Chapter 33E Neuroimaging
Interventional Neuroradiology: Neurological Endovascular Therapy in Hemorrhagic and Ischemic Strokes
Historical Background
Neurological endovascular therapy is a relatively new subspecialty dealing with a wide range of pathologies linked to central nervous system (CNS) hemorrhagic (Jabbour et al., 2009; Pearl et al., 2010) or ischemic (Santos-Franco et al., 2009; Thorisson and Johnson, 2009; Zenteno-Castellanos et al., 2009) disorders of arteries and veins (Caso et al., 2008) and of the head and neck (Gandhi et al., 2008; Sekhar et al., 2009; Turowski and Zanella, 2003). Working closely with different disciplines—stroke neurology, diagnostic and interventional neuroradiology, neurosurgery, neurointensive care, and neurorehabilitation (Connors et al., 2005; Qureshi et al., 2008)—endovascular therapy plays a key role in patient management.
Endovascular therapy has evolved in the last 25 years. Thanks to rapid technological developments, better knowledge of applied neuroanatomy, and greater understanding of the pathological processes, many procedures that were previously risky and often ineffective now produce excellent clinical results with low morbidity and mortality (Naggara et al., 2010). The implementation of neurointerventional procedures requires a multidisciplinary team including neuroanesthesiologists (Brekenfeld et al., 2010; Varma et al., 2007; Young, 2007), neurosurgeons, radiologists, critical care specialists (Bruder et al., 2008; Connolly et al., 2005), and neurologists, along with a well-trained staff of nurses (Galimany-Masclans et al., 2009; Wright, 2007) and technologists. Additionally, some studies comparing the length of stay and total hospital charges have recently favored interventional treatment over surgical procedures in selected cases (Hoh et al., 2010).
Since the first in vivo angiography performed by Edgas Moniz in 1927, this technique has evolved significantly from direct puncture of cervical vessels to transfemoral or transradial approaches to the neurovascular structures (Jo et al., 2010). In the late 1970s, most of the procedures were limited to the extracranial vasculature. In 1976, Kerber described a balloon catheter with a calibrated leak as a new system for super-selective angiography and occlusive catheter therapy. Flow-guided catheters were soon replaced by more soft, trackable devices. The use of latex/silicone detachable balloons and mechanically driven coils were also soon complemented by the addition of particles, liquid embolic agents, and electrically detachable coils.
The diagnostic and technical excellence brought by digital subtraction angiography was also improved with the development of new guide wires and hydrophilic microcatheters. New angiography techniques were incorporated, such as high-speed serial imaging, road mapping (Rossitti and Pfister, 2009), contrast injectors, and the availability of biplanar rotational (Dorfler et al., 2008) three-dimensional (3D) flat-panel angiography and C-arm flat-detector computed tomography (CT) (Kamran et al., 2010). Noninvasive imaging techniques such as computed tomography angiography (CTA) and magnetic resonance angiography (MRA) complement the use of digital 3D subtraction angiography, though the latter remains the diagnostic gold standard owing to its more accurate assessment of intracranial and spinal vascular anatomical and dynamic information (Mo et al., 2010). Interventional neuroradiology is a discipline that brings together three major branches of the neurosciences: observation through neuroradiology, technical mastery of neurosurgery, and clinical skills of neurology. Combining these three skill sets allows endovascular therapy to be both comprehensive and effective in the diagnosis and management of patients with vascular disorders of the CNS.
Intracranial Vascular Malformations
Brain Arteriovenous Malformations
As seen in Table 33E.1, arteriovenous malformations (AVMs) can be divided into two different groups (Chaloupka and Huddle, 1998). The group in the left column is suitable for neurointerventional management.
Table 33E.1 Types of Arteriovenous Malformations with and without Shunts
| ARTERIOVENOUS SHUNT | |
|---|---|
| Present | Absent |
| Arteriovenous malformation | Capillary vessel malformations (telangiectasias) |
| Pial arteriovenous fistulas | Developmental venous anomalies (venous angiomas) |
| Dural arteriovenous fistulas | Cavernous angiomas |
Epidemiology and Pathology
Brain AVMs are the more frequent type of vascular malformations and those which cause the most morbidity and mortality. The true incidence is difficult to estimate, but some retrospective population-based studies have shown that the incidence of symptomatic intracranial hemorrhage (ICH) due to any type of intracranial vascular malformations was 0.8 per 100,000 (Brown et al., 1996a). The New York Island Arteriovenous Malformation Study was the first ongoing prospective population-based survey to determine the incidence of AVM hemorrhage and associated morbidity and mortality rates in New York City. Initial results calculated an AVM detection rate of 1.34 per 100,000 person-years and an acute AVM hemorrhage rate of 0.51 per 100,000 person-years (Stapf et al., 2003).
Some 90% of brain AVMs are supratentorial, and 10% are infratentorial. An AVM is a complex tangled bundle of abnormal arteries and veins linked by one or more fistulas (Choi and Mohr, 2005). An important anatomical feature of this vascular conglomerate, also known as a nidus, is the lack of a capillary bed (Choi and Mohr, 2005). The nidus is surrounded by gliotic tissue with traces of hemosiderin and calcifications due to previous bleeds. Most AVMs harbor intracranial aneurysms, which can be intranidal, related to the AVM (distal or proximal arising from the feeder vessels), or located in different parts of the arterial circulation.
Natural History
The clinical presentation generally occurs between the second and fourth decades of life. The natural history of unruptured AVMs is unclear. A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA) is underway to address this issue (Stapf et al., 2006). About 50% of patients harboring a brain AVM present with hemorrhage (intraparenchymal, subarachnoid, or intraventricular) (Brown et al., 1996b). In the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage, symptomatic AVMs were found in 8.6% of all patients with nontraumatic subarachnoid hemorrhages. Seizures are the second most common presentation, followed by headache and progressive focal neurological deficit. The risk of hemorrhage is 1.3% to 3.9% yearly after diagnosis of an AVM in patients who present without ICH. For patients with a previous hemorrhage, the risk of rebleeding is between 6% and 17% in the first year (Fleetwood and Steinberg, 2002), diminishing thereafter.
Various angiographic and clinical factors predictive of bleeding have been identified in retrospective studies and include (Fleetwood and Steinberg, 2002): previous hemorrhage, deep venous drainage, unique venous drainage, venous stenosis or aneurysms, intranidal aneurysms, venous reflux into a venous sinus, small nidus size, high-feeding artery pressure, slow arterial filling, and deep/periventricular location.
Imaging and Classification
Several imaging findings in brain AVMs influence the patient’s therapeutic and clinical management decisions. The most important ones are those known to be associated with hemorrhage or risk of future hemorrhage (evidence of previous hemorrhage, intranidal aneurysms, venous stenosis, deep venous drainage, and deep location of the nidus) (Geibprasert et al., 2010). Magnetic resonance imaging (MRI) is more sensitive than CT in the diagnosis of an AVM and is useful in accurately identifying its location and relationship to functional regions. The most significant features are flow-void signal and hemosiderin deposits in T1- and T2-weighted images. Functional MRI plays an important role in interventional management because it facilitates the localization of functionally important brain areas adjacent to the AVM nidus (Schlosser et al., 1997). Although MRA provides useful information on AVM feeder arteries and draining veins, digital 3D cerebral angiography is the gold standard (Strozyk et al., 2009) for the acquisition of accurate anatomical and dynamic information. The addition of superselective catheterization and angiography of AVM arterial feeders adds key information on AVM angioarchitecture (identification of high-flow arteriovenous fistulas [AVFs], intranidal aneurysms, and selective stenosis of AVM-draining veins).
AVMs can be superficial (sulcal/gyral) or deep (deep parenchymal/choroid plexus). In the sulcal type, the nidus is located in the subpial space and has a conical or wedge-shaped morphology. In the gyral type, AVMs tend to be spherical, since they are covered with cortex. These AVMs have feeding arteries that continue beyond the lesion to supply healthy brain tissue (arteries “en passage”) (Choi and Mohr, 2005).
Morphological characteristics (size and location) and drainage patterns of the AVM are used to classify patients for the risk of persistent neurological deficits from surgery (Choi and Mohr, 2005).
The classification used in clinical practice for surgical management is the Spetzler-Martin grading scale based on three criteria: (1) size of the AVM, (2) venous drainage, and (3) location (eloquent parenchyma corresponds to sensorimotor cortex, areas of language, visual cortex, hypothalamus, thalamus, internal capsule, brainstem, cerebellar peduncles, and deep cerebellar nuclei). However, one of the original authors has redesigned the grading system into a three-tiered classification of cerebral AVMs (class A combines grades I and II, class B are grade III, and class C combines grades IV and V), offering simplification of the previous placement of patients into five categories, which is intended to provide a guide to treatment and be predictive of outcome (Spetzler and Ponce, 2010). Spetzler-Martin grades were specifically designed to classify surgical patients and do not apply when the patient is managed endovascularly. Risk assessment and outcome determination in brain AVM patients treated by endovascular techniques seem adequate and clinically feasible using other scales (Feliciano et al., 2010).
Treatment
Multimodality treatment is the best approach in patients with complex AVMs (Yuki et al., 2010). The present therapeutic approaches include radiosurgery (with latency to obliteration of 1 to 3 years) (Yen et al., 2010), surgery (Rubin et al., 2010), and embolization (Valle et al., 2008; Vinuela et al., 2005; Xu et al., 2010). Medium and large AVMs (Valle et al., 2008) or AVMs with large AVFs or intranidal aneurysms also require a multidisciplinary strategy. The endovascular occlusion of large AVFs or intranidal aneurysms associated with an AVM nidus decreases endovascular or surgical complications, mostly related to local and regional high venous pressure and intraoperative bleeding.
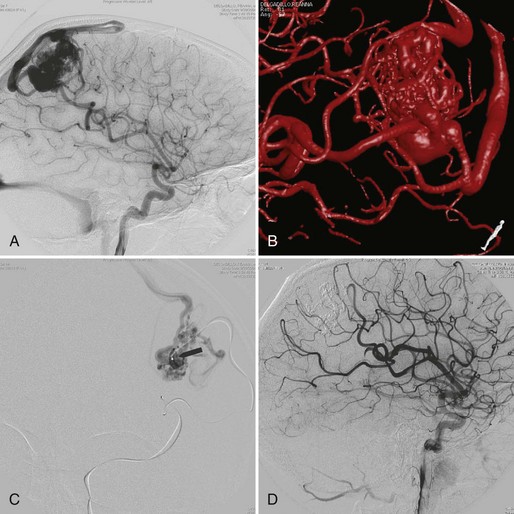
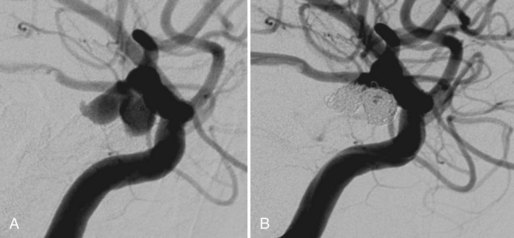
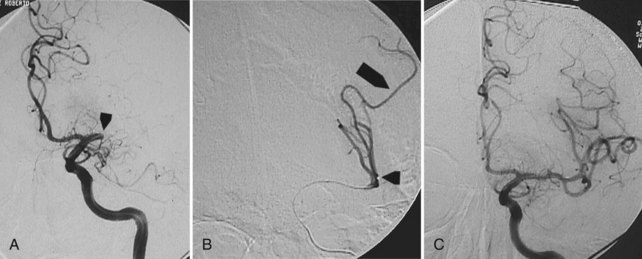
Embolization focuses on occlusion of surgically difficult-to-reach arteries (deep arteries), intranidal aneurysms, and AVFs (Yuki et al., 2010). A 48- to 72-hour interval is advised between AVM embolization and final surgical removal. If embolization is performed before radiosurgery (Shtraus et al., 2010), its primary goal is to reduce the size of the AVM, close fistulas, and treat intranidal aneurysms (see Fig. 33E.2). If an AVM is small and has few afferent pedicles, endovascular treatment can be complete and permanent (Oran et al., 2005) (Fig. 33E.1). Embolic materials include:
 Polyvinyl alcohol particles (PVAs): ranging from 14 to 1000 µm. PVAs cause a foreign body inflammatory reaction. Disadvantages are their adhesivity to the microcatheter and high recanalization rates (Sorimachi et al., 1999).
Polyvinyl alcohol particles (PVAs): ranging from 14 to 1000 µm. PVAs cause a foreign body inflammatory reaction. Disadvantages are their adhesivity to the microcatheter and high recanalization rates (Sorimachi et al., 1999).
 N-2-butyl-cyanoacrylate (NBCA) (Starke et al., 2009; Yu et al., 2004) causes an inflammatory reaction in arteries and surrounding tissue, leading to necrosis/fibrosis of the vessel. NBCA polymerizes in contact with ionic solutions. An iodized oil-based contrast agent (Lipiodol) is added to the NBCA to control its polymerization rate as well as to opacify the mixture for angiographic visualization (Calvo et al., 2001). The microcatheter must be flushed with 10% dextrose to prevent NBCA polymerization within it. Occlusion of cerebral AVMs with NBCA is generally permanent (Wikholm, 1995). It is essential to deliver the acrylic into the AVM nidus and not in the parent artery alone. Proximal arterial occlusion elicits early AVM recanalization by local collateral circulation, making the postembolization surgical AVM resection more difficult.
N-2-butyl-cyanoacrylate (NBCA) (Starke et al., 2009; Yu et al., 2004) causes an inflammatory reaction in arteries and surrounding tissue, leading to necrosis/fibrosis of the vessel. NBCA polymerizes in contact with ionic solutions. An iodized oil-based contrast agent (Lipiodol) is added to the NBCA to control its polymerization rate as well as to opacify the mixture for angiographic visualization (Calvo et al., 2001). The microcatheter must be flushed with 10% dextrose to prevent NBCA polymerization within it. Occlusion of cerebral AVMs with NBCA is generally permanent (Wikholm, 1995). It is essential to deliver the acrylic into the AVM nidus and not in the parent artery alone. Proximal arterial occlusion elicits early AVM recanalization by local collateral circulation, making the postembolization surgical AVM resection more difficult.
 Onyx (Hauck et al., 2009; Xu et al., 2010) is a copolymer of ethylene vinyl alcohol (EVOH) solved in dimethyl sulfoxide (DMSO). When the compound comes in contact with a liquid, it precipitates and forms a sponge-like material. The precipitation progresses centripetally, and the center remains fluid and continues its anterograde flow. Use of Onyx requires DMSO-compatible microcatheters, which are stiffer and often require guide wires for navigation (Weber et al., 2007). The injection of Onyx is slower and more controllable than the NBCA injection. In experienced hands, the percentage of complete AVM occlusion with Onyx reaches 50% (Maimon et al., 2010). NBCA is preferred in fistulous arteriovenous shunts, perforating arteries, leptomeningeal collaterals, and catheter positions distal from the nidus.
Onyx (Hauck et al., 2009; Xu et al., 2010) is a copolymer of ethylene vinyl alcohol (EVOH) solved in dimethyl sulfoxide (DMSO). When the compound comes in contact with a liquid, it precipitates and forms a sponge-like material. The precipitation progresses centripetally, and the center remains fluid and continues its anterograde flow. Use of Onyx requires DMSO-compatible microcatheters, which are stiffer and often require guide wires for navigation (Weber et al., 2007). The injection of Onyx is slower and more controllable than the NBCA injection. In experienced hands, the percentage of complete AVM occlusion with Onyx reaches 50% (Maimon et al., 2010). NBCA is preferred in fistulous arteriovenous shunts, perforating arteries, leptomeningeal collaterals, and catheter positions distal from the nidus.
 Microcoils are used to occlude high-flow AVFs in combination with AVM nidus. Their main role is to decrease untoward embolization of liquid embolic agents into the AVM venous drainage, dural venous sinus, and pulmonary circulation.
Microcoils are used to occlude high-flow AVFs in combination with AVM nidus. Their main role is to decrease untoward embolization of liquid embolic agents into the AVM venous drainage, dural venous sinus, and pulmonary circulation.
Dural Arteriovenous Fistulas
Dural arteriovenous fistulas (DAVFs) are characterized by discrete AVFs involving the intracranial meninges covering the venous sinuses. Although their etiology remains unknown, in most cases there is evidence that the fistula formation is preceded in some instances by trauma resulting in skull fracture, sinus thromboses, or venous outlet stenoses (Berenstein et al., 2004). They are often located in the cavernous sinus, transverse sigmoid sinus, superior sagittal sinus, foramen ovale, tentorium, and anterior or middle cranial fossae.
The clinical features associated with DAVFs depend on location of the lesion, extent of AV shunting, and associated recruitment pial veins. Symptoms may be benign (asymptomatic, tinnitus, ocular symptoms, cranial nerve palsies) or serious (ICH, focal neurological deficits, dementia, papilledema, and even death). These symptoms are associated with cortical venous reflux and/or development of intracranial hypertension. The risk of bleeding is 2% per year and depends on location and hemodynamics. Bleeding is always of venous origin. Several classifications have been proposed and compared (Davies et al., 1996). The most widely accepted ones are from Cognard et al. (1995) and Borden et al. (1995).
Therapeutic Approach to Cavernous Dural Arteriovenous Fistulas
Intermittent manual compression of the carotid artery may be effective in occluding the cavernous sinus. The ipsilateral carotid artery is compressed using the contralateral hand for approximately 5 minutes every waking hour for 1 to 3 days. If this is tolerated, the compression time is increased to 10 to 15 minutes of compression per waking hour. The compression produces concomitant partial obstruction of the ipsilateral carotid artery and jugular vein. This results in the transient reduction of arteriovenous shunting by decreasing arterial flow while simultaneously increasing the outlet venous pressure, thereby promoting spontaneous thrombosis within the cavernous sinus (Katsaridis, 2009).
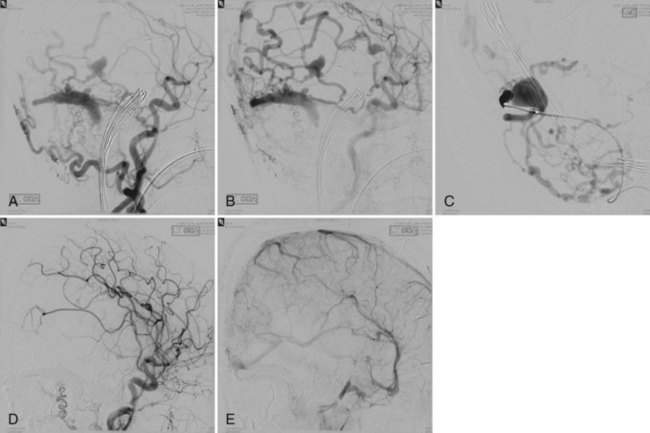
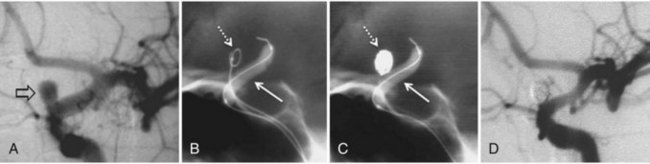
Treatment of DAVF may be endovascular (Kathleen et al., 2009; Katsaridis, 2009), radio-surgical, or surgical. The endovascular approach can be performed with detachable coils, cyanoacrylate, or Onyx (Cognard et al., 2008; Jiang et al., 2010) or via the venous route, packing the sinus with coils or Onyx (Lv et al., 2009) (Fig. 33E.2). The objective of the arterial approach is to close the fistula at the origin of the main draining vein. Occlusion of a meningeal fistula proximal to its draining vein elicits rapid development of arterial collaterals and fistula recanalization (“medusa head” angiographic appearance).
Carotid-Cavernous Fistulas
 Direct carotid cavernous fistula (CCF) (Barrow type A): related to trauma or a ruptured aneurysm of the intracavernous internal carotid artery. These are high-flow fistulas.
Direct carotid cavernous fistula (CCF) (Barrow type A): related to trauma or a ruptured aneurysm of the intracavernous internal carotid artery. These are high-flow fistulas.
 Indirect CCF (Barrow types B, C, and D): with abnormal arterial flow into the cavernous sinus from meningeal arteries arising from the internal and external carotid arteries. They are often spontaneous and occur more frequently in menopausal women.
Indirect CCF (Barrow types B, C, and D): with abnormal arterial flow into the cavernous sinus from meningeal arteries arising from the internal and external carotid arteries. They are often spontaneous and occur more frequently in menopausal women.
Symptoms and signs tend to be mild, and parenchymal hemorrhage is rare. Orbital pain, proptosis, chemosis, ophthalmoplegia, pulsating noise, increased intraocular pressure, and decrease in visual acuity are usually seen (Jabbour et al., 2009; Zenteno et al., 2010). The treatment of a CCF depends on the severity of clinical symptoms, its angiographic characteristics, and the risk it presents for ICH. In most instances, endovascular treatment is preferred.
 Intraarterial: detachable balloons (latex/silicone) (Teng et al., 2000), platinum coils, or stent-assisted coiling (Moron et al., 2005). Self-expandable covered stents look very promising but are still under investigation (Gomez et al., 2007). Cyanoacrylate embolization has a high rate of complete closure but can be associated with serious complications. Onyx injection into the cavernous sinus has also been used, with excellent clinical and angiographic results.
Intraarterial: detachable balloons (latex/silicone) (Teng et al., 2000), platinum coils, or stent-assisted coiling (Moron et al., 2005). Self-expandable covered stents look very promising but are still under investigation (Gomez et al., 2007). Cyanoacrylate embolization has a high rate of complete closure but can be associated with serious complications. Onyx injection into the cavernous sinus has also been used, with excellent clinical and angiographic results.
 Intravenous: embolization with coils or Onyx (Saraf et al., 2010) is the treatment of choice in indirect fistulas (Fig. 33E.3).
Intravenous: embolization with coils or Onyx (Saraf et al., 2010) is the treatment of choice in indirect fistulas (Fig. 33E.3).
 Parent vessel sacrifice: a last resort (Gemmete et al., 2009a, 2009b).
Parent vessel sacrifice: a last resort (Gemmete et al., 2009a, 2009b).
Brain High-Flow Arteriovenous Fistulas
Intracranial pial high-flow AVFs may be classified as:
Intracranial Pial Arteriovenous Fistula
An intracranial pial AVF is a rare cerebrovascular lesion that has only recently been recognized as a distinct pathological entity. According to a series reported by Halbach et al. (1989), pial AVFs account for 1.6% of all intracranial vascular malformations. Intracranial pial AVFs have a single or multiple arterial connections to a single venous channel. They differ from brain AVMs in that they lack a true nidus and differ from dural AVFs in that they derive their arterial supply from pial or cortical arteries and are not located within the dura mater (Hoh et al., 2001).
Pial AVFs can be congenital or may result from a traumatic injury (Lee et al., 2008). Little is known about their pathophysiological mechanisms. The clinical suspicion of pial AVFs should be followed by prompt appropriate treatment because of their natural history. They are associated with congestive heart failure, intracranial varices, increased intracranial pressure due to venous hypertension, and rarely with ICH.
Direct surgical exposure and occlusion of these vascular lesions is associated with high morbidity and mortality (Passacantilli et al., 2006). Today, most intracranial high-flow pediatric and adult AVFs are treated endovascularly. Accurate identification of the arteriovenous shunt and its precise occlusion with embolic materials make the neurointerventional approach the gold standard for this kind of cerebrovascular lesion.
Vein of Galen Aneurysmal Malformation
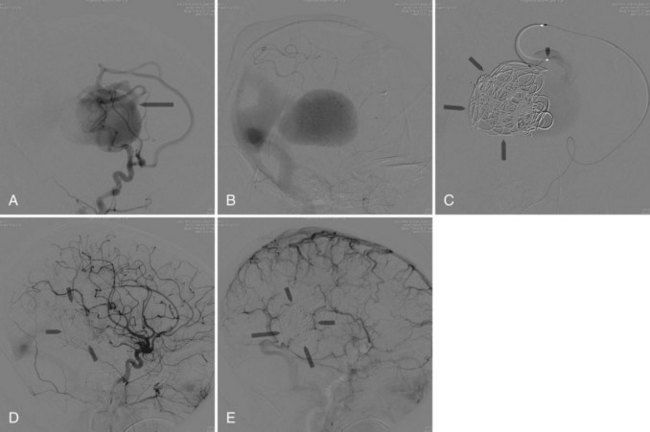
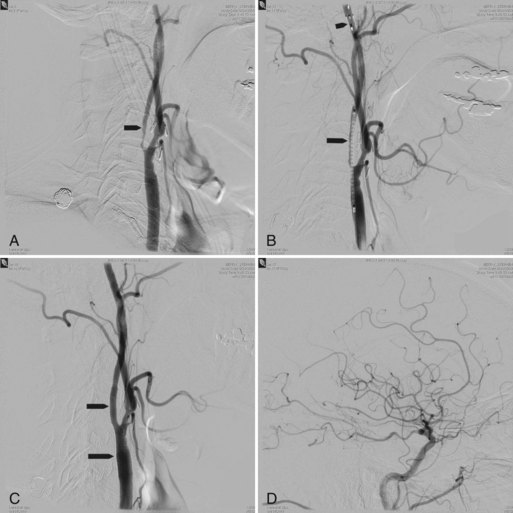
Vein of Galen aneurysmal malformations (VGAM) are rare intracranial AVFs that present almost exclusively in children. They are disproportionately represented in pediatric neurovascular disorders, accounting for up to 30% of intracranial vascular abnormalities (Gupta et al., 2006; Kumar et al., 2006). A VGAM consists of multiple AVFs draining into a dilated median prosencephalic vein of Markowski (Hoang et al., 2009). This embryonic vein does not drain normal tissue and does not communicate with normal cerebral veins. In many cases, the straight sinus is absent, and the vein drains directly into the superior sagittal sinus through the falcine sinus. VGAMs can be categorized into choroidal or mural, depending upon their arterial supply.
Clinical manifestations vary according to age:
 Infants: loud intracranial bruit, severe cyanosis, and cardiac failure.
Infants: loud intracranial bruit, severe cyanosis, and cardiac failure.
 Early childhood: hydrocephalus with or without congestive heart failure.
Early childhood: hydrocephalus with or without congestive heart failure.
The primary indication for treating neonates with VAGMs is congestive heart failure refractory to medical treatment (Horowitz et al., 2005). Elective embolization is performed to close the arteriovenous shunt by the arterial route (Bhattacharya and Thammaroj, 2003). Endovascular techniques include transarterial embolization with cyanoacrylate or Onyx, transvenous embolization with use of coils and Onyx, and combined techniques (Pearl et al., 2010) (Fig. 33E.4). Endovascular embolization has considerably improved outcomes in patients with VGAM. More recently, with the continued development and improvement of endovascular techniques, many patients are found to be neurologically normal on clinical follow-up, and mortality rates have dropped substantially when compared with microsurgical treatment (Khullar et al., 2010).
Intracranial Aneurysms
Epidemiology
Intracranial aneurysms are the most frequent cause of nontraumatic subarachnoid hemorrhage (SAH). Their prevalence in adults ranges from 0.4% to 6%, depending on whether data are collected retrospectively (e.g., from autopsy series) or prospectively (from angiographic series). The incidence of intracranial aneurysms is associated with age, gender, race, tobacco and alcohol consumption, hypertension, family history, and some hereditary disorders (polycystic kidney disease, Ehlers-Danlos syndrome, neurofibromatosis type 1, and Marfan syndrome). Global mortality of SAH can be as high as 25%, and morbidity among survivors is 50% (Locksley, 1966).
The main complications of aneurysmal SAH are:
 Vasospasm: from day 3 to 5 after bleeding. The main predictive factor is the amount of blood in the subarachnoid space (Fisher scale).
Vasospasm: from day 3 to 5 after bleeding. The main predictive factor is the amount of blood in the subarachnoid space (Fisher scale).
 Rebleeding: particularly in the first 24 hours after hemorrhage.
Rebleeding: particularly in the first 24 hours after hemorrhage.
 Medical complications (neurological, cardiac, pulmonary, digestive, electrolytic).
Medical complications (neurological, cardiac, pulmonary, digestive, electrolytic).
The most important predictive factor of the patient’s prognosis is the patient’s clinical status at admission: Glasgow Coma Scale, Hunt and Hess Scale, and the classification of the World Federation of Neurologic Surgeons (WFNS) (Teasdale et al., 1988). Comparing the high prevalence of brain aneurysms with the relatively low incidence of SAH, it seems that only a small number of aneurysms actually do rupture. However, neurological sequelae after aneurysmal rupture may justify treatment of asymptomatic aneurysms in selected cases (Locksley, 1966).
Diagnosis
The first diagnostic modality for patients with possible SAH should be unenhanced CT. If the head CT is negative and clinical suspicion is high, a lumbar puncture is mandatory. Noninvasive imaging techniques such as CTA and MRA may show an intracranial aneurysm, but their resolution and sensitivity are inferior to 2D or 3D digital subtraction angiography (Anxionnat et al., 2001). Both carotid and both vertebral arteries must be angiographically explored because more than 20% of patients have multiple aneurysms. If cerebral angiography is negative, it should be repeated 2 weeks later. Sometimes an intra-aneurysmal clot or local arterial vasospasm may hide a small ruptured saccular or dissecting aneurysm. External carotid angiography should also be performed, looking for a DAVF with intracranial pial venous drainage.
Treatment
Ruptured intracranial aneurysms must be treated endovascularly or surgically as soon as possible to avoid aneurysm rebleeding (Heros, 2006). In asymptomatic aneurysm found incidentally, the conservative versus active decision should be based on the benefit/risk ratio associated with treatment and the natural history of the aneurysm.
The International Study of Unruptured Intracranial Aneurysms (ISUIA) investigated the natural history of intracranial aneurysms according to the characteristics of the patient, aneurysm size, and morbidity and mortality of the treatment (Wiebers et al., 2003). In the subgroup of small aneurysms (up to 7 mm in diameter) diagnosed and managed conservatively, some of them remained stable and others grew, increasing their risk of rupturing. Some neurointerventional centers perform anatomical follow-up imaging studies (CTA or MRA) with aneurysm fluid dynamic evaluations using computer flow analysis (CFA). The goal of this new evaluation is to depict hemodynamic characteristics in aneurysms related to a higher incidence of aneurysm growth and/or rupture (Chien et al., 2009; Ford et al., 2008).
Intracranial aneurysms can be treated by endovascular embolization or surgical clipping. In the comparative ISAT (International Subarachnoid Aneurysm Trial) study, embolization yielded better results in terms of short-term morbidity and mortality when compared to open surgery (Molyneux et al., 2002). However, the mid-term follow-up showed that surgery had better anatomical results and a lower incidence of aneurysm rebleeding and recanalization (Molyneux et al., 2009).
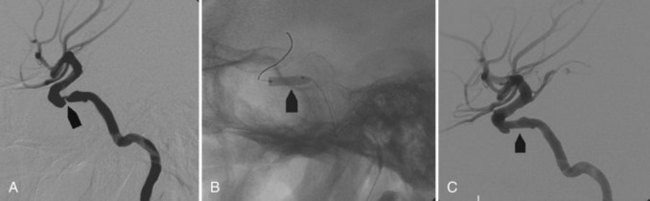
A turning point in the history of interventional neuroradiology occurred in the early 1990s with the advent of the Guglielmi Detachable Coils (GDC, Target). This device is a platinum coil released by electrolytic detachment when properly placed within the aneurysm. If necessary, it can be removed or placed in another position before detachment (Fig. 33E.5). Today more than 100 types of detachable coils are being manufactured. They differ in shape (spiral, 2D, 3D), stiffness, and coating (bioabsorbable polymer or hydrogel).
After embolization, poor packing of the coils may lead to aneurysm regrowth or recanalization (Nguyen et al., 2007). Alternative techniques have been developed to reduce aneurysm recanalization in small aneurysms with wide necks (>4 mm), large (>10 mm in diameter) and giant (>25 mm in diameter) aneurysms.
Balloon Remodeling Technique
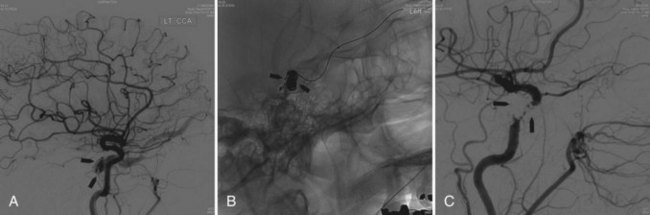
In the balloon remodeling technique, a small nondetachable balloon is inflated intermittently within the parent artery at the neck of the aneurysm to provide a transient support for coil placement, reducing untoward coil herniation into the parent artery and distal intracranial coil migration (Moret et al., 1997) (Fig. 33E.6).
Stent-Assisted Embolization
The placement of a stent across the neck of an aneurysms leads to:
1. Redistribution and decrease of blood flow into the aneurysm.
2. Later neo-intimal formation and endothelialization of the stent into the wall of the parent vessel, with permanent blockade of the aneurysm from the arterial circulation.
3. A permanent scaffold at the neck of the aneurysm to prevent late herniation of coils (Lubicz et al., 2009).
This technique is associated with an increased risk of thromboembolic events and acute stent thrombosis if antiplatelet drugs are not administered before the procedure. Antiplatelet medication must continue for several months after the procedure as well. Stents may be used alone or in combination with coils. They may be overlapped across the aneurysmal neck, or they can be deployed with a Y-configuration in terminal aneurysms (Doerfler et al., 2004). More recently, stent flow diverters have been developed. These special stents do not require coil embolization, because they elicit hemodynamic changes that produce aneurysm thrombosis between 3 and 10 days (Merlin, Silk, Pipeline embolization devices) (Lylyk et al., 2009) (Fig. 33E.7). As noted earlier, Onyx is a novel liquid embolic material (EVOH, DMSO, and micronized tantalum), and when it comes in contact with water or blood, the copolymer precipitates because of rapid diffusion of the DMSO solvent. This liquid embolizing agent may be used alone or in association with other devices (stents, coils, etc.). A temporary balloon occlusion is performed at the neck of the aneurysm while injecting Onyx through a microcatheter to decrease the chances of untoward Onyx migration into the parent artery. Onyx has been mostly used to embolize large and giant aneurysms. In experienced hands, it has shown satisfactory anatomical and clinical outcomes, but it requires a more complex technique than the aneurysm coil or stent embolizations (Molyneux et al., 2004).
Extracranial Carotid Atherosclerosis
Carotid angioplasty and stenting (CAS) is a valid alternative to surgery in selected patients. The first carotid angioplasty was published by Bockenheimer and Mathias in 1983. The first carotid angioplasty and stenting with distal embolic protective device (EPD) was published by Jacques Theron in 1996 (Theron et al., 1996). A review of the present literature on CAS finds numerous single-center studies with conflicting results, industry-sponsored registries, and randomized trials comparing CEA to CAS.
The SAPPHIRE trial (Stenting and Angioplasty with EPD in Patients at High Risk for Endarterectomy) was a prospective noninferiority design trial (a clinical trial that shows that a new treatment is equivalent to standard treatment) with randomization of high-risk asymptomatic and symptomatic patients to CAS or CEA (Yadav et al., 2004). Its primary endpoint was stroke/death/myocardial infarction (MI) at 30 days plus ipsilateral stroke or death at 1 year. This was the pivotal study leading to Centers for Medicare and Medicaid Services (CMS) approval for reimbursement of CAS with EPD in high-risk symptomatic octogenarian patients with angiographic evidence of greater than 70% ICA stenosis. Clinical follow-up at 3 years showed no significant differences between patients who underwent carotid stenting with an EPD and those who underwent carotid endarterectomy (Gurm et al., 2008).
The CREST trial (Carotid Revascularization Endarterectomy versus Stenting Trial) has being the largest prospective randomized carotid revascularization trial ever conducted (2502 symptomatic and asymptomatic patients) (Brott et al., 2010). Its primary endpoint was periprocedural stroke/death/MI and ipsilateral stroke at 4 years. Brott et al. reported that in the 30-day period following the procedure, the rate of stroke was 2.3% in the surgical patients and 4.1% in the stenting group. However, the heart attack rate was higher in the surgical group: 2.3% compared to 1.1% of the stenting group. The difference in heart attack and stroke between the groups was statistically significant.
Angioplasty with a balloon and placement of a stent create an intimal lesion favoring thrombosis, so patients must be on an appropriate postprocedural antiplatelet regime. During the procedure, the risk of stroke due to intracranial migration of fresh thrombus or a friable atheromatous plaque exists and may cause neurological deficits. To minimize this risk, several devices are available, distal protection filters being the most commonly used (see Fig. 33E.7). Carotid stents should be self-expandable; balloon-expandable stents have a higher collapse rate. The morphology can be straight or conical (corresponding to the differences in diameter between internal and common carotid arteries). The use of bioactive or drug-eluting stents is under current assessment for the prevention of restenosis.
Intracranial Arterial Atherosclerosis
The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study of arterial stenosis showed the potentially severe prognosis of ICS (Chimowitz et al., 2005). In patients entering the study with a stroke and 70% or greater arterial stenosis, the ipsilateral stroke rate was 23% per year. Patient enrollment was stopped after 569 patients because of concerns about the safety of patients assigned to warfarin (death, 4.3% versus 9.7%; hemorrhage, 2.9% versus 7.3%). The 2-year rate for ischemic stroke in this trial was 19.7% in the aspirin group and 17.2% in the warfarin group. These data indicated that intracranial stenosis is a high-risk disease for which alternative therapies are needed (e.g., aggressive management of risk factors, alternative antiplatelet regimens, intracranial angioplasty and stenting).
Intracranial angioplasty and stenting was first performed using coronary devices. The first stent manufactured for intracranial stenosis was the Wingspan intracranial stent. The Wingspan intracranial stent was manufactured by Boston Scientific Corporation for use in ICS (Fig. 33E.8). The first Wingspan Humanitarian Device Exemption (HDE) study was performed in 17 centers outside the United States and was a prospective single-arm study that incorporated 45 patients presenting with recurrent stroke attributable to atherosclerotic disease refractory to medical therapy and ICS of 50% or more. A U.S. multicenter study of Wingspan stents in 78 patients with 82 intracranial atheromatous lesions, 54 of which had 70% or greater stenosis, was published in 2007 (Fiorella et al., 2007). Of the 78 patients, 48 presented with a stroke ipsilateral to the ICS, 28 had a transient ischemic attack (TIA), and 59 failed antiplatelet therapy. Of the 82 lesions treated, there were 5 (6.1%) major periprocedural neurological complications, 4 of which ultimately led to patient death within 30 days of the procedure. The National Institutes of Health Registry on use of the Wingspan stent enrolled 129 patients with symptomatic 70% to 99% ICS. Its primary endpoint was stroke/death up to 30 days or any ipsilateral stroke beyond 30 days. These events occurred at 5% at 24 hours, 9.2% at 30 days, and 13.9% at 6 months (Zaidat et al., 2008). The technical success rate was 96.7%. The frequency of > or 50% restenosis on follow-up angiography was 13/52 (25%). Fiorella et al. (2009) noted a 27% restenosis rate (36/129 patients) after Wingspan stenting; 29 of those 36 cases required retreatment, and 9 required multiple endovascular angioplasties.
The SAMMPRIS trial (Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) was up and running in the United States starting in November 2008 (Registry, 2010). This was an investigator-initiated, phase III, multicenter, randomized, blindly adjudicated, clinical trial of angioplasty and stenting with aggressive medical management versus medical management alone. This trial’s main objective was to determine whether intracranial stenting (using the Wingspan self-expanding nitinol stent [Boston Scientific, Natick, Massachusetts]) and intensive medical therapy were superior to intensive medical therapy alone for preventing the primary endpoint (any stroke or death within 30 days after enrollment, any stroke or death after revascularization of the qualifying artery, any stroke or death within 30 days of re-angioplasty of symptomatic restenosis of the qualifying lesion, or stroke in the territory of the symptomatic intracranial artery beyond 30 days) during a mean follow-up of 2 years in high-risk patients with symptomatic stenosis of a major intracranial artery (middle cerebral, carotid, vertebral, basilar). Patients in the medical arm who underwent angioplasty for recurrent TIAs (i.e., crossovers) and who had a stroke or death within 30 days also met this endpoint. In April 2011, NINDS decided that enrollment in the study should be stopped and that the trial currently available indicates that aggressive medical management alone is superior to angioplasty combined with stenting in patients with recent symptoms and high grade intracranial arterial stenosis. At the time of the most recent data safety board review, 14% of patients treated with angioplasty combined with stenting experienced a stroke or died within the first 30 days after enrollment compared with 5.8% of patients treated with medical therapy alone, a highly significant difference.
Neurointerventional Management of Acute Stroke
Imaging of Stroke
Computed Tomography
The sensitivity for detection of acute ischemia within the first 6 hours after onset is below 50% for CT. Unenhanced CT is fast, readily and widely available, and may contribute not only to ruling out hemorrhage (a contraindication to thrombolytic therapy) but also to detection of early acute ischemia (Box 33E.1).
The Alberta Stroke Program Early CT Score (ASPECTS) was developed to offer the reliability and utility of a standard CT examination with a reproducible grading system to assess early ischemic changes (<3 hours from symptom onset) on pretreatment CT studies in patients with acute ischemic stroke of the anterior circulation (Pexman et al., 2001). It is a 10-point quantitative topographic CT scan score using a segmental assessment of MCA territory. One point is removed from the initial score of 10 if there is evidence of infarction in each of the 10 regions (M1, M2, M3, M4, M5, M6, caudate nucleus, lentiform nucleus, internal capsule, and insular cortex). The baseline ASPECTS correlates inversely with the National Institutes of Health Stroke Score (NIHSS), and as the ASPECTS score decreases, the likelihood of dependence, death, and symptomatic hemorrhage is increased.
Computed Tomographic Angiography
Computed tomographic angiography is best performed on a late-generation multislice CT scanner on which a fast thin-section volumetric spiral examination is performed during a time-optimized bolus of IV contrast material injection with opacification of blood vessels (Tomandl et al., 2003). Complete imaging of the craniocervical circulation from the aortic arch through the circle of Willis region can be performed in as little as 20 seconds. High-resolution 2D (multiplanar reformatted [MPR]) or 3D reconstructed images presented as maximum intensity projection (MIP) or shaded surface display (SSD) images (see Fig. 33E.7) can be obtained. CTA can be performed at the same time that a dedicated cranial CT examination is performed, as CTA requires relatively little patient cooperation, is a quick examination, and can identify sites of intracranial or extracranial vessel stenosis or occlusion as possible underlying causes of a patient’s acute symptoms. It can therefore potentially identify the source of an ischemic process to aid in the planning of (sometimes emergent) definitive therapy.
Computed Tomographic Perfusion Imaging
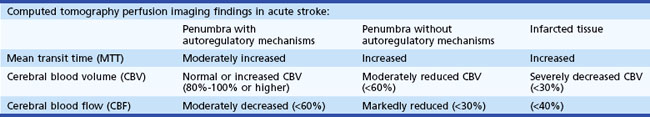
Computed tomography perfusion imaging is 75.7% to 86% accurate for detecting stroke and 94.4% accurate in determining the extent of stroke (Shetty and Lev, 2005). Table 33E.2 lists perfusion imaging parameters, and Table 33E.3 describes typical perfusion imaging findings with this technique.
| Cerebral blood volume | The volume of blood per unit of brain tissue; normal range = 4-5 mL /100 g |
| Cerebral blood flow | The volume of blood flow per unit of brain tissue per minute; normal range in gray matter = 50-60 mL /100 g/min |
| Mean transit time | Time difference between the arterial inflow and venous outflow |
| Time to peak enhancement | Time from the beginning of contrast material injection to the maximum concentration of contrast material within a region of interest (ROI) |
Magnetic Resonance Imaging in Acute Stroke Evaluation
Conventional spin-echo MRI is more sensitive and more specific than CT for the detection of acute cerebral ischemia within the first few hours after the onset of stroke. It has the additional benefit of depicting the pathological entity (stroke and its mimics) in multiple planes. The MR sequences typically used in the evaluation of acute stroke include T1-weighted spin-echo, T2-weighted fast spin-echo, fluid-attenuated inversion recovery, T2*-weighted gradient echo, and gadolinium-enhanced T1-weighted spin-echo sequences (Schellinger et al., 2001). Common MRI results in acute stroke can be found in Box 33E.2.
Perfusion-weighted MRI is used to identify areas of reversible ischemia.
Intravenous Thrombolysis
Intravenous thrombolysis is an IV therapeutic modality that was FDA approved in 1996 for patients arriving before 3 hours of onset of symptoms. This decision was based upon results published by the National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group (1995).
For every 100 patients treated with tPA, 32 benefit and 3 deteriorate. It is necessary to treat 8.3 patients to see a patient completely recovered, and 3.1 patients to observe clinical improvement (Saver 2004).
Del Zoppo reported in 1992 that IV recombinant tissue plasminogen activator (rtPA) is more efficient in smaller arteries such as the M2 and M3 portions of the MCA and less so in the ICA and M1 portion of the MCA (Saver, 2004). He reports an 8% recanalization rate in the ICA, 26% in MCA M1, 35% in M2, and 40% in M3.
Intraarterial Thrombolysis
The technique of intraarterial thrombosis requires intracranial navigation with a microcatheter and guide wire and location of the tip of the microcatheter distal to the clot (Fig. 33E.9). The contrast injection must be delivered gently, avoiding arterial perforators and distal emboli. A dose of 2 mg of rtPA is delivered distal to the clot before introducing the microcatheter tip into the middle of the clot. Then 10 to 20 mg of rtPA is delivered in situ over 60 to 120 minutes, checking with contrast injections every 15 to 20 minutes. A gentle mechanical manipulation of the clot with the guide wire can also be done.
The authors concluded that despite an increased frequency of early symptomatic ICH, treatment with intraarterial rpro-UK within 6 hours of the onset of acute ischemic stroke caused by MCA occlusion significantly improved clinical outcome at 90 days (Furlan et al., 1999).
Mechanical Thrombectomy
MERCI Retriever
The MERCI Retriever is an FDA-approved device intended to restore blood flow by removing intracranial thrombus in patients experiencing an ischemic stroke (Fig. 33E.10). It may be used alone or in combination with intraarterial rtPA.
The Multi-MERCI Trial involved 14 sites in the United States and Canada and recruited 111 patients up to 8 hours after onset of symptoms. It included anterior and posterior circulation arteries, intraarterial thrombolysis was permitted, and its primary endpoint was arterial recanalization. The study reported an overall Thrombolysis in Myocardial Infarction grading system (TIMI) 2+3 recanalization rate, 2.4% device-related complications, and 5.5% procedure-related complications. Total ICH at 24 hours was 40.2% (30.5% asymptomatic and 9.8% symptomatic). Some 36% of patients had a 90-day good outcome (modified Rankin Scale <2); mortality was 34% at 90 days. In the cases of successful arterial recanalization, good outcome was observed in 49% of patients and in 10% of patients with poor arterial recanalization (Smith et al., 2008).
Penumbra System
The Penumbra Pivotal Stroke Trial reported its results in the journal Stroke in 2009. A total of 125 target vessels in 125 patients were treated by the Penumbra system. Post procedure, 81.6% of the treated vessels were successfully revascularized to TIMI 2 to 3. There were 18 procedural events reported in 16 patients (12.8%); 3 patients (2.4%) had events that were considered serious. A total of 35 patients (28%) were found to have ICH on 24-hour CT, of which 14 (11.2%) were symptomatic. All-cause mortality was 32.8% at 90 days, with 25% of the patients achieving a modified Rankin Scale score of 2 or below. The authors concluded that their results suggest the Penumbra system allows safe and effective revascularization in patients experiencing ischemic stroke secondary to large-vessel occlusive disease who present within 8 hours from symptom onset.
1995 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581-1587.
2009 The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40(8):2761-2768.
Anxionnat R., Bracard S., Ducrocq X., et al. Intracranial aneurysms: clinical value of 3D digital subtraction angiography in the therapeutic decision and endovascular treatment. Radiology. 2001;218(3):799-808.
Berenstein A., Lasjounias P., TerBrugge K.G. Surgical Neuroangiography. Clinical and Endovascular Treatment Aspects, vol 2. Berlin: Springer. 2004.
Bhattacharya J.J., Thammaroj J. Vein of Galen malformations. J Neurol Neurosurg Psychiatry. 2003;74(Suppl. 1):i42-i44.
Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82(2):166-179.
Brekenfeld C., Mattle H.P., Schroth G. General is better than local anesthesia during endovascular procedures. Stroke. 2010;41(11):2716-2717.
Brott T.G., Hobson R.W.2nd, Howard G., et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11-23.
Brown R.D.Jr., Wiebers D.O., Torner J.C., et al. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted County, Minnesota. J Neurosurg. 1996;85(1):29-32.
Brown R.D.Jr., Wiebers D.O., Torner J.C., et al. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology. 1996;46(4):949-952.
Bruder N., Velly L., Codaccioni J.L. Modern approach to SAH in intensive care unit (ICU). Interv Neuroradiol. 2008;14(Suppl 1):13-16.
Calvo W.J., Lieber B.B., Hopkins L.N., et al. Europium fluorescence to visualize N-butyl-2-cyanoacrylate in embolized vessels of an arteriovenous malformation swine model. AJNR Am J Neuroradiol. 2001;22(4):691-697.
Caso V., Billeci A.M., Leys D. Interventional neuroradiology in the treatment of cerebral venous thrombosis. Front Neurol Neurosci. 2008;23:144-160.
Chaloupka J.C., Huddle D.C. Classification of vascular malformations of the central nervous system. Neuroimaging Clin N Am. 1998;8(2):295-321.
Chien A., Castro M.A., Tateshima S., et al. Quantitative hemodynamic analysis of brain aneurysms at different locations. AJNR Am J Neuroradiol. 2009;30(8):1507-1512.
Chimowitz M.I., Lynn M.J., Howlett-Smith H., et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305-1316.
Choi J.H., Mohr J.P. Brain arteriovenous malformations in adults. Lancet Neurol. 2005;4(5):299-308.
Cognard C., Gobin Y.P., Pierot L., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194(3):671-680.
Cognard C., Januel A.C., Silva N.A.Jr., et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008;29(2):235-241.
Connolly E.S.Jr., Lavine S.D., Meyers P.M., et al. Intensive care unit management of interventional neuroradiology patients. Neurosurg Clin N Am. 2005;16(3):541-545. vi
Connors J.J.3rd, Sacks D., Furlan A.J., et al. Training, competency, and credentialing standards for diagnostic cervicocerebral angiography, carotid stenting, and cerebrovascular intervention: a joint statement from the American Academy of Neurology, the American Association of Neurological Surgeons, the American Society of Interventional and Therapeutic Neuroradiology, the American Society of Neuroradiology, the Congress of Neurological Surgeons, the AANS/CNS Cerebrovascular Section, and the Society of Interventional Radiology. Neurology. 2005;64(2):190-198.
Davies M.A., TerBrugge K., Willinsky R., et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85(5):830-837.
Doerfler A., Wanke I., Egelhof T., et al. Double-stent method: therapeutic alternative for small wide-necked aneurysms. Technical note. J Neurosurg. 2004;100(1):150-154.
Dorfler A., Struffert T., Engelhorn T., et al. Rotational flat-panel computed tomography in diagnostic and interventional neuroradiology. Rofo. 2008;180(10):891-898.
Feliciano C.E., de Leon-Berra R., Hernandez-Gaitan M.S., et al. A proposal for a new arteriovenous malformation grading scale for neuroendovascular procedures and literature review. P R Health Sci J. 2010;29(2):117-120.
Fiorella D., Levy E.I., Turk A.S., et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38(3):881-887.
Fiorella D.J., Levy E.I., Turk A.S., et al. Target lesion revascularization after wingspan: assessment of safety and durability. Stroke. 2009;40(1):106-110.
Fleetwood I.G., Steinberg G.K. Arteriovenous malformations. Lancet. 2002;359(9309):863-873.
Ford M.D., Nikolov H.N., Milner J.S., et al. PIV-measured versus CFD-predicted flow dynamics in anatomically realistic cerebral aneurysm models. J Biomech Eng. 2008;130(2):021015.
Furlan A., Higashida R., Wechsler L., et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003-2011.
Galimany-Masclans J., Garcia-Sort R., Pernas-Canadell J.C. Nursing care in patients undergoing interventional neuroendovascular procedures. Enferm Clin. 2009;19(3):160-163.
Gandhi D., Gemmete J.J., Ansari S.A., et al. Interventional neuroradiology of the head and neck. AJNR Am J Neuroradiol. 2008;29(10):1806-1815.
Geibprasert S., Pongpech S., Jiarakongmun P., et al. Radiologic assessment of brain arteriovenous malformations: what clinicians need to know. Radiographics. 2010;30(2):483-501.
Gemmete J.J., Ansari S.A., Gandhi D. Endovascular treatment of carotid-cavernous fistulas. Neuroimaging Clin N Am. 2009;19(2):241-255.
Gemmete J.J., Ansari S.A., Gandhi D.M. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuroophthalmol. 2009;29(1):62-71.
Gomez F., Escobar W., Gomez A.M., et al. Treatment of carotid cavernous fistulas using covered stents: midterm results in seven patients. AJNR Am J Neuroradiol. 2007;28(9):1762-1768.
Gupta A.K., Rao V.R., Varma D.R., et al. Evaluation, management, and long-term follow up of vein of Galen malformations. J Neurosurg. 2006;105(1):26-33.
Gurm H.S., Yadav J.S., Fayad P., et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358(15):1572-1579.
Halbach V.V., Higashida R.T., Hieshima G.B., et al. Transarterial occlusion of solitary intracranial arteriovenous fistulas. AJNR. 1989;10:747-752.
Hauck E.F., Welch B.G., White J.A., et al. Preoperative embolization of cerebral arteriovenous malformations with Onyx. AJNR Am J Neuroradiol. 2009;30(3):492-495.
Heros R.C. Clip ligation or coil occlusion? J Neurosurg. 2006;104(3):341-343. discussion 343
Hoang S., Choudhri O., Edwards M., et al. Vein of Galen malformation. Neurosurg Focus. 2009;27(5):E8.
Hoh B.L., Chi Y.Y., Lawson M.F., et al. Length of stay and total hospital charges of clipping versus coiling for ruptured and unruptured adult cerebral aneurysms in the Nationwide Inpatient Sample database 2002 to 2006. Stroke. 2010;41(2):337-342.
Hoh B.L., Putman C.M., Budzik R.F., et al. Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery. 2001;49(6):1351-1363. discussion 1363-1364
Horowitz M.B., Jungreis C.A., Quisling R.G., et al. Vein of Galen aneurysms: a review and current perspective, in: Neuroendovascular Surgery. Karger: Basel; 2005. 216-231
Jabbour P.M., Tjoumakaris S.I., Rosenwasser R.H. Endovascular management of intracranial aneurysms. Neurosurg Clin N Am. 2009;20(4):383-398.
Jiang C., Lv X., Li Y., et al. Transarterial Onyx packing of the transverse-sigmoid sinus for dural arteriovenous fistulas. Eur J Radiol. 2010. Epub before print
Jo K.W., Park S.M., Kim S.D., et al. Is transradial cerebral angiography feasible and safe? A single center’s experience. J Korean Neurosurg Soc. 2010;47(5):332-337.
Kamran M., Nagaraja S., Byrne J.V. C-arm flat detector computed tomography: the technique and its applications in interventional neuro-radiology. Neuroradiology. 2010;52(4):319-327.
Kathleen A.M., Stavropoula I.T., Jason A., et al. Neuroendovascular management of dural arteriovenous malformations. Neurosurg Clin N Am. 2009;20(4):431.
Katsaridis V. Treatment of dural arteriovenous fistulas. Curr Treat Options Neurol. 2009;11(1):35-40.
Khullar D., Andeejani A.M., Bulsara K.R. Evolution of treatment options for vein of Galen malformations. J Neurosurg Pediatr. 2010;6(5):444-451.
Kumar J., Kumar A., Gupta S. Vein of Galen aneurysmal malformation. Arch Neurol. 2006;63(10):1500-1501.
Lee J.Y., Son Y.J., Kim J.E. Intracranial pial arteriovenous fistulas. J Korean Neurosurg Soc. 2008;44(2):101-104.
Locksley H.B. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1966;25:321-368.
Lubicz B., Bandeira A., Bruneau M., et al. Stenting is improving and stabilizing anatomical results of coiled intracranial aneurysms. Neuroradiology. 2009;51(6):419-425.
Lv X., Jiang C., Li Y., et al. Percutaneous transvenous packing of cavernous sinus with Onyx for cavernous dural arteriovenous fistula. Eur J Radiol. 2009;71(2):356-362.
Lylyk P., Miranda C., Ceratto R., et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632-642. discussion 642-643; quiz N6
Maimon S., Strauss I., Frolov V., et al. Brain arteriovenous malformation treatment using a combination of Onyx and a new detachable tip microcatheter, SONIC: short-term results. AJNR Am J Neuroradiol. 2010;31:947-954.
Mo D.P., Bao S.D., Li L., et al. Virtual reality system for diagnosis and therapeutic planning of cerebral aneurysms. Chin Med J (Engl). 2010;123(16):2206-2210.
Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274.
Molyneux A.J., Cekirge S., Saatci I., et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25(1):39-51.
Molyneux A.J., Kerr R.S., Birks J., et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8(5):427-433.
Moret J., Cognard C., Weill A., et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997;24(1):30-44.
Moron F.E., Klucznik R.P., Mawad M.E., et al. Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol. 2005;26(6):1399-1404.
Naggara O.N., White P.M., Guilbert F., et al. Endovascular treatment of intracranial unruptured aneurysms: systematic review and meta-analysis of the literature on safety and efficacy. Radiology. 2010;256(3):887-897.
Nguyen T.N., Hoh B.L., Amin-Hanjani S., et al. Comparison of ruptured vs unruptured aneurysms in recanalization after coil embolization. Surg Neurol. 2007;68(1):19-23.
Oran I., Parildar M., Derbent A. Ventricular/paraventricular small arteriovenous malformations: role of embolisation with cyanoacrylate. Neuroradiology. 2005;47(4):287-294.
Passacantilli E., Pichierri A., Guidetti G., et al. Surgical treatment of pial cerebellar arteriovenous fistulas with aneurysm of the main feeding artery. Surg Neurol. 2006;65(1):90-94.
Pearl M., Gomez J., Gregg L., et al. Endovascular management of vein of Galen aneurysmal malformations. Influence of the normal venous drainage on the choice of a treatment strategy. Childs Nerv Syst. 2010;26(10):1367-1379.
Pearl M., Gregg L., Gailloud P. Endovascular treatment of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):271-280.
Pexman J.H., Barber P.A., Hill M.D., et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22(8):1534-1542.
Qureshi A.I., Abou-Chebl A., Jovin T.G. Qualification requirements for performing neurointerventional procedures: a report of the Practice Guidelines Committee of the American Society of Neuroimaging and the Society of Vascular and Interventional Neurology. J Neuroimaging. 2008;18(4):433-447.
Registry, T.I.S. C.-S.T. The Internet Stroke Center Stroke Trials Registry-SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis. Available at http://www.strokecenter.org/trials/TrialDetail.aspx?tid=819, 2010.
Rossitti S., Pfister M. 3D road-mapping in the endovascular treatment of cerebral aneurysms and arteriovenous malformations. Interv Neuroradiol. 2009;15(3):283-290.
Rubin D., Santillan A., Greenfield J.P., et al. Surgical management of pediatric cerebral arteriovenous malformations. Childs Nerv Syst. 2010;26(10):1337-1344.
Santos-Franco J.A., Zenteno-Castellanos M.A., Jaramillo-Magana Jde J., et al. Endovascular management of carotid atherosclerosis. Section II: current therapeutic approaches and limitations. Gac Med Mex. 2009;145(5):415-425.
Saraf R., Shrivastava M., Kumar N., et al. Embolization of cranial dural arteriovenous fistulae with Onyx: indications, techniques, and outcomes. Indian J Radiol Imaging. 2010;20(1):26-33.
Saver J.L. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004;61(7):1066-1070.
Schellinger P.D., Fiebach J.B., Jansen O., et al. Stroke magnetic resonance imaging within 6 hours after onset of hyperacute cerebral ischemia. Ann Neurol. 2001;49(4):460-469.
Schlosser M.J., McCarthy G., Fulbright R.K., et al. Cerebral vascular malformations adjacent to sensorimotor and visual cortex. Functional magnetic resonance imaging studies before and after therapeutic intervention. Stroke. 1997;28(6):1130-1137.
Sekhar L.N., Biswas A., Hallam D., et al. Neuroendovascular management of tumors and vascular malformations of the head and neck. Neurosurg Clin N Am. 2009;20(4):453-485.
Shetty S.K., Lev M.H. CT perfusion in acute stroke. Neuroimaging Clin N Am. 2005;15(3):481-501. ix
Shtraus N., Schifter D., Corn B.W., et al. Radiosurgical treatment planning of AVM following embolization with Onyx: possible dosage error in treatment planning can be averted. J Neurooncol. 2010;98(2):271-276.
Smith W.S., Sung G., Saver J., et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205-1212.
Sorimachi T., Koike T., Takeuchi S., et al. Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: angiographic reappearance and complications. AJNR Am J Neuroradiol. 1999;20(7):1323-1328.
Spetzler R.F., Ponce F.A. A 3-tier classification of cerebral arteriovenous malformations. J Neurosurg. 2010.
Stapf C., Mast H., Sciacca R.R., et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke. 2003;34(5):e29-e33.
Stapf C., Mohr J.P., Choi J.H., et al. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Curr Opin Neurol. 2006;19(1):63-68.
Starke R.M., Komotar R.J., Otten M.L., et al. Adjuvant embolization with N-butyl cyanoacrylate in the treatment of cerebral arteriovenous malformations: outcomes, complications, and predictors of neurologic deficits. Stroke. 2009;40(8):2783-2790.
Strozyk D., Nogueira R.G., Lavine S.D. Endovascular treatment of intracranial arteriovenous malformation. Neurosurg Clin N Am. 2009;20(4):399-418.
Teasdale G.M., Drake C.G., Hunt W., et al. A universal subarachnoid hemorrhage scale: a report of a committee of the World Federation of Neurological Societies. J Neurol Neurosurg Psych. 1988;51(11):1457.
Teng M.M., Chang C.Y., Chiang J.H., et al. Double-balloon technique for embolization of carotid cavernous fistulas. AJNR Am J Neuroradiol. 2000;21(9):1753-1756.
Theron J.G., Payelle G.G., Coskun O., et al. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology. 1996;201(3):627-636.
Thorisson H.M., Johnson M.H. Endovascular management of extracranial carotid and vertebral disease. Neurosurg Clin N Am. 2009;20(4):487-506.
Tomandl B.F., Hastreiter P., Iserhardt-Bauer S., et al. Standardized evaluation of CT angiography with remote generation of 3D video sequences for the detection of intracranial aneurysms. Radiographics. 2003;23(2):e12.
Turowski B., Zanella F.E. Interventional neuroradiology of the head and neck. Neuroimaging Clin N Am. 2003;13(3):619-645.
Valle R.D., Zenteno M., Jaramillo J., et al. Definition of the key target volume in radiosurgical management of arteriovenous malformations: a new dynamic concept based on angiographic circulation time. J Neurosurg. 2008;109(Suppl):41-50.
Varma M.K., Price K., Jayakrishnan V., et al. Anaesthetic considerations for interventional neuroradiology. Br J Anaesth. 2007;99(1):75-85.
Vinuela F., Duckwiler G., Jahan R., et al. Therapeutic management of cerebral arteriovenous malformations. Present role of interventional neuroradiology. Interv Neuroradiol. 2005;11(Suppl 1):13-29.
Weber W., Kis B., Siekmann R., et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. AJNR Am J Neuroradiol. 2007;28(2):371-377.
Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103-110.
Wikholm G. Occlusion of cerebral arteriovenous malformations with N-butyl cyano-acrylate is permanent. AJNR Am J Neuroradiol. 1995;16(3):479-482.
Wright I. Cerebral aneurysm–treatment and perioperative nursing care. AORN J. 2007;85(6):1172-1182. quiz 1183-1186
Xu F., Ni W., Liao Y., et al. Onyx embolization for the treatment of brain arteriovenous malformations. Acta Neurochir (Wien). 2010.
Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493-1501.
Yen C.P., Monteith S.J., Nguyen J.H., et al. Gamma knife surgery for arteriovenous malformations in children. J Neurosurg Pediatr. 2010;6(5):426-434.
Young W.L. Anesthesia for endovascular neurosurgery and interventional neuroradiology. Anesthesiol Clin. 2007;25(3):391-412. vii
Yu S.C.H., Chan M.S.Y., Lam J.M.K., et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol. 2004;25(7):1139-1143.
Yuki I., Kim R.H., Duckwiler G., et al. Treatment of brain arteriovenous malformations with high-flow arteriovenous fistulas: risk and complications associated with endovascular embolization in multimodality treatment. Clinical article. J Neurosurg. 2010;113(4):715-722.
Zaidat O.O., Klucznik R., Alexander M.J., et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70(17):1518-1524.
Zenteno-Castellanos M.A., Santos-Franco J.A., Vega-Montesinos S., et al. Endovascular management of carotid atherosclerosis. Section I: basic considerations and diagnostic elements. Gac Med Mex. 2009;145(5):407-414.
Zenteno M., Santos-Franco J., Rodriguez-Parra V., et al. Management of direct carotid-cavernous sinus fistulas with the use of ethylene-vinyl alcohol (Onyx) only: preliminary results. J Neurosurg. 2010;112(3):595-602.