in muscle recruitment can lead to decreased stability of the joint because the musculature is the prime joint stabilizer owing to loss of ACL function. Beard and coworkers7 examined the effects of applying 100 N of anterior shear force on ACL-deficient knees and noted a reflexive activation of the hamstring muscles. Paterno and associates45 reported a significant difference in force production during a drop vertical jump in ACL-reconstructed knees compared with the contralateral limbs a mean of 27 months after ACL reconstruction. This study is one of many that show continued differences between ACL-reconstructed and uninvolved limbs for an extended period of time after surgery.28,30,53,59
Wilk and colleagues28 reported that 24 to 48 hours after ACL injury, proprioception was altered bilaterally according to measurements on a stability system. The uninvolved lower extremity’s ability to stabilize on a sway board (Biodex Stability System, Shirley, NY) was compromised for 6 to 8 weeks, with a gradual improvement in sway balance thereafter.
EFFECTS OF INJURY ON GAIT
After ACL injury, patients exhibit an alteration in gait patterns. Andriacchi and Birac1 and Berchuck and coworkers8 coined the term “quadriceps avoidance gait pattern” (see Chapter 6, Human Movement and Anterior Cruciate Ligament Function: Anterior Cruciate Ligament Deficiency and Gait Mechanics). Patients walk with greater hamstring activity, a flexed knee, and minimal to no quadriceps electromyographic activity. It has been clinically observed that these protective neuromuscular adaptations (quadriceps-avoidance gait) may persist for several months if not appropriately addressed in rehabilitation.
DURATION OF INJURY EFFECTS
Many theories exist regarding the length of time that a patient with an acute ACL injury experiences a decreased sense of proprioception and neuromuscular control; the exact duration remains unclear. Most sources cite anywhere from 1 to 3 years as the timeframe for altered proprioception of the knee joint. Harrison and associates25 studied the differences between the reconstructed and the uninvolved legs during single-leg stance in patients after ACL reconstruction. These researchers found no significant differences in postural sway (with eyes both open and closed) between the involved and the uninvolved lower extremities during single-leg stance 10 to 18 months after surgery. This finding suggests that proprioception can be restored in a shorter timeframe than expected. Other studies propose that proprioception and joint position sense take much longer to be reestablished. Fremerey and colleagues24 examined the differences of joint position sense at different time intervals and compared those data to information gathered preoperatively. This study showed that joint position sense of the knee was almost completely restored at the near-end range of knee flexion and knee extension 6 months postoperatively. However, the study also reported that proprioception at the midrange of knee motion was not fully restored at 6 months. In fact, some patients took over 3½ years to fully recover their joint position sense at midrange positions. This is critical to an athlete because a majority of activities that occur during competition do so at these midrange of motion positions. The lack of joint position sense at these levels may have a significant impact on the probability that the athlete will sustain a second injury.
Another theory also considered when studying the duration of decreased proprioception in patients with acute ACL injury is the preinjury level of activity of the individual. Roberts and coworkers47 demonstrated that patients whose preinjury activity levels were high had a faster recovery of joint position sense after ACL reconstruction.
STAGES OF MOTOR SKILL DEVELOPMENT
Individuals progress through four stages when learning a new motor skill: mobility, stability, controlled mobility, and skill.52Mobility refers to the availability of ROM to assume a posture or position and the presence of sufficient motor unit activity to initiate a movement. Individuals cannot perform a movement or skill if they do not exhibit adequate mobility. Stability refers to the ability to produce a co-contraction to provide tonic holding. Controlled mobility refers to movement added to a stable posture. Examples include standing and rocking back and forth, weight-shifting, ambulation, and balancing on an unstable platform. Skill is the highest level of motor control and refers to function and the ability to manipulate and perform tasks in an unstable environment, such as playing soccer or tennis.
Chmielewski and coworkers12 classified three stages of motor skill development: cognitive, associative, and autonomous. The cognitive stage exists when a new task or drill is introduced. During this time, errors are made, movement is rigid or stiff, and the individual requires more training to learn the new task. Next is the associative stage in which less time is spent thinking about the task/drill and the movement is improved but is still not automatic. The third stage is the autonomous stage, defined as when, after practice, the movement becomes automatic and efficient and, with time, more skilled. In sports, skills such as shooting a basketball, hitting a tennis serve, or swinging a golf club require these three stages of motor skill. There also appears to be a fourth stage, which the authors refer to as the refining stage. This occurs when the individual refines the task/drill to a level of perfection.
NEUROMUSCULAR-TRAINING PROGRAM
A variety of training methods are available to the clinician to implement a neuromuscular-training program. The training program should be multiphased, be progressive in levels of difficulty, and use both isolation and combined movements. The program must be adaptable and based on the patient’s own neuromuscular status, desired goals, and phase of the rehabilitation program (e.g., acute, subacute, advanced, or return to activity). Nashner and associates40 and Diener and colleagues19 reported that the individual must be challenged to make sufficient gains. When attempting to learn a new skill or task, the individual needs to exhibit a 50% to 60% failure rate to improve motor control. This must be done to enhance neuromuscular control and improve dynamic stability.
Joint Repositioning
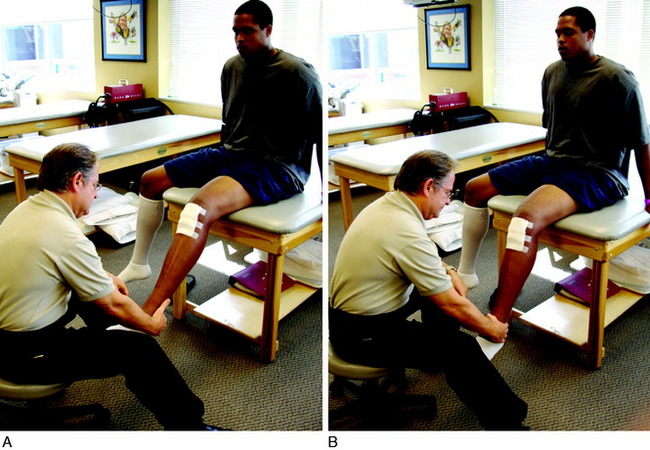
This technique, described by Lephart and associates in numerous publications,34,35 involves passively positioning the joint, followed by asking the patient to actively reposition her or his knee joint angle to that position. This is referred to as passive-then-active joint repositioning (Fig. 14-1). This technique can be performed either as a test or as a rehabilitation-training technique.
Balance Training
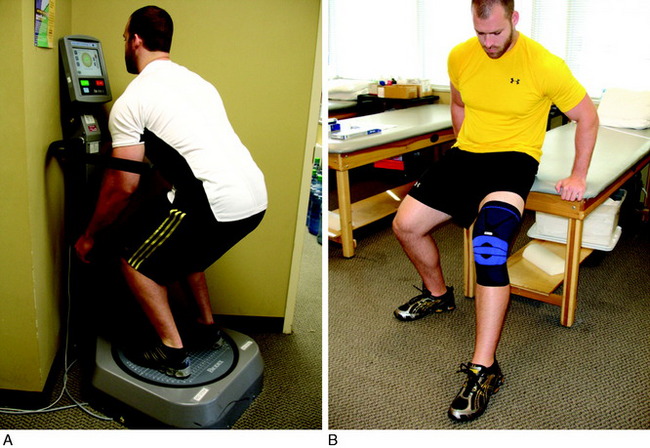
Balance-training exercises are used to facilitate the proprioceptive component of postural control and to reduce postural sway. Balance training may be performed on the floor, an unstable surface, or a balance-training device (Fig. 14-2). Balance training may be performed on a force platform to determine weight distribution (Fig. 14-3). An unstable surface is excellent to promote and improve balance. Such devices include wobble boards (Fig. 14-4), rocker boards (Fig. 14-5), or the Biodex Stability System (Biodex Medical Systems, Shirley, NY; see Fig. 14-2A). Balance training may also be performed on surfaces such as foam, air mattresses, or uneven surfaces to increase the neuromuscular challenges.
Perturbation Training
Perturbation training refers to the reactive motor responses to a change in postural position. Thus, an applied force is imparted to create a postural disturbance. An example of perturbation training is when an individual stands on a rocker board with a single-limb stance, and then the clinician applies an external force, by tapping either the board or the individual, which creates a disturbance. The patient is required to react to the stimulus and correct the postural change. Perturbation training should progress from a low level to advanced challenges. Lowlevel perturbations are predictable, low in force, and unchallenging. Conversely, as the changes in posture increase in load, speed, and force, they become more challenging. The program should progress from single-plane challenges to multiplane. Wilk and coworkers55 introduced perturbation training in the ACL-injured female athlete and emphasized this form of training as a “critical component” necessary for athletes to master, thus enabling them to successfully return to sports. Since that time, Fitzgerald and associates22 demonstrated that perturbation training results in improved outcome in the ACL-deficient knee in the return of the individual to sports. Wilk and coworkers55–57 and Snyder-Mackler and colleagues50 strongly recommended this form of training as an important aspect in ACL rehabilitation. Chmielewski and colleagues13,14 found that there was a restoration of knee joint kinematics and a reduction in quadriceps-hamstring muscle co-activation after perturbation training in patients with ACL-deficient knees.
Plyometric Exercises
Plyometric exercise is commonly referred to as jump training or stretch-shortening exercise drills. Wilk and coworkers57 referred to plyometric training as “reactive neuromuscular training.” Plyometrics are commonly used to increase performance in the advanced phase of rehabilitation and in injury prevention. Noyes and Barber-Westin designed and published on the beneficial effect of a 6-week neuromuscular-training program in female athletes (see Chapter 19, Decreasing the Risk of Anterior Cruciate Ligament Injuries in Female Athletes). An epidemiology study showed that this program may reduce the incidence of ACL injuries, and other knee injuries, in the female athlete.26 The specially designed preventive program, referred to as “Sportmetrics,”27 is discussed in Chapter 19, Decreasing the Risk of Anteror Cruciate Ligament Injuries in Female Athletes. This program and others have been used to reduce ACL injuries in athletes.
Technique Training
The clinician may offer both verbal and visual feedback to the patient. Phrases such as “land light as a feather,” be a “shock absorber,” and act as a “spring” can be used.27 Technique training has been reported to reduce ACL injury.39 Mandelbaum and associates36 emphasized proper technique in their ACL preventive program, which was highly successful in reducing ACL injuries in soccer players. The Sportsmetrics program emphasizes proper landing from plyometric jumps.27 If the exercise is not performed appropriately, the drill is stopped and the technique is corrected and practiced until the athlete exhibits correct landing technique.
REHABILITATION PROGRAM
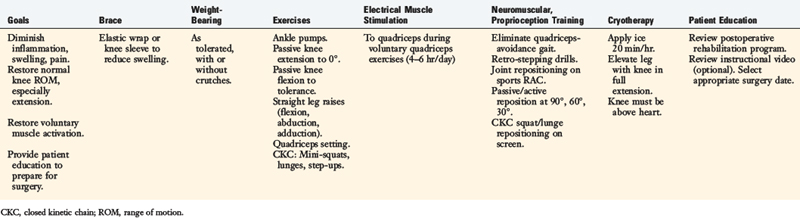
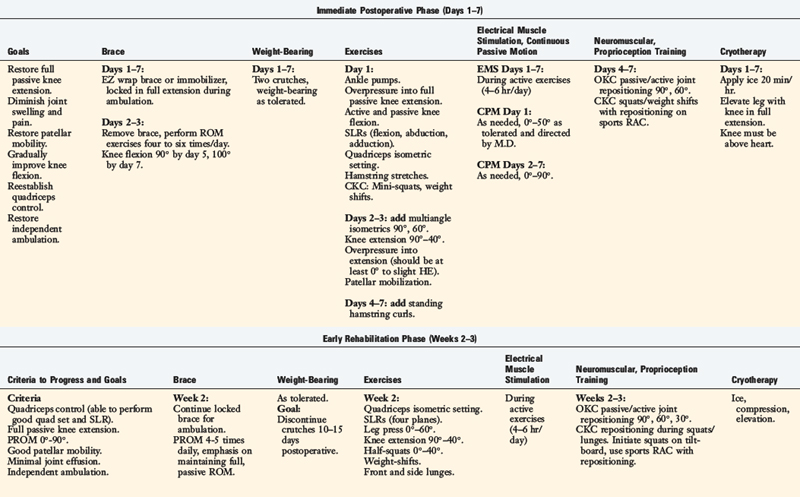
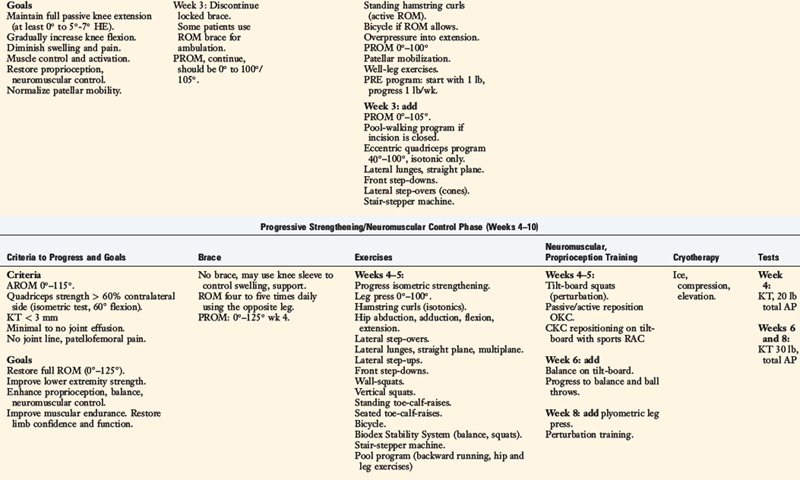
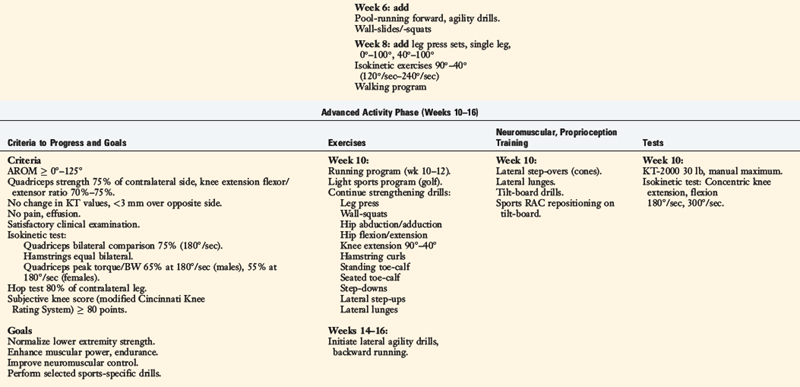
Rehabilitation after ACL reconstruction has changed significantly since the mid 1990s. Today’s programs emphasize immediate motion, immediate partial weight-bearing, immediate muscular training, closed kinetic chain exercises, and an earlier return to functional activities. The authors’ rehabilitation program after ACL injury is shown in Table 14-1, and after reconstruction using a bone–patellar tendon–bone autograft appears in Table 14-2.
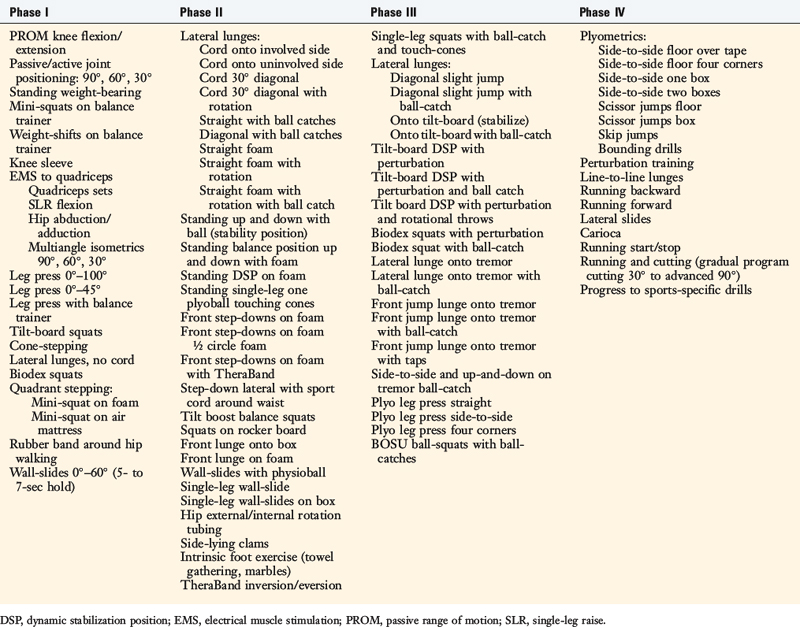
The program is based on 10 key components, listed in Table 14-3. The neuromuscular-training program is a four-phased program; examples of specific exercises and drills are found in Table 14-4. In this section, a brief discussion and examples of specific drills in each phase of the rehabilitation program are provided.
TABLE 14-3 Ten Key Components to the Neuromuscular-Training Program
Phase I: Acute Phase Drills
Immediately after injury or surgery, numerous neuromusculartraining drills and activities may be initiated. In this phase, most of the drills are isolated in nature and a blocked-training technique is employed. Performing passive ROM is beneficial to joint position sense because it stimulates the mechanoreceptors. Furthermore, passive/active reposition sense techniques may be performed to improve proprioception. These activities are usually performed with the eyes closed. Performing weight-bearing exercises is also safe and beneficial. Mini-squats (0°–40°) can be done on a force platform, which provides visual feedback to the patient regarding weight distribution, or on an unstable platform (Fig. 14-6). In addition, weight-shifting can be performed on the platform.
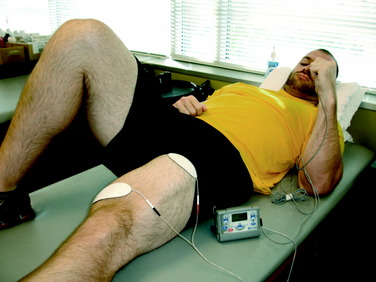
During the 2nd week after surgery, gait activities such as stepping over cones are initiated. This drill may be performed laterally or forward and backward (Figs. 14-7and 14-8). Immediately after injury or surgery, the use of electrical stimulation to the quadriceps (Fig. 14-9) is strongly recommended to prevent “quadriceps shutdown” and assist in muscle reeducation, hypertrophy, and strength gains.51
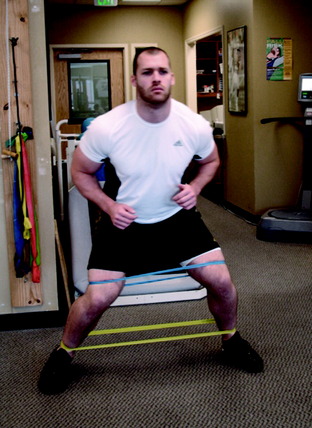
Lateral lunges are performed on a stable floor surface without resistance. The goal of this exercise is to train patients to land from the lunge with a flexed knee; thus improving dynamic stability through co-contracture of the hamstrings and quadriceps (Fig. 14-10). Ambulation with a rubber band around the patient’s hip is beneficial to improve hip muscular strength (Fig. 14-11). Another excellent exercise to improve stability through balance training is squats on a Biodex Stability System (see Fig. 14-2A). Lastly, patients are instructed to wear a compression sleeve when performing daily activities. Birmingham and colleagues10 reported that wearing an elastic sleeve improved proprioception by 25%. A simple compression sleeve (see Fig. 14-2B) can significantly improve a patient’s joint awareness.
Phase II: Dynamic Stability Drills
Lateral lunge exercises are progressed from using a resistance cord in a straight plane (Fig. 14-12) to using a resistance cord in the diagonal plane (Fig. 14-13) and, finally, with rotation (Fig. 14-14). This drill can be progressed to landing on a unilateral surface such as foam (Fig. 14-15) or an air mattress (Fig. 14-16). The specific goal of this drill is the unilateral landing of the lunge. The patient is instructed to land with the knee flexed to 25° to 30° and the hip flexed approximately 30° to 45°, because this promotes a stable joint through quadriceps and hamstring co-activation.56

FIGURE 14-16 Sportcord lateral lunges performed onto an air mattress to create an unstable landing surface.
Other drills include front step-downs and lateral step-ups. The front step-down is an excellent functional training drill (Fig. 14-17). Chmielewski and coworkers16 reported that the front step-down correlated to the patient’s functional level and patient satisfaction. This drill may be performed with half-circle foam under the patient’s foot or with a ball-catch (Fig. 14-18) to diminish the patient’s conscious awareness of his or her knee joint. Squats on an unstable surface, such as on a BOSU ball (Fig. 14-19) or a Biodex Stability System, are excellent in this phase. In addition, hip rotation strengthening exercises are strongly emphasized through a variety of exercise drills (see Table 14-2, Early Rehabilitation Phase [Weeks 2–3]) along with foot and ankle exercise drills. Lastly, endurance exercises are gradually implemented. Numerous authors33,43,60 have shown that once a joint is fatigued, proprioception is significantly decreased; in some cases, up to 75%. Wojtys and associates60 reported a significantly slower response time in the quadriceps, hamstrings, and gastrocnemius after fatigue.
Phase III: Neuromuscular Control Drills
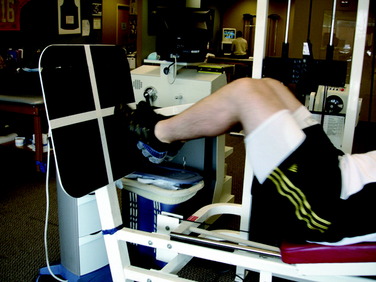
The third phase of the neuromuscular-training program implements random designed drills performed in combined functional movement planes. The majority of these drills are designed to combine knee stabilization drills with sports-specific activities and other techniques to create higher levels of functional demands. Perturbation-training drills are initiated on a rocker board. These drills are progressed from bilateral squatting with perturbation (Fig. 14-20), to single-leg holds at 30° of flexion, to single-leg squats from 0° to 45° with holds, to single-leg holds at 30° flexion with a ball-throw/-catch (Fig. 14-21). During these drills, the clinician taps the rocker board or the patient to create a postural disturbance. The patient is instructed to maintain a horizontal platform position and to “right” the board after the perturbation force. The perturbation drills can also be performed on a Biodex Stability System. Other drills that challenge the neuromuscular system are step lunges onto the tremor device (Fig. 14-22) or a tilt-board. Plyometrics are also initiated in this phase and are performed bilaterally. Leg press plyometrics are initiated before floor-jumping drills, because it is easier to control the patient’s body position. A preferred plyometric leg press drill is the four corners (Fig. 14-23), which combines proprioception and coordination.
Phase IV: Functional/Skills
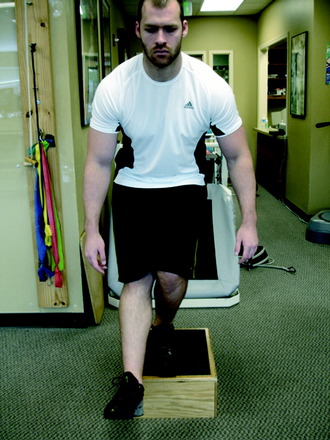
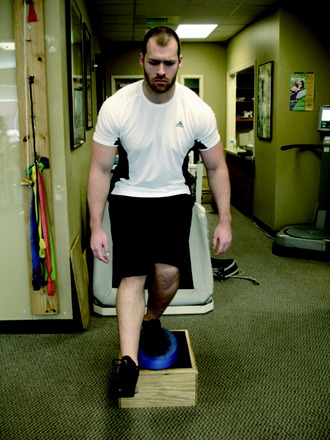
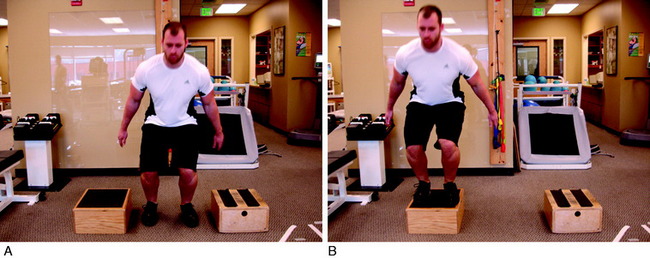
The final phase is the skill and the return to activity phase. During this phase, four types of neuromuscular-training drills are employed: plyometrics, perturbation, technique, and sports-specific progression. The plyometric drills are progressed to jumping on the floor and/or over a cone or hurdle and then to box jumps. These drills include one-box jumps (Fig. 14-24), two-box jumps (Fig. 14-25), and four-box jumps. A running program is initiated with backward running to promote knee flexion and muscle co-activation. Running backward also results in higher electromyographic activity of the quadriceps and hamstrings than that of running forward.23 From backward running, the patient progresses to side shuffles and cariocas, followed by forward running. Once forward running is achieved with a normal gait pattern, the patient is advanced to running and deceleration drills, and finally to running and cutting. After this progression is mastered, the patient may begin a sports-specific training program. Proper technique is critical during this phase. Any problems with technique should result in the rehabilitation professional stopping the drill to both protect the motor skill and promote proper technique. In addition, during this phase, a functional assessment test may be performed to compare one extremity with the other.2 Barber-Westin and colleagues3 reported on ACL-reconstructed and normal patients performing the functional hop test.
1 Andriacchi T.P., Birac D. Functional testing in the anterior cruciate ligament–deficient knee. Clin Orthop Relat Res. 1993;288:40-47.
2 Barber S.D., Noyes F.R., Mangine R., DeMaio M. Rehabilitation after ACL reconstruction: function testing. Orthopedics. 1992;15:969-974.
3 Barber-Westin S.D., Galloway M., Noyes F.R., et al. Assessment of lower limb neuromuscular control in prepubescent athletes. Am J Sports Med. 2005;33:1853-1860.
4 Barrack R.L., Skinner H.B., Buckley S.L. Proprioception in the anterior cruciate deficient knee. Am J Sports Med. 1989;17:1-6.
5 Barrett D.S., Cobb A.G., Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br. 1991;73:53-56.
6 Beard D.J., Dodd C.A.F., Trundle H.R., Simpson A.H.R.W. Proprioception enhancement for anterior cruciate ligament deficiency. A prospective randomised trial of two physiotherapy regimes. J Bone Joint Surg Br. 1994;76:654-659.
7 Beard D.J., Kyberd P.J., Fergusson C.M., Dodd C.A.F. Proprioception after rupture of the anterior cruciate ligament. An objective indication of the need for surgery? J Bone Joint Surg Br. 1993;75:311-315.
8 Berchuck M., Andriacchi T.P., Bach B.R., Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72:871-877.
9 Biedert R.M. Contribution of the three levels of nervous system motor control: spinal cord, lower brain, cerebral cortex. In: Lephart S., Fu F., editors. Proprioception and Neuromuscular Control in Joint Stability. Champaign, IL: Human Kinetics; 2000:23-29.
10 Birmingham T.B., Kramer J.F., Inglis J.T., et al. Effect of a neoprene sleeve on knee joint position sense during sitting open kinetic chain and supine closed kinetic chain tests. Am J Sports Med. 1998;26:562-566.
11 Brooks V., editor. The Neural Basis of Motor Control. New York: Oxford University Press, 1986.
12 Chmielewski T., Hewett T.E., Hurd W.J. Principles of neuromuscular control for injury prevention and rehabilitation. In: Magee D., Zachazewski J.E., Quillen W.S., editors. Scientific Foundations and Principles of Practice in Musculoskeletal Rehabilitation. St. Louis: Saunders; 2007:375-387.
13 Chmielewski T.L., Hurd W.J., Rudolph K.S., et al. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys Ther. 2005;85:740-749. discussion 750–754
14 Chmielewski T.L., Rudolph K.S., Snyder-Mackler L. Development of dynamic knee stability after acute ACL injury. J Electromyogr Kinesiol. 2002;12:267-274.
15 Chmielewski T.L., Stackhouse S., Axe M.J., Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22:925-930.
16 Chmielewski T.L., Wilk K.E., Snyder-Mackler L. Changes in weight-bearing following injury or surgical reconstruction of the ACL: relationship to quadriceps strength and function. Gait Posture. 2002;16:87-95.
17 Denti M., Randelli P., Lo Vetere D., et al. Motor control performance in the lower extremity: normals vs. anterior cruciate ligament reconstructed knees 5–8 years from the index surgery. Knee Surg Sports Traumatol Arthrosc. 2000;8:296-300.
18 Di Fabio R.P., Graf B., Badke M.B., et al. Effect of knee joint laxity on long-loop postural reflexes: evidence for a human capsular-hamstring reflex. Exp Brain Res. 1992;90:189-200.
19 Diener H.C., Horak F.B., Nashner L.M. Influence of stimulus parameters on human postural responses. J Neurophysiol. 1988;59:1888-1905.
20 Dutton M. Neuromuscular control. In: Dutton M., editor. Orthopaedic Examination, Evaluation and Intervention. New York: McGraw-Hill; 2004:55-57.
21 Evarts E.V. Motor cortex reflexes associated with learned movement. Science. 1973;179(72):501-503.
22 Fitzgerald G.K., Axe M.J., Snyder-Mackler L. The efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physical active individuals. Phys Ther. 2000;80:128-140.
23 Flynn T.W., Soutas-Little R.W. Mechanical power and muscle action during forward and backward running. J Orthop Sports Phys Ther. 1993;17:108-112.
24 Fremerey R.W., Lobenhoffer P., Zeichen J., et al. Proprioception after rehabilitation and reconstruction in knees with deficiency of the anterior cruciate ligament. J Bone Joint Surg Br. 2000;82:801-806.
25 Harrison E.L., Duenkel N., Dunlop R., Russell G. Evaluation of single-leg standing following anterior cruciate ligament surgery and rehabilitation. Phys Ther. 1994;74:245-252.
26 Hewett T.E., Lindenfeld T.N., Riccobene J.V., Noyes F.R. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999;27:699-706.
27 Hewett T.E., Stroupe A.L., Nance T.A., Noyes F.R. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24:765-773.
28 Hooks T.R., Wilk K.E., Reinold M.M. Comparison of proprioceptive deficits of the involved and noninvolved lower extremity following ACL injury and surgical reconstruction. J Orthop Sports Phys Ther. 2003;33:A59.
29 Houk J., Simon W. Responses of Golgi tendon organs to forces applied to muscle tendon. J Neurophysiol. 1967;30:1466-1481.
30 Hurd W.J., Axe M.J., Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 2, determinants of dynamic knee stability. Am J Sports Med. 2008;36:48-56.
31 Johansson H. Role of knee ligaments in proprioception and regulation of muscle stiffness. J Electromyogr Kinesiol. 1991;1:158-179.
32 Johansson H., Sjolander P., Sojka P. Receptors in the knee joint ligaments and their role in the biomechanics of the joint. Crit Rev Biomed Eng. 1991;18:341-368.
33 Lattanzio P.J., Petrella R.J., Sproule J.R., Fowler P.J. Effects of fatigue on knee proprioception. Clin J Sport Med. 1997;7:22-27.
34 Lephart S., Kocher M.S., Fu F., et al. Proprioception following anterior cruciate ligament reconstruction. J Sport Rehabil. 1992;1:188-196.
35 Lephart S.M., Pincivero D.M., Giraldo J.L., Fu F.H. The role of proprioception in the management and rehabilitation of athletic injuries. Am J Sports Med. 1997;25:130-137.
36 Mandelbaum B.R., Silvers H.J., Watanabe D.S., et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33:1003-1010.
37 Matthews P.B. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol. 1981;320:1-30.
38 Matthews P.B. Recent advances in the understanding of the muscle spindle. Sci Basis Med Annu Rev. 1971:99-128.
39 Myklebust G., Engebretsen L., Braekken I.H., et al. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13:71-78.
40 Nashner L.M., Shupert C.L., Horak F.B., Black F.O. Organization of posture controls: an analysis of sensory and mechanical constraints. Prog Brain Res. 1989;80:411-418.
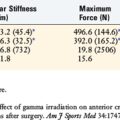
41 Noyes F.R., Berrios-Torres S., Barber-Westin S.D., Heckmann T.P. Prevention of permanent arthrofibrosis after anterior cruciate ligament reconstruction alone or combined with associated procedures: a prospective study in 443 knees. Knee Surg Sports Traumatol Arthrosc. 2000;8:196-206.
42 Noyes F.R., Mangine R.E., Barber S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med. 1987;15:149-160.
43 Nyland J.A., Shapiro R., Stine R.L., et al. Relationship of fatigued run and rapid stop to ground reaction forces, lower extremity kinematics, and muscle activation. J Orthop Sports Phys Ther. 1994;20:132-137.
44 Palmieri-Smith R.M., Kreinbrink J., Ashton-Miller J.A., Wojtys E.M. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35:1269-1275.
45 Paterno M.V., Ford K.R., Myer G.D., et al. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17:258-262.
46 Pope M.H., Johnson R.J., Brown D.W. The role of musculature in injuries to the medial collateral ligament. J Bone Joint Surg Am. 1979;61:398-402.
47 Roberts D., Andersson G., Friden T. Knee joint proprioception in ACL-deficient knees is related to cartilage injury, laxity and age: a retrospective study of 54 patients. Acta Orthop Scand. 2004;75:78-83.
48 Schmidt R., Lee T., editors. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics, 1999.
49 Skinner H.B., Wyatt M.P., Hodgdon J.A., et al. Effect of fatigue on joint position sense of the knee. J Orthop Res. 1986;4:112-118.
50 Snyder-Mackler L., Delitto A., Bailey S.L., Stralka S.W. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166-1173.
51 Snyder-Mackler L., Ladin Z., Schepsis A.A., et al. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1991;73:1025-1036.
52 Sullivan P.E., Markos P.D., Minor M.A., editors. An Integrated Approach to Therapeutic Exercise, Theory, and Clinical Application. Reston: Reston Publishing, 1982.
53 Swanik C.B., Lephart S.M., Giraldo J.L., et al. Reactive muscle firing of anterior cruciate ligament–injured females during functional activities. J Athl Train. 1999;34:121-129.
54 Wilk K.E. Rehabilitation of isolated and combined posterior cruciate ligament injuries. Clin Sports Med. 1994;13:649-677.
55 Wilk K.E., Arrigo C., Andrews J.R., Clancy W.G. Rehabilitation after anterior cruciate ligament reconstruction in the female athlete. J Athl Train. 1999;34:177-193.
56 Wilk K.E., Escamilla R.F., Fleisig G.S., et al. A comparison of tibiofemoral joint forces and electromyographic activity during open and closed kinetic chain exercises. Am J Sports Med. 1996;24:518-527.
57 Wilk K.E., Voight M.L., Keirns M.A., et al. Stretch-shortening drills for the upper extremities: theory and clinical application. J Orthop Sports Phys Ther. 1993;17:225-239.
58 Williams G.N., Chmielewski T., Rudolph K., et al. Dynamic knee stability: current theory and implications for clinicians and scientists. J Orthop Sports Phys Ther. 2001;31:546-566.
59 Wojtys E.M., Huston L.J. Neuromuscular performance in normal and anterior cruciate ligament–deficient lower extremities. Am J Sports Med. 1994;22:89-104.
60 Wojtys E.M., Wylie B.B., Huston L.J. The effects of muscle fatigue on neuromuscular function and anterior tibial translation in healthy knees. Am J Sports Med. 1996;24:615-621.