CHAPTER 100 NEUROLOGICAL COMPLICATIONS OF CANCER TREATMENTS
People with cancer frequently develop neurological symptoms. One of the dilemmas facing doctors is whether these symptoms are directly due to cancer affecting the nervous system, indirectly associated with cancer (e.g., paraneoplastic syndromes), due to causes other than cancer, or due to a complication of one of the treatments that the cancer patient has received (e.g., anticonvulsants, antidepressants, steroids, chemotherapy).
TIMING OF NEUROLOGICAL COMPLICATIONS
 Neurological complications of radiation therapy are rare during radiation therapy (because of the concomitant use of steroids), are most common weeks to a few months after completion of treatment and then are relatively uncommon until years later when late neurological complications start developing in long-term survivors.
Neurological complications of radiation therapy are rare during radiation therapy (because of the concomitant use of steroids), are most common weeks to a few months after completion of treatment and then are relatively uncommon until years later when late neurological complications start developing in long-term survivors. Complications of local cancer treatments (e.g., intracarotid or intrathecal chemotherapy), commonly start 2 to 3 days after each treatment and are direct toxic complications.
Complications of local cancer treatments (e.g., intracarotid or intrathecal chemotherapy), commonly start 2 to 3 days after each treatment and are direct toxic complications. Neurological complications of systemic cancer treatments (e.g., chemotherapy) can be as a direct effect (a neural toxic effect on a nerve) or an indirect effect (secondary to immunosuppression).
Neurological complications of systemic cancer treatments (e.g., chemotherapy) can be as a direct effect (a neural toxic effect on a nerve) or an indirect effect (secondary to immunosuppression). If chemotherapy is given systemically, neurological complications that start around the time of nadirs in blood counts are commonly due to indirect complications (bacterial, viral; e.g., shingles) or opportunistic infection. Neurological complications can start within a few weeks of completion of chemotherapy due to a direct cumulative drug toxic effect on the nerve.
If chemotherapy is given systemically, neurological complications that start around the time of nadirs in blood counts are commonly due to indirect complications (bacterial, viral; e.g., shingles) or opportunistic infection. Neurological complications can start within a few weeks of completion of chemotherapy due to a direct cumulative drug toxic effect on the nerve.NEUROLOGICAL COMPLICATIONS OF CHEMOTHERAPY
Neuropathy
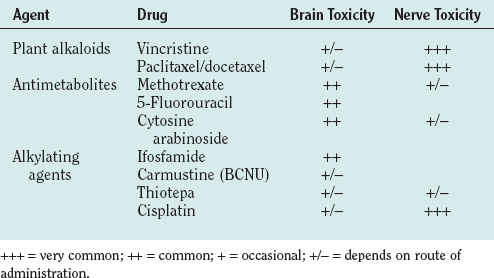
Neuropathies in patients with systemic cancer may be associated with dietary deficiency or cachexia but are also the commonest neurological side effect of anticancer drugs (Table 100-1). People with preexisting neuropathies, diabetes, or alcohol abuse are more prone to the chemotherapy-induced neuropathy. Drug-induced neuropathies develop during chemotherapy or within a few months (usually less than 4 months) after completion of chemotherapy. Neuropathies may progress after discontinuation of treatment for up to 2 to 3 months and then often stabilize or recover to some degree over the ensuing months or years. Acute severe neuropathies, leading to a Guillain-Barré–like syndrome, have been reported rarely after the administration of suramin and tacrolimus.
Encephalopathy
Confusion is a common occurence in cancer patients undergoing treatment. Treatable reversible causes of confusion, such as metabolic encephalopathy, infection, endocrine (hypothyroid, diabetes) deficiency states (vitamin B12/folate), and drug-related causes (e.g., tricyclics, anxiolytics, analgesics), must be sought. Most chemotherapeutic agents in usual dosages have limited penetration across the blood-brain barrier. Symptoms of encephalopathy are more likely to occur when high doses of drugs are used (e.g., methotrexate, cytosine arabinoside, 5-fluorouracil [5-FU]), when drugs are administered locally to brain tissue (e.g., Gliadel wafers, biological response modifiers, gene therapy), intrathecally (e.g., methotrexate, cytosine arabinoside, thiotepa) intra-arterially (e.g., nitrosourea or cisplatin), or if the blood-brain barrier is already damaged such as by a brain tumor (Table 100-2). Frequency of confusion is also associated with the mode of drug delivery (intratumoral > intrathecal > intracarotid > oral or intravenous) and the dose given. A more specific condition, reversible posterior leukoencephalopathy (RPLE), can be caused by immunosuppression or some chemotherapy agents (cyclosporin, tacrolimus, interferon α, L-asparginase, vincristine, and high-dose methotrexate). Patients develop confusion, visual disturbance, seizures, and hypertension, which may progress to blindness and coma. White matter changes in the posterior brain on magnetic resonance imaging are diagnostic of RPLE. Elimination of the offending drug and treatment of hypertension can result in recovery without residual neurological signs over a 2- to 3-week period.
| Side Effect | Drug |
|---|---|
| Encephalopathy | High dose: methotrexate, cytosine arabinoside, 5-fluorouracil, ifosfamide, paclitaxel, tamoxifen |
| Intra-arterial: BCNU/ACNU, platinum derivatives. | |
| Intrathecal: methotrexate, cytosine arabinoside, thiotepa | |
| Rare: platinum derivatives, interleukin 2, interferon α, L-asparginase | |
| Other: anticonvulsants, antidepressants, antinauseants | |
| Cerebellar ataxia | Cytosine arabinoside, 5-fluorouracil |
| Seizures | All the above (including anticonvulsants) |
| Cranial neuropathies | Cisplatin, vincristine, tamoxifen, interferon α |
| Meningitis | Intrathecal: methotrexate, cytosine arabinoside |
| Stroke | Cisplatin, bleomycin, L-asparginase, tamoxifen |
| Neuropathy | Common: platinum derviates, vinca alkaloids, taxols |
| Rare: cytarabine, daunorubicin, 5-fluorouracil, suramin, tacrolimus | |
| Muscle cramps | Platinum derivatives, taxols, vinca alkaloids |
| Lhermitte’s sign | Platinum derviatives, taxols |
CHEMOTHERAPEUTIC AGENTS
Vinca Alkaloids
Vinca alkaloids bind to tubulin and prevent its depolymerization from microtubules to dimers, disrupting normal spindle function and thus preventing cell division. This tubular toxic effect is believed to inhibit fast axonal transport and leads to axonal degeneration. Vincristine causes the most severe neuropathy, is usually the dose-limiting side effect, and occurs to some degree in all patients. Vindesine, vinblastine, and vinorelbine usually only give rise to mild neuropathies. Vincristine is used in the treatment of hematological malignancies, sarcomas, and brain tumors. After the administration of vincristine, mild paraesthesias in the fingertips and feet and muscle cramps are experienced, followed by weakness. Sural nerve biopsy shows primary axonal degeneration accompanied by segmental demyelination and reduction in both large and small fiber densities. It is usually slowly reversible months to years after withdrawal. Vincristine can cause an autonomic neuropathy, with abdominal pain and constipation (30%), urinary retention, or postural hypotension. Life-threatening paralytic ileus may occur. Some patients develop cranial nerve palsies, bilateral foot drop, or wrist drop, which can be severe and incompletely reversible. Vincristine administration intrathecally produces a fatal encephalopathy.
Methotrexate
A delayed reversible myelopathy can be seen after several intrathecal injections with methotrexate.
NEUROLOGICAL COMPLICATIONS OF CRANIAL IRRADIATION
Early Radiation Side Effects
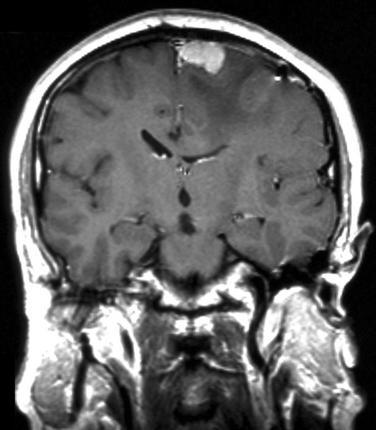
Patients with aggressive large intracerebral tumors who have not been given adequate doses of steroids are at increased risk of acute encephalopathy. Symptoms usually develop within 1 or 2 days of radiotherapy treatment and are usually at their worst within the first 2 weeks. Patients develop headaches, nausea and vomiting, and worsening of existing neurological deficits. Magnetic resonance imaging may reveal localized peritumoral white matter edema and mass effect (Fig. 100-1). Symptoms usually resolve after the steroid dose is increased.
Late Effects of Radiation
Radiation-Induced Cranial Neuropathy
The cranial nerves and retina are remarkably resistant to the effects of radiation, but after treatment for pituitary tumors, optic neuropathy or rarely retinopathy can occur. Painless visual loss begins 12 to 18 months after radiotherapy with a loss of acuity or visual field constriction. Ophthalmoscopy may reveal cotton-wool spots, hemorrhages, and optic nerve swelling. Steroids are ineffective in treating this complication. Optic neuropathy may also occur with radiation-related accelerated carotid atherosclerosis, producing carotid occlusion. Differential diagnosis for visual loss in cancer patients includes optic neuropathy due to intracarotid chemotherapy, high-dose tamoxifen, methotrexate, vincristine, or 5FU; paraneoplastic retinopathy; anterior ischemic optic neuropathy; glaucoma; and multiple sclerosis. Rarely, hypoglossal, accessory, vagal, glossopharyngeal, and acoustic nerve palsies occur, usually with radiation treatment for head and neck cancers. Hearing loss after radiotherapy occurs years after treatment for acoustic neuroma and may be related to ischemic damage of the cochlea. Damage to the other cranial nerves is rare.
Radiation Necrosis
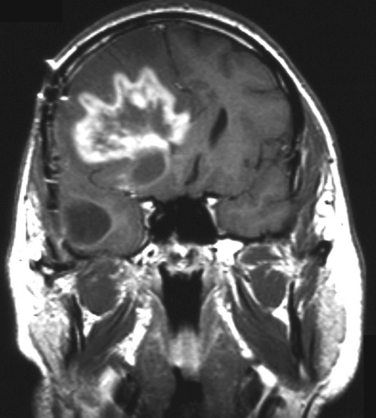
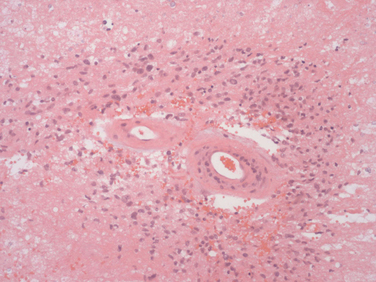
Radiation necrosis occurs in patients treated with high focal doses of radiation. Patients present from several months to 10 years after cranial radiation. In 2.8% of patients treated for malignant glioma, focal radiation necrosis develops, but among those surviving a year as many as 9% develop the condition. The most important factor is the total dose of radiation given equal to or greater than 6000 cGy. The clinical and radiological presentation is similar to that of recurrent intracerebral tumor, with progressive focal neurological signs associated often with headaches, papilloedema, and seizures, usually close to the original tumor site. Computed tomography and magnetic resonance imaging can be indistinguishable from tumor progression (Fig. 100-2). Single-photon emission computed tomography with thallium or positron emission tomography with 18F-FDG can help distinguish tumor from radionecrosis. Increased uptake supports active tumor and low uptake suggests radionecrosis. Pathological examination shows white matter necrosis and, in more severe cases, necrosis of gray and white matter. There is often extensive vascular damage with endothelial proliferation, fibrosis/fibrinoid necrosis, and luminal occlusion (Fig. 100-3). Treatment of radiation necrosis with high-dose oral steroids may produce an improvement in symptoms and imaging. Surgical debulking of the mass is justified in some patients, first, to confirm the diagnosis and, second, to reduce mass effect. The use of anticoagulants and hyperbaric oxygen is reported to be successful in case reports, although they are not routinely used in clinical practice.
Dementia Due to Radiation-Induced Leukoencephalopathy
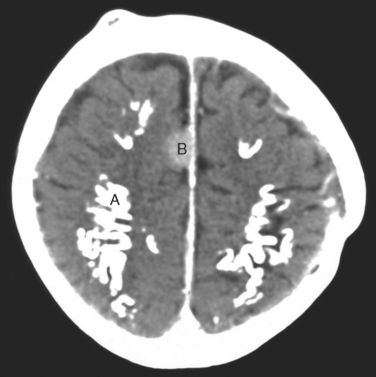
Up to one fifth of long-term survivors of cancer who have had cranial radiation therapy suffer from a slow or stepwise dementia with apraxia and ataxia, mimicking normal pressure hydrocephalus. This is one of the most common reasons for poor quality of life in long-term survivors of malignant glioma. It causes a subcortical dementia with slowness of thought, apathy, and unsteady walking (apraxia). It is sometimes severe enough to inhibit the patient initiating gait or even standing. Later, incontinence develops. Although it has been reported as occurring as early as 6 months after radiation, the more characteristic course is starting 2 to 3 years after radiation therapy and slowly deteriorates over the next 5 to 10 years, until the patient is totally dependent on caregivers. Imaging shows marked atrophy of the brain with enlargement of the ventricles or diffuse white matter changes, most marked on magnetic resonance imaging (Fig. 100-4). Later, there may be dystrophic calcification in the brain (Fig. 100-5).
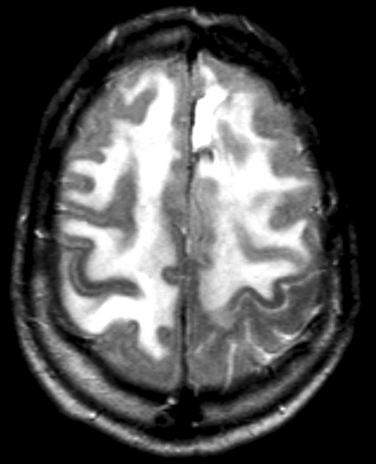
Figure 100-4 Severe radiation-induced leukoencephalopathy following radiation for a left frontoparietal anaplastic astrocytoma.
Transient Ischemic Attacks and Stroke
Stenosis of the carotid and vertebral arteries has been associated with radiotherapy to the head and neck and may manifest with stroke. Radiotherapy seems to result in an accelerated atherosclerosis, which can affect both intracranial and extracranial blood vessels. The atherosclerosis is due to a combination of radiation-induced endothelial damage and conventional risk factors for arterial disease. Short lesions can be treated with endovascular techniques. Moyamoya disease can be found in patients after radiotherapy treatment, which may manifest with hemorrhagic or ischemic complications.
Radiation-Induced Tumors
The most common radiation-induced tumors are meningiomas, gliomas, and sarcomas (see Fig. 100-5). The evidence is strongest for meningiomas (relative risk, 9.5) but gliomas (relative risk, 2.6) also have an increased frequency. The frequency is directly related to radiation dose and length of survival.
Cavernous Angiomas
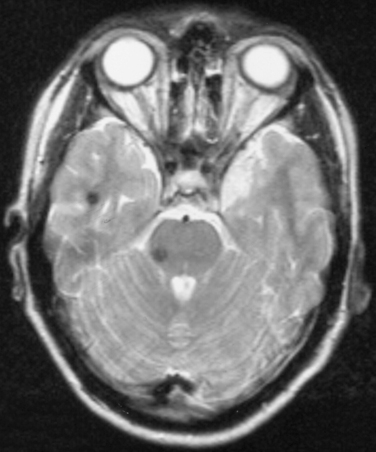
Cavernous angiomas are small capillary dilatations in the brain that may develop 4 to 8 years after cranial irradiation. They may manifest as asymptomatic abnormalities on imaging or may be associated with seizures or headache. They may be difficult to distinguish from tumor recurrence in some situations on computed tomography scanning, but magnetic resonance imaging demonstrates the lesion has no mass effect and there is a characteristic hemosiderin ring around them (Fig. 100-6).
NEUROLOGICAL COMPLICATIONS OF SPINAL CORD/PLEXUS IRRADIATION
Early Delayed Radiation Myelopathy
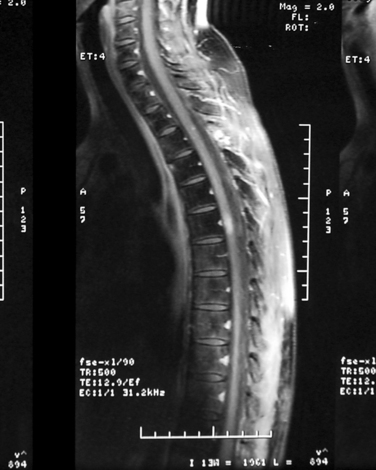
Subacute transient myelopathy is common after radiotherapy of the spine. It may occur in up to 5% to 15% of patients treated for Hodgkin’s disease where the cord is in the field. The only symptom experienced may be Lhermitte’s phenomenon. There are rarely findings on imaging, but some develop high signal in the cord with contrast enhancement (Fig. 100-7). The symptoms improve after 1 to 9 months. Steroids are rarely required.
Brachial and Lumbosacral Plexopathies
Radiation-induced plexopathies usually begin years after treatment and have a progressive course. They are usually associated with breast cancer or lymphoma. Brachial neuropathies usually manifest with numbness or paraesthesia of the fingers, followed by pain and sometimes weakness. The symptoms may stabilize, although they can progress. The condition is believed to be due to fibrosis of tissues surrounding the plexus and perhaps radiation-induced vascular occlusion of vessels supplying the plexus or nerves leading to nerve infarction. Distinguishing these plexopathies from tumor involvement or other causes of brachial plexopathy can be difficult, although Horner’s syndrome and early, severe pain are found more frequently in brachial plexus metastasis. Myokymia (spontaneous, semirhythmical bursts of electrical potentials) is found in about one half of patients with radiation-induced plexopathy. Lumbosacral plexopathy has similar clinical features but involves the lower limbs.
Belka C, Budach W, Kortmann RD, et al. Radiation induced CNS toxicity: molecular and cellular mechanisms. Br J Cancer. 2001;85:1233-1239.
Crossen JR, Garwood D, Glastein E, et al. Neurobehavioural sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627-642.
Keime-Guibert F, Napolitano M, Delattre J-Y. Neurological complications of radiotherapy and chemotherapy. J Neurology. 1998;245:695-708.
Verstappen CCP, Heimans JJ, Hoekman K, et al. Neurotoxic complications of chemotherapy in patients with cancer. Drugs. 2003;63:1549-1563.