Chapter 584 Neurologic Evaluation
History
The history should begin with the chief complaint, as well as a determination of the complaint’s relative significance within the context of normal development (Chapters 7–14). The latter step is critical because a 13 mo old who cannot walk may be perfectly normal, whereas a 4 yr old who cannot walk might have a serious pathology.
The most important component of a neurologic history is the developmental assessment (Chapters 6 and 14). Careful evaluation of a child’s social, cognitive, language, fine motor, and gross motor skills is required to distinguish normal development from either isolated or global (i.e., in two or more domains) developmental delay. An abnormality in development from birth suggests an intrauterine or perinatal cause, but a loss of skills (regression) over time strongly suggests an underlying degenerative disease of the CNS, such as an inborn error of metabolism. The ability of parents to recall the precise timing of their child’s developmental milestones is extremely variable. It is often helpful to request old photographs of the child or to review the baby book, where the milestones may have been dutifully recorded. In general, parents are aware when their child has a developmental problem, and the physician should show appropriate concern. Table 584-1 outlines the upper limits of normal for attaining specific developmental milestones. A comprehensive review of developmental screening tests and their interpretation is listed in Chapter 14.
Neurologic Examination
The neurologic examination begins at the outset of the interview. Indirect observation of the child’s appearance and movements can yield valuable information about the presence of an underlying disorder (Chapters 6 and 14). For instance, it may be obvious that the child has dysmorphic facies, an unusual posture, or an abnormality of motor function manifested by a hemiparesis or gait disturbance. The child’s behavior while playing and interacting with his or her parents may also be telling. A normal child usually plays independently early in the visit but will rapidly engage in the interview process. A child with attention-deficit/hyperactivity disorder might display impulsive behavior in the examining room, and a child with neurologic impairment might exhibit complete lack of awareness of the environment. Finally, note should be made of any unusual odors about the patient, because some metabolic disorders produce characteristic scents (e.g., the “musty” smell of phenylketonuria or the “sweaty feet” smell of isovaleric acidemia). If such an odor is present, it is important to determine whether it is persistent or transient, occurring only with illnesses.
The examination should be conducted in a nonthreatening, child-friendly setting. The child should be allowed to sit where he or she is most comfortable, whether it be on a parent’s lap or on the floor of the examination room. The physician should approach the child slowly, reserving any invasive or painful tests (e.g., measurement of head circumference, gag reflex) for the end of the examination. In the end, the more that the examination seems like a game, the better the child will cooperate. Because the neurologic examination of an infant requires a somewhat modified approach from that of an older child, the two groups are considered separately (Chapters 7, 8, and 88).
Head
Correct measurement of the head circumference is important. It should be performed at every visit for patients younger than 3 yr and should be recorded on a suitable head growth chart. To measure, a nondistensible plastic measuring tape is placed over the mid-forehead and extended circumferentially to include the most prominent portion of the occiput. If the patient’s head circumference is abnormal, it is important to document the head circumferences of the parents and siblings. Errors in the measurement of a newborn skull are common owing to scalp edema, overriding sutures, and the presence of cephalohematomas. The average rate of head growth in a healthy premature infant is 0.5 cm in the 1st 2 wk, 0.75 cm in the 3rd wk, and 1.0 cm in the 4th wk and every week thereafter until the 40th wk of development. The head circumference of an average term infant measures 34-35 cm at birth, 44 cm at 6 mo, and 47 cm at 1 yr of age (Chapters 7 and 8).
If the brain is not growing, the skull will not grow; therefore, a small head reflects a small brain, or microcephaly. Conversely, a large head may be associated with a large brain, or macrocephaly, which is most commonly familial but may be due to a disturbance of growth, neurocutaneous disorder (e.g., neurofibromatosis), chromosomal defect (e.g., Kleinfelter syndrome), or storage disorder. Alternatively, the head size may be increased secondary to hydrocephalus (Fig. 584-1) or chronic subdural hemorrhages. In the latter case, the skull tends to assume a square or boxlike shape, because the long-standing presence of fluid in the subdural space causes enlargement of the middle fossa.
The shape of the head should be documented carefully. Plagiocephaly, or flattening of the skull, can be seen in normal infants but may be particularly prominent in hypotonic or weak infants, who are less mobile. A variety of abnormal head shapes can be seen when cranial sutures fuse prematurely, as in the various forms of inherited craniosynostosis (Chapter 585.12).
Cranial Nerves
Optic Nerve (Cranial Nerve II)
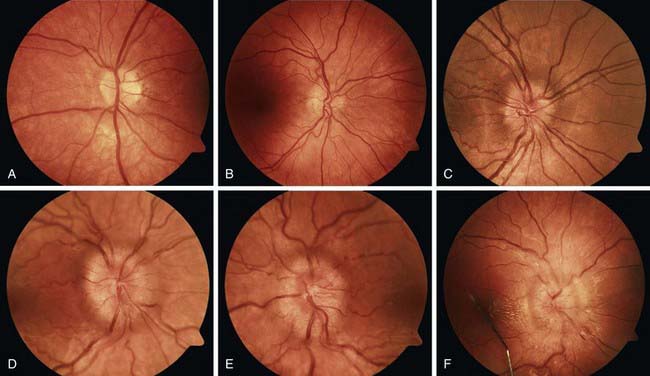
Disc edema refers to swelling of the optic disc, and papilledema specifically refers to swelling that is secondary to increased intracranial pressure. Papilledema rarely occurs in infancy because the skull sutures can separate to accommodate the expanding brain. In older children, papilledema may be graded according to the Frisen scale (Fig. 584-2). Disc edema must be differentiated from papillitis, or inflammation of the optic nerve. Both conditions manifest with enlargement of the blind spot, but visual acuity and color vision tend to be spared in early papilledema in contrast to what occurs in optic neuritis.
Oculomotor (III), Trochlear (IV), and Abducens Nerves (VI)
Disconjugate gaze can result from extraocular muscle weakness; cranial nerve (CN) III, IV, or VI palsies; or brainstem lesions that disrupt the medial longitudinal fasciculus. Infants who are <2 mo old can have slightly disconjugate gaze at rest, with one eye horizontally displaced from the other by 1 or 2 mm (strabismus). Vertical displacement of the eyes, known as skew deviation, is always abnormal and requires investigation. Strabismus is discussed further in Chapter 615.
Vestibulocochlear Nerve (Cranial Nerve VIII)
Because hearing is integral to normal language development, the physician should inquire directly about hearing problems. Parents’ concern is often a reliable indicator of hearing impairment and warrants a formal audiologic assessment with either audiometry or brainstem auditory evoked potential testing (Chapter 629). Even in the absence of parents’ concern, certain children warrant formal testing within the first month of life, including those with a family history of early life or syndromic deafness or a personal history of prematurity, severe asphyxia, exposure to ototoxic drugs, hyperbilirubinemia, congenital anomalies of the head or neck, bacterial meningitis, and congenital TORCH (toxoplasmosis, other infections, rubella, cytomegalovirus, herpes simplex virus) infections. For all other infants and children, a simple bedside assessment of hearing is usually sufficient. Newborns might have subtle responses to auditory stimuli, such as changes in breathing, cessation of movement, or opening of the eyes and/or mouth. If the same stimulus is presented repeatedly, normal neonates cease to respond, a phenomenon known as habituation. By 3-4 mo of age, infants begin to orient to the source of sound. Hearing-impaired toddlers are visually alert and appropriately responsive to physical stimuli but might have more frequent temper tantrums and abnormal speech and language development.
Motor Examination
Tone
There are 3 key tests for assessing postural tone in neonates: the traction response, vertical suspension, and horizontal suspension (Fig. 584-3; Chapters 88 and 91). To evaluate the traction response, the physician grasps the infant’s hands and gently pulls the infant to a sitting position. Normally, the infant’s head lags slightly behind his or her body and then falls forward upon reaching the sitting position. To test vertical suspension, the physician holds the infant by the axillae without gripping the thorax. The infant should remain suspended with his or her lower extremities held in flexion; a hypotonic infant will slip through the physician’s hands. With horizontal suspension, the physician holds the infant prone by placing a hand under the infant’s abdomen. The head should rise and the limbs should flex, but a hypotonic infant will drape over the physician’s hand, forming a U-shape. Assessing tone in the extremities is accomplished by observing the infant’s resting position and passively manipulating his or her limbs. When the upper extremity of a normal term infant is pulled gently across the chest, the elbow does not quite reach the midsternum (scarf sign), whereas the elbow of a hypotonic infant extends beyond the midline with ease. Measurement of the popliteal angle is a useful method for documenting tone in the lower extremities. The examiner flexes the hip and extends the knee. Normal term infants allow extension of the knee to ∼80 degrees. Similarly, tone can be evaluated by flexing the hip and knee to 90 degrees and then internally rotating the leg, in which case the heel should not pass the umbilicus.
Abnormalities of tone include spasticity, rigidity, and hypotonia. (Paratonia, which is rarely seen in the pediatric population, is not discussed here.) Spasticity is characterized by an initial resistance to passive movement, followed by a sudden release, referred to as the clasp-knife phenomenon. Because spasticity results from upper motor neuron dysfunction, it disproportionately affects the upper extremity flexors and lower extremity extensors and tends to occur in conjunction with disuse atrophy, hyperactive deep tendon reflexes, and extensor plantar reflexes (Babinski sign). In infants, spasticity of the lower extremities results in scissoring of the legs upon vertical suspension. Older children can present with prolonged commando crawling or toe-walking. Rigidity, seen with lesions of the basal ganglia, is characterized by resistance to passive movement that is equal in the flexors and extensors regardless of the velocity of movement (lead pipe). Patients with either spasticity or rigidity might exhibit opisthotonos, defined as severe hyperextension of the spine due to hypertonia of the paraspinal muscles (Fig. 584-4), though similar posturing can be seen in patients with Sandifer syndrome (gastroesophageal reflux or hiatal hernia associated with torsional dystonia). Hypotonia refers to abnormally diminished tone and is the most common abnormality of tone in neurologically compromised neonates. A hypotonic infant is floppy and often assumes a frog-leg posture at rest. Hypotonia can reflect pathology of the cerebral hemispheres, cerebellum, spinal cord, anterior horn cell, peripheral nerve, neuromuscular junction, or muscle.
Strength
Because infants and young children are not able to participate in formal strength testing, they are best assessed with functional measures. Proximal and distal strength of the upper extremities can be tested by having the child reach overhead for a toy and by watching him or her manipulate small objects. In infants <2 mo old, the physician can also take advantage of the palmar grasp reflex in assessing distal power and the Moro reflex in assessing proximal power. Infants with decreased strength in the lower extremities tend to have diminished spontaneous activity in their legs and are unable to support their body weight when held upright. Older children may have difficulty climbing or descending steps, jumping, or hopping. They might also use their hands to “climb up” their legs when asked to rise from a prone position, a maneuver called Gowers sign (Fig. 584-5).
Involuntary Movements
Most other involuntary movements including tics, dystonia, chorea, and athetosis stem from disorders of the basal ganglia. Tremor seems to be an exception, as it is thought to be mediated by the cerebellum. Further detail on the individual movement disorders is provided elsewhere in this text (Chapter 590).
Reflexes
Primitive Reflexes
Primitive reflexes appear and disappear at specific times during development (Table 584-2), and their absence or persistence beyond those times signifies CNS dysfunction. Although many primitive reflexes have been described, the Moro, grasp, tonic neck, and parachute reflexes are the most clinically relevant. The Moro reflex is elicited by supporting the infant in a semierect position and then allowing his or her head to fall backwards onto the examiner’s hand. A normal response consists of symmetric extension and abduction of the fingers and upper extremities, followed by flexion of the upper extremities and an audible cry. An asymmetric response can signify a fractured clavicle, brachial plexus injury, or hemiparesis. Absence of the Moro reflex in a term newborn is ominous, suggesting significant dysfunction of the CNS. The grasp response is elicited by placing a finger in the open palm of each hand; by 37 wk of gestation, the reflex is strong enough that the examiner can lift the infant from the bed with gentle traction. The tonic neck reflex is produced by manually rotating the infant’s head to one side and observing for the characteristic fencing posture (extension of the arm on the side to which the face is rotated and flexion of the contralateral arm). An obligatory tonic neck response, in which the infant becomes “stuck” in the fencing posture, is always abnormal and implies a CNS disorder. The parachute reflex, which occurs in slightly older infants, can be evoked by holding the infant’s trunk and then suddenly lowering the infant as if he or she were falling. The arms will spontaneously extend to break the infant’s fall, making this reflex a prerequisite to walking.
General Examination
Examination of other organ systems is essential because myriad systemic diseases affect the nervous system. Dysmorphic features can indicate a genetic syndrome (Chapter 102). Heart murmurs may be associated with rheumatic fever (Sydenham’s chorea), cardiac rhabdomyoma (tuberous sclerosis), cyanotic heart disease (cerebral abscess or thrombosis), and endocarditis (cerebral vascular occlusion). Hepatosplenomegaly can suggest an inborn error of metabolism, storage disease, HIV, or malignancy. Cutaneous lesions may be a feature of a neurocutaneous syndrome (Chapter 589).
Special Diagnostic Procedures
Lumbar Puncture and Cerebrospinal Fluid Examination
Normal CSF contains up to 5/mm3 white blood cells (WBCs), and a newborn can have as many as 15/mm3. Polymorphonuclear (PMN) cells are always abnormal in a child, but 1-2/mm3 may be present in a normal neonate. An elevated PMN count suggests bacterial meningitis or the early phase of aseptic meningitis (Chapter 595). CSF lymphocytosis can be seen in aseptic, tuberculous, or fungal meningitis; demyelinating diseases; brain or spinal cord tumor; immunologic disorders, including collagen vascular diseases; and chemical irritation (following myelogram, intrathecal methotrexate).
Neuroradiologic Procedures
CT is a valuable diagnostic tool in the evaluation of many neurologic emergencies, as well as some nonemergent conditions. It is a noninvasive, rapid procedure that can usually be performed without sedation. CT scans use conventional x-ray techniques, meaning that they produce ionizing radiation. Because children <10 yr of age are several times more sensitive to radiation than adults, it is important to consider the whether imaging is actually indicated and, if so, whether an ultrasound or MRI might be a more appropriate study. In the emergency setting, a noncontrast CT scan can demonstrate skull fractures, pneumocephalus, intracranial hemorrhages, hydrocephalus, and impending herniation. If the noncontrast scan reveals an abnormality and an MRI cannot be performed in a timely fashion, nonionic contrast should be used to highlight areas of breakdown in the blood-brain barrier (e.g., abscesses, tumors) and/or collections of abnormal blood vessels (e.g., arteriovenous malformations). CT is less useful for diagnosing acute infarcts in children, because radiographic changes might not be apparent for up to 24 hr. Some subtle signs of early (<24 hr) infarction include sulcal effacement, blurring of the gray-white junction, and the hyperdense middle cerebral artery (MCA) sign (increased attenuation in the MCA that is often associated with thrombosis). In the routine setting, CT imaging can be used to demonstrate intracranial calcifications or, with the addition of three-dimensional reformatting, to evaluate patients with craniofacial abnormalities or craniosynostosis. Although other pathologic processes may be visible on CT scan, MR is generally preferred because it provides a more-detailed view of the anatomy without exposure to ionizing radiation (Table 584-3).
Table 584-3 PREFERRED IMAGING PROCEDURES IN NEUROLOGIC DISEASES
ISCHEMIC INFARCTION* OR TRANSIENT ISCHEMIC ATTACK
INTRAPARENCHYMAL HEMORRHAGE
ARTERIOVENOUS MALFORMATION
CEREBRAL ANEURYSM
HYPOXIC-ISCHEMIC BRAIN INJURY
METABOLIC DISORDERS
HYDROCEPHALUS
HEADACHE
CT or MRI if a structural disorder is suspected
HEAD TRAUMA
EPILEPSY
BRAIN TUMOR
MRI without and with contrast
MULTIPLE SCLEROSIS
MENINGITIS OR ENCEPHALITIS
BRAIN ABSCESS
MOVEMENT DISORDERS
CTA, computed tomographic angiography; FLAIR, fluid-attenuated inversion recovery; ICP, intracranial pressure; MRA, magnetic resonance angiography; MRS, magnetic resonance spectroscopy; MRV, magnetic resonance venography; PET, positron emission tomography; SPECT, single-photon emission computed tomography; TCD, transcranial Doppler ultrasonography.
* Choice of studies differs from that in the adult population because tissue plasminogen activator (tPA) is not approved for use in children <18 yr of age.
Magnetic resonance imaging (MRI) is a noninvasive procedure that is well suited for detecting a variety of abnormalities, including those of the posterior fossa and spinal cord. MR scans are highly susceptible to patient motion artifact; therefore, most children <8 yr require sedation to ensure an adequate study. Because the American Academy of Pediatrics recommends that infants be kept NPO for ≥4 hr and older children for ≥6 hr before deep sedation, it is often difficult to obtain an MRI on an infant or young child in the acute setting. MRI can be used to evaluate for congenital or acquired brain lesions, migrational defects, dysmyelination or demyelination, post-traumatic gliosis, neoplasms, cerebral edema, and acute stroke (see Table 584-3). Paramagnetic MR contrast agents (e.g., gadolinium-DTPA) are efficacious in identifying areas of disruption in the blood-brain barrier, such as those occurring in primary and metastatic brain tumors, meningitis, cerebritis, abscesses, and active demyelination. MR angiography (MRA) and MR venography (MRV) provide detailed images of major intracranial vasculature structures and assist in the diagnosis of conditions such as stroke, vascular malformations, and cerebral venous sinus thrombosis. MRA is the procedure of choice for infants and young children owing to the lack of ionizing radiation and contrast; however, CTA may be preferable in older children because it is faster and can eliminate the need for sedation.
Avery RA, Shah SS, Licht DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010;363:891-893.
Barkovich JA. Pediatric neuroimaging, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005. pp 1–16
Beitzke D, Simbrunner J, Riccabona M. MRI in paediatric hypoxic-ischemic disease, metabolic disorders, and malformations—A review. Eur J Radiol. 2008;68:199-213.
Campbell WW. DeJong’s the neurologic examination, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2005.
Chiappa KH. Evoked potentials in clinical medicine, ed 3. Philadelphia: Lippincott–Raven; 1997.
DeRoos ST, Chillag KL, Keeler M, et al. Effects of sleep deprivation on the pediatric electroencephalogram. Pediatrics. 2009;123:703-708.
Jan MMS. Neurological examination of difficult and poorly cooperative children. J Child Neurol. 2007;22:1209-1213.
Portnoy JM, Olson LC. Normal cerebrospinal fluid values in children: another look. Pediatrics. 1985;75:484-487.

 to 3 yr of age can identify the objects on a pediatric eye chart (Allen chart) at a distance of 15-20 ft.
to 3 yr of age can identify the objects on a pediatric eye chart (Allen chart) at a distance of 15-20 ft.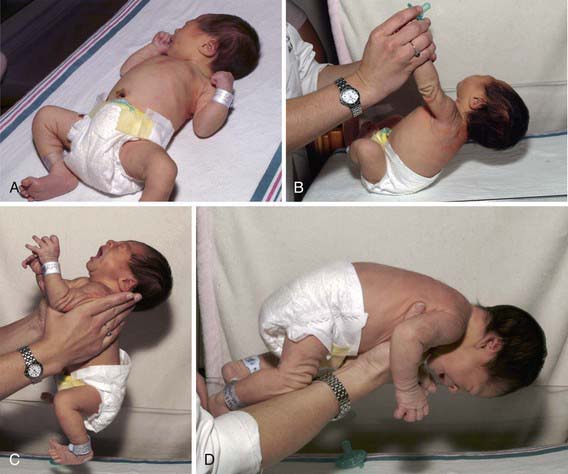


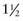 to 3 in, sharp, beveled spinal needle with a properly fitting stylet is introduced in the midsagittal plane and directed slightly cephalad. The physician should pause frequently, remove the stylet, and assess for CSF flow. Although a pop can occur as the needle penetrates the dura, it is more common to experience a subtle change in resistance.
to 3 in, sharp, beveled spinal needle with a properly fitting stylet is introduced in the midsagittal plane and directed slightly cephalad. The physician should pause frequently, remove the stylet, and assess for CSF flow. Although a pop can occur as the needle penetrates the dura, it is more common to experience a subtle change in resistance.




