5 Neurology of sensory and receptor systems
Introduction
Sensory information is essential for a variety of reasons such as to construct a perception of ourselves in the universe, muscle movement control, internal organ and blood flow functionality, maintaining arousal, and developing and maintaining survival and plasticity in neural networks.
Reception and sensation of afferent stimulation
The physical properties of the stimuli detected by our receptors often differ dramatically from the perceptions that we form from these stimuli. For example, our retinal cells detect electromagnetic radiation patterns but we perceive the Mona Lisa. Our ears detect sound wave variations and patterns but we perceive a Mozart symphony. Our nociceptors detect chemical imbalances but we perceive pain. This amazing ability of our brain to process this information in an individually unique but universally characteristic fashion is one of the fundamental attributes that determines our humanism. Colours, sounds, feelings, pain, and all other human perceptions do not exist outside of the human mind. Thus our perception of our universe is not a true representation of our physical universe but a mental construct composed by our mind’s interpretation of all of the integrated nervous input available to it at any given moment (Martin & Jessell 1995). It is important to remember this when a patient presents with symptoms that cannot be explained by our traditional understanding of neuroscience or neuroanatomy. The patient’s perception is their reality. For example, in people with fibromyalgia, the detection of movement by their joint receptors is perceived as pain in their mind. To this patient this perception of pain is very real.
The sensory neurons
Somatic sensation
Somatic sensibility has four major types of modalities:
1. Discriminative touch—Discrimination of size, shape, texture, and movement across the skin.
2. Proprioception—The sense of static and kinetic position of the limbs and body without visual input.
3. Nociception—The signalling of tissue damage or chemical irritation, typically perceived as pain or an itch.
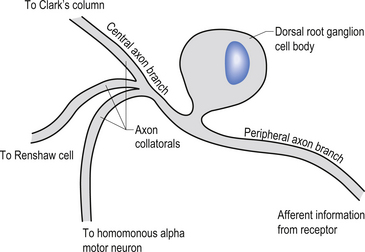
The cell bodies of the primary afferent neurons of the spinal cord live in the dorsal root ganglia situated adjacent to the spinal cord in the immediate vicinity of the intervertebral foramen from which they enter the spinal canal (Schwartz 1995). The primary afferent neurons are classified as pseudounipolar cells because they give rise to only one axon that transmits information from the periphery to the spinal cord. The segment of the axon that transmits information from the peripheral receptor towards the cell body is called the peripheral axon branch, and the portion of the axon transmitting information away from the cell body towards the spinal cord is called the central axon branch. The dorsal root neurons do not form dendrites as most other neurons do; thus any modulatory activity occurring at these neurons must occur at the cell body or presynaptically on the central process as it enters the grey matter spinal cord. Most of the central processes of the primary afferent develop collateral axon branches as they enter the spinal cord (Fig. 5.1). These collateral branches synapse with a variety of other neurons depending on the type of modality that the primary afferent cell is relying. The collateral branching and synapse formation of each type of primary afferent will be described in detail as each individual modality is described below.
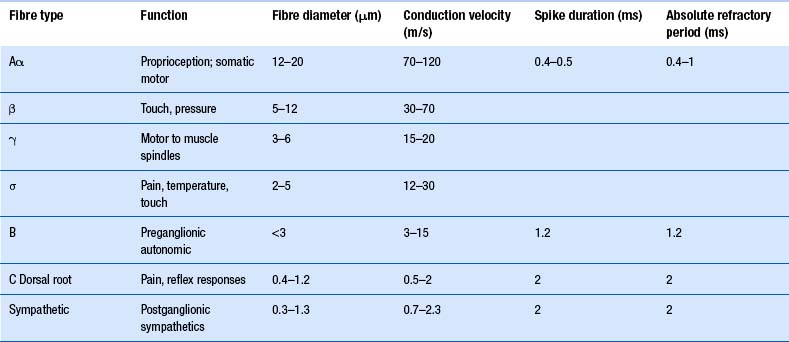
The axons of the various primary afferents display a variety of different diameters and myelination densities. Functionally, these morphological differences result in axons that transmit impulses at different rates of speed or conduction velocities. Table 5.1 outlines the various conduction velocities and diameters of different primary afferent axons.
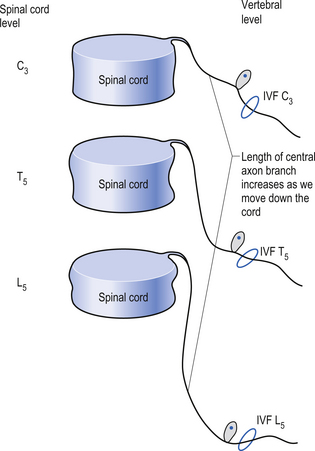
Because of different growth rate patterns between the spinal cord and the vertebral column the dorsal root ganglion cells below T3 are located at increasingly further distances from the segmental spinal cord levels that they supply. This results in the progressive lengthening of the central processes of the primary afferents and in the formation of the cauda equina after the spinal cord ends at the level of L1–L2 in most people. It is these central processes that may experience compression from a posterior central or posterior lateral vertebral disc herniation (Figs 5.2 and 5.3).
Receptors of primary afferent input
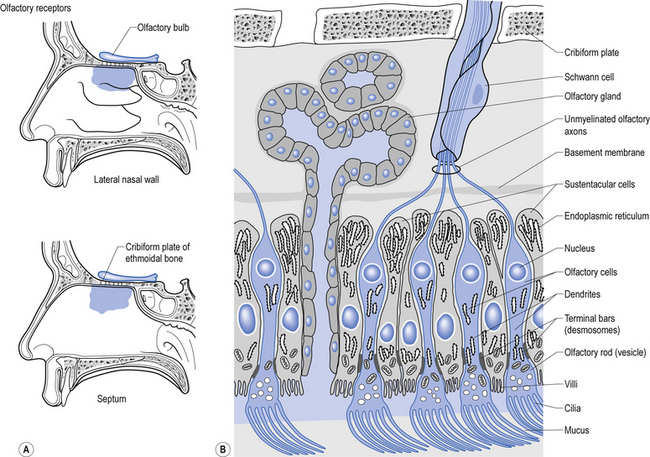
Structurally, receptors can be classified into three basic morphological forms: neuroepithelial receptors, visual receptors, and primary afferent receptors. In the case of the neuroepithelial receptor, the sensory sensitive detection apparatus is contained in the neuron cell body itself. These types of neurons are situated near the sensory surface with their axons projecting back towards the central nervous system. The unique quality of these receptors is that the neuron cell body has derived from epithelial tissue and remains in that tissue as a receptor organ. In humans the only known example of this type of sensory reception can be found in the sensory cells of the olfactory epithelium. Here, the axons of these neurons which form the olfactory nerve or 1st cranial nerve (CN I) traverse the cribriform plate of the ethmoid bone to synapse in the glomeruli of the olfactory bulb (Williams & Warwick 1984) (Fig. 5.4).
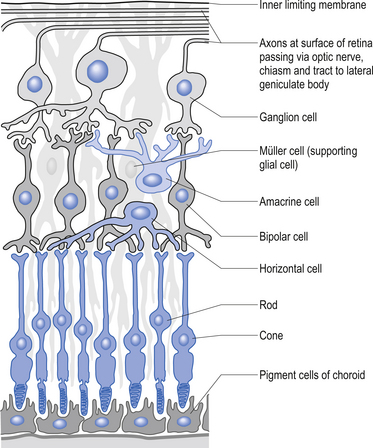
The second type of receptor can be classed as visual receptors. These receptors are similar to the neuroepithelial receptors in that the receptive apparatus is in their cell bodies; however, in this case, their cell bodies are derived from neuroectoderm of the fetal ventricles which form the fetal brain and migrate to the retinal areas (Fig. 5.5).
In the third type of receptor, which may be classified as a primary afferent receptor, the cell body is located near to or in the central nervous system. Long peripheral processes extend to the receptive area and form the specialised receptive structures that act as the receptors. All cutaneous receptors and most proprioceptors are composed of this type of receptor (Williams & Warwick 1984) (see Fig. 5.1).
The muscle spindle
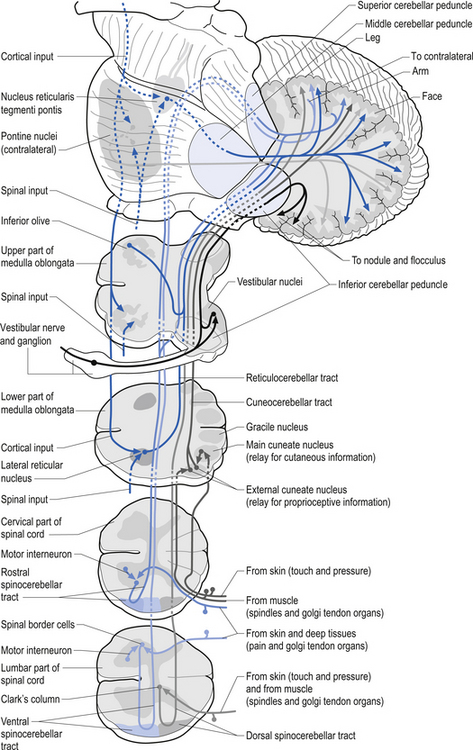
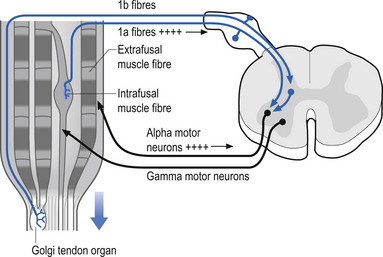
Muscle spindles provide the nervous system with information concerning the instantaneous static length of a muscle at any given moment and the rate of change of muscle length during any movement. The output of the muscle spindle is simultaneously transmitted to a variety of interneuron networks in the spinal cord. This information is then relayed to areas in the brainstem, cerebellum, and basal ganglia. The axons of the primary afferent neurons that relay muscle spindle information to the spinal cord form many collateral branches as they enter the spinal cord. Some of these collaterals proceed without synapsing on an interneuron to synapse directly (monosynaptic connection) on the alpha motor neuron pools of the muscle fibres from which they are relaying information (homonymous muscle), resulting in an excitatory stimulus (Fleshman et al. 1981). Other collaterals first synapse (polysynaptic connection) on interneurons in lamina VIII of the grey matter of the spinal cord, referred to as Clark’s column. These interneurons then project to the brainstem reticular formation nuclei, various cortical areas of the cerebellum via mossy fibres, and the basal ganglia (Cram & Kasman 1998). The spinocerebellar tracts are formed from the axons of these interneurons in Clark’s columns. The anterior spinocerebellar tracts receive axons from both ipsilateral and contralateral lamina VIII neurons. The posterior spinocerebellar tracts receive axons from only ipsilateral lamina VIII neurons. This is an example of reciprocal innervation that occurs when vital information transfer is required (Fig. 5.6). All regions other than the segmental interneurons and their associated alpha motor neurons receiving input from the primary afferents at that level are referred to as suprasegmental to the input. Most suprasegmental integration is involved with modulation of movement, or in feedback control of the accuracy of the movement generated. Some controversy exists as to whether the receptive input of muscle spindles can be consciously perceived. Since muscle spindles do not project directly to the somatosensory cortex, their activity was thought not to be consciously perceived. However, some research has indicated that conscious perception is possible (Williams & Warwick 1984).
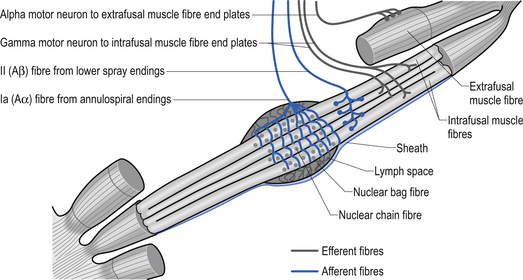
The specialised receptors on the muscle spindle afferent axons are associated with the intrafugal fibres of the muscle. These intrafugal fibres are interposed between the major contractile tissues referred to as the extrafugal fibres of the muscle. The intrafugal fibres are surrounded by a sheath that separates them from the extrafugal fibres, which is filled with a fluid rich in hyaluronic acid. The intrafugal fibres can be classified into nuclear bag and nuclear chain fibres. Nuclear bag fibres have their nuclei clustered in the central area of the fibre, giving them a fusiform appearance. Within the nuclear bag fibres a further distinction between static and dynamic fibre types can also be made. The nuclear bag fibres are much longer than the nuclear chain fibres and extend beyond the capsule of the spindle to attach to the endomysium of the surrounding extrafugal fibres. The nuclear chain fibres have their nuclei lined up centrally along the midline of the length of the fibre and are attached to the nuclear bag fibres at their poles. Sensory endings in the muscle spindles give rise to two types of afferent axons emerging from a muscle fibre. The large myelinated, type Ia afferent fibres – also known as annulospiral endings – innervate all types of primary sensory endings in an intrafugal muscle fibre and are rapidly adapting and extremely sensitive to changes in muscle fibre length (Banks et al. 1981). The type II afferent fibres – also known as flower spray endings – emerge from the sensory endings of nuclear chain and static nuclear bag fibre types and are slow adapting and highly sensitive to static muscle length (Cooper & Daniel 1963; Boyd 1980) (Fig. 5.7). Human muscle spindle sensory endings also respond to tendon percussive stimulation, vibration, and other forms of rhythmic stretch (Burke 1981). Most types of human muscle tissue have one nuclear bag dynamic fibre, one nuclear bag static fibre, and a number of nuclear chain fibres per muscle spindle (Ghez & Gorden 1995). When the intrafugal fibres become stretched, the nerve endings depolarise and an increase in action potential firing occurs. This process is known as ’loading’ the spindle. ’Unloading’ the spindle occurs when the intrafugal fibre returns toward its normal length. This results in a decrease in the action potential frequency of firing in the afferent nerve fibres.
Gamma motor fibres can change the sensitivity and gain of the muscle spindle
The intrafugal fibres contain specialised areas of contractibility innervated by efferent motor fibres referred to as gamma motor fibres (Matthews 1981). Each intrafugal fibre receives motor innervation from more than one gamma motor axon. This is termed polyneuronal innervation and is peculiar for the most part to the intrafugal fibres of muscle. (Polyneuronal innervation does occur in extrafugal fibres in neonates before functional maturation has occurred and occasionally in the early stages of reinnervation of muscle tissue following injury (Burke & Lance 1992).) These gamma motor fibres release acetylcholine at their synapses and arise from gamma motor neurons that live in lamina IX of the anterior grey matter of the spinal cord of the same segmental level as the alpha motor neurons supplying the extrafugal fibres from the homonymous muscle (Hunt 1974). The contractile elements of the nuclear bag fibres are rich in mitochondria and oxidative enzymes, whereas the nuclear chain fibres have more extensive sarcoplasmic reticulum and T-fibre development but fewer mitochondria and oxidative enzymes. This results in a slower contraction of nuclear bag fibres than nuclear chain fibres when the spindle is stimulated (Williams & Warwick 1984).
Two classes of somatic sensation can be distinguished neurologically
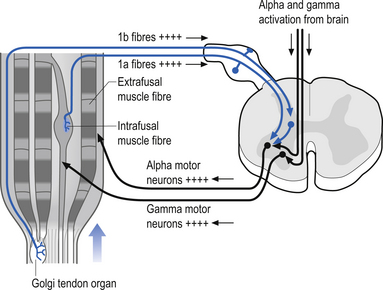
The gamma motor input to the intrafugal fibres is involved with adjusting the appropriate length of intrafugal fibres in order to maintain an accurate measure of the degree of contraction of the muscle. If the length of the intrafugal fibre was not constantly adjusted as the extrafugal fibres contracted, it would become slack and fail to accurately record further contraction or the static state of the muscle at that instant. Loading of the intrafugal fibres may occur by increasing the stretch on the extrafugal fibres or by increasing the activity of the contractile elements of the intrafugal fibres by increasing the gamma motor activity. Increasing the gamma motor activity results in a high specificity of muscle activity with a small amplitude of sway variation in maintaining extrafugal to intrafugal muscle length within a tight oscillating pattern. The degree of oscillation occurring in a muscle is referred to as gain. Thus with increasing gamma activation the specificity of movement or sensitivity increases and the gain decreases. This interaction provides for the occurrence of a reflex compensation for small irregularities of movement that may occur between the suprasegmental programming and the performance of the actual movement (Burke & Lance 1992). This modulation circuit is referred to as the motor servo mechanism (see Chapter 6). In order to ensure that this system performs properly there is an interactive connection between the alpha and gamma motor neurons that results in a co-activation of both systems simultaneously. So important is knowing the moment-to-moment state of our muscle position to the nervous system that nearly 30% of all descending efferent motor control systems are associated with the gamma motor modulation of spindle fibres, and the feedback information from these fibres is transmitted from the spinal cord to the suprasegmental areas via redundant pathways. As a general rule only information crucial to the nervous system is transmitted by more than one pathway. As previously mentioned, when information is transmitted through more than one pathway it is referred to as redundant transmission. To exemplify this point, the information from the muscle spindles below vertebral level of T1 travels up the spinal cord via the ipsilateral dorsal or posterior spinocerebellar tract and bilaterally in the ventral or anterior spinocerebellar tracts to the cerebellum. The axons of the posterior spinocerebellar tracts and the ipsilateral anterior spinocerebellar tracts pass through the ipsilateral inferior cerebellar peduncles to reach the ipsilateral cerebellum. The axons of the contralateral anterior spinocerebellar tracts cross back to the side of their origin in the superior cerebellar peduncles and thus maintain the rule that cerebellar input from muscle spindles occurs ipsilaterally. The paravertebral muscles of the spine have one of the highest concentrations of spindle receptors in the body and the upper cervical region of the spinal cord has the highest density of muscle spindle receptors in the spine (Kulkarni et al. 2001).
The muscle spindles of humans are usually well developed at birth. The development of the spindles in the fetus depends solely on the presence of the primary afferent axon. After the spindles have formed they can tolerate periods of denervation; however, they often undergo marked changes in function and reactivity even when reinnervation occurs by the original axon (Milburn 1973). The altered functional activity in muscle spindles following injury can contribute to asymmetries in cortical afferent input, resulting in hemispheric asymmetry. This presents one of the clinical challenges facing a patient following axon injuries or demyelination conditions.
Golgi tendon organs
These specialised receptors are found extensively in the collagen fibres of the muscle tendon junction. These receptors align themselves in series with the muscle fibres in such a way that stretching the tendon results in depolarisation of the receptors (Barker 1974). Some tendon organs are activated with even the weakest of contractions. The total discharge frequency of the organ increases with the strength of contraction of the homonymous muscle. The tendon organs are generally silent in relaxed muscle. There is some controversy as to when the tendon organs actually become activated. It has been the prevailing view that tendon organs become active whenever the muscle is stretched either by active contraction or by passive stretch (Matthews 1972). However, another opinion which states that tendon organs usually will not activate even if the muscle is stretched unless the muscle contracts during the stretch has recently arisen. Thus, the newest theory is that these organs monitor muscle contraction in conjunction with tendon tension and not just tendon tension in isolation (Houk & Crago 1980). One of the actions of stimulating the tendon organs of a muscle is a reflex inhibitory feedback stimulus on the alpha motor neurons controlling the homonymous muscle (Fig. 5.8).
The sensory organs of Golgi tendon organs give rise to type Ib afferent fibres, which are myelinated but slightly smaller in diameter than the type Ia fibres of the muscle spindles. The fibres intertwine between the collagenous fibres of the tendon muscle junction where they are unmyelinated and highly branching. As they emerge from the collagen fibre network they bundle together and form a single myelinated axon. Usually, each individual tendon organ gives rise to one type Ib axon (Schoultz & Swett 1974; Ghez & Gorden 1995). Although the receptor information from the Golgi tendon organs does not reach the somatosensory cortex directly, and thus is not consciously perceived, the information supplied by the Golgi tendon organs is very important in the integration of inhibition of motor activity. This is especially apparent when learning how to perform a new motor function. For example, when learning how to hit a tennis ball, the player learns after many attempts not only how to perform the coordinated actions of the muscles involved in the correct swing, but also the ’feel’ of the muscle tensions involved in the correct swing (see Chapter 6).
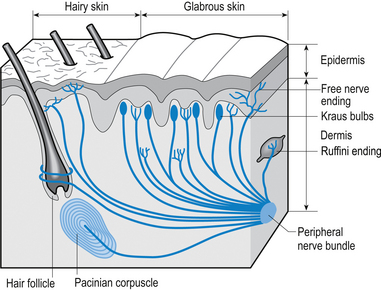
Tactile (Meissner’s) corpuscles
These receptors are present on all parts of the hands and feet, on the forearm, the lips, and the tip of the tongue. The corpuscles consist of a capsule and a core portion. The capsule is formed by layers or lamellae between which substantial amounts of collagen have been laid down. The core consists of epidermally derived cells and nerve fibres. Each corpuscle is supplied by several large, heavily myelinated nerve (Aα) fibres and a few small unmyelinated (C) fibres. These receptors are low threshold, rapidly adapting and thus provide information concerning the change of mechanical pressure on the overlying skin. Interestingly, these receptors are formed in abundance at birth and over our lifetimes reduce in number by some 80% unless constantly maintained by stimulation (Cauna 1966) (Fig. 5.9).
Pacinian corpuscles
These receptors are sensitive to changes in pressure in a variety of tissues in the body. Pacinian corpuscles are found on the palmar aspects of the hands and feet, the genital organs of both sexes, and buried deep in most muscle tissues. These receptors are extremely sensitive to mechanical disturbances and are particularly sensitive to vibration (Gray & Sato 1953). In muscle tissue they give feedback on the internal pressure changes inside the muscle fibres during both stretch and contraction.
Each corpuscle consists of a capsule and a central core containing a nerve fibre. The capsule is lamellar in nature, with the lamellae separated by layers of collagen. The amounts of collagen deposited between the lamellae increases with age, decreasing the sensitivity of the receptors. Each corpuscle is supplied by one thickly myelinated nerve fibre of the Aα type (Williams & Warwick 1984) (see Fig. 5.9).
Merkel cell endings
These receptors are found immediately under the epidermis or around the base of certain hair follicles. The nerve fibres expand at their periphery into flattened disc-like structures that come into close association with a non-neural type of cell called the Merkel cell. The Merkel cells have a number of interdigitating processes that contact other neighbouring cells. The nerve/Merkel cell units together are called Merkel discs and are sensitive to vertical pressure. The receptors are slow adapting and transmit information via large, myelinated (Aα) afferent fibres (English 1977) (see Fig. 5.9).
Ruffini endings
The Ruffini system of receptors including the Ruffini endings and Ruffini nuclei are found in each and every joint complex and in the dermis of hairy skin. These receptors respond to changes in stress levels in collagen. In joint capsules they are very sensitive to capsular deformation and may be activated by changes in the degree of arc about a joint. In most cases involving peripheral joints they can detect a 1° change of arc about the joint. In the case of certain types of receptors in the upper cervical spine as little as a 0.4° change of arc has been shown to cause activation. This is probably due to the high concentration of joint receptors in the upper cervical spine region which has been shown to contain the highest concentration of joint receptors in the spine (Vele 1970). These receptors are slowly adapting and utilise large, myelinated (Aα) afferent fibres for transmission (Chambers et al. 1972) (see Fig. 5.9).
Joint receptors
A complex system of specialised receptors that supply feedback concerning the static and dynamic position of joints has been described by Wyke (1966). His description involves a classification of four types of joint receptors, which are described as follows.
Type I or tonic receptors have a low threshold and are slow adapting. These receptors are active both at rest and during changes of joint arc. Thus when the joint arc changes, these receptors show an initial burst of activity and continue to fire at a reduced but specific frequency until another change in joint angle occurs. These types of receptors are multibranched, encapsulated Ruffini ending type receptors. These receptors appear to be the most abundant type of joint receptor and are most highly concentrated in the weight-bearing joints where static postural information is crucial. It is thought that the information transferred by these receptors may reach conscious perception (Skoglung 1973).
Type II joint receptors are similar to Pacinian corpuscles but much smaller in size and have a low threshold but are rapidly adapting. Their activity increases at both the initiation and cessation of movement. They are highly sensitive to movement and pressure changes in the capsule and are thought to detect duration of movement. The information from these receptors is thought not to reach conscious awareness (Skoglung 1973).
Type III receptors have a high threshold and are slowly adapting to stimuli. They are inactive in non-moving joints and fire only at extremes of joint motion. They are similar in structure to the Golgi tendon organs of tendons and cause a reflex inhibition of the homonymous muscle when highly stimulated (Williams & Warwick 1984) (Fig. 5.10).
Type IV receptors are nociceptive in function and have a high threshold and do not adapt. These types of receptors are free nerve endings that invade the synovial layers, blood vessels, and fat pads surrounding the joint and are normally inactive. They seem to be most sensitive to excessive joint movement and all causes of damaging stimuli that result in the perception of joint pain (Wyke 1966; Newton 1982; Fitz-Ritzon 1988; McLain & Picker 1998; Eriksen 2004).
This diverse variety of receptors in the joint capsule allows these receptors to supply the nervous system with a continuous input stream regarding joint position at any given moment in time. Some of the input from this system of receptors can be volitionally perceived although most of the time we are not consciously aware of the position of each of our joints (Fitz-Ritzon 1988).
Free nerve endings
Three different types of nociceptors can be distinguished by the various stimuli to which they respond. These are mechanical, thermal, and polymodal receptor types (Gardner et al. 2000). Mechanical receptors are responsive to sharp penetration of objects into the skin or tissues. They are also sensitive to pinching or squeezing actions. The axons are mechanical nociceptors, are myelinated, and thus are the fastest conducting fibres of the nociceptors receptors. Thermal receptors are excited by extremes of temperature. The two different types of thermal receptors respond to cold and heat. Polymodal receptors respond to a variety of stimuli including mechanical, chemical, and temperature extremes. These receptors evoke the sensation of deep burning pain when their activation becomes perceived consciously.
Some of the nociceptive input may reach the somatosensory cortex where it is consciously perceived most commonly as pain (Cauna 1966). Nociceptive information is conveyed from the spinal cord to the thalamus and then to the sensory cortex. The spinal tracts that convey this information to the thalamus or hypothalamus include the spinothalamic, spinoreticular, spinomesencephalic, cervicothalamic, and spinohypothalamic tracts. The most prominent nociceptive tract is the spinothalamic tract of which both anterior and lateral divisions participate in nociceptive signal transmission (Basbaum & Jessell 2000).
How do we begin to understand the complexity of the integration that must be occurring for even the most primitive type of movement?
The muscle stretch reflex occurs because the group Ia (primary afferent) fibres from the muscle spindles have an overall excitatory effect on the motor neuron pools of the homonymous and heteronymous (synergistic) muscles and an overall inhibitory effect on the antagonistic motor neuron pools. The excitatory effects are produced through a number of pathways including monosynaptic and polysynaptic connections (Jankowska et al. 1981). The spindle afferent fibres branch to form collateral fibres on entering the grey area of the spinal cord. Some of these fibres proceed to synapse directly on the homonymous and heteronymous alpha motor neuron pools, forming an excitatory monosynaptic connection. Other collaterals synapse on interneurons called Ia inhibitory interneurons. The spindle collaterals excite the Ia inhibitory interneuron pools that in turn cause an inhibitory stimulus to be received by the antagonist motor neuron pools. A collateral axon from the alpha motor neuron also synapses with another interneuron called a Renshaw cell that in turn inhibits both the original alpha motor neuron and the Ia interneuron. Thus the inhibitory pathway from the agonist to the antagonist is momentarily opened and that from the antagonist to the agonist is momentarily closed until the activity of the Renshaw cell returns the cycle to neutral (Crone et al. 1987) (see Fig. 5.10). The overall response produced by this activity of stretching the spindle fibres is an increase in the probability of both a contraction of the agonist and inhibition of the antagonist muscles surrounding the joint in question. This is the response normally observed when we strike the tendon of a muscle with a reflex hammer. The impact of the hammer results in a momentary lengthening of the spindle fibres, which sets in motion the events previously described. This sequence of events can be modulated by input from other neurons in either segmental or suprasegmental pools via presynaptic modulation at the primary afferent central process, modulation of interneuron pool central integrative states, and modulation of the alpha and gamma motor neurons directly (Pearson & Gorden 1991).
Activation of the Golgi tendon organs leads to an inhibitory stimulus at the homonymous muscle. This inhibition is accomplished through the excitation of an interneuron called a Ib inhibitory interneuron. When the afferent fibre of the Golgi tendon organ enters the grey area of the spinal cord it synapses on a Ib interneuron, which then in turn synapses on the homonymous alpha motor neuron, increasing the probability of the cell not reaching threshold. The integration of this reflex is complicated because the Ib interneuron pool also receives modulatory input from other interneuron pools that receive their stimulus from joint receptors, muscle spindles, and cutaneous skin receptors and from descending supraspinal neurons (see Fig. 5.8).
Clinical application
Activation of both of the above reflex mechanisms may be lost because of a neuropathy or dysfunction affecting the large afferent fibres. Any situations in which the motor neurons are deprived of the primary afferent loops results in abolishment of the myotactic (stretch and Golgi) reflexes (Sherrington 1906). Muscle stretch reflexes may be absent even in the presence of upper motor neuron disease, which usually manifests as a hyperactive reflex, if afferent dysfunction coexists. Examples of conditions that afferent dysfunction and upper motor neuron lesions may coexist include subacute combined degeneration and Friedreich’s ataxia (Williams & Warwick 1984). Posterior root compression by extruded disc material or tumour may impair the conduction of large-diameter fibres before affecting the smaller, slower fibres. Thus stretch reflexes or vibration sense may be lost before loss of pain and temperature sensation. Other compressive or traumatic lesions of the spinal cord may abolish these reflexes by interrupting the reflex arc at the segmental root level, thus identifying the level of the lesion (Nakashima et al. 1989).
Case 5.2
5.2.4
Loss of proprioception, pain, and other modalities from the knee joint has resulted in a continued dysfunction of motion in the joint, resulting in excessive destruction of the joint’s structure. Neurological signs such as altered gait, loss of deep tendon reflexes, and loss of pain sensation should alert you to the possibility of a neurotrophic or Charcot’s joint (Yochum & Rowe 1996).
References
Boyd I. The isolated mammillian muscle spindle. Trends Neurosci.. 1980;3:258-265.
Matthews P. Mammillian muscle receptors and their control actions. London: Arnold, 1972.
Milburn A. The early development of muscle spindles in the rat. J. Cell. Sci.. 1973;12:175-195.
Wyke B. The neurology of joints. Proc. R. Coll. Surg. Engl.. 1966;41:25-50. 1967, 235