105 Neurologic Procedures
• Topical anesthetics (especially in children), liberal use of subcutaneous lidocaine, and small doses of anxiolytic agents can expedite performance of lumbar puncture in an anxious patient.
• To obtain an accurate opening pressure, patients should lie on their side with the head supported by a pillow and the legs straight. Cerebrospinal fluid should be allowed to equilibrate in the manometer before reading.
• A smaller-gauge needle with the bevel of the needle angled parallel to the dural fibers or use of a small-diameter blunt-tipped needle will decrease the frequency of post–dural puncture headache.
Lumbar Puncture
EPs perform LP to diagnose and treat a variety of neurologic complaints. Currently, this procedure is used mainly in the ED for assessment of headache or altered mental status. CSF analysis and opening pressure (OP) measurements obtained from the LP can make or exclude the diagnosis of meningitis, subarachnoid hemorrhage (SAH), pseudotumor cerebri, and other diseases with high morbidity. Although performance of LP is considered relatively safe, patients with a suspected mass lesion or ventricular obstruction may have a theoretic risk for herniation; in such cases neuroimaging may be warranted before LP.1
Procedure
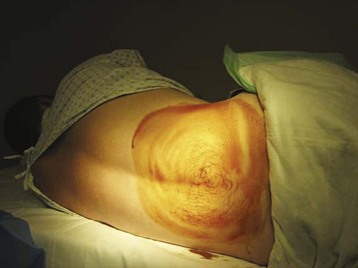
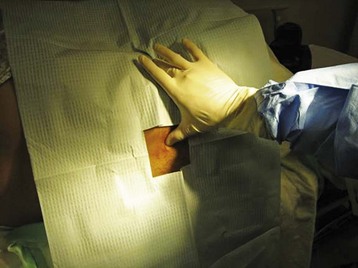
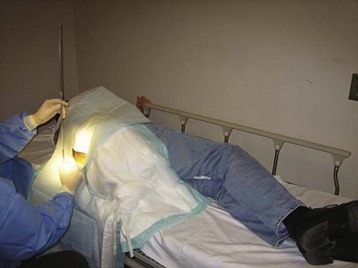
The iliac crest is located and the thumbs used to palpate and identify the spinous processes in the midline of the back. This is roughly the L4-L5 interspace and is an appropriate interspace to use. In adults, the L3-L4 interspace can also be used because both lie below the termination of the spinal cord within the spinal canal. An absorbent towel is placed under the patient, and the patient’s back is cleansed with the skin preparation solution (povidone-iodine or chlorhexidine) by making circular motions to scrub the back in gradually expanding circles, starting at the midline of the L4-L5 interspace and out to the iliac crests and lower thoracic spine; the skin should be allowed to dry between wipes (Figs. 105-1 and 105-2). The procedure may be facilitated by having the patient sit and lean over a bedside table while being stabilized by a helper, but this prevents measurement of OP. In anatomically challenging patients, ultrasonography and fluoroscopic guidance may aid in the identification of landmarks.
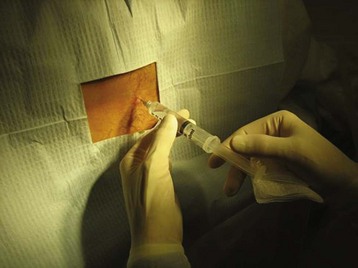
Once the patient and physician are in positions of comfort, the landmarks are reassessed and lidocaine is injected subcutaneously with a 27- or 25-gauge needle at the identified interspace to anesthetize the skin (Fig. 105.3). In children, topical application of anesthetic before injection may reduce the initial discomfort. After 1 to 2 minutes the deeper tissues are anesthetized by advancing a longer, 22-gauge needle slowly and injecting lidocaine. Achieving sufficient anesthesia is critical to success of the procedure.
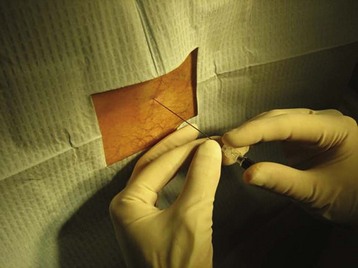
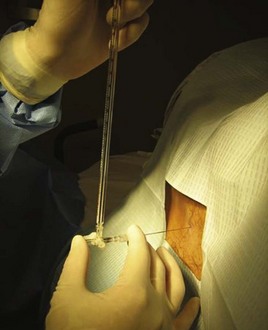
Once anesthesia is achieved, the landmarks are checked yet again. A 22-gauge spinal needle is used to advance into the previously identified space (Fig. 105.4). The bevel of the needle is kept parallel to the dural fibers, which run cephalad to caudad, to minimize the incidence of post–dural puncture headache (PDPH). The needle is advanced slowly while controlling it at the skin with the nondominant hand. Angling the needle slightly cephalad may be required because of the orientation of the spinous processes. The widely discussed “pop” of the needle penetrating the dura is not always felt; after traversing the ligamentum flavum, removal of the introducer every few millimeters of advancement may identify CSF. It is important to keep the orientation of the needle in the midline to avoid nerve roots as they exit the spinal canal.
Once CSF is observed, the manometer is attached to measure OP (Fig. 105.5). The patient’s legs are straightened while the patient is lying on the side, and the EP waits for CSF to equilibrate in the manometer column (slight respiratory variation will be noted) (Fig. 105.6). The fluid meniscus is the OP, which in the absence of obstructive hydrocephalus is equivalent to ICP.
Complications
PDPH is the most common complication associated with LP, with a rough incidence of 5% to 40%. Factors associated with PDPH include the technique and the type and gauge of needle used.2 PDPH develops about 2 days after the performance of a dural puncture, is worse with standing, and is partially relieved by lying down. The exact cause of this complication is uncertain, but it possibly due to continued leakage of CSF through the dural rent created by the needle. The headache is typically self-limited but can last up to a week and be fairly debilitating.
Several strategies can be used to reduce the incidence of PDPH, including orientation of the bevel of sharp-tipped needles parallel to the dural fibers to avoid cross-cutting of the fibers, the use of atraumatic blunt-tipped spinal needles (Pajunk, Sprotte) with a noncutting tip and side port, and reinsertion of the introducer into the noncutting needle before removal of the needle.3–5 The patient’s position following dural puncture or the periprocedural use of intravenous (IV) fluids is not associated with the development or prevention of PDPH. PDPH can be treated by reassurance and administration of IV fluids, analgesics, and IV caffeine. Severe headaches may require the placement of a blood patch, which is curative in 80% to 90% of cases.
Cerebrospinal Fluid Analysis
CSF cell counts must be determined in the setting of infection, primarily to assess the number and type of leukocytes present in the CSF.6 If the CSF obtained during LP is obviously bloody or blood-tinged, cell counts should be performed on multiple tubes and the number of leukocytes corrected for the number of erythrocytes present.7 The typical anecdotal correction applied in the past is 1 leukocyte for every 500 erythrocytes in a traumatic tap. More than 5 leukocytes per high-power field in CSF is considered abnormal, although CNS infections typically yield higher values. Lower leukocyte counts still above the cutoff may also signify CNS vasculitis, viral infection, or malignancy, depending on the cell type present.
Intracranial Pressure Monitoring
Placement of an invasive ICP monitor is typically performed in the setting of traumatic brain injury (TBI).8 Patients with suspected TBI and an associated Glasgow Coma Scale score of 7 to 8 or less often require ICP monitoring because an adequate neurologic examination may be difficult to perform in these patients. Sustained elevations in ICP, greater than 15 to 20 mm Hg, are associated with worse clinical outcomes. To assess ICP in patients with an unreliable neurologic examination, a monitor can be placed directly overlying the dura or into the ventricles.9 When performed on an emergency basis in the ED, these devices are typically placed by neurosurgeons in sterile fashion at the bedside. Furthermore, placement of an invasive monitor should be reserved for patients with some chance for meaningful recovery from their injuries.10
Arendt K, Demaerschalk BM, Wingerchuk DM, et al. Atraumatic lumbar puncture needles: after all these years, are we still missing the point? Neurologist. 2009;15:17–20.
Ellenby MS, Tegtmeyer K, Lai S, et al. Videos in clinical medicine. Lumbar puncture. N Engl J Med. 2006;355(13):e12.
Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296:2012–2022.
1 Ellenby M, Tegtmeyer K, Lai S, et al. Lumbar puncture. N Engl J Med. 2006;355:e12.
2 Turnbull DK, Shepherd DB. Post–dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–729.
3 Straus S, Thorpe K, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296:2012–2022.
4 Novak RW. Lack of validity of standard corrections for white blood cell counts of blood-contaminated cerebrospinal fluid in infants. Am J Clin Pathol. 1984;82:95–97.
5 Strupp M, Schueler O, Straube A, et al. “Atraumatic” Sprotte needle reduces the incidence of post–lumbar puncture headaches. Neurology. 2001;57:2310–2312.
6 Vallejo MC, Mandell GL, Sabo DP, et al. Postdural puncture headache: a randomized comparison of five spinal needles in obstetric patients. Anesth Analg. 2000;91:916–920.
7 Luostarinen L, Heinonen T, Luostarinen M, et al. Diagnostic lumbar puncture. Comparative study between 22-gauge pencil point and sharp bevel needle. J Headache Pain. 2005;6:400–404.
8 Jordan KG. Neurophysiologic monitoring in the neuroscience intensive care unit. Neurol Clin. 1995;13:579–626.
9 Lang EW, Chesnut RM. Intracranial pressure: monitoring and management. Neurosurg Clin North Am. 1994;5:573–605.
10 Rajshekhar V, Harbaugh RE. Results of routine ventriculostomy with external ventricular drainage for acute hydrocephalus following subarachnoid haemorrhage. Acta Neurochir (Wien). 1992;115:8–14.