15 Neuroimmune functional interactions
Overview of the immune system
The immune system is a complex system of interacting components including physical barriers, bone marrow, lymphoid tissues, leukocytes, and soluble mediators. These elements function together to recognise, engulf, and destroy invading microbes, tumour cells, and any substance recognised as non-self. For the immune system to mount an effective response to invading antigens an intricate series of cellular events must occur. The antigen must be recognised and, if deemed necessary, bound and processed by antigen-presenting cells, which must then communicate with activated T and B cells. The T-helper cells must then assist in the activation and formation of B cells and cytotoxic T cells. Activated cells must then undergo a series of proliferative steps that involve activation of second and third messengers and selective genetic proliferation that result in an adequate response to the antigen presenting. Once an antigen has presented, a memory cell must be produced to enable a more efficient and deadly defence should the antigen present again in the future (Roitt 1994). To further complicate matters, all of these complex activities must be accomplished in a controlled and selective manner so as not to destroy cells or tissues not contaminated or of use to the host.
Barriers resisting infection
The simplest way for an organism to avoid infection or invasion by a foreign antigen is to prevent entry being gained into their body in the first place. Humans are no exception. In humans, the major physical barrier of defence is the skin, which, when intact, is virtually impermeable to most infectious agents (Roitt 1994). In addition, a large variety of microorganisms cannot survive long on the skin due to the low pH which results from the presence of lactic acid, and fatty acids in the sweat (Abbas et al. 1997).
Mucous secreted by the membranes lining the inner surfaces of the body acts as a protective barrier that blocks the adhesion of bacteria to epithelial cells. Other microbes become trapped in the mucous and are removed via the mechanical action of coughing, sneezing, or swallowing (Roitt 1994).
Many secreted body fluids contain bactericidal components such as acid in gastric juice, spermine and zinc in semen, and lactoperoxide in milk. The washing action of tears and saliva which both contain lysozyme is also a barrier to microbial invasion (Youmans 1980). Finally, the normal bacterial flora of the body acts as a form of microbial antagonism, which is effective in suppressing the growth of many pathologic bacteria and fungi (Sommers 1980).
Cells of the immune system
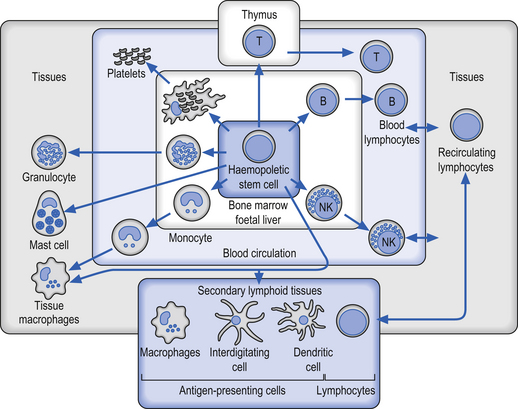
Although all of the components of the immune system must function in a multifactorial interactive process in order to function effectively, the most crucial cell types involved are the leukocytes or white blood cells (WBCs), which form the mobile foot soldiers of the immune system (Fig. 15.1). Leukocytes normally account for about 1% of total blood volume. In normal circumstances the WBC number between 4000 and 11 000 per cubic millimetre of blood, with an average of 7000 (Marieb 1995; Guyton & Hall 1996).
Immunology definitions
• Neutrophils—are mobile phagocytic cells that engulf and destroy unwanted matter.
• Eosinophils—secrete chemicals that destroy parasites and are involved in allergic reactions.
• Basophils—are also involved in allergic reactions.
• B lymphocytes—transform into plasma cells to secrete antibodies and prepare foreign matter for destruction indirectly.
• T lymphocytes—are involved in cell-mediated immunity to directly destroy cells by non-phagocytic means that have been damaged by viruses or mutations.
• Macrophages—are derived from circulating monocytes and become localised phagocytic specialists.
• Non-specific immune responses—include inflammation, interferon, NK cells, and the complement system. These operate even when there has been no previous exposure to an offending material.
• Specific immune responses—include antibody-mediated immunity by B cells and cell-mediated immunity by T cells.
Neutrophils, or polymorphonuclear leukocytes, are derived from pleuripotent haematopoietic stem cells and eventually differentiate from myeloid cells in the bone marrow. Neutrophils are short-lived cells with a lifespan of hours to days, but are present in large numbers in the bone marrow, peripheral blood, and marginal pool, which is a reserve of cells adherent to the walls of postcapillary venules. These cells are crucial to the host defence against bacteria and some fungi. Neutrophils and monocytes can move from the bloodstream into the tissues by a process called diapedesis. In this process the leukocytes squeeze through tiny pores in the vessel walls by assuming the size and shape of the pores. Once in the tissues the cells move around by amoeboid-like motion (Guyton & Hall 1996).
Monocytes are derived from myeloid precursor cells in the bone marrow, which migrate through the circulation to the tissues where they mature as macrophages. Monocytes have very little contribution to immunity until they have matured into macrophages. Often, in people who are actively fighting a serious infection, the numbers of monocytes in the blood will increase but have little involvement in the immunological processes until they are activated and mature into macrophages. Macrophages are highly mobile and are actively phagocytic. These cells have lifespans ranging from months to years depending on how often and to what severity they are called upon to fight antigens (Guyton & Hall 1996). These cells have three important immunological roles:
1. They process antigens and present the essential cell membrane fragments of partially digested antigens, called epitopes, to lymphocytes which then initiate the process of cell-mediated immunity against the antigen.
2. They secrete many immunologically active substances such as cytokines, complement, and prostaglandins.
3. They are themselves activated by T lymphocytes to phagocytose bacteria and intercellular parasites.
Lymphocytes are the primary cells of the cellular immune response. These cells originally derive from pluripotent stem cells in the bone marrow and eventually differentiate into T cells, B cells, non-T cells, and non-B cells in the various lymphoid tissues of the body. Lymphocytes develop in the thymus and populate the germinal centres in the lymph nodes and spleen. Although there are large numbers of lymphocytes in the body very few are present normally in the peripheral blood. Usually the only lymphocytes present in the blood are those travelling to a specific lymphoid tissue or those travelling to the site of an infection. About 80% of the lymphocytes present in peripheral blood are T cells, which have many important functions including (Simon 1991):
1. The regulation of the immune response;
2. The production of lymphokines;
There are three major populations of T cells that are antigen-bearing: helper T cells, cytotoxic T cells, and suppressor T cells. Both the helper and suppressor T cells are involved in the regulation aspect of the immune response, mainly the initiation and termination, respectively. Recent understanding of the structural differences in the membrane glycoproteins of these cells has led to a new classification system. CD4 or T4 cells express a specific glycoprotein structural receptor on their membranes specific for primary helper T cells. Two classes of helper T cells have also been distinguished and are referred to as Th1 and Th2 classes. These cells show different levels of activation and cytokine production that regulates the shift between cellular and humeral immunity processes (see below). The CD4 receptor moiety is the suspected attachment site for the HIV virus, which exclusively targets helper T cells. CD8 or T8 cells express a specific glycoprotein structural receptor on their membranes specific for both cytotoxic and suppresser T cells populations (Marieb 1995).
B lymphocytes develop in the bone marrow and undergo a secondary differentiation when exposed to an antigen to become non-dividing plasma cells which secrete immunoglobulins or antibodies. Plasma cells develop an elaborate intercellular rough endoplasmic reticulum which is capable of secreting huge amounts of antibody. Non-T, non-B cells do not carry the surface marker glycoproteins of either T or B cells. The major cell type of this class is the natural killer cells, which are capable of killing a large variety of non-specific targets without the presence of antibody or without the prior sensitisation of antibodies present (Simon 1991). These cells are augmented by interferons, which are a family of broad-spectrum antiviral agents synthesised by cells when they become infected with a viral agent (Heaney & Golde 1998).
Innate and specific immunity
1. Physical and chemical barriers; and
2. Blood proteins including compliment and mediators of inflammatory neutrophils, macrophages, and natural killer cells (Abbas et al. 1997).
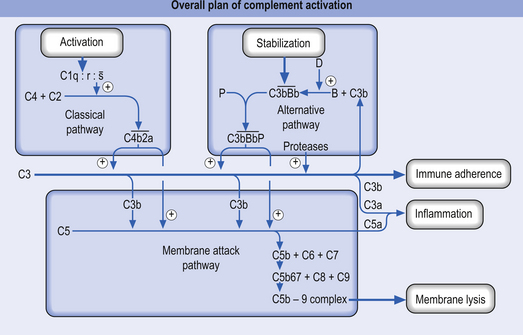
The complement system is a collection of a variety of proteins (approximately 20) present in the plasma and paracapillary tissue spaces. Many of these proteins exist in the form of precursors that can activate a cascade of reactions that terminate in the death or destruction of a target pathogen (Fig. 15.2). In normal circumstances the precursors remain inactive in the plasma unless they are activated in one of two ways:
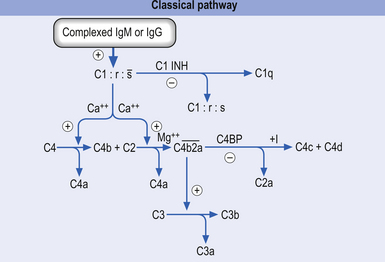
1. The classical activation pathway—initiated by antigen antibody binding. When the antibody binds an antigen it undergoes a change in its structure that results in the activation of the C1 precursor protein of complement. C1 activation results in a feedforward cascade that amplifies as it progresses so that a small initial stimulus results in a much larger reaction with the formation of multiple end products (Fig. 15.3).
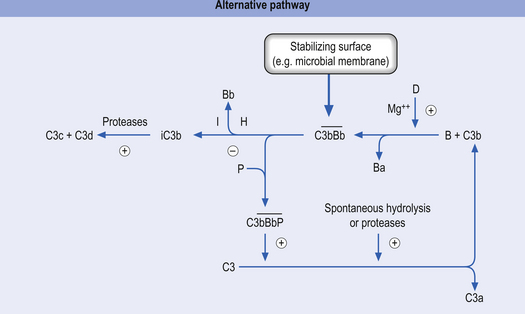
2. The alternative activation pathway—initiated by the activation of precursor proteins B and D, which enter the previous cascade at the C3 precursor level. The activation of B and D precursors is achieved when they come into contact with large polysaccharide molecules usually present on the membranes of pathogens and no antibody formation is necessary for this activation to occur. The end result is the same as the classical activation pathway (Fig. 15.4).
The characteristics of adaptive or specific immunity are specificity for distinct molecules, specialisation, and ‘memory’ capability that allows a more vigorous response to repeated exposure to the same microbe. The components of specific immunity are the lymphocytes and their products. Foreign substances that induce specific responses such as the production of antibodies are called antigens. These two systems do not function in isolation but act in an integrated fashion. Innate immunity not only provides early defence against microbes, but also plays an important role in the induction of specific immune responses. One mechanism that illustrates this cooperative effort occurs when a macrophage is exposed to an inflammatory stimulus; it secretes protein hormones called cytokines that promote activation of the lymphocytes specific for the microbial antigens. Another mechanism of interaction occurs when macrophages that have ingested microbes secrete a particular cytokine which stimulates development of T lymphocytes particularly effective at activating macrophage activity. Thus, the interactions between innate and specific immunity are bidirectional (Roitt 1994).
Humoral response
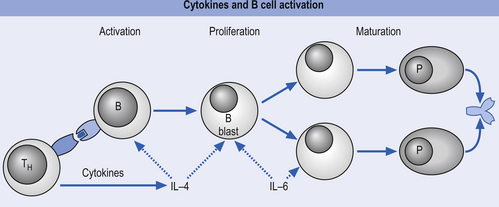
The primary humoral responses occur when an antigen binds to the surface receptors of a B-lymphocytic cell, causing activation of a variety of second and third messengers that eventually result in the activation and replication of cellular DNA to initiate synthesis of antibodies or immunoglobulins (Igs). The activation of surface receptors causes the B lymphocyte to multiply into a series of clones that mature into plasma cells capable of secreting antibodies (Igs) against the antigen (Fig. 15.5). Some of these B lymphocytes become memory cells, which are capable of storing the memory of the assaulting antigen in case re-exposure occurs in the future. This results in the secondary humoral response, which involves the IgM antibodies and is much more vigorous and rapid than the primary response. The antibodies produced combine with the specific antigen that stimulated their production and form an antigen–antibody complex that allows other cells such as macrophages, natural killer cells, and neutrophils to recognise and destroy the antigen-bearing complex.
Antigens
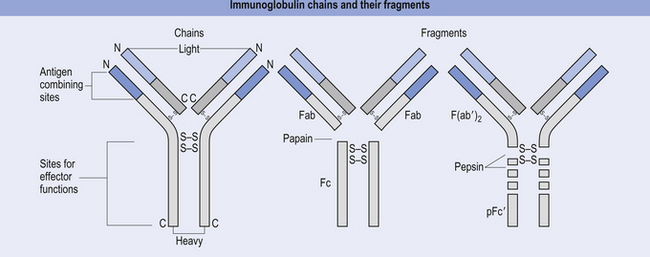
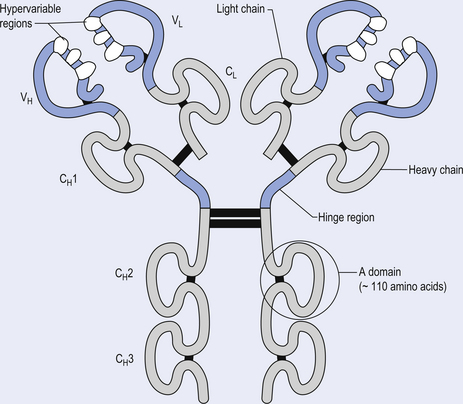
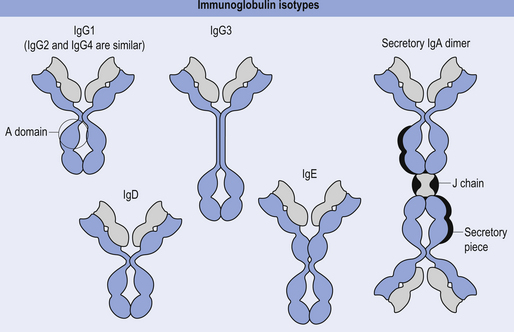
The antibody molecule or immunoglobulin (Ig) is composed of two identical heavy and two identical light chain peptides held together by interchain disulfide bonds (Figs 15.6 and 15.7). Five classes of antibody have been identified, each with a variety of subgroups also identified. These classes of antibody are IgG, IgA, IgM, IgE, and IgD.
IgG
This immunoglobulin is the most abundant immunoglobulin of the internal body fluids, especially in extravascular fluid where it combats microorganisms and their toxins. When IgG complexes with a bacteria or antigen the classic complement cascade is triggered, which results in chemotactic attraction of polymorphonuclear (PMN) cells, which then can adhere to the bacteria or antigen through surface receptors that recognise segments of the IgG antibody, called constant regions, and stimulate the PMN cell to ingest the bacteria through the process of phagocytosis.
IgG is the only antibody that can cross the human placenta such that it provides a major line of defence for the first few weeks of the baby’s life (Fig. 15.8).
IgA
This antibody only appears in the seromucous secretions such as saliva, tears, nasal fluids, sweat, colostrum, and secretions of the lung, gastrointestinal, and genitourinary tracts, where it has the job of defending the body against attack by microorganisms. IgA is synthesised by plasma cells, and functions by inhibiting the adherence of coated microorganisms to the surface of mucosal cells, thereby preventing entry into the body tissues. IgA can also activate the alternative (not classic) complement pathway (Fig.15.8).
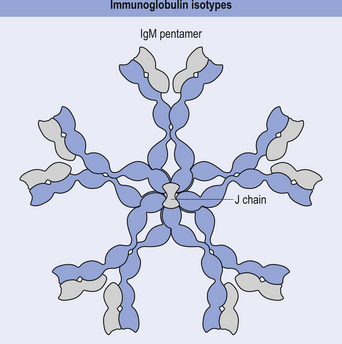
IgM
This antibody is formed as a pentamer of IgG molecules and is the largest of all the immunoglobulins. For this reason it is very effective at agglutinating bacteria and initiating the classic complement pathway (Fig. 15.9).
Cell-mediated immune response
Cell-mediated immunity involves the T-lymphocyte cell series which, unlike B lymphocytes, are unable to recognise free antigens but can only respond to processed fragments of protein antigens displayed on the surfaces of the body’s own cells. The T-lymphocyte attack is directed against body cells infected with viruses, bacteria or intracellular parasites, and cells recognised as non-self such as transplanted or infused tissue (Marieb 1995).
Discrimination of self from non-self is one of the most remarkable properties of every normal individual’s immune system. This ability is called self-tolerance. Self-tolerance is maintained partly by the elimination of lymphocytes that may express receptor specific for self-antigens and partly by functional inactivation of self-reactive lymphocytes after their encounter with self-antigens. The T lymphocyte recognises ‘self’ and ‘non-self’ by proteins on the cell membrane called major histocompatibility complex (MHC).
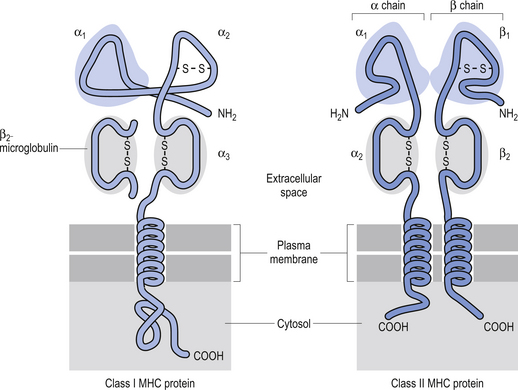
The MHC is a region of highly polymorphic genes whose product proteins are expressed on the surfaces of a variety of cells. This allows T lymphocytes the ability to survey the body for the presence of peptides derived from foreign proteins. There are two different types of MHC gene products called class I and class II MHC molecules. Any given T lymphocyte recognises foreign peptides bound to only one class I or one class II MHC molecule (Fig. 15.10) (Abbas et al. 1997).
Class I MHC proteins are present on all cells of the body except red blood cells. These allow the T cells to recognise ‘self’. Class II MHC proteins are present only on B cells, some T cells, and antigen-presenting cells such as macrophages. The proteins of class II MHC are composed of pieces of foreign antigen that have been phagocytosed and broken down by intracellular mechanisms and recycled back to the plasma membrane. The role of MHC proteins in the immune response is extremely important because they provide the means for signalling the immune system cells that infected or cancerous cells are present but camouflaged inside our own cells (Roitt 1994).
One form of communication between the brain and the immune system probably occurs via the autonomic nervous system
The parasympathetic system communicates via several cranial nerves including the oculomotor (CN III) nerve, the trigeminal (CN V) nerve, the facial (CN VII) nerve, and the vagus (CN X) nerve. The vagus nerve and sacral nerve roots compose the major output route of parasympathetic enteric system control (Furness & Costa 1980). The neurotransmitter released both pre- and postsynaptically is acetylcholine. Functionally, the neurological output from the parasympathetic system is the integrated end product of a complex interactive network of neurons spread throughout the mesencephalon, pons, and medulla. This complex interactive network receives modulatory input from wide areas of the neuraxis including all areas of cortex, limbic system, hypothalamus, cerebellum, thalamus, vestibular nuclei, basal ganglia, and spinal cord (Walberg 1960; Angaut & Brodal 1967; Brodal 1969; Brown 1974; Webster 1978). The relationship of the parasympathetic outflow to the immune system has received very little study to date and as a consequence very little is known about the influence of parasympathetic or the enteric system on immune function.
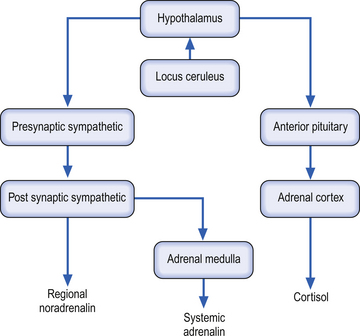
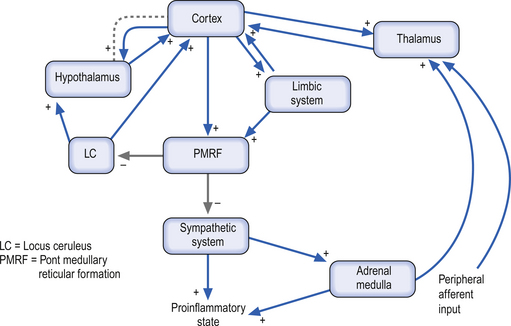
The sympathetic system enjoys a wide-ranging distribution to virtually every tissue of the body. The presynaptic neurons live in a region of the grey matter of the spinal cord called the intermediomedial and intermediolateral cell columns. The output of the preganglionic neurons of the sympathetic system is the summation of a complex interactive process involving segmental afferent input from dorsal root ganglion and suprasegmental input from the hypothalamus, limbic system, and all areas of cortex via the mesencephalic and pontomedullary reticular formations (Donovan 1970; Webster 1978; Williams & Warwick 1984). Most postganglionic fibres of the sympathetic nervous system release norepinephrine as their neurotransmitter. The chromaffin cells of the adrenal medulla, which are embryological homologues of the paravertebral ganglion cells, are also innervated by preganglionic sympathetic fibres which fail to synapse in the paravertebral ganglia. When stimulated, these cells release a neurotransmitter/neurohormone that is a mixture of epinephrine and norepinephrine with a 4:1 predominance of epinephrine (Elenkov et al. 2000).
Both epinephrine and norepinephrine are manufactured via the tyrosine–dihydroxyphenylalanine (DOPA)–dopamine pathway and are called catecholamines. When the body is in a neutral environment, catecholamines contribute to the maintenance of homeostasis by regulating a variety of functions such as cellular fuel metabolism, heart rate, blood vessel tone, blood pressure and flow dynamics, thermogenesis and as explained below, certain aspects of immune function. When a disturbance in the homeostatic state is detected, both the sympathetic nervous system and the hypothalamus–pituitary–adrenal axial system become activated in the attempt to restore homeostasis via the resulting increase in both systemic (adrenal) and peripheral (postganglionic activation) levels of catecholamines and glucocorticoids. In the 1930s, Hans Selye described this series of events or reactions as the general adaptation syndrome or generalised stress response (Selye 1936). Centrally, two principal mechanisms are involved in this general stress response; these are the production and release of corticotrophin-releasing hormone produced in the paraventricular nucleus of the hypothalamus and increased norepinephrine release from the locus ceruleus norepinephrine-releasing system in the brainstem. Functionally, these two systems cause mutual activation of each other through reciprocal innervation pathways (Chrousos & Gold 1992). Activation of the locus ceruleus results in an increased release of catecholamines, of which the majority is norepinephrine, to wide areas of cerebral cortex, subthalamic, and hypothalamic areas. The activation of these areas results in an increased release of catecholamines from the postganglionic sympathetic fibres as well as from the adrenal medulla (Fig. 15.11).
Catecholamine-releasing nerve fibres have been found in a wide range of cells and tissues including thymus, spleen, lymph nodes, tonsils, bone marrow, mucosa-associated lymphoid tissue (MALT), gut-associated lymphoid tissue (GALT), and the parenchyma of lymphoid tissues not associated with blood vessels. Generally, these areas of adrenogenic innervation appear to be in areas with high concentrations of T lymphocytes, macrophages, and plasma cells. This is in contrast to areas of high concentrations of developing B lymphocytes, which seem to be poorly innervated by these fibres (Felten et al. 1985). The appearance of these fibres occurs early in the development of these cell types, suggesting a possible role in the maturation and development process of these cell types and in immune system maturation (Elenkov et al. 2000). Current understanding of synaptic transmission processes suggests that the majority of the interactions between the above described cell types and the nerve fibres in the lymphoid tissues occur via an alternate form of synaptic transmission recently termed ‘nonsynaptic transmission’ (Vizi & Labos 1991; Vizi 2000). In this alternate form of neurotransmission, neurotransmitters are released from postsynaptic sympathetic neurons and diffuse over relatively large distances before interacting with receptors on the various cell types previously described. Thus three main classes of neurochemical interactions in sympathetic/immune communication may be identified. These include fast synaptic transmission, moderately fast nonsynaptic transmission, and slow neurohormonal transmission. Nonsynaptic transmission may also play a role in the norepinephrine regulation of blood flow in various tissues and also in modulating lymphocyte trafficking through the body (Villaro et al. 1987).
Neuroimmune interactions
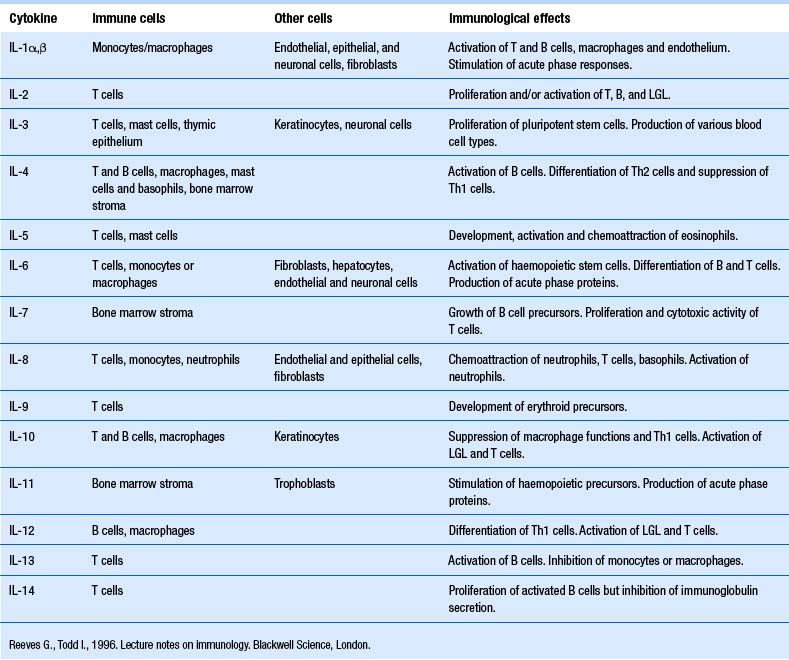
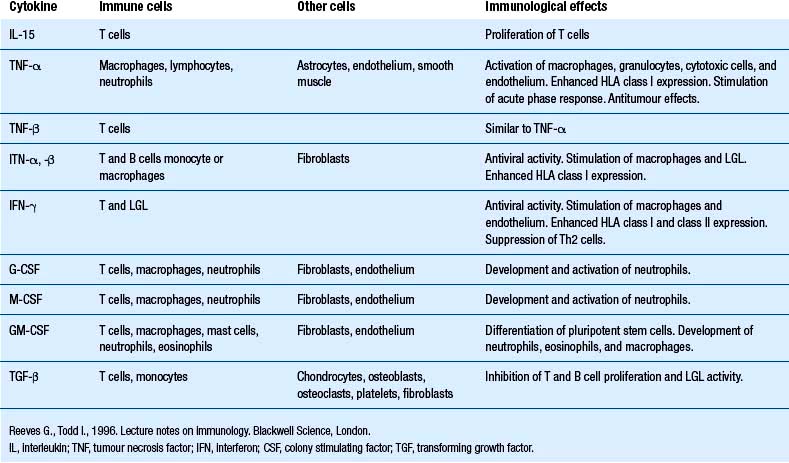
Our understanding of the bidirectional communication between the nervous and the immune system has developed over the past 25 or so years to the point that it is clear that these two systems have a well-developed bidirectional communications system, involving neurotransmitters and cytokines (Besedovsky et al. 1981; Ader & Cohen 1982). Cytokines are a group of chemical mediators utilised by cells as a form of communication. A vast number of cytokines have been identified to date (Tables 15.1 and 15.2).
Cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumour necrosis factor-alpha (TNF-α) can signal the brain via a complex pathway involving the activation of both the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis. The involvement of both fast-acting and slow-acting mechanisms suggests that both acute and chronic types of interactions are possible and, in fact, occur functionally (Berkenbosch et al. 1989; Elenkov et al. 1996).
How do the immune system and nervous systems communicate?
• Glucocorticoids (cortisol) secreted from the adrenal cortex
• Catecholamines (noradrenalin) secreted from the sympathetic nerve terminals
• Catecholamines (adrenalin) secreted from the adrenal medulla
• Hormones (ACTH) secreted from the pituitary gland
• Cytokines, lymphokines, interleukins, interferons, anti-necrosis factors produced by immune cells.
Communication between sympathetic neurons and other cells is mediated by two principal types of receptors. These principal types of receptors have been classified as alpha (α) and beta (β) adrenergic receptors. Both classes have subsequently been subclassified to include beta 1, 2, and 3 and alpha 1 and 2 subtypes, with even more subtypes in each class known to exist. A crucial discovery in relating the nervous system to the immune system occurred when it was observed that beta adrenergic receptors are found on all types of lymphoid cells. The quantity across various cell types seems to vary, with natural killer cells having the greatest density and helper T lymphocytes having the lowest density (Khan et al. 1986; Maisel et al. 1990). Recent investigations have demonstrated that two subclasses of helper T lymphocytes show different receptor characteristics with the helper type-1 lymphocytes expressing beta-2 adrenergic receptors on their membrane but these same receptors are not expressed on type-2 helper lymphocyte membranes (Sanders 1998). As we shall discuss later, this receptor variation may play an important part in the different functional reactions of these two lymphocyte subclasses.
Once activated, the beta adrenergic receptors activate a chain of G-proteins that act as intracellular effectors, which in turn stimulate the activation of a series of successive enzymes such as adenylate cyclase, cyclic adenosine monophosphate (cAMP), inositol-1,4,5-triposphate (IP3), and diacylglycerol (DAG) (see Chapter 3). Variations in the intracellular concentrations of these various ‘second messenger’ enzymes result in different functional outcomes. Activity of different receptors can also alter the production and activation thresholds of second messengers within the cell. For example, the activation of adrenergic–G-protein complexes usually results in inhibition of adenylate cyclase and a subsequent decrease in the production of cAMP in the cell. cAMP has been shown to modulate a variety of transcription factors important in the expression of various genes, including those genes involved in the production of a variety of cytokines produced in lymphocytes. Thus activation of beta-2 adrenergic receptors by catecholamines usually results in a decreased transcription rate of genes responsible for the production of TNF-α and IL-12. The same receptor activation pathway also stimulates the activation of genes that transcribe the production enzymes for IL-10 (Elenkov et al. 1995; Hasko et al. 1998). This series of events is important in the activation shift between cellular and humoral immunity, as outlined below.
The T-lymphocyte subclasses, T-helper type 1 (Th1) and T-helper type 2 (Th2), are both components of the cellular or acquired immunity response but may activate or inhibit activity of other immune responses via cytokine mechanisms. Th1 lymphocytes primarily produce and release interferon gamma (IFN-γ), IL-2, and TNF-α, which promote cellular immunity processes, whereas Th2 lymphocytes produce and release a different set of cytokines, namely IL-1, IL-4, IL-10, and IL-13, which promote humoral immunity processes (Abbas et al. 1996; Grohmann et al. 2000). Unactivated CD41 lymphocytes (Th0) are lymphocytes that have not been exposed to an antigen and are referred to as antigen inexperienced or naive. These cells are bipotential and have the capability to develop into either Th1 or Th2 lymphocytes. Which development pathway these cells follow will depend to a large extent on the cytokines released by the antigen-presenting cells that initially induce their activation. For instance, IL-12 produced by a macrophage presenting an antigen acts in concert with other signalling mechanisms to induce the development of Th1-type lymphocytes. Because Th1 lymphocytes are involved in the production of IL-12, TNF-α, and IFN-γ, these lymphocytes are considered to promote the process of inflammation and are classed functionally as proinflammatory lymphocytes. In contrast, the Th2 lymphocytes promote anti-inflammatory processes and are termed as such (Trinchieri 1995; Mosmann & Sad 1996).
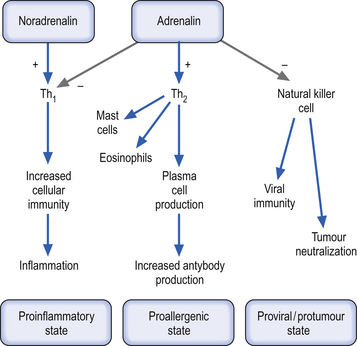
The cytokines produced in Th1-type lymphocytes inhibit the response and activation of Th2-type lymphocytes and vice versa. Thus IL-4 and IL-10 inhibit Th1 responses and IL-12 inhibits Th2 responses. The Th2 production of IL-10 promotes the stimulation of humoral immunity by stimulating both the growth and activation of mast cells and eosinophils and the activation and maturation of B lymphocytes to plasma cells. This cytokine also stimulates the plasma cell into the process of immunoglobulin switching from IgG to IgE, all of which inhibit the production of Th1 proinflammatory cytokines and promote anti-inflammatory states in the region. The activation of catecholamine receptors on T-helper cells seems to inhibit type-1 activities and favour type-2 activities. This results in a functional shift from cellular immunity towards humoral immunity. The major mechanism involved in this shift is the inhibition of IL-12 via stimulation of beta-2 adrenergic receptors. Activation of these same receptors also results in the inhibition of TNF-α, another proinflammatory cytokine, while at the same time promoting the production of IL-10, one of the most potent anti-inflammatory cytokines (Suberville et al. 1996) (Fig. 15.12).
The discussion thus far has focused on the systemic effects of catecholamine release on the balance between cellular and humoral immunity in T-helper lymphocytes. Locally, these responses may be different to that discussed earlier. In local responses in specific tissue areas release of catecholamines may result in predominately alpha receptor stimulation, which promotes the activation of inflammatory responses through the stimulation and recruitment of polymorphonuclear leukocytes in the regions involved. This proinflammatory process occurs through a complex series of interactions involving chemotactic cytokines called chemokines. Other components of the immune system seem to be modulated by the activity of catecholamines. Natural killer cells seem to be inhibited by catecholamine release and, in fact, may be the most sensitive cell type to the circulating concentration of catecholamines due to the large number of beta-2 adrenergic receptors on their membranes (Irwin 1994). The effect of catecholamines on macrophage function is complex and appears to be somewhat dependent on the state of activation of the macrophage at the time of interaction. Some evidence suggests that naive or non-antigen challenged macrophages may respond more aggressively to alpha adrenergic receptor stimulation, which results in increased activation of the macrophage. Activated macrophages show increased receptiveness to beta-adrenergic receptor stimulation, which results in a reduction in activation or an inhibition of activity. The final activation state of a macrophage may also depend on the presence of other cytokines or proinflammatory mediators in the immediate environment of the cell (Baker & Fuller 1995). The effect of catecholamines on cytotoxic (CD8+) lymphocytes is sketchy at best. Some indication that catecholamines may stimulate the development of CD8+ lymphocytes but inhibit their functional activity at the same time has been postulated (Benschop et al. 1996). Neutrophil function appears to be inhibited by catecholamines over a wide range of activities including phagocytosis, chemotaxis, release of lysosomal enzymes, and superoxide formation (Zurier et al. 1974; Gibson-Berry et al. 1993).
Can immune function be modulated by different areas of cortex?
There is widespread evidence that asymmetric lateralisation of cortical functioning does occur. Several of the lateralised functions are also known to be involved in brain neuroimmune modulation such as emotional arousal, sympathetic innervation, neurotransmitter concentrations, and neuroendocrine activity (Wittling 1998). It would seem from the widespread evidence of cortical functional hemispheric dominance in other systems that modulation of the immune system would also fall under asymmetric cortical control.
A growing body of evidence indicates that immune system function is modulated by different areas of the cortex in an asymmetrical fashion. For instance, a variety of studies have demonstrated that the rostral portions of the frontal cortical areas are differentially activated when the individual is exposed to different emotional stimulus and that the activation state experienced altered the immune response of the individual (Kang et al. 1991). The left frontal cortex appears to be activated during the expression or experience of positive emotional states, whereas the right frontal cortex seems to be activated during the expression or experience of negative emotional states (Davidson 1984; Leventhal & Tomarken 1986; Silberman & Weingartner 1986; Davidson & Tomarken 1989). One of the difficulties in this type of research is the huge individual variability of immune responses in individuals that occurs to a variety of cognitive stimulus. A few studies have tried to specifically address this problem and the results indicate that individual responses can be significantly correlated to immunological change. For instance, individuals with depression seem to show a range of immune activation, which is dependent on the severity of the depressive symptoms (Irwin et al. 1990). The severity of symptoms in depression has been linked to the activation levels in the left frontal cortex (Robinson et al. 1984). Those patients with left frontal cortex lesions but sparing of the right frontal cortex showed the most severe depressive symptoms, which suggests that the asymmetrical activation levels between the right and left cortical areas may also be important in the modulation of immune response (Davidson et al. 1990). Another study showed that individual personality traits were predictive of natural killer cell activity both before and after a stressful event (Kiecolt-Glaser et al. 1984). Another study found that natural killer cell activity was significantly increased in human females with extreme left frontal cortical activation when compared to females with extreme right cortical frontal activation (Kang et al. 1991). The level of hemispheric activation in these women was determined by electroencephalographic (EEG) determinants of regional alpha power density. This measurement has been shown to be inversely related to emotional or cognitive brain activation (Davidson 1988).
A variety of animal studies have also provided direct evidence of the relationship between cerebral asymmetry and immune system function (Barneoud et al. 1987; Neveu 1988). Partial ablation of the left frontoparietal cortex in mice, which results functionally in relative right cortical activation, resulted in decreased immune responses and partial right cortical ablation, which would result functionally in a left cortical activation, showed no change or a reduced immune response (Renoux et al. 1983; Neveu et al. 1986).
A variety of further studies have found several consistent findings relating to ablation of cortical areas and resultant immune dysfunction (Renoux et al. 1983; Biziere et al. 1985; Renoux & Biziere 1986; Barneoud et al. 1988). These finds show the following:
1. Development of the lymphoid organs including the spleen and thymus occurs with left cortical lesions, whereas increased development of the spleen and thymus occurs with right cortical lesions.
2. Activation of T cells is significantly diminished in lesions involving the left cortex and elevated with lesions of the right cortex.
These findings indicate that T-cell-mediated immunity is modulated asymmetrically by both hemispheres, with each hemisphere acting in opposition to the other. Increased activity of the left cortex seems to enhance the responsiveness of a variety of T-cell-dependent immune parameters, whereas increased right cortical activity seems to be immunosuppressive. B-cell activity was found not to be affected by cortical activation asymmetry (Neveu et al. 1988; LaHoste et al. 1989).
In summary, most studies have shown that changes in hemispheric activation because of either ablation of cortical areas or modulation in physiological activation levels result in changes in immunological response activity. Both hemispheres seem to be active in the modulation of immune response, with the left hemisphere enhancing cellular immune responses and the right inhibiting those responses. Some evidence does suggest that the involvement of the right hemisphere may not act directly on immune components but may modulate the activity of the left hemisphere which does act directly to regulate immune function (Renoux et al. 1983).
Cerebellar–hypothalamic communication may also be important in immune system function
The hypothalamo-neurohypophyseal system as well as the autonomic nervous system is involved in homeostatic responses associated with changes in head position and orthostatic reflex. The responses induced by body tilt on earth are thought to be attributed to changes in inputs from baroreceptors, vestibular organs, and proprioreceptors normally required for postural control. The information from these organs is sent to the hypothalamus, which thereby influences both neuroendocrine and autonomic systems as well as various kinds of emotional behaviour. The fastigial input to the hypothalamus suggested that the fastigial nucleus plays a significant role in these homeostatic responses through its connections with the brainstem and the hypothalamus (Katafuchi et al. 1995).
Does immune activity equate to appropriate immune function?
The complex nature of neuroimmune interactions has made interpreting the impact of these reactions on the health and well-being of the person in question very difficult. For example, in some cases an increase in certain cytokines may be appropriate and in other cases cause the person great despair. If we are just measuring the concentration of that cytokine without regard to appropriateness of its action, the true meaning of the increase may well be lost. It is also important to understand which aspects of immune function are being measured and if they are actually measuring immune function or just quantitative aspects of cell mobilisation. For instance, an increase in total lymphocyte count may not indicate the actual activity of those cells, which must undergo a complex interactive process of activation in order to actually perform their immune functions. A simple measure of concentration may be misleading.
Clinical implications
It is clear from the previous discussion that the interactions between the nervous and immune systems are complicated and are multifactorial in nature. Inappropriate interaction via efferent or afferent loops of this communication system may result in dysfunction or disease (Fig. 15.13).
Inappropriate levels of systemic catecholamines have been associated with a variety of clinical conditions associated with immune dysfunction including (Elam et al. 1992; Jarek et al. 1993; Abbas et al. 1996; Li et al. 1997):
1. Both onset and progression of a variety of infectious diseases;
2. Helicobacter pylori, both onset and progression;
3. Increased susceptibility to the common cold;
4. Increased complications following major trauma;
8. Autoimmune thyroid disease;
9. Crohn’s disease; fibromyalgia;
Several studies have investigated the effect of changes in spinal afferentiation as a result of manipulation on the activity of the sympathetic nervous system (Korr 1979; Sato 1992; Chiu & Wright 1996). Suprasegmental changes, especially in brain function, have demonstrated the central influence of altered afferentiation of segmental spinal levels (Thomas & Wood 1992; Carrick 1997; Kelly et al. 2000). Changes in immune system function can be mediated through spinal afferent mechanisms. These mechanisms may operate via suprasegmental or segmental levels by modulating the activity of the sympathetic nervous system (Beck 2003).
Case 15.1
Case 15.2
References
Beck R.W. Psychoneuroimmunology. Beirman R., editor, Handbook of Clinical Diagnosis. 2003:27-35.
Brodal A. Neurological Anatomy. London: Oxford University Press, 1969.
Brown L.T. Corticorubral projections in the rat. J. Comp. Neurol.. 1974;154:149-168.
Donovan B.T. Mammillian Neuroendocrinology. New York: McGraw-Hill, 1970.
Furness J.B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5:1-20.
Guyton A., Hall J. Textbook of Medical Physiology. Philadelphia: Saunders, 1996.
Heaney M., Golde D. Soluble receptors in human disease. J. Leukoc. Biol.. 1998;64:135-146.
Leventhal H., Tomarken A.J. Emotion: today’s problems. Annu. Rev. Psychol.. 1986;37:565-610.
Marieb E. Human Anatomy and Physiology. New York: Benjamen/Cummings, 1995. pp. 594–595
Roitt I. Essential Immunology, eight ed. London: Blackwell Scientific, 1994.
Williams P.L., Warwick R. Gray’s Anatomy. Edinburgh: Churchill Livingstone, 1984.