Neurologic Disorders
Acute Bacterial Meningitis
Despite advances in medical care, acute bacterial meningitis (ABM) remains a potentially life-threatening emergency. Nationwide mortality rates for treated cases are 20 to 30% in neonates and adults and 2% in infants and children.1 With the recommendation in 1991 that all infants, starting at 2 months of age, receive the conjugated vaccine against Haemophilus influenzae type b, the incidence of bacterial meningitis caused by this organism has been reduced in children by more than 99%. However, recent data show that the highest incidence is still found in children younger than 2 months.1,2 Even with the best care, 10 to 30% of all survivors of bacterial meningitis of all types demonstrate persistent, functionally important disabilities.3,4 These include hearing deficits, seizures, learning and behavioral problems, and cognitive impairments.
Neonates
Group B streptococci account for more than three quarters of the cases of neonatal meningitis; Escherichia coli (and other coliforms), Listeria monocytogenes, Streptococcus pneumoniae, Neisseria meningitidis, and H. influenzae are responsible for another one fourth of cases.2 The incidence of early-onset group B streptococcal disease has declined as a result of intrapartum prophylaxis. However, late-onset disease (after 7 days of life) has not. Staphylococcus epidermidis, Staphylococcus aureus, S. pneumoniae, N. meningitidis, group D streptococci, Ureaplasma urealyticum, H. influenzae type b, and nontypable strains are less common pathogens in neonatal infection. L. monocytogenes rarely is the agent of meningitis in neonates but is important because of its prevalence within some immigrant groups, its association with unpasteurized dairy products, and its resistance to cephalosporins.5
Infants and Children
Beyond the neonatal period, in the United States, S. pneumoniae, N. meningitidis, and H. influenzae account for 90% of the documented cases of ABM.2 Unusual pathogens include Salmonella species, Campylobacter species, L. monocytogenes, group G streptococci, Francisella tularensis, group B beta-hemolytic streptococci, and several anaerobic organisms.1,2 Recent advances involving the heptavalent pneumococcal conjugate vaccine (PCV7), recommended for all children younger than 2 years, have dramatically reduced the number of cases of bacterial meningitis caused by invasive pneumococcal disease.6 It is anticipated that the newly released 13-valent pneumococcal conjugate vaccine (PCV13) will have an even greater impact on the reduction of invasive pneumococcal disease.7
Principles of Disease
Bacterial meningitis is thought to result from infection of the respiratory tract, development of bacteremia, invasion of the meninges, and inflammation of the meninges and brain.5 Invasion of the meninges by bacterial pathogens is facilitated by mechanical disturbances. These include recent neurosurgical procedures, such as ventriculoperitoneal shunt placement and lumbar puncture, and skull fracture with a persistent cerebrospinal fluid (CSF) leak. Congenital or developmental central nervous system (CNS) abnormalities also predispose affected patients to bacterial seeding of the CNS. These include intracranial cysts and epidermoid or dermoid tumors, often with an associated congenital dermal sinus track.
If bacteria gain entrance into the subarachnoid space, they replicate. Microbial traversal of the blood-brain barrier occurs by microbial interactions with host receptors. Each organism may gain entry in a specific manner. For example, E. coli penetration into the brain involves binding to and invasion of the human brain microvascular endothelial cells, which are part of the blood-brain barrier.8 Host inflammatory mediators and cytokines, including interleukin-1, tumor necrosis factor, and platelet-activating factor, may also be produced and secreted by CNS macrophages and endothelial cells. As a result of an inflammatory response, vascular and parenchymatous cerebral changes occur. These include vasculitis, microthrombus formation, occlusion of venous sinuses, reduced blood flow, increased permeability of the blood-brain barrier, increased intracranial pressure (ICP), diffuse cerebral edema, and intracerebral hemorrhage. Changes involved in microbial invasion of the blood-brain barrier may be different from those involved in the release of inflammatory mediators and cytokines in response to meningitis-causing pathogens.8
Clinical Features
In three fourths of children ultimately diagnosed with ABM, the clinical presentation is subacute, evolving during 2 to 5 days. At onset, affected children typically exhibit a variety of symptoms and signs, such as fever, malaise, decreased interest in surroundings, irritability, alteration in sleeping pattern, anorexia, nausea, vomiting, or diarrhea. These findings are nonspecific, seen with equal frequency in children who are suffering from trivial and self-limited illnesses.8,9 If the patient is examined early in the course, physical signs may be subtle or lacking.
Children with this insidious presentation have a better prognosis than that of patients with ABM who present with a rapid progression of signs and symptoms. In one fourth of children with ABM, an acute illness with manifestations such as vomiting, fever, and lethargy develops in less than 24 hours. In such patients, the diagnosis is rarely missed.10 Those with fulminant illness exhibit a higher risk for death as well as immediate and long-term complications.
Neonatal Period (Up to the Age of 1 Month)
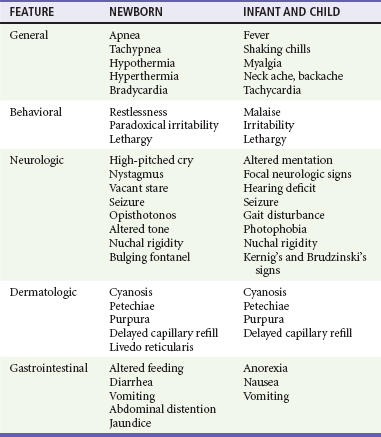
The clinical findings with ABM in the neonate are nonspecific and may include altered vital signs, behavioral changes, neurologic aberrations, dermatologic lesions, and gastrointestinal manifestations (Table 175-1). In the first 30 days of life, patients who present to an ED with a temperature of 38° C (100.4° F) or higher are found to have serious bacterial illness (SBI) and ABM in 4 to 15% and 1 to 2% of cases, respectively.11 The absence of fever does not eliminate the possibility of SBI because more than half of neonates with meningitis are afebrile or exhibit hypothermia. Other vital sign changes include tachycardia, bradycardia, tachypnea, and apnea. Apnea with cessation of breathing for longer than 20 seconds may signify either seizure activity or a nonspecific medullary respiratory center dysfunction.
Rashes are infrequent manifestations of neonatal meningitis.12 One clue may be generalized pallor accompanied by indistinctly outlined truncal patches of blue discoloration (livedo reticularis). Difficulty in feeding and unusual stooling patterns are nonspecific gastrointestinal features. Nonprojectile vomiting may appear in the course of meningitis, becoming projectile only after ICP has increased. Increased stool volume, irritability during defecation, abdominal distention, hepatomegaly, and jaundice can develop with meningitis.
One Year to 5 Years
The cardinal features in this age group are fever, headache, vomiting, and stiff neck. Obtundation and lethargy also are common findings with meningitis but are nonspecific. After the first year of life, neck stiffness with difficulty in flexing the neck is reliably seen during the acute phase of meningitis. A patient rarely may exhibit torticollis.13 The clinician can attempt to elicit nuchal rigidity by forced flexion of the neck with the child in the supine position. However, this method is less reliable than attempting neck flexion with the child seated with both legs outstretched. Both of these maneuvers involve passive motion that many children voluntarily resist. More reliable methods that have fewer false-negative and false-positive results involve active neck motion on the part of the child. In a toddler, a distracting object can be used to attract eye contact, and the active range of neck motion can be determined. In addition, the neck stiffness that accompanies ABM may be displayed in both the prone and supine positions when the child’s shoulders are placed at the edge of the examining table and minimal support of the occiput or forehead is provided manually. Presence of Kernig’s sign (flexion of the hip 90 degrees with subsequent pain on extension of the leg) and Brudzinski’s sign (involuntary flexion of the hips and knees after passive flexion of the neck performed with the patient supine) is less reliable. Kernig’s and Brudzinski’s signs are seen in approximately 27% and 51%, respectively, of children 1 to 5 years of age with ABM.14
Diagnostic Strategies
Indications: Lumbar puncture is indicated in any child in whom bacterial meningitis is suspected (Box 175-1). Patients to be considered candidates for this procedure are those with classic signs and symptoms, such as fever, stiff neck, and photophobia, but in infants and children, classic signs often are absent. In a study by Walsh-Kelly and colleagues, nuchal rigidity was present in only 27% of infants younger than 6 months with bacterial meningitis. By 12 months of age, 71% showed nuchal rigidity; this rate rose to more than 95% by 19 months of age.15 Therefore, the decision to perform a lumbar puncture in children with suspected meningitis should be based primarily on the constellation of presenting signs and symptoms.
Nuchal Signs.: A lumbar puncture should be performed in ill children who exhibit nuchal rigidity or Kernig’s sign or Brudzinski’s sign and who have suspected meningitis. These three signs of meningeal irritation can also be seen in non-ABM, such as subarachnoid hemorrhage and trauma.
Suspected Sepsis in Young Infants.: A bacterial infection is the most likely etiologic disorder in any infant younger than 3 months who is ill.11 Current practice advocates that the possibility of severe bacterial infection be pursued with an ill young infant. No distinguishing features differentiate sepsis from meningitis in this age group. Because 50% of neonates with acute meningitis are bacteremic at the time of the initial evaluation, and because an intracranial infection subsequently develops in 25% of bacteremic neonates, blood cultures and lumbar puncture should be undertaken concomitantly.16,17 In infants older than 1 month but younger than 3 months, history and physical examination, even if augmented with selective laboratory investigations (excluding lumbar puncture), may fail to identify those infants with SBI. A lumbar puncture should be undertaken unless there is a contraindication as part of the sepsis evaluation in this age group.8,18,19
Toxic Appearance.: A lumbar puncture also should be considered in any child with toxic appearance. Such observations include a blunted response to social overtures, poor perfusion, altered motor tone, and abnormal cry. Febrile pediatric patients of all ages with a toxic appearance have an increased incidence of severe bacterial infections, including ABM.8
When obvious clues to the source of an apparent infectious process exist and an initial lumbar puncture yields normal findings in the face of increasing severity of illness, additional information can be obtained by repeating the lumbar puncture. The CSF composition may change from normal cellularity to marked pleocytosis within as little as 30 minutes.20 Thus later reexamination of the CSF may be invaluable in establishing the nature of an occult intracranial infection.
Febrile Illness after Intimate Contact.: All children who have prolonged and close physical involvement with persons found to have a serious bacteremic illness require prompt medical evaluation. In particular, a strong indication for lumbar puncture is the development of fever after intimate contact with high-risk patients with meningococcal disease (higher risk: household contacts, children younger than 2 years, history of direct exposure to secretions from index case or having slept in same dwelling 7 days before onset of illness, and passengers seated next to an index case on a flight longer than 8 hours) or Haemophilus disease (higher risk: household with at least one contact younger than 4 years who is either unimmunized or poorly immunized, a child younger than 12 months who has not received the primary series of immunizations, an immunocompromised child of any age regardless of H. influenzae type b immunization status, and a child who attends daycare where two or more cases have been seen in a 60-day period). Even in the absence of pleocytosis, the emergency physician should consider admission and antibiotic treatment when a child has been exposed to meningococcemia and is symptomatic (e.g., fever, headache) as absence of meningitis is a poor prognosis in this disease.
Febrile Seizures.: Children 6 months to 5 years of age who have experienced a simple febrile seizure and who appear well (alert, active, playful) are extremely unlikely to have meningitis. In general, patients who exhibit complex features of the febrile seizure are at increased risk for meningitis. Lumbar puncture should be considered in patients who have characteristics that increase their risk for ABM or who show signs and symptoms of ABM. The historical features are age between 6 and 12 months; exposure to another child with meningitis or serious bacterial infection; deficiency in H. influenzae or S. pneumoniae immunizations, or immunization status cannot be determined; and pretreatment with antibiotics. Children with febrile seizures who exhibit any clinical sign associated with increased risk for intracranial infection, including lethargy, decreased motor tone, doll’s eye sign, inability to fix and follow, decreased response to painful stimuli, nuchal rigidity, full fontanel, petechiae, and poor skin perfusion, should undergo lumbar puncture.21
Fever and Petechiae.: Trivial to life-threatening infectious disease can cause fever and petechiae. No current optimal strategy has emerged for evaluation of febrile children with petechial eruptions. Well-appearing febrile children with localized petechiae, especially above the nipple line and with no purpura, are unlikely to have invasive bacterial disease. Patients at highest risk for invasive bacterial disease are more likely to appear ill or to have generalized petechiae and purpura or signs of meningeal irritation.22 In the high-risk group, unless it is contraindicated by coagulopathy, lumbar puncture should be performed to exclude concurrent meningitis.23
Sepsis Suspected in an Abnormal Host.: Immunocompromised pediatric patients are at risk for the development of both opportunistic infections and invasive illness from pathogens common to all children. Immunologically incompetent children may not exhibit the typical manifestations of intracranial infections, including nuchal rigidity. However, meningitis should be suspected in an immune-impaired child who experiences only a change in mental status. CSF parameters in these patients are less sensitive indicators of the presence of bacterial meningitis compared with normal host response to a bacterial invasion of the meninges. A lumbar puncture nonetheless should be performed in the process of excluding meningitis in a compromised host.
Penetration of Dura.: Fracture through the paranasal sinuses or anterior or middle cranial fossa and penetrating nasal injury or instrumentation may lead to dural tears. Such defects constitute a potential portal of entry for microorganisms into the CNS. Evidence of CNS infection generally is seen within 2 weeks of the initial injury, but clinical manifestations may be delayed for years. Persistent CSF otorrhea or rhinorrhea may be encountered but is not a clinical prerequisite for post-traumatic meningitis. When patients with a recent or remote history significant for craniofacial trauma develop any constellation of symptoms suggestive of meningitis, lumbar puncture should be performed after a computed tomography (CT) scan.
Acute Hearing Loss.: Hearing loss has been identified in up to one third of patients with ABM. Hearing impairment may be identified in the acute phase or become apparent only in the post-meningitic period. Of significance, hearing loss may precede the onset of systemic complaints and herald the appearance of meningitis. This is especially true for patients with an inner ear fistula or basilar skull fracture. Acute hearing loss should be an indication for lumbar puncture in patients with traumatic injury, which may have directly inoculated organisms into the labyrinth or basilar subarachnoid space, and in patients who have symptoms suggestive of meningitis.
Contraindications: Most children with ABM exhibit some mental status changes, but rapid onset and progression to deep coma (Glasgow Coma Scale score of less than 8) usually reflect increased ICP, and lumbar puncture is contraindicated.24 Herniation of the temporal lobes through the tentorium or herniation of the cerebellar tonsils through the foramen magnum rarely occurs in patients with meningitis and increased ICP. Focal neurologic signs suggestive of an abscess are further contraindications to lumbar puncture in children.
Positioning and Precautions: When it is not contraindicated, emergency lumbar puncture with CSF examination should be performed to confirm a presumptive diagnosis of ABM. Patients with suspected bacterial meningitis traditionally were placed in the lateral decubitus position with the spine flexed and the knees drawn upward toward the chest, with shoulders and back perpendicular to the table. Studies with ultrasonography have called this positioning into question and reveal that the interspinous space is greatest in the sitting position with the hips flexed, and neck flexion did not result in increase in the interspinous space.25 These data reveal that forward head flexion may not be necessary. Opening pressure should be obtained if possible with the patient in the lateral decubitus position. In circumstances in which opening pressure measurement cannot be obtained, an alternative position is sitting with the thighs flexed toward the abdomen.
Standard Cerebrospinal Fluid Interpretation
A standard CSF examination includes bacterial culture and Gram’s stain from tube 1, protein and glucose assessment from tube 2, and blood cell count from tube 3. If there is not an adequate amount of CSF collected, the most important is the specimen sent for culture and Gram’s stain. In cases of culture-proven bacterial meningitis, up to 6% of patients have normal glucose and protein levels, few white blood cells, and negative result on Gram’s staining. Patients who have received antibiotics before their first lumbar puncture merit special mention. Although the CSF can be sterilized after the administration of antibiotics in the ED, especially in cases of pneumococcal and meningococcal disease, the CSF profile often is unaffected for more than 12 to 24 hours after therapy.6
Glucose.: The CSF glucose concentration should be interpreted in relation to a concomitant serum glucose value. The normal steady-state ratio of CSF glucose to serum glucose is approximately 0.6. Hypoglycorrhachia, defined as a ratio below 0.4, is characteristically found in cases of ABM with common pathogens and with Mycobacterium tuberculosis. Hypoglycorrhachia also may be associated with viral meningitis, although CSF glucose concentration in such cases typically is normal.
Protein.: The normal CSF protein range is 55 to 80 mg/dL in newborns (65-150 mg/dL in preterm babies) and 5 to 40 mg/dL in children. Normal to modest elevations in protein concentration occur in the course of viral meningitis. Higher levels of protein are encountered with ABM. After a traumatic lumbar puncture, each 800 to 1000 red blood cells elevate the protein level by 1 mg/dL.
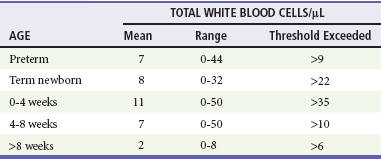
Cellularity.: A representative cellularity profile is presented in Table 175-2. Unfortunately, these numbers have been largely derived from healthy or nonsystemically ill children.26 The standard “panic values” that have been quoted often are more than 2 SDs above the mean or are figures that are seen in only 5 to 10% of studied populations. Cellularity that exceeds these typical threshold values can be seen with conditions that indirectly influence the CNS; however, such cases should be considered to represent possible ABM and treated accordingly. These conditions include generalized seizure, shigellosis, and parameningeal foci of infections such as otitis media, sinusitis, and mastoiditis. Patients with suspected sepsis or with foci of infection distant from the CNS, such as pneumonia, also may have increased cellularity. Classically in ABM, the total CSF white blood cell count ranges from 1000 to 20,000/mm3. A white blood cell count higher than 2000/mm3 is present in more than one third of cases of ABM. Significant pleocytosis also can accompany aseptic meningitis in as many as 95% of cases.27
Of note, the practice of “correcting” for white blood cells in bloody CSF samples may have little scientific support. Underestimation of the white blood cell count may occur in the setting of culture-positive meningitis. Furthermore, consideration should be given to other illnesses, such as HSV encephalitis, in which an increased number of red blood cells may be seen in the CSF.5
Gram’s Stain.: The probability of visualizing bacteria with Gram’s staining depends on the number of bacterial organisms present at the time of sampling.8 One fourth of the smears yield positive results with 103 or more colony-forming units (CFUs) per milliliter; 60% of the smears show positive results with 103 to 105 CFUs/mL, and 97% show positive results with 106 CFUs/mL. A positive result on Gram’s staining necessitates immediate antibiotic therapy, even in the absence of increased white blood cells and protein levels or decreased glucose level in the CSF.
Other Cerebrospinal Fluid Tests
Antigen Detection.: Commercial antigen detection kits are available for the common pathogens causing ABM. Microbial antigens in CSF are detected by coagglutination, latex agglutination, countercurrent immunoelectrophoresis, enzyme-linked immunosorbent assay, and centrifugation-augmented solid-phase immunoassay. Because antigens remain measurable after antibiotic treatment, antigen detection proves to be most effective in cases of partially treated meningitis or in patients in whom the CSF Gram’s stain and culture results are negative and ABM is strongly suspected. In any meningitic state, either untreated or pretreated, the CSF antigen detection kits (commercially available to detect multiple organisms in one packaged product) are most sensitive when more than 500 white blood cells are present in the CSF. The antigens typically are present at high levels in the CSF for several days, even after parenteral antibiotics have been initiated. A positive CSF assay result provides reliable bacteriologic diagnosis. A negative antigen test result cannot exclude the diagnosis of ABM.
Cytokines.: Assayed concentrations of C-reactive protein, various interleukins, tumor necrosis factor, and prostaglandins are elevated with ABM. These assays require sophisticated techniques and expensive equipment and take several hours to complete. For these reasons, the assays are of little utility in the ED setting.
Differential Considerations
Any of the manifestations of ABM can be expressed in a single patient. The most common presentation involves a combination of thermal instability, altered behavior, blunted mental status, and neck stiffness. The differential diagnosis for this constellation comprises infectious, metabolic, traumatic, and miscellaneous conditions (Box 175-2).
Many conditions cause a child to appear ill. Any infectious agent can cause clinical illness in children, particularly in the first months of life. Infants with infectious disease exhibit altered affect, including significant changes in mental status, strongly suggesting an intracranial pathologic process. Beyond infancy, children may exhibit a toxic appearance with any focal infection or with extensions from a primary focus leading to systemic invasion.10 In addition to infection, a change in mental status may be noted with endocrinopathy, hypoglycemia, electrolyte imbalance, metabolic disease, uremia, seizure, unintentional trauma, abusive head injury, intussusception, or exposure to toxins.
Viral Meningitis
In the first few years of life, the signs and symptoms of bacterial and viral meningitis can be indistinguishable.28 Beyond 1 year of age, a majority of children with viral meningitis have a relatively mild illness compared with children with ABM. Fever and retro-orbital or frontal headache are frequent symptoms of both diseases. Gastrointestinal disturbances, such as anorexia, nausea, and diarrhea, are more common with viral meningitis. Generalized myalgia also may be a feature, and patients often complain of neck ache or neck stiffness. However, reduction in active neck flexion is uncommon.
Modest elevation of PMNs in CSF specimens coupled with normal glucose level, normal to slightly elevated protein concentration, and negative result on Gram’s staining is anticipated with viral meningitis. These laboratory findings can confirm a clinical suspicion of viral meningitis. However, atypical features, such as hypoglycorrhachia and significant pleocytosis, can be found in 20% of encounters. This blurs the distinction between the two states and may alter management. Decision rules have been proposed to distinguish viral from bacterial meningitis, but their usefulness in clinical practice may be limited if they cannot identify every case of ABM. Nigrovic and colleagues, however, demonstrated a low risk (0.1%) for bacterial meningitis in children who lack the following criteria: positive result of CSF Gram’s stain, CSF absolute neutrophil count of at least 1000 cells/µL, CSF protein concentration of at least 80 mg/dL, peripheral blood absolute neutrophil count of at least 10,000 cells/µL, and history of seizure before or at the time of presentation.27
Management
Antibiotic Therapy
A major consideration in the choice of antibiotics is the emergence of antimicrobial-resistant organisms, including S. pneumoniae resistant to second- and third-generation cephalosporins and gram-negative bacilli resistant to many β-lactam drugs. Thus empirical treatment in areas where there are resistant strains of S. pneumoniae needs to include vancomycin.8 The four clinical categories of patients and their management are as follows.
Nontoxic, low risk: In a non-pretreated patient with no evidence of systemic toxicity in whom the clinical picture is suggestive of viral meningitis, if the CSF indices confirm a clinical suspicion of viral meningitis (negative result on Gram’s stain, few white blood cells in the CSF, and normal CSF protein and glucose levels), antibiotics can be withheld in consultation with the patient’s primary care physician or a neurologist. Another rarely considered option is to discharge the patient and to schedule repeated lumbar puncture within 12 hours after antibiotic therapy. These strategies are not without risk because it often is difficult to distinguish viral meningitis from other infections, such as Rocky Mountain spotted fever and ABM. If biochemical parameters or cytologic assessment findings resemble those in ABM, it is appropriate to begin empirical antibiotic therapy and to hospitalize the patient for observation.
Nontoxic, high risk: A patient with no evidence of toxicity but with high-risk historical factors (such as pretreatment with antibiotics, exposure to an invasive organism [H. influenzae or N. meningitidis], or age younger than 1 year) prompting increased concern for ABM requires blood culture and lumbar puncture for CSF analysis. If the spinal fluid is cloudy, antibiotic therapy should begin immediately rather than being withheld pending complete CSF analysis. In symptomatic patients exposed to a person with meningococcemia, appropriate management consists of a full range of cultures, empirical antibiotic therapy, and hospitalization for observation until the results of all cultures are known because the absence of meningitis does not rule out the presence of meningococcal disease.
Critical, stable: For the patient with classic signs of ABM who has a protected airway, adequate ventilation, normal perfusion, and no evidence of coagulopathy and is assessed as being in critical but stable condition, a blood culture with phlebotomy and a second culture with placement of an intravenous catheter, urinalysis and culture, blood chemistries, and CBC are recommended, after which a lumbar puncture is performed. Antibiotics should be administered immediately after lumbar puncture and before the results of the CSF or other studies become available from the laboratory.
Critical, unstable: A patient with a CNS syndrome, abnormal vital signs for age, unprotected airway, ongoing seizure activity, focal neurologic deficit, or coagulopathy is considered to be in critical and unstable condition. In these patients, the clear risk of an adverse outcome (i.e., respiratory failure) as a result of diagnostic lumbar puncture is magnified, especially if the procedure is carried out before the patient is stabilized. Although the cause and effect relationship is controversial, patients with clinical signs of increased ICP may experience fatal cerebral herniation (even if CT findings are normal) after a lumbar puncture.24 Two blood culture samples and blood and urine specimens for antigen detection should be obtained. Antibiotics should be administered and the lumbar puncture deferred.
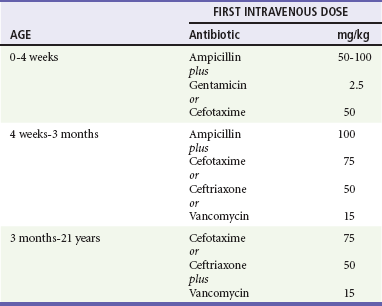
ED antimicrobial treatment decisions are empirically based. The choice of agents should be based on knowledge of the prevalent organisms responsible for intracranial infections and the regional patterns of their antimicrobial susceptibility. The initial regimen chosen for treatment should be broad enough to provide coverage for the various pathogens typical for the age group being treated (Table 175-3).8
In the newborn, no single antibiotic has bactericidal activity against all of the possible organisms commonly encountered. The most widely used combination therapy consists of ampicillin with an aminoglycoside or ampicillin plus cefotaxime, which is equally effective. For infants older than 1 month and younger than 3 months, in the absence of any evidence for presence of unusual organisms, conventional therapy is ampicillin and a third-generation cephalosporin. Beyond the age of 3 months, monotherapy with a third-generation cephalosporin provides adequate coverage, except in cases involving resistant strains of S. pneumoniae. When gram-positive cocci are identified on the CSF Gram’s stain, the combination of a broad-spectrum cephalosporin (e.g., cefotaxime, ceftriaxone) and vancomycin is now routinely recommended.8
Steroid Therapy
The role of dexamethasone therapy for ABM has long been the focus of clinical interest. Consensus opinion suggests that for infants beyond 8 weeks of age, dexamethasone may mitigate some neurologic sequelae.1
Use of dexamethasone is not without risk. The most commonly reported deleterious effect of dexamethasone is gastrointestinal bleeding. The anti-inflammatory effects of dexamethasone have led to a false sense of clinical improvement, overshadowing the failure of the chosen antibiotic to eradicate an invasive organism. Reduced penetration of vancomycin with dexamethasone therapy has been demonstrated.29 As a potentially universal distribution of cephalosporin-resistant and penicillin-resistant S. pneumoniae approaches, reducing the penetrating effect of vancomycin may cause undue harm. In summary, it is best to adhere to the latest recommendations of the American Academy of Pediatrics (AAP) and to limit the use of dexamethasone to the presumptive treatment of pneumococcal meningitis in infants who are older than 6 weeks after consideration of potential benefits and risks. The AAP recommendation is that dexamethasone may also be beneficial for infants and children with H. influenzae meningitis to decrease the risk of neurologic sequelae, including hearing loss, if it is given before or concurrently with the first dose of an antimicrobial agent.8,30
Acyclovir
The issue of empirical treatment of a neonate or young infant (younger than 3 months) with acyclovir is controversial because identification of infected infants is challenging. Acyclovir should be administered in an ill or febrile infant with a history of maternal HSV infection, presence of vesicles on the skin, seizure, or focal neurologic signs. Use of acyclovir also should be considered in atypical presentations of sepsis or meningitis.5 The dose of acyclovir is 20 mg/kg intravenously every 8 hours for 21 days in full-term immunocompetent infants (<35 weeks postconceptual age: 40 mg/kg/day divided q12hr IV for 14-21 days and ≥35 weeks postconceptual age: 60 mg/kg/day divided q8hr IV for 14-21 days). The dose for immunocompromised infants is 750 to 1500 mg/m2/24 hours divided every 8 hours for 7 to 14 days. PCR assay for HSV should be performed in these cases. Treatment may be initiated on the basis of the criteria outlined earlier before results of PCR tests.
Seizures
Principles of Disease
The young (<5 years), immature nervous system is remarkably susceptible to seizures. Early in development, excitatory activity predominates and inhibitory systems are undeveloped.32 This is known as the period of vulnerability. A paucity of synaptic connections and alterations in the synthesis of neurotransmitters also may play a role.
Seizures and Brain Damage: It is well known that children with epilepsy are at a significant risk for cognitive impairment and behavioral abnormalities. It is difficult to distinguish the relative contributions of the effect of the seizures from the underlying CNS disease and from the effect of anticonvulsants. A single prolonged seizure (>30 minutes) has been shown to damage the brain, particularly the temporal lobes and hippocampus.33 In addition, a growing body of evidence points to the lasting effect of repetitive, brief seizures in early childhood.34
Clinical Features
Classification of Seizure Type
Seizures are classified into two main types: partial (consciousness is maintained) and generalized (consciousness is lost) (Box 175-3). There are two types of partial, or focal, seizures: complex and simple. In complex partial seizures, the patient experiences a change in level of awareness and may exhibit bizarre behaviors, including staring, lip smacking, wandering, or picking at clothing. In simple partial seizures, the patient experiences no change in mentation.
Classification of Epilepsy: Epileptic Syndromes
An epileptic syndrome is characterized by a triad of age at onset, seizure type, and electroencephalographic findings. Identification of the epileptic syndrome provides information on prognosis and informs management decisions. Several of the more common epileptic syndromes of infancy and childhood are reviewed (Box 175-4).
Infantile Spasms: Infantile spasms are manifested during the first year of life with rapid flexor or extensor spasms that appear in clusters. The electroencephalogram (EEG) shows hypsarrhythmia, an abnormal pattern characterized by high-voltage slow waves and disorganized spike activity.
Approximately two thirds of children with infantile spasms have an underlying CNS disorder, such as a congenital brain malformation or tuberous sclerosis. The outcome is poor; only half will attain remission of seizures, and the large majority will be mentally retarded. Treatment is notoriously difficult. In the United States, a course of ACTH has traditionally been the treatment of choice; prednisolone treatment is being used with increasing frequency.35 Vigabatrin was recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of infantile spasms and is particularly useful in the setting of tuberous sclerosis.
Childhood and Juvenile Absence Epilepsy: Childhood and juvenile absence epilepsy begins between 4 and 12 years of age and is characterized by frequent absence seizures; generalized convulsive seizures occur in up to 50% of the patients. The diagnosis is frequently missed for prolonged periods; the parent or physician may assume the child is daydreaming or purposely not responding. Bursts of spike-and-wave activity at 3/second on the EEG during an absence spell are indicative of this syndrome. Hyperventilation can trigger absence seiziures and can be used to diagnose absence epilepsy. Treatment is with ethosuximide; if convulsions also occur, valproic acid or lamotrigine are used instead.36 The prognosis is excellent, and the majority of children outgrow this disorder by 16 years of age.
Benign Epilepsy with Centrotemporal Spikes: This epilepsy syndrome typically begins between 3 and 13 years of age. The seizures occur most commonly during sleep and involve the facial muscles; secondary generalization frequently occurs. The typical electroencephalographic pattern is one of central temporal (rolandic) spikes, particularly during sleep. The prognosis is excellent; the seizures typically cease by 16 years of age. Considerable controversy exists as to whether anticonvulsant treatment of a patient with brief, infrequent seizures is warranted.37
Lennox-Gastaut Syndrome: Developmental disability, multiple seizure types and a “slow” spike and wave pattern on EEG in a young child (2-5 years) are the characteristics of Lennox Gastaut Syndrome. Infantile spasms commonly evolves into Lennnox Gastaut. Valproic acid, lamotrigine, topiramate, felbamate, and rufinamide have shown promise for the treatment of Lennox-Gastaut syndrome,38 but treatment is notoriously difficult.
Febrile Seizures: A febrile seizure is defined as a seizure occurring in the presence of fever without CNS infection or other cause. A simple febrile seizure is generalized, lasts less than 15 minutes, and occurs in a neurologically and developmentally normal child between 6 months and 6 years of age.39 Simple febrile seizures typically occur early in the course of the febrile illness. Temperature does not need to be markedly elevated; almost half the children with a febrile seizure have a documented temperature below 39° C. Complex febrile seizures are diagnosed when multiple seizures occur during the same illness, the seizures are prolonged (longer than 15 minutes), or the seizures have a focal component.
Febrile seizures are common, occurring in 2 to 5% of children. Thirty percent of children with a simple febrile seizure will have a recurrence; of these, half will have a third event.40 The younger the age at onset, the higher the likelihood of recurrence. Children with simple febrile seizures have a 2 to 3% chance for development of epilepsy (compared with a 1% rate of epilepsy in the general population). Those with complex febrile seizures have a significantly higher risk. Treatment with long-term anticonvulsants does not affect the later risk of epilepsy.41
Rectal diazepam (Diastat) is safe and effective for the termination of prolonged or repetitive febrile seizures and should be available for home use in the child with recurrent, prolonged febrile seizures.42 Long-term anticonvulsant use is rarely warranted.39 The use of antipyretics, such as acetaminophen and ibuprofen, does not decrease the likelihood of recurrence.43 Reassurance and education are the mainstays of management (see the section on management of febrile seizure and the discussion about lumbar puncture).
Neonatal Seizures: The frequency of seizures is higher in the first month of life than at any other time in childhood. Neonatal seizures may be subtle; apnea, sustained eye deviation, chewing, or limb bicycling movements may be the only apparent signs. There is a high incidence of subclinical electrographic seizures.44 Focal clonic movements are often associated with an underlying structural lesion.
Although there are many causes of neonatal seizures, a relatively few account for a majority of cases. These include hypoxic-ischemic encephalopathy, intracranial infection, congenital brain malformation, cerebrovascular events, and metabolic disturbances, particularly hypoglycemia and hypocalcemia (Box 175-5). Although inborn errors of metabolism are rare, early treatment can be lifesaving.
Neonatal seizures may predispose to the development of cognitive and behavioral difficulties and to an increased risk of epilepsy later in life, although the prognosis ultimately depends on the etiology of the seizure.44
Status Epilepticus: Convulsive status epilepticus is a true neurologic emergency; it is associated with high morbidity and mortality rates that increase with duration of the seizure activity. Status epilepticus is defined as greater than 30 minutes of either continuous seizure activity or repeated brief seizures without full recovery of consciousness between seizures. In clinical practice, any seizure lasting more than 5 minutes warrants intervention.45 Although the diagnosis of convulsive status epilepticus usually is obvious, the duration of the seizures often is underestimated because the intensity of the jerking tends to diminish with time. The importance of careful observation cannot be overstated.
Status epilepticus occurs significantly more frequently in children than in adults, particularly in those younger than 1 year.46 Febrile illness is by far the most common precipitant of status epilepticus in children. Medication change, toxic ingestion, idiopathic epilepsy, metabolic derangements, and congenital abnormalities are the next most common. Although morbidity and mortality rates are significantly lower than in adults, they are still significant at 30% and 4%, respectively.
Etiology of Seizures and Differential Considerations
The first task of the ED evaluation is to determine whether the event in question was truly a seizure. Box 175-6 lists disorders that may be mistaken for seizures.
Breath-holding spells occur in 4 to 5% of children, primarily between the ages of 6 and 18 months. They are triggered by pain or emotional upset. The child becomes pale or cyanotic and may lose consciousness. If so, the child is typically limp, but a brief period of clonic movements or opisthotonos may occur. The average attack lasts approximately 40 seconds.47
Etiology of Seizures in Children
After the diagnosis of seizure is made, the seizure type and the etiology should be determined. Seizures can be divided into three main etiologic categories: acute symptomatic, remote symptomatic, and idiopathic seizures (Box 175-7).
Acute Symptomatic Seizures: Fever is the most common cause of acute symptomatic seizures in children. Meningitis should always be considered in any patient with seizures and fever. The section below on febrile seizures further discusses this important issue. In general, a child whose mental status is normal both before and after the seizure is unlikely to have meningitis.
Post-traumatic seizures occur in as many as 15% of children after head injury. Impact seizures, occurring within 1 hour of a head trauma, are not associated with significant injury or with the later development of epilepsy; however, it may be difficult to distinguish these from seizures associated with intracranial injury, and a CT scan of the head without contrast enhancement is indicated. Early post-traumatic seizures (occurring within the first week of injury) may arise from cerebral edema or intracranial hemorrhage, laceration, or contusion. Phenytoin is effective in preventing early post-traumatic seizures but does not influence the development of epilepsy.48
Numerous drugs are known to cause seizures. Cyclic antidepressants, cocaine and other stimulants, antihistamines, and isoniazid are the most common agents of drug-induced seizures. Drugs that may be associated with seizures are summarized in Box 175-8. Seizures also may occur during drug withdrawal, usually within 48 hours of drug cessation. Drug withdrawal in the neonate is a significant issue. Withdrawal of benzodiazepines and barbiturates leads to an abstinence syndrome similar to ethanol withdrawal.
Reflex seizures are precipitated by a specific, identifiable stimulus. For example, flashing lights on television or video games may induce seizures in photosensitive children.49
Diagnostic Strategies
Events leading up to the attack: What was the patient doing at the time? Did the patient become confused or complain of dizziness, a bad smell, flashing lights, or anything else?
The event itself: Did the patient get stiff or limp? Did the patient shake? Was there a loss of consciousness? Did the eyes or head turn in one direction? Was there incontinence? Having the observer demonstrate the movements can be helpful.
Events directly after the attack: Was the patient lethargic or confused and for how long? Did the patient remember the event? The occurrence of postictal confusion, headache, or fatigue can help differentiate a seizure from a nonepileptic event.
Possible precipitants of seizures: Has there been recent fever, illness, rash, head trauma, drug use, or medication or supplement use?
Risk factors for epilepsy: Is there a past history of meningitis, head injury, febrile seizures, congenital anomalies, or developmental delay? a family history of epilepsy? presence of unusual color or number of birthmarks?
Previous history of abnormal movements, staring spells, or myoclonus: It is not uncommon for a patient with a first generalized tonic-clonic seizure to have underlying, undiagnosed absence epilepsy.
Management
The management of four categories of seizures—febrile seizures, afebrile seizures, neonatal seizures, and status epilepticus—is described next. Box 175-9 provides an overview of the approach to a child with a seizure.
Febrile Seizures
In general, a child with fever between 6 months and 5 years of age who presents after a brief seizure (<15 minutes) with a normal neurologic examination can be assumed to have had a simple febrile seizure.39 Blood and urine testing may be required as part of the work up for the fever, but is not required for work up of a febrile seizure. According to the newer AAP recommendations, lumbar puncture is not necessary in children older than 12 to 18 months in whom the clinical findings are not suggestive of meningitis. Lumbar puncture should be considered in children between 6 and 12 months of age whose vaccination status for H. influenzae type b or S. pneumoniae is incomplete and in children who have been pretreated with antibiotics.21 Lumbar puncture should be strongly considered in children younger than 6 to 12 months because signs of meningitis can be subtle in this age group. Electroencephalography and neuroimaging generally are not required.
Afebrile Seizures
Laboratory Studies.: Laboratory tests generally include determinations of calcium, glucose, urea, electrolytes, and magnesium.50 The results of such studies are rarely abnormal in the child who is neurologically normal after a brief seizure.51 If drug exposure is a possibility, toxicology screen is sent. Anticonvulsant levels are measured if applicable.
Radiography and Other Imaging.: Consensus is lacking in regard to the need for neuroimaging in children who arrive in the ED after a first seizure. Emergent imaging should be performed in a patient with new focal deficits, persistent altered mental status, recent trauma, persistent headache, or partial-onset seizures.52 Because a generalized convulsion may have begun as a focal onset seizure, it is crucial to obtain a careful history from both an eyewitness and the patient. Children with generalized unprovoked seizures and normal examination findings do not necessarily require acute imaging. At follow-up evaluation, further evidence, such as a focal abnormality on the EEG, may indicate a need for neuroimaging, which can be done at that time. Children with a history of epilepsy do not need neuroimaging unless there is a change in clinical status.
Special Procedures.: The EEG is the most important laboratory test for evaluation of the patient with an unprovoked seizure. It rarely needs to be performed in the acute situation unless nonconvulsive status epilepticus is suspected. The EEG helps determine the seizure type, specific epilepsy syndrome, and risk for recurrence.51 Transient postictal slowing may occur for several days after a seizure.
Treatment of Specific Causes of Acute Seizures.: Hypoglycemia in children older than 2 months is treated with an intravenous bolus of 10% dextrose, 2.5 mL/kg. Neonates are treated with 2 mL/kg of 10% dextrose solution. Severe symptomatic hyponatremia may be treated with the intravenous administration of 3% saline (2 mL/kg infused during 30 minutes) to raise the serum sodium level by 3 to 7 mEq/L. The remainder of the correction should occur slowly during the next 24 hours. Hypernatremia is corrected slowly during 48 hours. Hypocalcemia is treated with 10% calcium gluconate, 100 mg/kg intravenously; the patient should be on a cardiac monitor during the infusion. Toxic ingestions are treated based on the specific toxin involved. One specific example is the treatment of isoniazid poisoning with pyridoxine. The dose of pyridoxine is 1 g IV for every gram of INH ingested. When the quantity of INH ingested is unknown, 5 g IV may be administered to an adult and 70 mg/kg (maximum 5 g) to a child.
Empirical treatment with antibiotics is ordered if meningitis is a possibility. (see Table 175-3). The patient with altered mental status and focal seizures is treated additionally for herpes encephalitis with acyclovir, 20 mg/kg per dose (10 mg/kg per dose in children older than 12 years) every 8 hours. In patients with acute significant brain injury, such as depressed skull fracture or intracerebral hematoma, anticonvulsant treatment reduces the incidence of early seizure, and should be given. Although there is no consensus on the ideal drug, phenytoin is most commonly used as it does not cause significant sedation and is conveniently loaded intravenously. The optimal duration of therapy is not clear and depends in part upon the severity of injury.
Neonatal Seizures
The common underlying causes of neonatal seizures differ from those in older children and adults (see Box 175-5). The diagnostic assessment of neonatal seizures is detailed in Box 175-10. It includes metabolic testing, CSF analysis, and neuroimaging. Glucose, calcium, magnesium, and electrolyte levels and CBC are determined immediately. If findings are normal, lactic acid, ammonia, and pH determinations should be considered. As clinical assessment for meningitis is not reliable in young infants, lumbar puncture is performed and fluid sent for cells, protein and glucose determinations, culture, and herpes PCR assay. Head CT or MRI should be obtained. In the unstable neonate, a head ultrasound scan may be performed at the bedside to rule out a neurosurgical emergency, until more definitive imaging can be obtained.
Phenobarbital is typically the drug of choice for neonatal seizures. A loading dose of 20 mg/kg is given; additional doses of 5 to 10 mg/kg may be given if necessary. If seizures continue, fosphenytoin 20 PE/kg may be loaded. Lorazepam 0.1 mg/kg intravenously may be useful but is associated with hypotension and respiratory depression. Refractory seizures may be treated with midazolam infusion.34 Continuous electroencephalographic recording is necessary at this point. If seizures are refractory to medical treatment, empirical treatment with pyridoxine, 100 mg intravenously, is indicated. This will abort seizures in the rare child with pyridoxine dependency syndrome.
Status Epilepticus
Monitor heart rate, blood pressure, respiratory rate, and pulse oximetry and treat hyperthermia with antipyretics and cooling blankets. Place an intravenous line and send blood samples for electrolyte values, glucose concentration (including rapid blood glucose test), calcium and magnesium levels, renal function tests, liver function tests, antiepileptic levels (if indicated), and CBC. Urine should be sent for toxicology.53
Correct any metabolic abnormalities (see previous section on afebrile seizures). If necessary, an intraosseous line may be used.
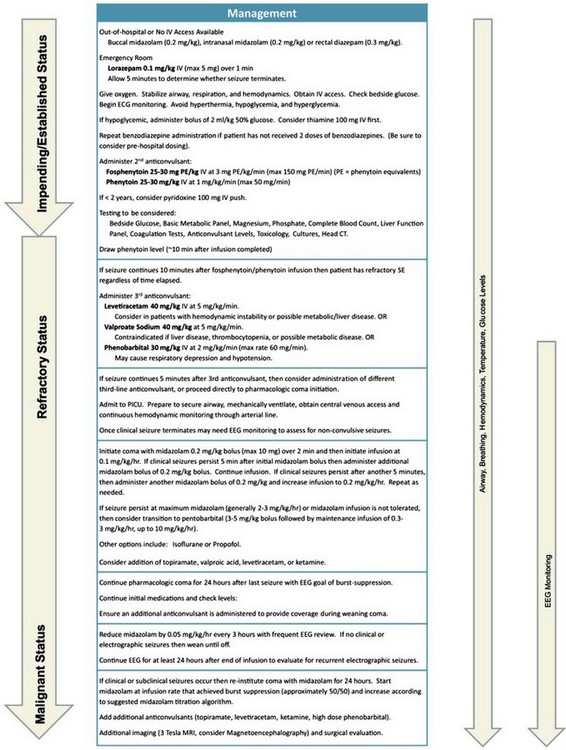
Begin anticonvulsant treatment as quickly as possible. Figure 175-1 presents a protocol for treatment of status epilepticus in a child.54 Benzodiazepines, particularly lorazepam and diazepam, are the initial drugs used in the treatment of status epilepticus. They diffuse quickly into the CNS, rapidly terminating seizure activity 70% of the time. The recommended pediatric intravenous dosage for lorazepam is 0.1 mg/kg (maximum 4 mg) over 1 minute. Hypotension, respiratory depression, and impaired consciousness may occur.
Rectal diazepam may be used if intravenous access cannot be obtained. The dosage is 0.5 mg/kg (maximum 20 mg). If Diastat, a rectal preparation of diazepam, is not available, the intravenous preparation may be instilled through a lubricated feeding tube inserted 4 to 6 cm into the rectum. Buccal and intranasal midazolam preparations at a dosage of 0.2 mg/kg are also effective.55
Midazolam is a safe and effective benzodiazepine used to treat status epilepticus that is refractory to conventional agents. A bolus of 0.2 mg/kg over 2 minutes is followed by infusion of 0.1 mg/kg per hour.56 The dose is increased every 15 minutes as necessary to a maximum of 3 mg/kg per hour. Tachyphylaxis may occur, necessitating high doses.
Propofol also has been shown to be highly effective in the treatment of refractory status. An initial intravenous bolus of 1 to 2 mg/kg is followed by infusion of 2 to 10 mg/kg per hour.57 Propofol is contraindicated in children on the ketogenic diet.
Nonconvulsive status epilepticus is more difficult to recognize and often requires electroencephalography for diagnosis. Treatment is with benzodiazepines or intravenous valproic acid.58
Once the seizure has been effectively terminated, neuroimaging and lumbar puncture are indicated.
Disposition
Children with simple febrile seizures can almost always be sent home. Warden and Hauser and their colleagues outlined advice to be given to families in the ED59,60 (Box 175-11).
Initiating Anticonvulsant Therapy
Two thirds of children with a first unprovoked seizure never experience a recurrence.60 The risk for recurrence is increased with the presence of neuroimaging or electroencephalographic abnormalities, developmental delay, family history of epilepsy, remote symptomatic seizure, occurrence of the first seizure during sleep, or Todd’s paralysis. If none of these risk factors is present, the 5-year recurrence risk is only 21%.
There is no evidence that early treatment with anticonvulsant medications after a single seizure alters the later risk of epilepsy,61 nor is there evidence to show that a single self-limited seizure causes neurologic sequelae.
In the past 20 years, several new anticonvulsants have been approved by the FDA. These include gabapentin, felbamate, lamotrigine, topiramate, zonisamide, oxcarbazepine, levetiracetam, vigabatrin, lacosamide, rufinamide, and clobazam. Their role in the management of pediatric epilepsy is becoming increasingly important. Table 175-4 provides a summary of the commonly used anticonvulsants in children.
Table 175-4
Summary of Commonly Used Anticonvulsants in Children
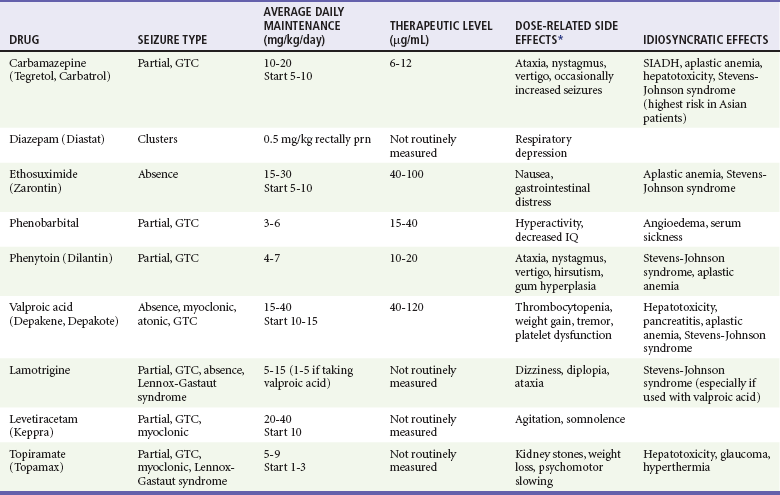
GTC, generalized tonic-clonic; SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
*Not including sedation and dizziness.
Modified from Flomin O, Nield L, Kamat D: Seizure medications: A review for the primary care pediatrician. Clin Pediatr 44:383, 2005; and Bourgeois BF: Broader is better: The ranks of broad-spectrum antiepileptic drugs are growing. Neurology 69:1734, 2007.
Headaches in Children
Principles of Disease
Several pathophysiologic theories have emerged to explain the mechanisms of headache. However, there is still ongoing debate as to whether the vascular component of migraines or neurogenic inflammation is the sole basis for the clinical expression of headache. The vascular theory proposes that headaches are produced by dilation of intracranial or extracranial arteries. The change in vessel diameter triggers the auras before and the pain during a headache; the dilation may be relieved by vasoconstrictive drugs, such as ergots. The neuronal theory proposes that headaches are due to a primary dysfunction of the brain; a wave of spreading neuronal depression is accompanied by decreased cerebral blood flow. Serotonin (5-hydroxytryptamine [5-HT]) may be a key mediator in this cascade of events. Serotonin agonists have been shown to relieve migraine pain. The trigeminovascular theory is an integrated hypothesis that combines elements of both theories. The trigeminal nerve makes synaptic connections with cerebral blood vessels, and the headache is an expression of the nerve–blood vessel interaction. Another theory that combines elements of the vascular and neuronal theories suggests that an initial electrical phenomenon beginning in the cerebrum initiates a cascade of events that affects the vasculature, with the release of biochemical substances leading to neurogenic inflammation.63,64
Clinical Features
Headaches can be classified into five temporal patterns: acute, acute recurrent, chronic progressive, chronic nonprogressive, and mixed. An acute headache is new in onset and different from previous headaches; it can herald a broad range of conditions ranging from a viral illness to a subarachnoid hemorrhage. Acute recurrent headaches can be expressed as periodic events separated by pain-free intervals. Chronic progressive headaches occur during weeks to months. They can signify serious medical disorders, such as brain tumors or arteriovenous malformations. Chronic nonprogressive headaches usually occur for years and are classified as primary headaches (as opposed to secondary symptomatic headaches). Mixed headaches are acute recurrent headaches (migraine) superimposed on a pattern of daily chronic nonprogressive headaches.63,65
The primary goal of the ED evaluation is to differentiate symptomatic headaches from primary headaches, such as migraine or tension headaches. The history is critical in investigating children’s complaints of headache and is the most important component of the evaluation to determine the correct diagnosis. Both the patient and family members should be asked about specific factors related to the headache. Rothner has created a patient questionnaire to generate a “headache database” from which clinicians can formulate a diagnosis, which is presented in Box 175-12.65
In addition to the important questions in Box 175-12, the clinician must focus on a detailed history of the neurologic system to identify any related symptoms (e.g., lethargy, ataxia, seizures, weakness, visual disturbances) and a general review of other organ systems. Warning signs of symptomatic headaches include recent onset, occurrence with straining or athletic endeavors, association with neurologic symptoms, change in headache pattern, nocturnal awakening, and bilateral occipital headaches. Additional information related to the past medical history (e.g., history of recent head trauma, neurologic or psychiatric disorders, hospital admissions, medications) also is important to obtain.
Differential Considerations and Headache Syndromes
The list of differential considerations for headache is extensive (Box 175-13).66
Chronic Progressive Headache
Headaches may be the first symptom of a brain tumor and as such occur with increasing frequency with age. In a study by Flores and colleagues, 18% of 0- to 5-year-olds, 52% of 6- to 10-year-olds, and 68% of 11- to 20-year-olds who had brain tumors presented with headaches. Only 38% of primary brain tumors were diagnosed within the first month after the onset of symptoms. More than 50% of these patients had at least three associated symptoms or signs, such as nausea, vomiting, visual effects, problems with walking, weakness, changes in personality or school performance, or speech changes. The most common neurologic findings were papilledema, abnormal eye movements, ataxia, abnormal tendon reflexes, and abnormalities on the visual examination.67
Clinical findings with pseudotumor cerebri (idiopathic intracranial hypertension) are secondary to the increased ICP and include papilledema, sixth cranial nerve palsy, and visual field obstruction. This condition is more common in females and in younger children is associated with obesity; presence of otitis media or head trauma; use of vitamin A, steroids, birth control pills, or tetracycline; presence of menstrual irregularities or diseases that produce obstruction of the CSF pathways; and obstruction of the major venous sinuses. By definition, neuroimaging findings are normal. The lumbar puncture usually demonstrates elevated pressure, often higher than 20 cm H2O, and normal CSF protein and glucose levels. Neuroimaging should precede lumbar puncture when increased ICP is suspected from mass or injury. Treatment can include diuretics and repeated lumbar puncture for removal of CSF.68 Hydrocephalus may be related to a previous episode of meningitis, subarachnoid hemorrhage, or head injury or may be congenital.
Migraine Headache
The diagnosis of migraine is based on symptoms of recurrent headaches separated by pain-free intervals. A revised classification of migraine syndromes has been produced for pediatric migraine (Box 175-14).69
Migraine with an aura, previously known as classic migraine, is diagnosed when at least two attacks fulfilling the diagnosis of migraine occur accompanied by a variety of sensory warning symptoms, such as flickering lights, loss of vision, and tingling or numbness. The symptoms develop during 5 minutes or more and completely resolve within 60 minutes.69,70
Diagnostic Strategies
Radiology
CT of the head and cranial MRI are both excellent studies to evaluate patients with headaches but are often not needed emergently.71 In general, MRI provides superior anatomic detail compared with a CT scan. MRI is particularly useful in the detection of abnormalities in the sella turcica, posterior fossa, and cervicomedullary junction. MRI also is better at detection of arteriovenous malformations and low-grade tumors. However, a patient cannot be closely monitored during the MRI study, and MRI is not always available in the ED. CT scan is superior to MRI in the detection of acute blood and skull fractures. Therefore, CT is usually the modality of choice in the ED if neuroimaging is required. However, most patients presenting with headache do not require neuroimaging. Indications for the use of neuroimaging are presented in Box 175-15.68
Management
Treatment of primary childhood headaches requires attention to initial pharmacologic management as well as reassurance, removal of potential triggering factors, and initiation of a behavioral management program.64,70,72,73,75,76 Nonmedical interventions may have some impact and should be strongly considered. These include avoidance of triggers, placement of the child in a darkened room with minimal or no extraneous noise, avoidance of hypoglycemia by feeding during a migraine, avoidance of caffeinated beverage (except for possible use as a migraine medication during an acute migraine), application of a cool compress on the forehead, use of a gentle fan, and breathing exercises and relaxation techniques.64 A headache diary is important to help the patient identify potential triggers and the effects of medications. Common triggers include reduction in sleep, perimenstrual stress, missed meals, and foods (such as chocolate, processed meats, alcohol, hard cheeses, red wine, monosodium glutamate, yeast extracts, nuts, figs, aspartame, and sauerkraut). Children with primary headaches are not hospitalized for care unless the diagnosis is uncertain and a serious cause of secondary headache is being considered.
Migraine
Treatment of migraine headache includes analgesics, vasoconstrictors, sedatives, triptans, antiemetics, anti-inflammatory agents, and other modalities.64,72,73,75,76 The patient should be placed in a quiet and safe environment. Simple analgesics and rest are the first line of treatment for patients with migraine as well as nonmigraine headache. Medications include acetaminophen (pediatric: oral or rectal, 10-15 mg/kg/dose po/pr q4-6h, maximum dosage 90 mg/kg/24 hours; adolescent: 325-650 mg/dose, maximum dosage, 4 g/24 hours) and the nonsteroidal anti-inflammatory drugs (aspirin, 10-15 mg/kg/dose, 650-1000 mg for patients 12 years of age and older, maximum dosage 4 g/24 hours; ibuprofen 5-10 mg/kg/dose q6-8h; or 400 mg/dose for those 12 years of age and older, maximum daily dose of 1200 mg per day; or naproxen 2.5-5.0 mg/kg/ dose q8-12h, 250-300 mg for those 12 years of age and older to a maximum daily dosage of 1000 mg/24 hours). Some children respond well to small doses of caffeine.
Vasoconstrictor drugs are used less commonly in the adolescent population than in the adult population. Antiemetic agents, although not as well studied in the pediatric as in the adult population, also may be considered. This group of dopamine receptor antagonists includes metoclopramide (in children older than 14 years: 10 mg intravenously × 1; children younger than 14 years: 0.1 to 0.2 mg/kg per dose intravenously), promethazine (in children: 0.25 to 0.5 mg/kg per dose intramuscularly or rectally every 4 to 6 hours as needed; in adults: 12.5 to 25 mg every 4 to 6 hours as needed), and prochlorperazine (in children weighing more than 10 kg or older than 2 years: 0.4 mg/kg per 24 hours orally or rectally, divided three to four times per day, or 0.1 to 0.15 mg/kg per dose intramuscularly, three to four times per day). Dystonic reactions with antiemetics are more common in children, so these agents should be used with caution in this population. Brousseau, in one of the few pediatric randomized clinical trials examining the efficacy of prochlorperazine compared with ketorolac for the treatment of acute migraine, found that by 60 minutes, a greater reduction in headache pain was obtained in the prochlorperazine group, even though 30% of patients in each group experienced some recurrence of headache symptoms.77 In older patients who may be treated for nausea without sedation, ondansetron (a serotonin receptor antagonist) can be considered (0.15 mg/kg per dose intravenously or 4 to 8 mg/day orally).
Sumatriptan is a selective 5-HT1 receptor agonist that can mediate cerebral vasoconstriction and block inflammatory response; it can be administered orally, intranasally, or subcutaneously. A review of medication trials of triptans in children with migraine highlighted the important finding that randomized clinical trials found no difference in outcome between the control and the experimental groups.72 However, nasal sumatriptan (5 to 20 mg, repeated in 2 hours, to a maximum daily dose of 40 mg) has been shown to be effective in the adolescent population, along with oral preparations of 25 to 50 mg (repeated in 2 hours, to a maximum daily dose of 200 mg).73 Sumatriptan should be avoided in persons with cardiac disorders or hypertension and in patients who have received an ergot alkaloid in the preceding 24 hours. Other newer 5-HT1 agents, such as zolmitriptan (Zomig) at doses of 2.5 mg intranasally and 5 mg orally compare favorably with sumatriptan. In a recent randomized clinical trial, the onset of significant pain relief was seen at 15 minutes asfter treatment with 5 mg of zolmitriptan nasal spray and at 1 hour produced a higher headache response rate compared with placebo (58.1% vs 43.3%).74 Cluster headaches, which are brief and may not respond to oral therapy, can be treated with inhalation of oxygen at 4 to 5 L/minute, the ergot alkaloid Cafergot, or sumatriptan.65 The most recently released triptan is a combination of sumatriptan and naproxen sodium (Treximet). It is approved for the treatment of migraine in adults only, although there is a recently completed adolescent clinical trial that could result in its approval.64
Pediatric Ataxia
Ataxia comes from the Greek word ataktos, meaning “lacking order,” and describes a pathologic abnormality of organization or modulation of movement.78
Clinical Features
Most children with ataxia are seen in the first few days after onset, usually because of a refusal to walk, unsteadiness of arm movements, or sudden development of a wide-based “drunken” gait. The history should identify any recent infection, injury, inadvertent drug ingestion, or other family members with the same problem. Mental status usually is normal in cases of postinfectious ataxia. If it is abnormal, the possibility of ingestion, acute disseminated encephalomyelitis, or stroke should be considered. Nystagmus is common if the cerebellum is affected. Papilledema or cranial nerve palsies suggest hydrocephalus or a central nervous system lesion.79
Differential Considerations
In children, 40% of ataxia cases are caused by acute cerebellar ataxia (Box 175-16). Boys are more commonly affected, with the highest incidence at the ages of 2 to 4 years. Seventy percent of patients have a history of recent illness with multiple causative agents, but varicella virus is the most common, with up to 26% of cases associated with varicella. Coxsackievirus and echovirus are other etiologic agents. The disease is thought to be due to an autoimmune phenomenon leading to cerebellar demyelination. Symptoms and signs are maximal at onset, with the extremities more seriously affected than the trunk, and range from unsteadiness and wide-based gait to complete inability to walk. Mental status is normal, and nystagmus is common. Fever and seizures are uncommon outcomes.79
Acute postinfectious demyelinating encephalomyelitis can also cause ataxia and occurs in the recovery phase of a viral illness or vaccination. It is distinguished from acute cerebellar ataxia by alteration in consciousness and multifocal neurologic deficits as well as by fever and the frequent occurrence of seizures. Brainstem encephalitis can involve the cerebellum, causing ataxia in association with focal neurologic abnormalities and respiratory irregularities. Etiologic agents include Epstein-Barr virus, L. monocytogenes, and enteroviruses.78,79
The opsoclonus-myoclonus syndrome consists of ataxia, rapid chaotic multidirectional eye movements, and myoclonic jerks of the extremities, head, trunk, and face. Most commonly, this is seen as a presenting manifestation of neuroblastoma or ganglioneuroblastoma in which the ataxia is thought to be due to a paraneoplastic autoimmune phenomenon involving cross-reactivity of tumor and cerebellar antigens. Spontaneous vertebral artery dissections also have been reported in children without a known history of trauma.78
Ataxia can be seen in patients with basilar migraine and can be associated with vertigo, hemiparesis, cranial nerve dysfunction, nausea, vomiting, or headache.78
Loss of sensory input to the cerebellum can cause a sensory ataxia. Clinical manifestations will include a Romberg sign, decreased deep tendon reflexes, and impaired proprioception and vibration sense. Fifteen percent of patients with Guillain-Barré syndrome have sensory ataxia. In the Miller Fisher variant of Guillain-Barré syndrome, the triad of ataxia, areflexia, and ophthalmoplegia of vertical gaze is characteristic.78
Friedreich‘s ataxia is a disorder of autosomal recessive inheritance characterized by progressive gait and limb disturbance. It is caused by mutation in the gene encoding a mitochondrial protein, frataxin, that results in loss of gene function. Affected patients demonstrate dysarthria, lower limb areflexia, proprioceptive sensory loss, and high-arched feet (pes cavus). Most patients are unable to walk by the age of 30 years. Death is most commonly due to cardiomyopathy, causing intractable congestive heart failure, and respiratory compromise from severe kyphoscoliosis.78
Ataxia-telangiectasia is a disorder of recessive inheritance caused by gene mutation manifested as a truncal ataxia in infancy that leaves most patients wheelchair bound by the age of 12 years. Oculocutaneous telangiectasias usually appear by the age of 3 to 5 years. These patients also demonstrate dysarthria, nystagmus, dystonic posturing, myoclonic jerks, and accelerated aging. They have deficient immunoglobulin A and decreased immunoglobulin E and M levels. They suffer from frequent sinus and pulmonary infections and have a 50- to 100-fold risk for development of leukemia and lymphoma. The mean age at death from this disorder is 20 years.80
Diagnosis
The key to making the diagnosis is to take a complete history and to perform a thorough physical examination. In particular, testing of cerebellar function can be accomplished by assessment of the child for dysmetria and dysdiadochokinesia.81 Urine and serum toxicologic studies are the laboratory tests most likely to prove diagnostic. CT and MRI findings usually are normal in patients with postinfectious ataxia, but demyelination, tumor, hydrocephalus, or traumatic injuries may be identified. CSF analysis may show mild pleocytosis or lymphocytosis in acute postinfectious ataxia; findings are normal in most other cases. Electroencephalography is recommended for patients with altered consciousness and fluctuating clinical signs. A pattern of epileptiform discharges or slowing is seen in 66% of children with acute cerebellar ataxia. Electroencephalography also can diagnose nonconvulsive or convulsive seizures. The electroencephalographic pattern is normal in the opsoclonus-myoclonus syndrome. Electromyography may be helpful if sensory ataxia is suspected and may help diagnose Guillain-Barré syndrome. Urinary catecholamines can be assayed for diagnosis of neuroblastoma. Testing for newborn errors of metabolism includes a CBC; liver function tests; measurement of blood ammonia, lactate, pyruvate, and ketone levels; and determination of acid-base status. Other tests include plasma and urinary amino acid assays, urine organic acid assay, determination of serum biotinidase level, and CSF lactate assays. Genetic or other specialized testing also may be required. In the absence of developmental delay or positive family histories, however, these investigations for inborn errors of metabolism are unlikely to be helpful.81
Pediatric Vertigo
Diagnostic Strategies
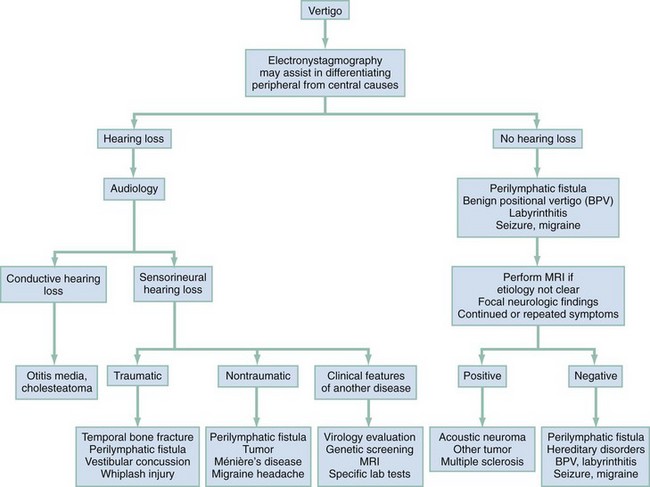
For determination of the cause of vertigo, patients can be divided into those who have hearing loss and those who have normal hearing. In the group with hearing loss, further characterization of the loss as conductive or sensorineural in character and identification of the presence of trauma, and whether the causative disorder involves the CNS or the peripheral nervous system from the labyrinth or eighth cranial nerve, will help in diagnosis (Fig. 175-2).
Differential Considerations
Although vertigo is not as common in the pediatric age group as in adults, it has many potential causes (Box 175-17).82 It usually is helpful to separate those conditions that cause vertigo into those with and those without associated hearing loss.
Benign paroxysmal vertigo of childhood is defined by the repeated occurrence of vertiginous episodes lasting seconds to minutes, with occasional vomiting. This entity usually remits spontaneously within months to years. The most frequent cause of benign paroxysmal vertigo of childhood is a migraine headache,80 with vertigo occurring as the aura of an episode.
Ménière’s disease, a syndrome with a combination of vertigo, fluctuating hearing loss, and tinnitus, is responsible for 1.5 to 4% of cases of pediatric vertigo.83,84
Vestibular neuronitis is manifested with vertigo without hearing loss. A viral infection is thought to be the cause. Sixty percent of patients have a preceding cold. It is manifested with severe vertigo that resolves in a few days, after which the child will have vertigo only with rapid head movements, which persists for weeks or months until central compensation occurs.85
Labyrinthitis is an inflammatory process involving the inner ear membranous labyrinth. It is manifested with vertigo, hearing loss, and tinnitus. Viruses such as cytomegalovirus, rubella virus, and rubeola virus are etiologic agents. Bacterial labyrinthitis also can occur, usually in association with meningitis. Lyme disease and syphilis may cause vertigo if the inner ear is affected.86 Neurofibromatosis can be manifested with vertigo if it involves the superior vestibular nerve; café au lait spots should be looked for. Other genetic syndromes also are associated with vertigo (see Box 175-17).86
Ototoxic drugs, such as aminoglycosides and chemotherapeutic drugs, can be a cause of vertigo, usually in association with hearing loss (Box 175-18). Cerebellar and brainstem lesions on occasion also are manifested with vertigo. Cranial nerve deficits associated with vertigo may indicate a lesion or tumor.85,86
In post-traumatic vertigo, disturbances of cochlear and vestibular functions are the most common etiology of late-onset vertigo from head injury in children. If labyrinthine damage is caused by trauma (labyrinthine concussion), vertigo, gait unsteadiness, and swaying toward the affected side are present along with nausea and vomiting. Symptoms and signs subside during a period of 4 to 6 weeks. Signs of vestibular dysfunction may be present many years after the injury. Positional nystagmus with the quick component toward the uninvolved side usually is present. Vestibular concussions can cause vertigo without hearing loss after a blow to the parietal-occipital or temporal-parietal region. It normally is continuous for several days, followed by improvement, but it may recur intermittently with certain head positions. Displacement of an otolith is thought to be responsible.87
Temporal bone fracture (especially transverse) may cause vertigo by injury to the eighth cranial nerve or the otic capsule; it also may cause sensorineural hearing loss.87 Whiplash injuries to the neck also can cause vertigo; up to 50% of patients experience hearing loss or tinnitus. Post-traumatic migraine and seizures also can cause vertigo and occur in 5 to 7% of patients with closed head injuries.88
Perilymphatic fistula is manifested with sensorineural hearing loss of sudden onset. It frequently is associated with congenital cranial deformities and congenital abnormalities of the ear. However, barotrauma (flying, extreme coughing, retching, or straining) or direct trauma also can be a cause. There is leakage of fluid from the oval or round window into the middle ear. Having the patient perform a Valsalva maneuver may precipitate vertigo. Hennebert’s sign, which consists of nystagmus and vertigo after negative pressure is induced in the ear by a pneumatic otoscope, may be a feature.85 Treatment usually consists of surgical repair.
Ocular disorders, such as convergence insufficiency, strabismus, and amblyopia, have been reported to cause a sensation of vertigo. Treatment of these disorders will eliminate the vertigo in children.87
Diagnostic Strategies
Vertical nystagmus is usually from a central cause. Nystagmus from peripheral causes tends to be extinguished or to diminish after rapidly repeated stimulation, whereas central nystagmus does not. Helpful laboratory tests in the vertiginous patient include glucose and electrolyte assessment, thyroid function tests, and viral titers or serologic studies (e.g., for Lyme disease or syphilis), as the physical examination dictates.86 The Hallpike-Dix positioning maneuver may help diagnose benign positional vertigo.85 A CT or MRI scan is indicated only in cases of central vertigo.
Management
Management of the vertiginous patient depends on the underlying cause, which may not be evident in the ED.89 For acute symptomatic relief, vestibular suppressants such as meclizine and diazepam may be helpful. Otoliths causing inappropriate stimulation may be restored to their proper place by Epley’s maneuver.82 Vestibular rehabilitation exercises that encourage patients to move the head while viewing a stimulus may assist in vestibular adaptation and reduce symptoms. Follow-up, including examination, testing, and treatment by a neurologist or an otolaryngologist for vertiginous patients, is recommended.90 Highly symptomatic children may need to be admitted to the hospital.
Syncope
Syncope is defined as a sudden, reversible, typically brief loss of consciousness and muscle tone (between 20 and 60 seconds) with spontaneous and complete recovery after a brief duration. Presyncope is defined as having a sensation of passing out but remaining conscious with a transient loss of postural tone. The exact incidence of syncopal episodes is not known, but 15 to 25% of children and adolescents experience one episode by young adulthood.91 The peak incidence occurs between 15 and 19 years of age, and females are affected more frequently than males. The presentation of these events can be frightening to the patient and those who witness them. Gordon and co-workers found that in a group of pediatric patients presenting to the ED, a mean of six diagnostic tests were performed per patient, 40% of these patients were admitted to the hospital, and total charges averaged $3000 per patient, yet significant pathologic conditions were found in less than 10% of the patients.92
Clinical Features
Patients can experience syncope during intercurrent illnesses, with mild dehydration, during menstruation, or after vigorous exertion. In those with situational syncope, typical triggers may include emotion, pain, hyperventilation, shower, cough, swallowing, urination, or defecation. Before the event, they may experience dizziness, lightheadedness, weakness, nausea, chest discomfort, palpitations, blurred vision, headache, tremulousness, and anxiety. After the event, loss of consciousness is brief (usually for less than 20 seconds), and the patient is described as ashen appearing, with sweating and coolness of the extremities.93 Other examples of conditions associated with neurally mediated syncope are postural orthostatic tachycardia syndrome, chronic fatigue syndrome, and pandysautonomia.94 Patients with postural orthostatic tachycardia syndrome and pandysautonomia experience orthostatic palpitations rather than straightforward syncope, do not appear healthy between events, and complain of fatigue and exercise intolerance. Patients with pandysautonomia also exhibit thermoregulatory impairment, sweating, and bowel dysfunction. Although cardiac causes of syncope are rare, they can be life-threatening and should be suspected when the episode occurred during exercise or physical activity. Noncardiac causes include seizures. Helpful features to differentiate seizures from syncope include the following: seizures usually occur in a supine rather than in an upright position; convulsions occur before rather than after the loss of consciousness; and warm, flushed, or cyanotic-appearing skin, rather than pale and sweaty skin, is characteristic.
Diagnosis
Evaluation of the patient begins by obtaining a description of the event, previous episodes, and past personal and family history. A detailed description of the event and particular note of any presyncopal symptoms or signs, such as sweating, nausea, pallor, visual changes, or onset with a rapid change in position, can suggest vasovagal syncope. A thorough physical examination including a detailed neurologic examination is essential. A baseline electrocardiogram should be obtained to rule out structural heart disease or an arrhythmia. If the history, physical examination, and electrocardiogram are typical for a neurally mediated event, further testing is not usually required. Additional testing is warranted with family history of sudden death, early infarction, or arrhythmia; previous personal history of fatigue with a known arrhythmia or heart defect; syncope preceded by chest pain or palpitations; occurrence of syncope during exercise; occurrence of syncope without a prodrome; recurrent syncope (more than two or three episodes); and a syncopal event with neurologic sequelae.95
Management and Disposition
Syncope is a common pediatric problem and usually has a benign, neurally mediated cause. Patients with a potential serious cause, such as a cardiac or neurologic condition, should be treated and admitted.95 Certain groups of patients may have an increased risk for cardiac syncope and should eventually be referred to a pediatric cardiologist for evaluation. These include athletes, patients with eating disorders or chronic fatigue, persons who use illicit substances, and survivors of congenital heart disease. Athletes with suspected hypertrophic cardiomyopathy, aortic stenosis, or other potentially critical cardiac conditions should be restricted from exercise until they are evaluated by a cardiologist.91
The approach to management of patients with neurocardiogenic syncope is primarily one of education, involving avoidance of potential triggering events (hot, crowded environments; volume depletion) and recognition of the early signs and symptoms to allow timely maneuvers to abort the syncopal episode (e.g., assumption of a supine position, ingestion of fluids, leg crossing). The patient and the family should be reassured that these events are not life-threatening. A simple approach is to encourage liberal fluid intake and to increase dietary salt intake. In a subgroup of patients with recurrent syncope or recurrent dizziness, however, a trial of an appropriate medication may be warranted. No large pediatric randomized studies have been conducted to investigate this application of such medications, which include beta-blockers, fludrocortisone, selective serotonin reuptake inhibitors, and alpha-agonists.96
References
1. Saez-Lorens, X, McCracken, GH. Bacterial meningitis in children. Lancet. 2003;361:2139.
2. Thigpen, MC, et al. Bacterial meningitis in the United States 1998-2007. N Engl J Med. 2011;364:2016.
3. Kaplan, SL. Clinical manifestations, diagnosis, and prognostic factors of bacterial meningitis. Infect Dis Clin North Am. 1999;13:579.
4. Grimwood, K, et al. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics. 1995;95:646.
5. Peters, ML. Suspected meningitis in the emergency department: Diagnosis and management. Clin Pediatr Emerg Med. 2003;4:186.
6. Peters, TR, Poehling, KA. Invasive pneumococcal disease: The target is moving. JAMA. 2007;297:1825.
7. Bryant, KA, et al. Safety and immunogenicity of a 13 valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866.
8. Kim, KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10:32.
9. Bundy, DG. Several clinical signs and symptoms are associated with the likelihood of bacterial meningitis in children; the most reliable diagnostic combination is uncertain. Evid Based Med. 2011;16:89.
10. Bonadio, WA. Assessing patient clinical appearance in the evaluation of the febrile child. Am J Emerg Med. 1995;13:321.
11. Baker, MD, Bell, LM. Unpredictability of serious bacterial infection in febrile infants from birth to one month of age. Arch Pediatr Adolesc Med. 1999;153:508.
12. Arango, CA, Rathore, MH. Neonatal meningococcal meningitis: Case reports and review of literature. Pediatr Infect Dis J. 1996;15:1134.
13. McIntosh, D, et al. Torticollis and bacterial meningitis. Pediatr Infect Dis J. 1993;12:160.
14. Amarilyo, G, et al. Diagnostic accuracy of clinical symptoms and signs in children with meningitis. Pediatr Emerg Care. 2011;27:196–199.
15. Walsh-Kelly, C, et al. Clinical predictors of bacterial versus aseptic meningitis in childhood. Ann Emerg Med. 1992;21:910.
16. Kellogg, JA, et al. Frequency of low level bacteremia in infants from birth to 2 months of age. Pediatr Infect Dis J. 1997;16:381.
17. Schelonka, RL, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129:275.
18. Baker, MD, Avner, JR, Bell, LM. Failure of infant observation scales in detecting serious illness in febrile, 4- to 8-week-old infants. Pediatrics. 1990;85:1040.
19. Jaskiewicz, JA, et al. Febrile infants at low risk for serious bacterial infection: An appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94:390.
20. Korones, DN, Shapiro, ED. Occult pneumococcal bacteremia: What happens to the child who appears well at reevaluation? Pediatr Infect Dis J. 1994;13:382.
21. Subcommittee on Febrile Seizures, American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127:389.
22. Mandl, KD, Stack, KM, Fleisher, GR. Incidence of bacteremia in infants and children with fever and petechiae. J Pediatr. 1997;131:398.
23. Cnota, JF, Barton, LL, Rhee, KH. Purpura fulminans associated with Streptococcus pneumoniae infection in a child. Pediatr Emerg Care. 1999;15:187.
24. Shetty, AK, et al. Fatal cerebral herniation after lumbar puncture in a patient with normal CT scan. Pediatrics. 1999;103:1284.
25. Abo, A, et al. Positioning for lumbar puncture in children evaluated by bedside ultrasound. Pediatr Emerg Care. 2010;125:e1149–e1153.
26. Bonadio, WA. The cerebrospinal fluid: Physiologic aspects and alterations associated with bacterial meningitis. Pediatr Infect Dis J. 1992;11:423.
27. Nigrovic, LE, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;52:297.
28. Rorabaugh, ML, et al. Aseptic meningitis in infants younger than 2 years of age: Acute illness and neurologic complications. Pediatrics. 1993;92:206.
29. Chandy, JC. Treatment failure with the use of a third-generation cephalosporin for penicillin-resistant pneumococcal meningitis: Case report and review. Clin Infect Dis. 1994;18:188.
30. American Academy of Pediatrics. Pneumoccocal infections. In Red Book: 2009 Report of the Committee on Infectious Diseases, 39th ed, Elk Grove Village, Ill: American Academy of Pediatrics; 2009:524.
31. Friedman, MJ, Sharieff, GQ. Seizures in children. Pediatr Clin North Am. 2006;53:257.
32. Volpe, JV. Neonatal seizures. In Volpe JV, ed.: Neurology of the Newborn, 3rd ed, Philadelphia: WB Saunders, 1995.
33. Homes, GL. Do seizures cause brain damage? Epilepsia. 1991;32:514.
34. Levene, M. The clinical conundrum of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2002;86:F75.
35. Mackay, M, Weiss, S, Snead, OC, 3rd. Treatment of infantile spasms: An evidence-based approach. Int Rev Neurobiol. 2002;49:157.
36. Glauser, TA, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790–799.
37. Peters, JM, Camfield, CS, Camfield, PR. Population study of benign rolandic epilepsy: Is treatment needed? Neurology. 2001;59:476.
38. Hancock, E, Cross, H. Treatment of Lennox-Gastaut syndrome. Cochrane Database Syst Rev. (3):2003.
39. Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures, American Academy of Pediatrics. Febrile seizures: Clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2011;127:389–394.
40. Sadleir, LG, Scheffer, IE. Febrile seizures. BMJ. 2007;334:307.
41. Shinnar, S, Glauser, TA. Febrile seizures. J Child Neurol. 2002;17S:S44.
42. Dreifuss, FE, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338:1869.
43. Warden, CR, et al. Evaluation and management of febrile seizures in the out-of-hospital and emergency department settings. Ann Emerg Med. 2003;41:215.
44. Booth, D, Evans, DJ. Anticonvulsants for neonates with seizures. Cochrane Database Syst Rev. (4):2004.
45. Appleton, RC, Martland, TP, Scott, R, Whitehouse, W. The treatment of convulsive status epilepticus in children. The Status Epilepticus Working Party. Arch Dis Child. 2000;83:415.
46. Riviello, JJ, Jr., et al. Practice parameter: Diagnostic assessment of the child with status epilepticus (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:1542.
47. Dimario, FJ. Breath-holding spells in childhood. Am J Dis Child. 1992;146:125.
48. The Brain Trauma Foundation, the American Association of Neurological Surgeons, the Joint Section on Neurotrauma and Critical Care. Role of antiseizure prophylaxis following head injury. J Neurotrauma. 2001;17:549.
49. Ritaccio, AL. Reflex seizures. Neurol Clin. 1994;12:57.
50. Scott, RC, Kirkham, FJ. Clinical update: Childhood convulsive status epilepticus. Lancet. 2007;370:724.
51. Hirtz, D, et al. Practice parameter: Treatment of the child with a first unprovoked seizure: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2003;60:166–175.
52. Hirtz, D, et al. Practice parameter: Evaluating a first nonfebrile seizure in children: Report of the Quality Standards Subcommittee of the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society. Neurology. 2000;55:616–623.
53. Scott, RC, Kirkham, FJ. Clinical update: Childhood convulsive status epilepticus. Lancet. 2007;370:724.
54. Abend, ND, Gutierrez-Colina, AM, Dlugos, DJ. Medical treatment of pediatric status epilepticus. Semin Pediatr Neurol. 2010;17:169.
55. Wolfe, TR, Macfarlane, TC. Intranasal midazolam therapy for pediatric status epilepticus. Am J Emerg Med. 2006;24:343.
56. Lowenstein, DH, Alldredge, BK. Status epilepticus. N Engl J Med. 1998;338:970.
57. Stecker, MM, et al. Treatment of refractory status epilepticus with propofol: Clinical and pharmacokinetic findings. Epilepsia. 1998;39:18.
58. Aldenkamp, A, Vigevano, F, Arzimanoglou, A, Covanis, A. Role of valproate across the ages: Treatment of epilepsy in children. Acta Neurol Scand Suppl. 2006;184:1.
59. Warden, CR, et al. Evaluation and management of febrile seizures in the out-of-hospital and emergency department settings. Ann Emerg Med. 2003;41:215.
60. Hauser, WA, et al. Risk of recurrent seizures after two unprovoked seizures. N Engl J Med. 1998;338:429.
61. Musicco, M, Beghi, E, Solari, A. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology. 1997;49:991.
62. Reference deleted in proofs.
63. May, A, Goadsby, PJ. The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cerebr Blood Flow Metab. 1999;19:115.
64. Lewis, KS. Pediatric headache. Semin Pediatr Neurol. 2010;17:224.
65. Lewis, DW, Rothner, DA. Headache in children and adolescents. In Johnson RT, Griffin JW, McArthur JC, eds.: Current Therapy in Neurological Diseases, 6th ed, Philadelphia: Mosby, 2002.
66. Rothner, DA. Headaches. In: Swaiman KF, Ashwal S, eds. Pediatric Neurology: Principles and Practice. 3rd ed. St. Louis: Mosby; 1999:747–758.
67. Flores, LE, et al. Delay in the diagnosis of pediatric brain tumors. Am J Dis Child. 1986;140:684.
68. Qureshi, F, Lewis, D. Managing headache in the pediatric emergency department. Clin Pediatr Emerg Med. 2003;4:159.
69. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. Cephalgia. 2004;24(Suppl 1):1.
70. Hershey, AD, et al. Headaches. Curr Opin Pediatr. 2007;19:663.
71. Lewis, DW, et al. Practice parameter: Evaluation of children and adolescents with recurrent headache. Neurology. 2002;59:490.
72. Lewis, D, et al. Practice parameter: Pharmacologic treatment of migraine headache in children. Report of the American Academy of Neurology Quality Standards Committee and Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215.
73. Walker, DM, Teach, SJ. Emergency department treatment of primary headaches in children and adolescents. Curr Opin Pediatr. 2008;20:248.
74. Lewis, DW, et al. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics. 2007;120:390.
75. Richer, LP, et al. Treatment of children with migraine in emergency departments: National practice variation study. Pediatrics. 2010;126:e150.
76. Winner, P, et al. A randomized, double blind, placebo controlled study of sumatriptan nasal spray in the acute treatment of acute migraine in adolescents. Pediatrics. 2000;106:989.
77. Brousseau, DC, et al. Treatment of pediatric migraine headaches: A randomized double blind trial of prochlorperazine versus ketorolac. Ann Emerg Med. 2004;43:256.
78. Ryan, M, Engle, E. Acute ataxia in childhood. J Child Neurol. 2003;18:309.
79. Adams, C, et al. Autoantibodies in childhood post-varicella acute cerebellar ataxia. Can J Neurol Sci. 2000;27:316.
80. Paulson, H, Ammache, Z. Ataxia and hereditary disorders. Neurol Clin. 2001;19:759.
81. Avery M, First L, eds. Pediatric Medicine. 2nd ed. Baltimore:Williams & Wilkins; 1993:785–787.
82. Rudolph C, Rudolph A, eds. Rudolph’s Pediatrics. 21st ed. New York:McGraw-Hill; 2003:1246–1247.
83. Weisleder, P, Fife, TD. Dizziness and headache: A common association in children and adolescents. J Child Neurol. 2001;16:727.
84. Balkany, TJ, Finkel, RS. The dizzy child. Ear Hearing. 1986;7:138.
85. Tusa, R, et al. Dizziness in childhood. J Child Neurol. 1994;9:261.
86. Papp, J, Dorsey, S. A preschool age child with first-time seizure and ataxia. J Emerg Med. 2009;36:30.
87. Meyerhoff, W, et al. Ménière’s disease in children. Laryngoscope. 1978;88:1504.
88. Akagi, H, et al. Ménière’s disease in children. J Pediatr Otorhinolaryngol. 2001;6:259.
89. Eviatar, L. Dizziness in children. Otolaryngol Clin North Am. 1994;27:557.
90. Anoh-Tanon, MJ, et al. Vertigo is an underestimated symptom of ocular disorders: Dizzy children do not always need an MRI. Pediatr Neurol. 2000;23:49.
91. Fischer, J, Cho, C. Pediatric syncope: Cases from the emergency department. Emerg Med Clin North Am. 2010;28:501.
92. Gordon, TA, et al. A retrospective analysis of the cost effective workup of syncope in children. Cleve Clin J Med. 1987;54:391–394.
93. Driscoll, DJ, Jacobsen, SJ, Porter, CJ, Wollan, PC. Syncope in children and adolescents. J Am Coll Cardiol. 1997;29:1039.
94. DiVasta, A, Alexander, M. Fainting freshmen and sinking sophomores: Cardiovascular issues of the adolescent. Curr Opin Pediatr. 2004;16:350.
95. Ouyang, H, Quinn, J. Diagnosis and evaluation of syncope in the emergency department. Emerg Med Clin North Am. 2010;28:471.
96. Johnsrude, CL. Current approach to pediatric syncope. Pediatr Cardiol. 2000;21:522.