CHAPTER 112 NEUROLOGY OF PREGNANCY AND THE PUERPERIUM
The hormonal changes of pregnancy mediate a multitude of physiological effects that promote successful gestation and birth and influence disease states. These physiological changes interact with many common neurological disorders. In addition, neurological injury and dysfunction constitute the most threatening aspect of preeclampsia-eclampsia, a condition unique to pregnancy. Here, we first review those aspects of the physiology of pregnancy that influence neurological disease and discuss selected important neurological disorders, focusing on their clinical recognition and available therapies.
PHYSIOLOGY OF PREGNANCY
Hormonal Changes
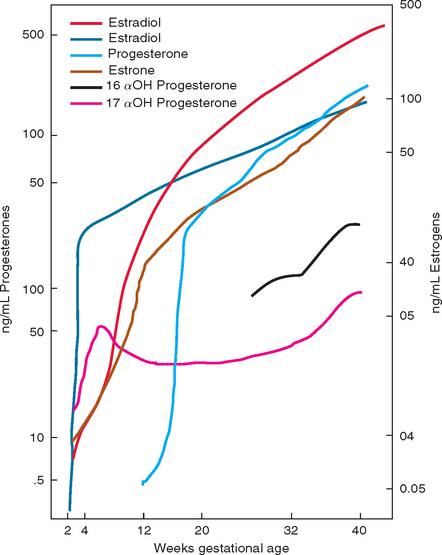
The three estrogens that are essential for the maintenance and growth of the fetus and the placenta are estradiol, estrone, and estriol. These hormones increase most dramatically in the first 16 weeks of pregnancy and then continue to increase at a slower rate until term. Estrogens, primarily estriol, increase uterine blood flow and are believed to be the trigger for parturition. Estrogens enhance myometrial irritability and contractility. They play a role in the preparation of the breast for lactation and of the cervix for labor and delivery. Through actions on the fetal pituitary, they may play a role in triggering the onset of labor. Estrogens increase neuronal excitability and lower the seizure threshold. This effect occurs through several mechanisms that inhibit the inhibitory action of γ-aminobutyric acid (GABA), including negative allosteric modulation of GABA transmission by binding to the GABA receptor, transcription regulation causing decreased synthesis of messenger RNA encoding the GABA precursor GABA-amino-decarboxylase, and decreased synthesis of GABA receptor subunits.1 Estrogens also have immunomodulatory effects (see Immune Changes).
Progesterone and 17α-hydroxyprogesterone are the two progestational steroid hormones most important in pregnancy. Progesterones act in many ways in opposition to estrogens. They relax myometrial musculature and decrease uterine irritability. They maintain the endometrium supporting the developing fetus, and they decrease uterine blood flow. Progesterones have immunosuppressive effects locally at the site of implantation, allowing placentation to occur without immunological rejection (see Immune Changes). The pathway for fetal steroid production is fed completely by progesterone. Near delivery, progesterone inhibits initiation of uterine contractions by stabilizing cell membranes and preventing prostaglandin formation. In contrast to estrogens, progesterones have an anticonvulsant effect, through positive allosteric modulation of GABA.1
After delivery, the levels of all estrogens, progesterones, α-fetoprotein, and β-HCG return to normal within about 6 to 8 weeks. Normal ovulatory cycles return in approximately 10 weeks in a nonlactating woman and in about 17 weeks in a lactating woman. Normal menses returns in 12 weeks in women who are not breastfeeding. In lactating women, the timing of return is more variable. Return of menses does not always signal return of ovulation. Figure 112-1 summarizes some of the major hormonal changes of pregnancy and the puerperium.
Fluid, Hemodynamic, Cardiovascular, and Endothelial Changes
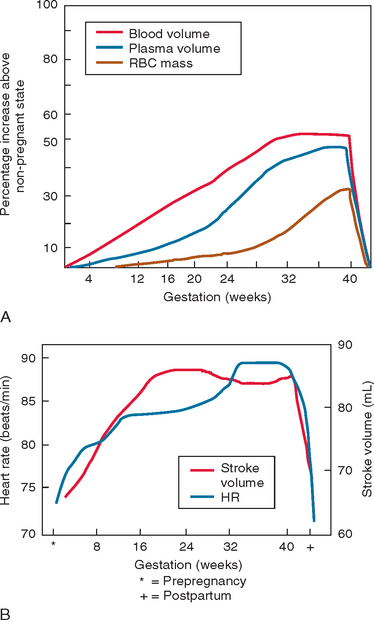
Blood pressure is decreased during pregnancy as a result of a decrease in systemic vascular resistance. Decreases in blood pressure, more so in diastolic than in systolic pressure, are first noted in the seventh week of pregnancy. The blood pressure reaches its nadir at 24 to 32 weeks and then increases progressively to prepregnancy levels at term. Venous compliance increases throughout pregnancy, leading to decreased blood flow, increased stasis, and a dampened ability to react to orthostatic changes. The mechanisms underlying the decrease in systemic vascular resistance have not been well established, but it is presumed that the effects of prostaglandins, progesterone, and nitric oxide lead to vasodilation via both arterial and venous relaxation. There is also evidence that pregnant women are refractory to the hypertensive effects of angiotensin II. Women with preeclampsia-eclampsia do not show this decreased response to angiotensin II, and it has been hypothesized that this dysregulation plays a primary role in the pathogenesis of this disorder. Figure 112-2 summarizes the hemodynamic changes of pregnancy and the puerperium.
Changes in Coagulability
In contrast to the elevation of procoagulant factors, the levels of some coagulation inhibitors fall during pregnancy, contributing to an overall state of hypercoagulability. The coagulation inhibitor antithrombin III is significantly decreased, with the lowest levels in the third trimester.2 Total and free levels of protein S are significantly decreased during the first and second trimesters. Although levels of protein C are largely unchanged, by the third trimester almost one third of women have functional activated protein C resistance.3
A study demonstrated that compared with pregnant controls, at all stages of gestation, protein C and activated protein C levels are lower in pregnant women with hypertension or a history of miscarriages.4 It has been postulated that these effects on protein C levels may contribute to the pathogenesis of preeclampsia-eclampsia.
Coagulation factors, coagulation inhibitors, platelets, and the regulation of fibrinolysis return to prepregnancy levels within a few weeks after delivery, with the exception of levels of protein S, antithrombin III, and von Willebrand factor. von Willebrand factor initially increases after delivery, while protein S and antithrombin III decrease even further.2 By 8 weeks, almost all factors have normalized.
Immune Changes
Once the blastocyst contacts the uterine wall, the developing placenta acts to create an antigenically neutral barrier between the mother and the developing fetus. Controversy continues over whether there is systemic or localized immunosuppression that allows development of the fetus to proceed uninterrupted. The syncytiotrophoblasts, which make up the placenta closest to the mother, lack identifying major histocompatibility complex class I molecules, creating functional immunological blindness to the developing fetus. In addition, the synciotiotrophoblasts elaborate humoral factors, such as transforming growth factor β-2, that contribute to the immunosuppressed state by inhibiting cytotoxic T cells and natural killer cells. Progesterone has local immunosuppressive effects, inhibits T lymphocytes, and is found in higher concentrations in trophoblastic tissues. Estrogen decreases T cell proliferation and T helper cells. T helper type 1 (Th1) and T helper type 2 (Th2) cytokines are balanced during pregnancy. Th1 cytokines make interleukin 2, interferon α, and lymphotoxin, all of which promote cell-mediated immunity. Th2 cytokines make interleukins 4, 5, 6, and 10, which play roles in antibody-mediated and allergic responses. Overall, it is believed that pregnancy favors a state dominated by Th2 over Th1.
CLINICAL TOPICS
Migraine
After puberty, migraine affects women three times more often than men and occurs in almost 20% of women across their lifetime. Headache during pregnancy is usually the result of a benign condition, most commonly migraine, but pregnancy predisposes women to several disorders that must be considered in the diagnostic evaluation. In addition, pregnant women are, of course, subject to the many uncommon disorders that may present with headache. Table 112-1 lists some common causes of headache during pregnancy and the puerperium.
TABLE 112-1 Some Causes of Headache Related to Pregnancy and Puerperium
Epidemiology
Several studies have looked at the pattern of migraine in pregnancy with consistent findings. The effect of pregnancy does not parallel that of oral contraceptive agents. In a case-control study of 100 women with migraine with aura and 200 age-matched controls, whereas oral contraceptives worsened headaches in 25% of women, pregnancy lessened migraine in 77%.5 In a prospective study of 49 women, migraine frequency decreased by about 50% in the first trimester, with 10.6% of patients experiencing complete remittance.6 In the second trimester, 87% of women reported a decrease in migraine frequency with 53% remission, and in the third trimester, 87% of women reported a decrease in migraine frequency with 79% remission. Migraine returned in 4% of women by day 2 postpartum, 34% by 1 week, and 36% by 1 month. Women who breastfed had a lower rate of early headache recurrence after delivery. Women with menstrual migraine, defined strictly7 as headache that occurs on the first day of menses, have the greatest improvement in headaches during pregnancy. Pregnancy appears to affect the pattern of migraine with and without aura differently. In a self-reporting study comparing 88 women with migraine with aura and 180 women with migraine without aura, women with aura were more likely to have premenstrual syndrome and headaches exacerbated by oral contraceptive agents, and they were more likely to get relief from headaches during pregnancy.8 Women with migraine without aura had more menstrual migraine.
Pathophysiology
There is little precise information about the etiology and triggers of headaches during pregnancy. Although it has always been postulated that a change in the estrogen levels plays a role in the pattern of migraines in pregnancy, experimental studies have not made this relationship clear. It has been shown that estradiol leads to a decrease in excitatory neurotransmitters such as dopamine and norepinephrine and an increase in inhibitory neurotransmitters such as serotonin, GABA, and endorphins.9 Estradiol increases during the course of pregnancy. This increase is physiologically advantageous in preparation for the pain of childbirth, and it may underlie the lessening of headaches through the course of pregnancy.
Treatment
Counseling about preventive interventions and safe medications is important in all women with headaches. The importance of nonpharmacological interventions, such as regular sleep and meals, avoidance of dehydration, avoidance of known headache triggers, regular exercise, meditation, and biofeedback, should be stressed as the first line of treatment during pregnancy. In some studies, these interventions have been shown to be as efficacious as medications.10 However, in many women with significant migraine, help from medications will be desirable.
The choice of medications for migraines in pregnant women is dictated mainly by the potential for adverse side effects. Acute medications are given at the start of a headache to ameliorate or abort the headache pain. In most cases, women are motivated to minimize medication use during pregnancy, and intermittent dosing of safe agents provides adequate relief. If medication is needed for acute pain, acetaminophen should be the first-line of treatment. Combinations of acetaminophen with caffeine and butalbital may also be safely used, if they are not taken so frequently that they promote the transformed migraine of caffeine dependence or excessive barbiturate intake. If these agents are ineffective, then opiates and antiemetics are third-line agents. Opiates (category B) should not be used more than a few times a week, because if used to excess they can cause constipation and dependence in the mother and withdrawal in the baby at the time of birth. The antiemetics metoclopromide and prochloperazine (category B) can be as effective as analgesics in alleviating acute migraine symptoms.
Potent inhibitors of prostaglandin synthesis and strong vasoconstrictors should be avoided in pregnancy. Aspirin in analgesic doses should be avoided. Aspirin interferes with implantation during conception, it increases the risk of bleeding throughout pregnancy, and it promotes premature closure of the ductus arteriosus in late pregnancy. Nonsteroidal anti-inflammatory agents should be avoided. They also may prevent implantation, and they promote early closure of the ductus arteriosus. Although some authors recommend them as acceptable during the first and second trimesters, other choices are preferred. There have been some reports of cleft palate associated with codeine use in the first trimester, and although a causal link is not well supported, it is best to avoid it during pregnancy. Ergots should be strictly avoided due to their potent vasoconstricting effects and long half-lives. Serotonin agonists, “triptan” agents, should be avoided in pregnancy based on their potent vasoconstrictive effects and the lack of sufficiently powered data to argue for their safety. Registry data offer no clear evidence of an increased rate of congenital malformations in women who have taken these agents during pregnancy, but these data are not controlled and numbers of patients are too small to draw clear conclusions. A Danish study found an increased rate of preterm delivery and lower birth weight among users of sumatriptan.11 A Swedish registry study found only statistically insignificant trends of preterm delivery and low birth weight and no increase in the rate of congenital malformations.12
Valproic acid and carbamazepine can cause neural tube and other major fetal malformations, and they should not be used for headache prevention during pregnancy.13–15 Gabapentin is contraindicated given its effects on bony development and overall growth in laboratory animal studies. Experience with newer antiepileptic agents is limited, and they, too, are best avoided as migraine prophylactic agents.
Cerebrovascular Issues
Epidemiology
There have been no well-designed, long-term prospective studies evaluating the incidence of stroke in pregnancy and the puerperium. Comparisons of available retrospective data are limited because authors have defined the patient groups differently, sometimes including all or only late pregnancy, all or part of the postpartum period, or including or excluding women with spontaneous or therapeutic abortions, and they have defined stroke differently, including or excluding venous sinus thrombosis, subarachnoid hemorrhage, and transient ischemic attack. Estimates of incidence of stroke in pregnancy range from a low of 4.3 to a high of 210 cases per 100,000. In recent years, several retrospective studies have examined stroke in pregnancy shedding some new light on its epidemiology. These studies agree with older ones that there is an increased risk of stroke in pregnancy and the puerperium compared with the nonpregnant state and that the distribution between ischemic and hemorrhagic stroke is roughly equal. In public hospitals in the Ile de France studied from 1989 to 1992, there were 4.3 ischemic strokes per 100,000 women and 4.6 hemorrhagic strokes per 100,000 women.16 This study included only 2 postpartum weeks, and it excluded cerebral venous sinus thrombosis, subarachnoid hemorrhage, and transient ischemic attacks from the definition of stroke. A population-based retrospective study of hospitals in the Baltimore-Washington, DC, area from 1988 through 1991 was able to compare the rate of stroke in pregnant and nonpregnant women aged 15 to 44 years to assess the risk attributable to pregnancy. This study found 11 cerebral infarctions and 9 intracerebral hemorrhages per 100,000 deliveries. There was no increased risk of ischemic stroke during pregnancy (relative risk [RR], 0.7) and only a small trend of increase in the risk of intracerebral hemorrhage (RR, 2.5; confidence interval, 1.0 to 6.4) but a significantly increased risk of stroke of both types in the 6 weeks postpartum (ischemic stroke RR, 8.7; confidence interval, 4.6 to 16.7; hemorrhagic stroke RR, 28.3; confidence interval, 13.0 to 61.4).17 The study population was almost 40% African American. It included women having abortions and stillbirths, and it considered venous sinus thrombosis in the ischemic stroke category. Another study looked at women aged 15 to 44 years from a single hospital in Taiwan during the period from 1984 to 2002 identifying first-ever cerebral infarct (including both cerebral venous thrombosis and arterial infarcts), cerebral hemorrhage, and subarachnoid hemorrhage. This study found a higher rate of strokes of 46.2 per 100,000 pregnancies (26 per 100,000 ischemic and 20.1 per 100,000 hemorrhagic), with the highest rate in the postpartum period.18 The high rate of rheumatic heart disease in the Taiwanese population likely accounts for the higher rate of ischemic strokes and emphasizes the difference in populations across these studies.
Pathophysiology of Ischemic Stroke in Pregnancy
Strokes during pregnancy can be divided into those with mechanisms unique to pregnancy and those with more common mechanisms that are influenced by the pregnant state. The categories used in analyses differ among the available studies and missing categories and possible overlap of general and more specific categories make judgments tentative. However, it appears that the most common causes of ischemic stroke in pregnant and postpartum women are eclampsia and cerebral venous thrombosis, followed by cardioembolism and a variety of other mechanisms that are found in large studies of nonpregnant young patients with ischemic stroke. The French study mentioned earlier looked retrospectively at 348,295 deliveries from 1989 to 1992 in 63 public maternity wards in the Ile de France region for strokes that occurred through the second postpartum week.16 Cases of venous sinus thrombosis were excluded. The etiologies of the 15 ischemic strokes were found to be preeclampsia-eclampsia (7), unknown (5), dissection (1), amniotic fluid embolism (1), and protein S deficiency (1). The Baltimore-Washington, DC, study looked at all women aged 15 to 44 years who were discharged from 46 area hospitals during the period 1988 through 1991 and found 17 cerebral infarctions, of which they reported the cause in 16.17 Ischemic strokes were due to undetermined causes (6), preeclampsia-eclampsia (4), primary central nervous system vasculopathy (2), carotid dissection (1), thrombotic thrombocytopenic purpura (1), cortical vein thrombosis (1), and postherpetic vasculitis (1). If “primary central nervous system vasculopathy” represents eclampsia, which is likely (see later), then 6 of the 16 were related to eclampsia. A review of all deliveries at a Toronto hospital from January 1980 through June 1997 found 21 infarctions in pregnant and postpartum women among 50,711 deliveries.19 Eight of the 21 infarctions were venous. Of 13 arterial infarctions, 6 had unknown causes, and 4 were attributed to cardioembolism. Of the infarctions with unknown causes reported, two of six arterial infarctions and two of five venous infarctions occurred in women with preeclampsia. The study from Taiwan for first-ever cerebral infarction reported strokes among all women aged 15 to 44 years from a single hospital from 1984 through 2002.18 Among 49,796 pregnancies, 27 ischemic strokes (16 arterial, 11 venous) were related to pregnancy and the 6 weeks postpartum. The identified causes of the 16 arterial strokes were cardioembolism (9 [7 rheumatic and 2 nonrheumatic]), protein S deficiency (3), unknown (2), preeclampsia-eclampsia (1), and aneurysm (1). Of 11 patients identified with cerebral venous sinus thrombosis, identified causes were protein S deficiency (4), undetermined (4), and other coagulopathies (3) (systemic lupus erythematosus, protein C deficiency, and antiphospholipid syndrome).
Peripartum cerebral angiopathy or “angiitis” has been reported as an entity in the clinical literature. The diagnosis is typically based on the presence of headache, visual symptoms, confusion, seizures, or focal neurological signs in a peripartum woman with a cerebral angiogram showing multiple segmental narrowings. Cases have not been reported with biopsy-proved angiitis, and the description just given is consistent with eclampsia, suggesting that most reported cases are, in fact, cases of eclampsia. Some cases have been associated temporally with the use of sympathomimetic agents or bromocriptine. Unless there is clear evidence of angiitis, the best treatment is removal of any potentially offending agents, the administration of magnesium sulfate, and control of blood pressure and seizures as for other cases of eclampsia.
Pathophysiology of Hemorrhagic Stroke in Pregnancy
Subarachnoid hemorrhage has been cited as the third most common cause of nonobstetrical death in pregnancy.20 Different classification of intracerebral hemorrhage including or excluding subarachnoid hemorrhage as an etiology of stroke makes comparison of studies of intracranial hemorrhage tentative.16 Sharshar and colleagues’ 1995 study of women in the Ile de France found 16 hemorrhagic strokes among 348,295 deliveries. The identified causes were eclampsia (7), unknown (3), arteriovenous malformations (2), aneurysm (2), and cavernous angioma (2).16 Kittner and colleagues’ 1996 study of women in the Baltimore-Washington, DC, area found 14 intracerebral hemorrhages among 234,023 pregnancies (including 140,167 live births, 1076 stillbirths, and 92,780 spontaneous or induced abortions). They reported the cause in 13. The identified causes were indeterminate (4), arteriovenous malformations (3), preeclampsia-eclampsia (2), cocaine use (2), primary central nervous system vasculopathy (1), and sarcoid vasculopathy (1).17 The Baltimore-Washington, DC, population-based study of risk supports the suggestion that risk of hemorrhage is increased in the postpartum period, but no hemorrhages in this study were attributed to aneurysm. There remains some controversy about whether or not there is an increased risk of hemorrhage from aneurysms and arteriovenous malformations in the peripartum period. Although studies of total rates do not find such an increase, an analysis of the risk of rupture per day suggests a several-fold increase on the day of delivery.21,22 The increased blood volume during the second trimester and the increase in blood pressure and mobilization of fluids at the time of delivery likely lead to a slightly increased likelihood of rupture of arteriovenous malformation and aneurysms at these times.23 For both arteriovenous malformations and aneurysms, the increasing levels of estrogen as pregnancy progresses and their vasodilating effects may also contribute to an increased susceptibility to blood vessel rupture.
Management of Stroke During Pregnancy
Warfarin (category X) crosses the placenta and can cause fatal fetal hemorrhage and developmental malformations, so it is contraindicated during pregnancy. In women who require full anticoagulation for mechanical heart valves, cerebral venous sinus thrombosis, a hypercoagulable syndrome, or other indications, heparins are the preferred treatment, because these large molecules do not cross the placenta. Both unfractionated heparin (category C) and low-molecular-weight heparin (category B) can be administered subcutaneously throughout pregnancy, and unfractionated heparin can be given intravenously as well. Intravenous heparin can be stopped around the time of delivery and restarted immediately afterward to minimized bleeding risks. Fondaparinux, an antithrombin III–mediated factor Xa inhibitor, is classified as category B. Because there is limited experience with its use in pregnancy, its use should be reserved for women who cannot tolerate heparins. Ginsberg and colleagues reviewed the use of antithrombotic agents during pregnancy in detail.24
Based on theoretical considerations, tissue plasminogen activator (tPA) is contraindicated in pregnancy, unless it is thought that the benefits largely outweigh the risks in the setting of a major stroke. In fact, a few pregnant women have received tPA with no apparent adverse outcome.25,26
Treatment of arteriovenous malformations without hemorrhage during pregnancy is less clear. Based on a series reported in 1974, Robinson and colleagues27 argue for a high likelihood of bleeding during pregnancy.27 Later series dispute this increase risk. Horton and colleagues21 found a hemorrhage rate of 3.5% per year during pregnancy and the puerperium, a rate similar to that of nonpregnant women with arteriovenous malformation and no history of prior hemorrhage. Robinson reported on arteriovenous malformations discovered after hemorrhage, whereas Horton and colleagues population included all arteriovenous malformations. This may account for some of the differences in their findings. Weir and Macdonald22 have argued that pregnancy increases the bleeding rate of arteriovenous malformations more than of aneurysms based on the relatively low ratio of aneurysms to arteriovenous malformations found as the cause of intracranial hemorrhage during pregnancy (1.3 in pregnancy versus 8.4 in all patients with intracranial hemorrhage). Both Horton and colleagues21 and Dias and Sekhar28 found that vaginal delivery added no risk of hemorrhage in patients with arteriovenous malformations. Based on these findings, vaginal delivery with epidural anesthesia is a reasonable choice for women with arteriovenous malformations.
Prognosis
The risk of maternal death from ischemic stroke is low and less than that from hemorrhagic stroke. Combining the French and Toronto studies mentioned, there were no maternal deaths among 36 women with ischemic stroke.16 Hemorrhagic stroke is the third most common cause of nonobstetrical death in pregnant women, and 7 of the 29 women with hemorrhage in these two studies died.20 Among 20 women with ischemic stroke before delivery and known outcomes, there were 4 premature deliveries, 2 stillbirths, 1 miscarriage, and 1 termination. Of 26 women with intracerebral hemorrhage before delivery, there were 7 premature deliveries, 3 fetal deaths due to maternal death, 2 stillbirths, and 1 termination.
The risk of recurrent stroke has been investigated in two recent studies.29,30 In these studies of young women with a history of stroke, the risk of recurrent stroke in subsequent pregnancies was very low (0% to 1.8%), with the greatest risk during the postpartum period in one study. Yet, in one study, more than 75% of young women who had had a stroke did not have subsequent pregnancy due to fear of having another stroke in pregnancy, to medical advice against another pregnancy, or to physical limitations resulting from the stroke.29
Preeclampsia-Eclampsia
Preeclampsia-eclampsia is a systemic disorder of mid to late pregnancy characterized by hypertension and proteinuria. The criteria for diagnosis recommended by the National High Blood Pressure Program Working Group on High Blood Pressure in Pregnancy are given in Table 112-2. Endothelial cell dysfunction plays a major role in the pathogenesis of preeclampsia and likely accounts for many of the additional clinical features that may be a part of the full syndrome, including thrombocytopenia, microangiopathic hemolytic anemia, and hepatic and renal dysfunction. The acronym HELLP has been applied to the combination of hemolytic anemia, elevated liver function tests, and low platelets in the setting of preeclampsia-eclampsia.31 Eclampsia is defined as preeclampsia complicated by seizures. More recent definitions accept preeclampsia with encephalopathy but without seizures as defining eclampsia. Which terminological convention is chosen does not affect our understanding of the pathophysiology.
TABLE 112-2 Criteria for the Diagnosis of Preeclampsia and Eclampsia
* In the absence of proteinuria, preeclampsia should be strongly considered when gestational hypertension is accompanied by headache, blurred vision, abdominal pain low platelet counts, or elevated liver enzyme values.
† In the absence of seizures, coma or other features suggesting severe encephalopathy are sometimes termed eclampsia, although this extension of the definition remains non-standard.
From Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183: S1-S22.
Epidemiology
Preeclampsia affects 3% to 5% of pregnant women.32 It is a major cause of maternal and infant mortality and morbidity. Approximately 1 in 2000 pregnancies in the United States is complicated by eclamptic seizures, and the incidence is much higher in developing countries.33,34 Risk factors include obesity and poor nutrition, nulliparity, multiple gestations, age greater than 35 years, extrauterine and molar pregnancies, preexisting hypertension, diabetes mellitus, and thrombophilia.
Pathophysiology
Although the pathophysiology of preeclampsia-eclampsia is still not clear, abnormal placentation leading to impaired placental perfusion is likely to be the event that triggers downstream events in the disease process. Remodeling of the spiral arteries creates the low-resistance system that perfuses the intervillous space. This process is abnormal in preeclampsia leading to placental hypoperfusion. Women with preeclampsia have increased systemic vascular tone and heightened sensitivity to mediators of vasoconstriction. This increased vascular tone underlies the systemic hypertension, vasospasm, and decreased organ perfusion. There is also much evidence for a disorder of endothelial dysfunction. This endothelial cell dysfunction promotes the instability of vasomotor tone and hypertension as well as the increased vascular permeability, edema, and proteinuria characteristic of preeclampsia. The mechanism by which the placental insufficiency leads to vasomotor hyperreactivity and endothelial dysfunction is not well established. Proposed mechanisms include autoantibodies and oxidative stress, prompting activation of neutrophils and monocytes and release of inflammatory mediators. There is also clinical evidence for genetic predisposition.32 Population data argue for maternal susceptibility, yet some findings, such as a lack of concordance between monozygotic twins, an increased risk with changed paternity, and an increased risk in partners of fathers whose mothers had preeclampsia, point to a fetal contribution to susceptibility as well.35–37
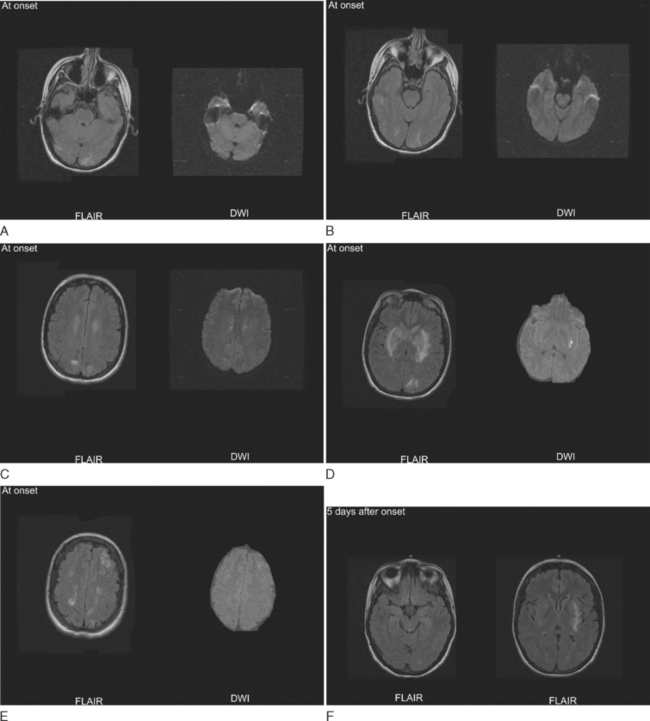
Almost all women with preeclampsia have absolute hypertension. Although the degree of hypertension may be mild, there is usually a significant elevation of blood pressure compared with the premorbid blood pressure during pregnancy.38 If systemic hypertension and endothelial cell dysfunction reach a threshold of adequate severity, then there is a breakdown of cerebral autoregulatory vasoconstriction resulting in cerebral hyperperfusion and vasogenic edema of the brain. The endothelial dysfunction probably allows for edema to form at lower absolute perfusion pressures than in other forms of hypertensive encephalopathy. The nature and distribution of the brain lesions, as defined by computed tomography, magnetic resonance imaging, and single-photon emission computed tomography, are the same as in patients with hypertensive encephalopathy.38 Hyperperfusion is most pronounced posteriorly, and edema is most prominent in the subcortical white matter of the occipital lobes and other structures fed by the posterior circulation (Fig. 112-3).39,40 This pattern of hyperperfusion and edema is believed to reflect the relative low density of vasoconstricting sympathetic receptors in the posterior circulation.41 Yet, gray matter and anterior circulation structures are often involved, especially in severe cases. If limited to vasogenic edema, the lesions are reversible, but intraparenchymal hemorrhage and, less commonly, infarctions due to vasospasm and thrombosis may occur. Seizures may occur as a result of the subcortical and cortical edema. Hence, the defining seizures of eclampsia appear to be the severe end of the spectrum of dysfunction caused by hyperperfusion and brain edema.
Clinical Presentation
Headache and visual phenomena consistent with occipital lobe edema are the most common symptoms of eclamptic encephalopathy. Seizures may be focal or secondarily generalized. Some women have encephalopathic features without seizures, such as confusion, aphasia, cognitive deficits, and depressed level of consciousness. Although hypertensive encephalopathy and seizure are most commonly preceded by typical features of preeclampsia, it is not uncommon for women to present with seizures in late pregnancy and in the early postpartum period with only mild hypertension.42
Diagnostic Evaluation and Differential Diagnosis
The diagnosis of preeclampsia is based on the fulfillment of the clinical criteria of hypertension and proteinuria. The diagnosis of eclampsia is based on the finding of encephalopathy or seizures in late pregnancy or the postpartum period with computed tomography scanning or magnetic resonance imaging that shows evidence of characteristic edema and that rules out alternative brain lesions. Magnetic resonance imaging is more sensitive for detection of edema than computed tomography, and diffusion-weighted imaging is useful to distinguish reversible edema from ischemic stroke. Technetium single-photon emission computed tomography imaging may demonstrate hyperperfusion characteristic of hypertensive encephalopathy, but this is not usually necessary for diagnosis. Patients with intracerebral or cerebellar hemorrhage should have vascular imaging to rule out an underlying vascular anomaly. Almost all women with eclampsia have hypertension, although the elevation may be mild or only relatively raised from a low baseline blood pressure of pregnancy.38
Management
The mainstays of management are the rapid control of hypertension and the administration of magnesium sulfate. Mean arterial blood pressure should usually be lowered by 15% to 20%. This is best achieved rapidly with intravenous medications such as labetalol, hydralazine, or nicardipine. Nitroprusside and nitroglycerin can cause fetal cyanide toxicity, so they should be avoided during pregnancy. Angiotensin-converting enzyme inhibitors are also contraindicated during pregnancy (category D).43 Most eclamptic women have intravascular volume contraction and will benefit from volume replacement, but invasive hemodynamic monitoring may be useful to guide fluid therapy in severely ill women with refractory hypertension, oliguria, or pulmonary edema.44,45
Magnesium sulfate therapy now has strong support from clinical trials both to prevent further seizures in women with eclampsia and to prevent seizures in women with preeclampsia.46,47 Therapy can start with a loading dose of 4 to 6 g of magnesium sulfate followed by a 2 g/hr infusion. A supplemental loading dose of 2 g may be given if seizures recur shortly after the initial load. Tendon reflexes, respiratory function, and urine output should be followed closely. It is common practice to follow serum levels of magnesium, although it has not been established that this helps to guide therapy. It is most important to follow women for signs of neurological depression and to adjust magnesium sulfate doses accordingly. At levels of 8 to 10 mEq/L, tendon reflexes are typically depressed. At levels above 10 to 12 mEq/L, there is a high risk of respiratory depression. When patellar reflexes are lost, magnesium sulfate should be discontinued. If respirations are depressed, calcium gluconate should be given. Doses of magnesium sulfate should be adjusted for renal insufficiency, for example, by reducing the loading dose to 4 g and halving the maintenance dose. Although recent data argue that magnesium sulfate is better than phenytoin or diazepam for eclamptic seizures, refractory seizures should be treated aggressively with traditional antiepileptic drugs in addition to magnesium sulfate.
Epilepsy
Effect of Pregnancy on Seizure Frequency
Epilepsy affects approximately 1% of the population, and about 1 million women in their reproductive years have epilepsy. It is estimated that 0.3% to 0.5% of all births occur in epileptic women. Seizure frequency seems to increase by approximately 25% to 33% during pregnancy.48 Older data suggest that the greatest increase occurs in the first trimester, but more recent data suggest a similar effect throughout pregnancy.49,50 Approximately 1% to 2% of women have a seizure during labor and delivery, and another 1% to 2% have a seizure within 24 hours after delivery. There are many possible explanations for this high rate of seizures. Many of these women do not have epilepsy but rather eclampsia or other symptomatic seizures. Among epileptics, the proconvulsant effect of the increased estrogen levels, third trimester and postpartum sleep deprivation, and decreasing levels of antiepileptic drugs due to more rapid metabolic inactivation and increased total body water may account for some of the increase in seizure frequency. Noncompliance with medication due to fear of side effects or an inability to tolerate oral medications during early pregnancy and the delivery period may also contribute.
Consequences of Seizures During Pregnancy
Prolonged generalized tonic-clonic activity during pregnancy has been shown to lead to maternal acidosis, hypoxia, and decreased fetal heart rate.51 Trauma from a seizure can lead to poor fetal and maternal outcomes, including premature rupture of membranes, infection, placenta abruption, placenta previa, and miscarriage. Most reports of poor outcome are from literature dating from the mid-twentieth century. More recent studies have found that complications of seizures are rare.52 Abruptio placenta was seen in 1% to 5% women after minor injury and in 20% to 50% after major seizure-induced injury. The effects of nonconvulsive status epilepticus during pregnancy are not well known.
Management
Treatment of women with epilepsy was outlined in the American Academy of Neurology Consensus Guidelines in 1998.53 Counseling and treatment should be undertaken long before planned conception. It is recommended that women be treated with an antiepileptic drug most appropriate to their seizure type and that they be maintained on a single agent whenever possible. If antiepileptic drug withdrawal before conception is planned, then it is best accomplished six months before the planned date. Folic acid, 0.36 to 5 mg daily, is recommended, because it is safe, and, although not conclusive, studies suggest that folate supplementation lowers the risk of teratogenicity attributable to antiepileptic drugs.
Selection of the proper antiepileptic drug is determined largely by standard considerations based on seizure type and individualized experience with effectiveness and tolerance. Development of congenital anomalies, fetal loss, and stillbirth all occur at an increased rate in women with epilepsy. A huge volume of data addresses the risk of fetal malformations induced by early exposure to antiepileptic drugs. Epileptic women taking antiepileptic drugs have a rate of major congenital malformations of about 4% to 6%. This is higher than the rate of 2% to 3% in the normal population. The baseline rate of major congenital malformations in epileptic women not taking antiepileptic drugs also may be elevated, but studies have not consistently found this increased background rate.54 Lack of controlled data greatly limits conclusions about the risk of particular antiepileptic drugs. The risk of an adverse outcome increases with increasing number of antiepileptic drugs; therefore, the recommendation to minimize polypharmacy is strong.
Although there may be little difference in the risk conferred by the commonly used agents, certain risks are notable. The developing fetus is most sensitive to induction of malformations by exogenous agents between days 21 and 56 of gestation. A 1982 French study documented an increase in neural tube defects in women on antiepileptic drugs, especially valproate and carbamazepine.55 Women taking carbamazepine and valproate were noted to have an increased rate of spina bifida aperta.13–15 These congenital malformations are thought to be associated with the low serum and red blood cell folate levels that lead to hyperhomocystinemia. A family history of neural tube defects further increases the risk, so these drugs should be especially avoided in women with a positive family history. From the small population evaluated so far, the Gabapentin Pregnancy Registry has found a rate of malformations fetal similar to the rate in the general population.56 A review of the International Lamotrigine Pregnancy Registry, again with a small sample, found a 2.9% risk of major congenital malformations, also comparable to that of the general population and to registries of other drugs.57,58 Valproic acid alone may confer a greater risk of malformations than other agents, and the combination of valproic acid, carbamazepine, and phenobarbital may confer a particularly high risk.54
During labor and delivery, antiepileptic drugs should be given in oral or intravenous form. Many newer antiepileptic drugs that are not available in intravenous form are long acting and may sustain the mother throughout delivery. If a woman taking an exclusively oral agent has labor extending beyond the duration of action of the oral agent, then she may be given intravenous lorazepam in doses of 1 mg every 8 hours during delivery. In such a case, the pediatrician should be notified to prepare for the possible delivery of a sluggish baby.
Multiple Sclerosis and Myasthenia Gravis
Data concerning the course of multiple sclerosis during pregnancy are conflicting. Women with multiple sclerosis usually remain stable during pregnancy. They may even improve on average. It has been reported that the risk of multiple sclerosis attacks increases by two- to three-fold during the postpartum period.59 However, a prospective study did not confirm this, finding a decreased risk of attacks during the third trimester and no increase in the postpartum period.60
It has been suggested that remissions of multiple sclerosis during late pregnancy result from the physiological immune permissive state that allows implantation and maintenance of the pregnancy.61,62 This effect is consistent with the known suppression of T-cell immunity during pregnancy discussed above.
There are no clear guidelines for therapy and prevention of attacks during pregnancy. Although short-term courses of low or moderate doses of corticosteroids are safe in mid to late pregnancy, use during the first trimester may increase the risk of fetal malformations, virilization, and adrenal suppression. Therefore, if corticosteroids are to be given during pregnancy, therapy should be delayed until after the first trimester, and low doses and short courses should be used. Stronger immunosuppressive agents, such as mitoxantrone, azathioprine, and cyclophosphamide, should be reserved for after the pregnancy, and women should be advised that these agents may cause infertility. Although experience with the use of interferons (category C) during pregnancy is still limited, these agents are abortifacients in animals, and interferon β has been associated with fetal loss and low birth weight in humans.63 The manufacturers of interferon β1b (Betaseron) and interferon β1a (Avonex, Rebif) recommend that patients avoid them when planning and during pregnancy. A review of the known experience with glatiramer acetate (Copaxone) (category B) during pregnancy suggested no added risk of congenital malformations or spontaneous abortions.64 This agent may be recommended to women with multiple sclerosis who require disease modifying treatment while planning pregnancies.
Beyenburg S, Stoffel-Wagner B, Bauer J, et al. Neuroactive steroids and seizure susceptibility. Epilepsy Res. 2001;44:141-153.
Birk K, Rudick R. Pregnancy and multiple sclerosis. Arch Neurol. 1986;43:719-726.
Ed Mancall EL, Munset TH. Neurologic Disorders and Pregnancy. Continuum: Lifelong Learning in Neurology. 2000;6:8-63.
Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768-774.
Roberts JM, Cooper DW. Pathogenesis and genetics of preeclampsia. Lancet. 2001;357:53-56.
Yerby MS, Kaplan P. Risks and management of pregnancy in women with epilepsy. Cleve Clin J Med. 2004;71:S25-S37.
1 Beyenburg S, Stoffel-Wagner B, Bauer J, et al. Neuroactive steroids and seizure susceptibility. Epilepsy Res. 2001;44:141-153.
2 Wickstrom K, Edelstam G, Lowbeer C, et al. Reference intervals for plasma levels of fibronectin, von Willebrand factor, free protein S and antithrombin during third-trimester pregnancy. Scand J Clin Lab Invest. 2004;64:31-40.
3 Clark P, Brennand J, Conkie JA, et al. Activated protein C sensitivity, protein C, protein S, and coagulation in normal pregnancy. Thromb Haemost. 1998;79:1166-1170.
4 Vodnik T, Ignjatovic S, Majkic-Singh N. Changes in the plasma levels of protein C system parameters in pregnancy. Scand J Clin Lab Invest. 2003;63:481-488.
5 Granella F, Sances G, Pucci E, et al. Migraine with aura and reproductive life events: a case control study. Cephalalgia. 2000;20:701-707.
6 Sances G, Granella F, Nappi RE, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 2003;23:197-205.
7 MacGregor EA. “Menstrual” migraine: towards a definition. Cephalalgia. 1996;16:11-21.
8 Cupini LM, Matteis M, Troisi E, et al. Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia. 1995;15:140-144.
9 Lagrange AH, Ronnekleiv OK, Kelly MJ. The potency of μ-opioid hyperpolarization of hypothalamic arcuate neurons is rapidly attenuated by 17-estradiol. J Neurosci. 1994;14:6196-6204.
10 Marcus DA. Nonpharmacologic treatment of migraine. TEN. 2001;3:50-55.
11 Olesen C, Steffensen FH, Sorensen HT, et al. Pregnancy outcome following prescription for sumatriptan. Headache. 2000;40:20-24.
12 Kallen B, Lygner PE. Delivery outcome in women who used drugs for migraine during pregnancy with special reference to sumatriptan. Headache. 2001;41:351-356.
13 Lindhout D, Meinardi H. Spina bifida and in-utero exposure to valproate. Lancet. 1984;2:396.
14 Lindhout D, Schmidt D. In-utero exposure to valproate and neural tube defects. Lancet. 1986;1:1392-1393.
15 Rosa FW. Spinal bifida in infants of women treated with carbamazepine during pregnancy. N Engl J Med. 1991;324:674-677.
16 Sharshar T, Lamy C, Mas JL, for the Stroke in Pregnancy Study Group. Incidence and causes of stroke associated with pregnancy and puerperium: a study in public hospitals of Ile de France. Stroke. 1995;26:930-936.
17 Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768-774.
18 Jeng JS, Tang SC, Yip PK. Incidence and etiologies of stroke during pregnancy and puerperium as evidenced in Taiwanese women. Cerebrovasc Dis. 2004;18:290-295.
19 Jaigobin C, Silver FL. Stroke and pregnancy. Stroke. 2000;31:2948-2951.
20 Barno A, Freeman DW. Maternal deaths due to spontaneous subarachnoid hemorrhage. Am J Obstet Gynecol. 1976;125:384-392.
21 Horton JC, Chambers WA, Lyons SL, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1990;27:867-872.
22 Weir B, Macdonald RL. Management of intracranial aneurysms and arteriovenous malformations during pregnancy. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1996:2421-2427.
23 Maymon R, Fejgin M. Intracranial hemorrhage during pregnancy and puerperium. Obstet Gynecol Surv. 1990;45:157-159.
24 Ginsberg JS, Greer I, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 2001;119:122S-131S.
25 Ahearn GS, Hadjiliadis D, Govert JA, et al. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissue plasminogen activator: a case report and review of treatment options. Arch Intern Med. 2002;162:1221-1227.
26 Nassar AH, Abdallah ME, Moukarbel GV, et al. Sequential use of thrombotic agents for thrombosed mitral valve prosthesis during pregnancy. J Perinatal Med. 2003;31:257-260.
27 Robinson JL, Hall CS, Sedzimir CB. Arteriovenous malformations, aneurysms, and pregnancy. J Neurosurg. 1974;41:63-70.
28 Dias MS, Sekhar LN. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery. 1990;27:855-865.
29 Lamy C, Hamon JB, Coste J, et al. Ischemic stroke in young women: risk of recurrence during subsequent pregnancies. French Study Group on Stroke in Pregnancy. Neurology. 2000;55:269-274.
30 Coppage KH, Hinton AC, Moldenhauer J, et al. Maternal and perinatal outcome in women with a history of stroke. Am J Obstet Gynecol. 2004;190:1331-1334.
31 Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
32 Roberts JM, Cooper DW. Pathogenesis and genetics of preeclampsia. Lancet. 2001;357:53-56.
33 Saftlas AF, Olson DR, Franks AL, et al. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163:460-465.
34 Aagaard-Tillery KM, Belfort MA. Eclampsia: morbidity, mortality, and management. Clin Obstet Gynecol. 2005;48:12-33.
35 Thornton JG, Macdonald AM. Twin mothers, pregnancy, hypertension and preeclampsia. Br J Obstet Gynaecol. 1999;106:570-575.
36 Lachmeijer AM, Aarnoudse JG, ten Kate LP, et al. Concordance for preeclampsia in monozygous twins. Br J Obstet Gynaecol. 1998;105:1315-1317.
37 Esplin MS, Fausett MB, Fraser A, et al. Paternal and maternal components of the predisposition to preeclampsia. N Engl J Med. 2001;344:867-872.
38 Schwartz RB, Feske SK, Polak JF, et al. Clinical and neuroradiolgraphic correlates in preeclampsia-eclampsia: insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371-376.
39 Mantello MT, Schwartz RB, Jones KM, et al. Imaging of neurologic complications associated with pregnancy. AJR Am J Roentgenol. 1993;160:843-847.
40 Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
41 Beausang Linder M, Bill A. Cerebral circulation in acute arterial hypertension: protective effects of sympathetic nervous activity. Acta Physiol Scand. 1981;111:193-199.
42 Higgins JR, de Swiet M. Blood-pressure measurement and classification in pregnancy. Lancet. 2001;357:131-135.
43 Cunningham FG, MacDonald PC, Grant NF, et al. Hypertensive Disorders of Pregnancy. Williams Obstetrics, 20th edition, Stamford, Connecticut: Appleton and Lange; 1997:715.
44 Clark SL, Cotton DB. Clinical indications for pulmonary artery catheterization in the patient with severe preeclampsia. Am J Obstet Gynecol. 1988;158:453-458.
45 Cotton DB, Lee W, Huhta JC, et al. Hemodynamic profile of severe pregnancy-induced hypertension. Am J Obstet Gynecol. 1988;158:523-529.
46 Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the collaborative trial. Lancet. 1995;345:1455-1463.
47 Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate and phenytoin for the prevention of eclampsia. N Engl J Med. 1995;333:201-205.
48 Yerby MS, Kaplan P. Risks and management of pregnancy in women with epilepsy. Cleve Clin J Med. 2004;71:S25-S37.
49 Knight AH, Rhind EG. Epilepsy and pregnancy: a study of 153 pregnancies in 59 patients. Epilepsia. 1975;16:99-110.
50 Costa ACL, Lopes-Cendes I, Guerreiro CAM. Seizure frequency during pregnancy and the puerperium. Int J Gynecol Obstret. 2005;88:148-149.
51 Hiilesmaa VK. Pregnancy and birth in women with epilepsy. Neurology. 1992;42(Suppl):8-11.
52 Bardy AH. Seizure frequency in epileptic women during pregnancy and puerperium: a prospective study. In: Janz D, Bossi L, Dam M, et al, editors. Epilepsy, Pregnancy, and the Child. New York: Raven Press; 1982:27-31.
53 The Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: Management issues for women with epilepsy (summary statement): Report of the Quality Standards Committee of the American Academy of Neurology. Epilepsia. 1998;39:1226-1231.
54 Holmes LB, Harvey EA, Coull BA, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132-1138.
55 Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2:937.
56 Montouris G. Gabapentin exposure in pregnancy: results from Gabapentin Pregnancy Registry. Epilepsy Behav. 2003;4:310-317.
57 Cunnington MC. International Lamotrigine Pregnancy Registry update for the Epilepsy Foundation. Epilepsia. 2004;45:1468.
58 Cunnington MC, Tennis P, the International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64:955-960.
59 Birk K, Ford C, Smeltzer S, et al. The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol. 1990;47:738-742.
60 Sadovnick AD, Eisen K, Hashimoto SA, et al. Pregnancy and multiple sclerosis: a prospective study. Arch Neurol. 1994;51:1120-1124.
61 Birk K, Rudick R. Pregnancy and multiple sclerosis. Arch Neurol. 1986;43:719-726.
62 Davis RK, Maslow AS. Multiple sclerosis in pregnancy: a review. Obstet Gynecol Surv. 1992;47:290-296.
63 Boskovic R, Wide R, Wolpin J, et al. The reproductive effects of beta-interferon therapy in pregnancy: a longitudinal cohort. Neurology. 2005;65:807-811.
64 Coyle PK, Johnson K, Pardo L, Stark Y. Pregnancy outcomes in patients with multiple sclerosis treated with glatiramer acetate (Copaxone). Mult Scler. 2003;9(suppl 1):564.