Neuroimaging in Pediatric Epilepsy
Epilepsy is a common pediatric neurological disorder. In North America, the overall annual incidence of epilepsy is approximately 50 per 100,000 persons. The incidence is highest for children younger than 5 years and for elderly persons.1,2 Children are at higher risk for the development of epilepsy than are adults. Atypical, idiopathic, and focal epilepsy, as well as epileptic syndromes, require evaluation with magnetic resonance imaging (MRI). In approximately 30% of children with epilepsy, the disease becomes refractory to medical therapy.3 In children with refractory epilepsy, neuroimaging is critical for identification of an epileptogenic substrate that is responsible for the epilepsy, particularly in children undergoing surgery. Patients without a lesion (that is, patients who have normal MRI findings) have been reported to have poorer outcomes of epilepsy surgery compared with patients who have a lesion identified upon neuroimaging.4 Epileptogenic substrates include malformations of cortical development (MCDs), developmental tumors, anoxic-ischemic injuries, prior cerebrovascular disease, neurocutaneous syndromes, and Rasmussen encephalitis. Concurrent lesions such as MCD, hippocampal sclerosis, and developmental tumors can occur in approximately 13% to 20% of cases.5,6 Another important role of imaging in children with intractable epilepsy is to identify the location of eloquent cortex and white matter tracts as part of the presurgical evaluation (see Chapter 27).
The MRI epilepsy protocol should include volumetric T1-weighted imaging, T2-weighted imaging, fluid attenuated inversion recovery (FLAIR), proton density, and inversion recovery sequences in at least two orthogonal planes, covering the entire brain. Volumetric gradient echo T1-weighted acquisitions with 1- to 1.5-mm section thickness provide excellent gray/white matter contrast and are useful for better anatomic delineation. This sequence can be reformatted into any orthogonal or nonorthogonal plane without the penalty of increased scan time and can be used for anatomic integration of functional data, stereotactic electrode placement, and neuronavigation.7–9 In persons with temporal lobe epilepsy, the coronal plane should be acquired perpendicular to the long axis of the hippocampus. Injection of contrast material does not improve the detection of a lesion but may help characterize a lesion once it is found. In cases in which an epileptogenic focus is not identified, further evaluation with dedicated higher resolution MRI, image postprocessing, or additional imaging techniques including diffusion tensor imaging, magnetic resonance spectroscopy, and functional imaging with interictal positron emission tomography (PET) or ictal/interictal single photon emission computed tomography (SPECT), along with magnetoencephalography, may help in identifying a lesion or the epileptogenic zone.10,11
Malformations of Cortical Development
MCDs are a major cause of drug-resistant epilepsy,12 in particular focal cortical dysplasia (FCD), hemimegalencephaly, and tuberous sclerosis. Other MCDs such as lissencephaly, gray matter heterotopia, polymicrogyria, and schizencephaly also are associated with epilepsy.13–16
Focal Cortical Dysplasia
Overview: FCD is an MCD that is intrinsically epileptogenic. It is one of the most common causes of intractable epilepsy in children and accounts for up to 39% of surgical cases.17,18 The mechanism of epilepsy is still unclear. Possibilities include abnormal firing from the dysplastic neurons rather than from balloon cells,19 dysfunction of synaptic circuits with abnormal synchronization of the neuronal population, and abnormal organization of the inhibitory interneurons.20 Numerous classifications of FCD have been proposed.13,21–23 Recently, a consensus classification has been proposed by the International League Against Epilepsy (Table 38-1).24
Table 38-1
Histopathologic Classification of Focal Cortical Dysplasia
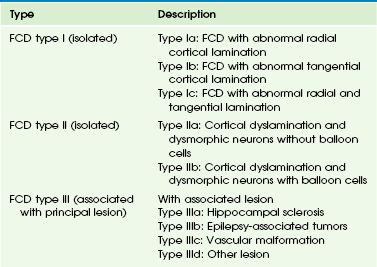
FCD, Focal cortical dysplasia.
From Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52(1):158-174.
Imaging: FCD can be located in any cortex of the cerebral hemisphere and can have variable size, from one gyrus to more than one lobe. The MRI features of FCD are increased cortical thickness, blurring of the cortical–white matter junction, increased T2 or FLAIR signal in the cortex and subcortical white matter, high T1 signal in the cortex, and an abnormal sulcation and gyration pattern. Taylor FCD or FCD type IIB more commonly involves the extratemporal cortex and is more likely to demonstrate a high T2 and FLAIR signal that tapers toward the ventricle.21,25,26 Non-Taylor FCD (type I FCD and mild MCDs) is more likely to be located in the temporal lobe and to demonstrate hypoplasia or atrophy of the white matter and mild increased signal. FCD, in particular non-Taylor FCD, is associated with hippocampal sclerosis.27 The MRI appearance of FCD may change with brain maturation. Longitudinal MRI studies in infants with FCD have shown that findings of the early study may be normal but later imaging may demonstrate a high T2 signal in the white matter, a high T1 signal in the cortex, and blurring of the cortical/subcortical white matter junction.28 The high signal in the white matter may be a result of abnormal myelination, either because of the underlying disease or as a result of seizures. Thus even when the MRI findings appear normal in a neonate or an infant with refractory partial seizures or infantile spasm, a repeat study is recommended (Fig. 38-1).28 In contrast to the high T2/FLAIR signal in the white matter of FCD in children, the white matter adjacent to the dysplastic cortex may demonstrate a low T2 and high T1 signal in neonates and infants, which is thought to be secondary to early white matter myelination as a result of repeated seizures.29
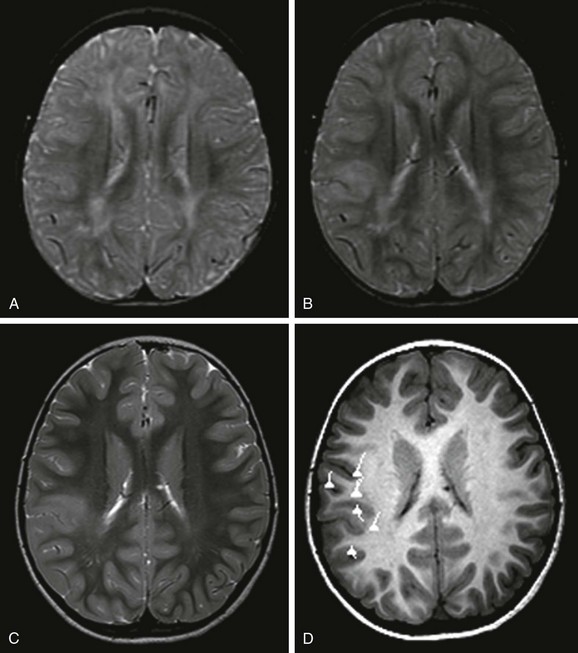
Figure 38-1 A 5-year-old girl with focal cortical dysplasia.
An axial T2-weighted image at 12 months (A) does not show a lesion. However, at 19 months (B) and 5 years of age (C), axial T2-weighted images demonstrate an abnormal signal in the cortex and subcortical white matter that is associated with blurring of the gray/white matter junction. D, Magnetoencephalography projected onto an axial T1-weighted image demonstrates that the dipoles, indicative of the epileptogenic zone, are located in and around the lesion. Surgical resection of the lesion and epileptogenic zone confirms the presence of focal cortical dysplasia type IIB.
Treatment: Good seizure control or a seizure-free outcome is achieved in up to 50% to 70% of patients with FCD after surgical resection of the FCD.30–32 This outcome compares favorably with the outcome of patients who have hippocampal sclerosis33 and a low-grade neoplasm.34 The reported surgical outcomes of persons with subtypes of FCD are variable. Authors of some studies reported a better surgical outcome in persons with Taylor FCD,21,35,36 whereas other authors reported better surgical outcomes in persons with other subtypes of FCD, including type I FCD and mild MCD.37,38 Differences in outcomes of persons with subtypes of FCD may in part be related to the preponderance of type I FCD and mild MCD in the temporal lobe.37
Hemimegalencephaly
Overview: Hemimegalencephaly is an MCD that is characterized by one hemisphere being larger than the contralateral side. It may be sporadic or it may be associated with a variety of syndromes, including proteus syndrome, epidermal nevus syndrome, hypomelanosis of Ito, linear nevus sebaceous syndrome, neurofibromatosis type I, and tuberous sclerosis. The sporadic form is considered a hemispheric variant of FCD.39 On histology, the appearance is similar to FCD with abnormal gyration of the cortex, dyslamination, blurring of the gray/white matter junction, giant neurons in both gray and white matter, and balloon cells in 50% of cases. The most common clinical presentation is early intractable epilepsy; other clinical presentations include hemiparesis, hemianopia, and mental retardation.
Imaging: The affected hemisphere is larger than the contralateral side. The cortex is dysplastic and thick, with broad gyri and shallow sulci (e-Fig. 38-2). The ipsilateral lateral ventricle may be enlarged. The ipsilateral white matter demonstrates variable signal changes, depending on the age of the patient. Neonates and infants usually demonstrate a high T1 and low T2 signal in the white matter suggestive of early myelination, whereas in older children the white matter shows a low T1 signal and a high T2 signal with associated cystic changes and calcification.40–42 With recurrent seizures or status epilepticus, the enlarged hemisphere later may become atrophic.43
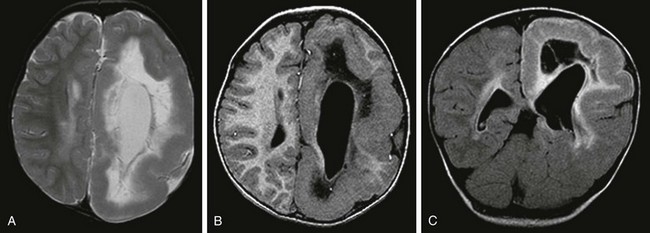
e-Figure 38-2 A 2-year-old with hemimegalencephaly.
An axial T2-weighted image (A), an axial volumetric T1-weighted image (B), and a coronal fluid attenuated inversion recovery (FLAIR) image (C) demonstrate diffuse enlargement of the left cerebral hemisphere that is associated with an abnormally thick cortex, an abnormal sulcation and gyration pattern, and a dysmorphic and enlarged left lateral ventricle. An abnormal increased T2 and FLAIR signal and a reduced T1 signal in the white matter of the affected hemisphere also are noted.
Tuberous Sclerosis Complex
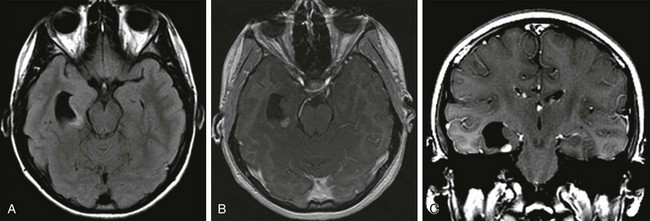
Overview and Imaging: Tuberous sclerosis complex is an autosomal-dominant neurocutaneous syndrome characterized by multisystem involvement including the brain, eyes, heart, kidney, skin, and lung. Seizures occur in 80% to 90% of patients, and seizures are intractable in 25% to 30% of patients.44 Children with medically refractory epilepsy usually have multiple cortical/subcortical tubers that exhibit broad gyri, a thick cortex, and an abnormal signal in the cortex and subcortical white matter. The cortical/subcortical tubers occasionally may demonstrate calcification and cystic degeneration. A combination of structural and functional imaging such as fluorodeoxyglucose (FDG)-PET, ictal/interictal SPECT, and magnetoencephalography can be used to identify the epileptogenic tubers45–48 (Fig. 38-3). Cerebellar tubers also occur and more commonly are seen in patients with a high cerebral tuber burden. The subependymal nodules often calcify. Another manifestation of tuberous sclerosis complex is subependymal giant cell astrocytomas, which commonly occur near the foramen of Monro.
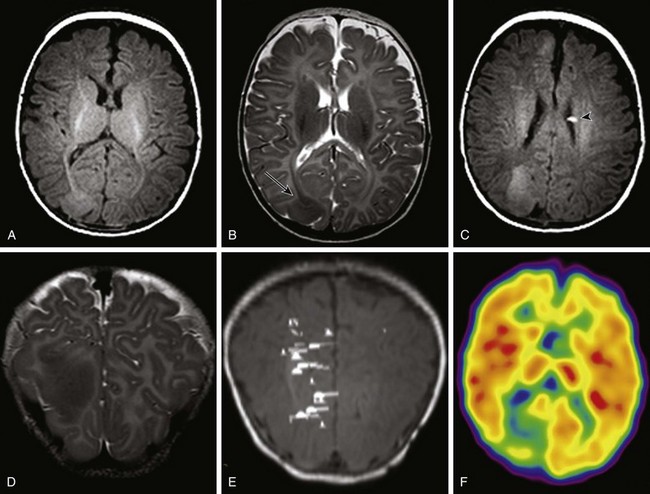
Figure 38-3 A 3-month-old with tuberous sclerosis complex and right occipital lobe intractable epilepsy.
Axial T1-weighted images (A and C), an axial T2-weighted image (B), and a coronal T2-weighted image (D) indicate several cortical/subcortical tubers in the right frontal and occipital lobes (arrow) that demonstrate a high T1 and lower T2 signal. Several subependymal nodules also are present (arrowhead). E, Magnetoencephalography dipoles projected onto a coronal T1 image demonstrate a dipole cluster in the right occipital lobe. F, A fluorodeoxyglucose positron emission tomography study shows hypometabolism (blue and green) that is more extensive than the right occipital tuber.
Sturge-Weber Syndrome
Overview and Imaging: Sturge-Weber syndrome, or encephalotrigeminal angiomatosis, is a phakomatosis characterized by a facial capillary vascular malformation or “port wine stain” in the territory of the trigeminal nerve, ipsilateral leptomeningeal angiomatosis, and angiomatosis of the choroid of the ipsilateral eye. Seizures usually are the initial neurological manifestation in the first year of life. Children with early onset of epilepsy are more likely to have hemiparesis, status epilepticus, and developmental delay compared with persons whose seizures start later in life.49 Imaging findings include leptomeningeal angiomatosis, enlargement of the choroid plexus, parenchymal atrophy, calvarial changes, and calcification (Fig. 38-4). Leptomeningeal angiomatosis and choroid plexus hyperplasia appear earlier in the course of the disease, whereas atrophy and calcification usually are evident later. Contrast-enhanced MRI is considered the most sensitive sequence for depicting leptomeningeal enhancement and revealing subtle bilateral hemispheric involvement, compensatory venous drainage, enlarged choroid plexus in the atria, and cerebellar and eye involvement.50–52
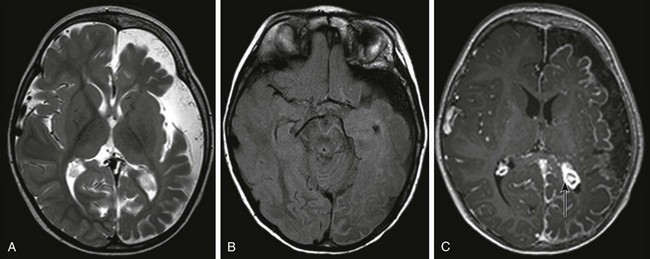
Figure 38-4 A 15-month-old boy with Sturge-Weber syndrome.
An axial T2-weighted image (A) and an axial fluid attenuated inversion recovery (FLAIR) image (B) show volume loss in the left cerebral hemisphere with enlargement of subarachnoid spaces and a high FLAIR signal in the left cerebral sulci. C, An axial T1-weighted postcontrast image shows pial enhancement over the left cerebral hemisphere, as well as an enlarged choroid plexus in the left trigone (arrow).
Mesial Temporal Sclerosis
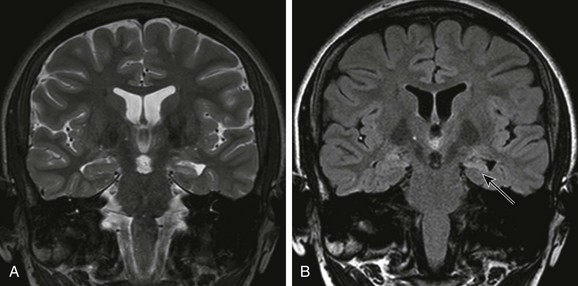
Overview and Imaging: Mesial temporal sclerosis is encountered less frequently in children than in adults.53–56 The etiology of hippocampal sclerosis is unknown, but a link with febrile seizures has been suggested.57,58 Hippocampal sclerosis is characterized by neuronal loss and gliosis in the hippocampus.59 MRI features include atrophy and increased T2 and FLAIR signal in the hippocampus (Fig. 38-5).59–62 Other findings include loss of interdigitations of the hippocampal head,63 atrophy of the ipsilateral mamillary body and fornix,64 dilatation of the ipsilateral temporal horn, volume loss of the temporal lobe, and atrophy of the collateral white matter between the hippocampus and collateral sulcus.62 Functional imaging with FDG-PET usually reveals hypometabolic activity that is larger than the hippocampus abnormality65,66 and can identify mesial temporal metabolic abnormalities in patients with normal MRI.67
Epilepsy-Associated Developmental Tumors
Epilepsy-associated tumors may account for up to two thirds of the surgical pathologic substrate72–75 in epilepsy surgery. These tumors originate in and develop from the cortex and present clinically with seizures. Tumors associated with epilepsy usually are associated with benign biological behavior and a low proliferation index. Only a small percentage of these tumors may undergo malignant transformation.76 The tumors include gangliogliomas, gangliocytomas, desmoplastic infantile gangliogliomas, dysembryoplastic neuroepithelial tumors (DNETs), pleomorphic xanthoastrocytomas (PXAs), and low-grade astrocytomas.77 FCD may be present concurrently.
The most common clinical presentation of gangliogliomas is seizures, which often are complex, partial, and medically intractable.78 Tumors are larger in children than in adults,79 and they are located mostly in the temporomesial (50%) or temporolateral (29%) location.80 Gangliogliomas are composed of glia and neurons and may present as a solid mass in 43% of cases, a cyst in 5% of cases, and a mixed lesion in 52% of cases.76,81 Because they are cortical, they may cause remodeling of the adjacent bone. Most gangliogliomas (38%) are hypodense on computed tomography (CT), but isodense (15%), hyperdense (15%), or mixed density masses (32%) can be found,82 and calcification is seen in 30% to 50% of cases. On MRI, the solid part of the tumor may appear hypointense or isointense to gray matter on T1-weighted images and hyperintense on T2-weighted images (Fig. 38-6). Enhancement is present in up to 60% of cases; it can be nodular, ringlike, or solid. Gangliocytomas, a variant of gangliogliomas without any glial component, affect older children and young adults. On MRI, a gangliocytoma may demonstrate a low T1 and a high T2 signal and show enhancement.83,84
Dysembryoplastic Neuroepithelial Tumor
A DNET is a pathologically benign supratentorial cortical tumor that shows predilection for the temporal lobes (in 62% to 86% of cases).85 DNETs also frequently affect the frontal lobes. The lesion can have a well-demarcated margin (50%), or the margins may be slightly blurred. The tumor may be associated with broadening of the gyri, effacement of the sulci, distortion of the ventricles, and remodeling of the overlying skull.85,86 On CT, a DNET tends to be hypodense with a cystic appearance.87 Calcification has been reported in 20% to 36% of cases.86 On MRI, a DNET demonstrates a low T1 and a high T2 signal, often with a multicystic and “bubbly” appearance,85 and it may have a thin rim of high signal on a FLAIR sequence (Fig. 38-7).88,89 Approximately one third of cases shows nodular, patchy, or ring enhancement. The tumor may extend to the ventricle in 30% of cases. Hemorrhagic changes are rare but have been described.88,90
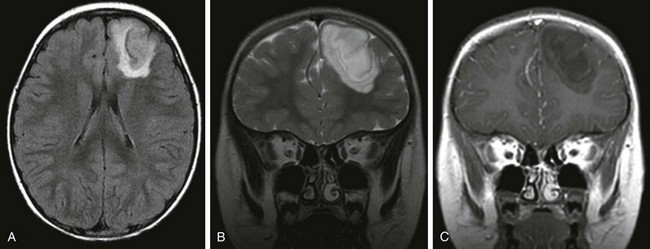
Figure 38-7 A 4-year-old boy with a dysembryoplastic neuroepithelial tumor.
An axial fluid attenuated inversion recovery (FLAIR) image (A), a coronal T2-weighted image (B), and a gadolinium-enhanced coronal T1-weighted image (C) demonstrate a well-defined tumor in the right frontal lobe involving the cortex and white matter. The tumor extends to the margin of the frontal horn of the left lateral ventricle. The tumor has a rim of high FLAIR signal and does not show enhancement.
Pleomorphic Xanthoastrocytoma
PXA is a rare localized glioma of astrocytic origin affecting children and young adults.91 It is mostly temporal (49%) and less commonly parietal, frontal, and occipital in location.92,93 Upon imaging, a PXA appears as a large hemispheric cystic and solid mass close to the cortex. CT shows that the tumor is predominantly hypodense with a mixed-density enhancing nodule. Upon MRI, the cystic portion of the tumor is isointense to CSF, and the solid tumor is hypointense to isointense to gray matter on T1-weighted images and hyperintense to isointense on T2-weighted images. Enhancement of the nodular solid component and the adjacent meninges is seen (“dural tail”) (e-Fig. 38-8).94 PXA may be associated with FCD.95,96
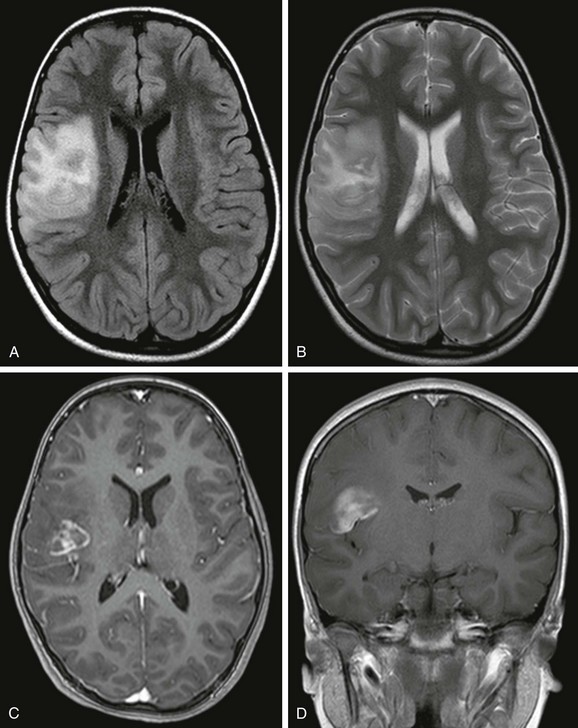
e-Figure 38-8 A 6-year-old with a pleomorphic xanthoastrocytoma.
An axial fluid attenuated inversion recovery image (A), an axial T2-weighted image (B), an axial image (C), and a coronal T1-weighted postcontrast image (D) demonstrate a mass in the right inferior frontal gyrus that involves the cortex and subcortical white matter. The tumor demonstrates enhancement medially, and there is surrounding edema.
Treatment and Follow-up: Patients with a ganglioglioma have the best surgical outcome with respect to epilepsy control compared with other types of epilepsy-associated tumors.74,80,97 DNETs have a less favorable seizure outcome compared with gangliogliomas, which could be related to longer duration of epilepsy, incomplete resection of the tumor, or the presence of FCD beyond the margins of the resected DNET.97–99 The prognosis of patients with PXA is generally good, but the tumor may recur, and malignant degeneration occurs in 20% of cases.100
Hypothalamic Hamartoma
Overview and Imaging: Hypothalamic hamartoma, also known as hamartoma of the tuber cinereum, is a congenital malformation of the hypothalamus that may be asymptomatic or may manifest with precocious puberty or gelastic seizures.101 Intrahypothalamic hamartomas that invade the third ventricle are associated with early occurrence of epilepsy, whereas the parahypothalamic hamartomas that do not involve the third ventricle more commonly present with central precocious puberty.102,103 MRI demonstrates a mass at the tuber cinereum that is T1 hypointense relative to the gray matter, T2 isointense or hyperintense relative to gray matter, and hyperintense on FLAIR. No enhancement occurs, and no calcification is seen (e-Fig. 38-9). Careful examination of the hypothalamic region on MRI is needed because hamartomas can be small and nonpedunculated.
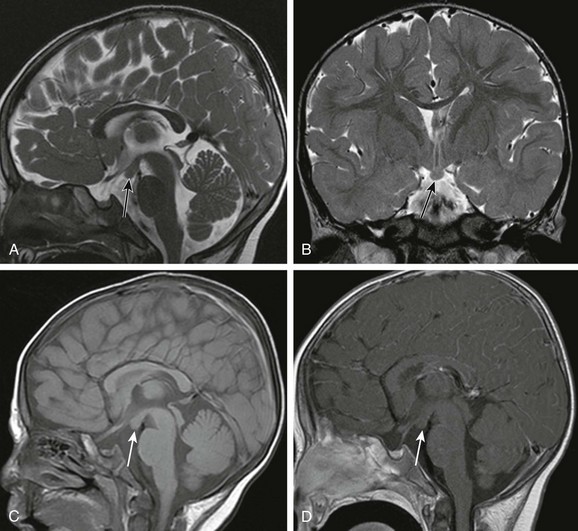
e-Figure 38-9 A 2-year-old with a hypothalamic hamartoma (arrows).
A sagittal T2-weighted image (A), a coronal T2-weighted image (B), and a sagittal T1-weighted image (C) show a focal lesion in the tuber cinereum that has a similar signal to gray matter on T1- and T2-weighted sequences. No enhancement of the lesion is seen on the sagittal T1-weighted postcontrast image (D).
Rasmussen Encephalitis
Overview and Imaging: Rasmussen encephalitis is a rare, chronic, progressive encephalitis characterized by severe refractory epilepsy and unilateral brain atrophy.105 Clinically, seizures begin abruptly in previously healthy children and include partial seizures and epilepsia partialis continua. With disease progression, hemiparesis or hemiplegia and cognitive decline develop. The disease might have an autoimmune basis.106–108 The histological findings include chronic encephalitis with lymphocytic infiltrations and perivascular cuffing; in the later stage of the disease, nonspecific findings such as atrophy and gliosis with minimal inflammatory cellular infiltrate are found.109 In the acute stages, findings of MRI can be entirely normal110,111 or demonstrate cortical swelling with a low T1 and hyperintense T2 and FLAIR signal, with no enhancement. With disease progression, MRI shows progressive volume loss and a progressive abnormal signal in the white matter and cortex (Fig. 38-10).112 Rasmussen encephalitis is predominantly a unilateral disease, and the frontal and temporal lobes more commonly are involved.
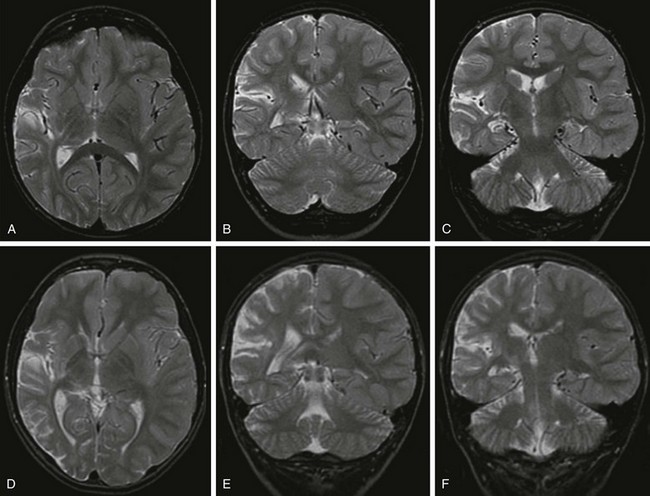
Figure 38-10 A 5-year-old boy with Rasmussen encephalitis presenting with epilepsia partialis continua.
At presentation (A, B, and C), axial and coronal T2-weighted images demonstrate mild volume loss in the right Sylvian and temporal lobe. Three years later (D, E, and F), axial and coronal T2-weighted images demonstrate progressive volume loss in the right Sylvian, temporal, and parietal lobes with ex vacuo dilation of the right lateral ventricle.
Treatment and Follow-up: Treatment options include medical treatment such as intravenous immunoglobulin, plasmapheresis, or immunosuppressive medications. However, functional hemispherectomy or hemispherotomy is frequently necessary for seizure control and to prevent progression of cognitive impairment and spread of seizures to the contralateral hemisphere.
Cavernomas
Overview and Imaging: In children, seizures are the most common symptom of cavernomas at presentation.113–115 Cavernomas are more commonly supratentorial (80%) and can have variable size. On MRI, cavernomas have a combination of mixed high and low T1 and T2 signals surrounding the hemosiderin rim,116 and they are best depicted on T2* gradient echo imaging. Calcification may be seen on CT.
Meningioangiomatosis
Overview and Imaging: Meningioangiomatosis is a rare lesion characterized by vascular proliferation involving the cortex and leptomeninges. The lesion can be sporadic or can occur in association with neurofibromatosis type 2. Sporadic lesions are solitary and typically present with refractory epilepsy. Lesions associated with neurofibromatosis type 2 often are multiple.119 On CT, the lesion is hypodense and may contain calcification. On MRI, the cortex may have a hypointense T1 and T2 signal as a result of calcification, and the subcortical white matter may have a hyperintense T2 signal. Enhancement may be seen after the administration of contrast material (Fig. 38-11). Meningioangiomatosis may mimic other disorders such as tumors, FCD, and arteriovenous malformation.120 Treatment is surgical resection. However, patients with meningioangiomatosis tend to have poorer outcome with respect to seizure control compared with patients who have tumors or hippocampal sclerosis.121
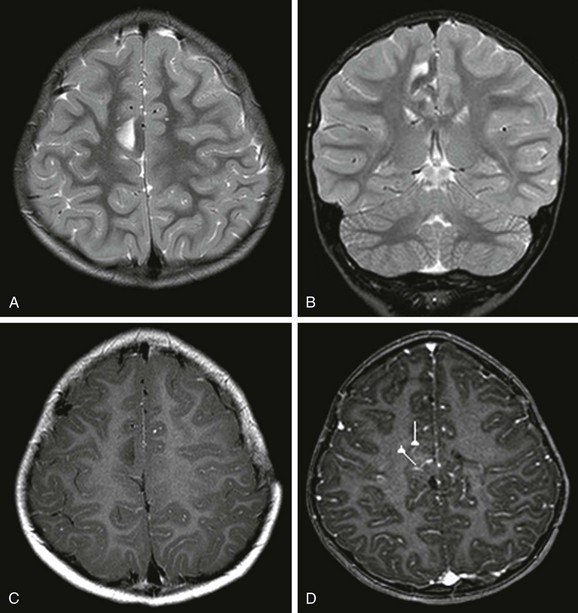
Figure 38-11 A 5-year-old boy with meningioangiomatosis and right frontal lobe epilepsy.
An axial image (A) and a coronal T2-weighted image (B) show a low T2 signal in the cortex and a high T2 signal in the subcortical white matter of the lesion in the right mesial frontal lobe. C, An axial T1-weighted postcontrast image shows no enhancement of the lesion. D, Magnetoencephalography dipoles projected onto an axial T1-weighted postcontrast image show dipoles around the lesion.
Colombo, N, Salamon, N, Raybaud, C, et al. Imaging of malformations of cortical development. Epileptic Disord. 2009;11(3):194–205.
Duncan, JS. Epilepsy and imaging. Brain. 1997;120:339–377.
Rastogi, S, Lee, C, Salamon, N. Neuroimaging in pediatric epilepsy: a multimodality approach. Radiographics. 2008;28(4):1079–1095.
Knowlton, RC. Multimodality imaging in partial epilepsies. Curr Opin Neurol. 2004;17:165–172.
Widjaja, E, Raybaud, C. Advances in neuroimaging in patients with epilepsy. Neurosurg Focus. 2008;25(3):E3.
References
1. Theodore, WH, Spencer, SS, Wiebe, S, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006;47(10):1700–1722.
2. Hauser, WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35(suppl 2):S1–S6.
3. Snead, OC, 3rd. Surgical treatment of medically refractory epilepsy in childhood. Brain Dev. 2001;23(4):199–207.
4. Tellez-Zenteno, JF, Hernandez Ronquillo, L, Moien-Afshari, F, et al. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2-3):310–318.
5. Berg, AT, Testa, FM, Levy, SR, et al. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics. 2000;106(3):527–532.
6. Gaillard, WD, Chiron, C, Cross, JH, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50(9):2147–2153.
7. Bastos, AC, Comeau, RM, Andermann, F, et al. Diagnosis of subtle focal dysplastic lesions: curvilinear reformatting from three-dimensional magnetic resonance imaging. Ann Neurol. 1999;46(1):88–94.
8. Bastos, AC, Korah, IP, Cendes, F, et al. Curvilinear reconstruction of 3D magnetic resonance imaging in patients with partial epilepsy: a pilot study. Magn Reson Imaging. 1995;13(8):1107–1112.
9. Montenegro, MA, Li, LM, Guerreiro, MM, et al. Focal cortical dysplasia: improving diagnosis and localization with magnetic resonance imaging multiplanar and curvilinear reconstruction. J Neuroimaging. 2002;12(3):224–230.
10. Widjaja, E, Raybaud, C. Advances in neuroimaging in patients with epilepsy. Neurosurg Focus. 2008;25(3):E3.
11. Grondin, R, Chuang, S, Otsubo, H, et al. The role of magnetoencephalography in pediatric epilepsy surgery. Childs Nerv Syst. 2006;22(8):779–785.
12. Guerrini, R, Barba, C. Malformations of cortical development and aberrant cortical networks: epileptogenesis and functional organization. J Clin Neurophysiol. 2010;27(6):372–379.
13. Barkovich, AJ, Kuzniecky, RI, Jackson, GD, et al. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65(12):1873–1887.
14. Cascino, GD, Buchhalter, JR, Sirven, JI, et al. Peri-ictal SPECT and surgical treatment for intractable epilepsy related to schizencephaly. Neurology. 2004;63(12):2426–2428.
15. Dubeau, F, Tyvaert, L. Understanding the epileptogenicity of lesions: a correlation between intracranial EEG and EEG/fMRI. Epilepsia. 2010;51(suppl 1):54–58.
16. Kobayashi, E, Bagshaw, AP, Grova, C, et al. Grey matter heterotopia: what EEG-fMRI can tell us about epileptogenicity of neuronal migration disorders. Brain. 2006;129(pt 2):366–374.
17. Jay, V, Becker, LE, Otsubo, HP, et al. Pathology of temporal lobectomy for refractory seizures in children. Review of 20 cases including some unique malformative lesions. J Neurosurg. 1993;79:53–61.
18. Farrel, M, DeRosa, M, Curran, J, et al. Neuropathological findings in cortical resection (including hemispherectomies) performed for the treatment of intractable childhood epilepsy. Acta Neuropathol. 1992;83:246–259.
19. Cepeda, C, Andre, VM, Vinters, HV, et al. Are cytomegalic neurons and balloon cells generators of epileptic activity in pediatric cortical dysplasia? Epilepsia. 2005;46(suppl 5):82–88.
20. Guerrini, R, Sicca, F, Parmeggiani, L. Epilepsy and malformations of the cerebral cortex. Epileptic Disord. 2003;5(suppl 2):S9–S26.
21. Tassi, L, Colombo, N, Garbelli, R, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125(pt 8):1719–1732.
22. Palmini, A, Najm, I, Avanzini, G, et al. Terminology and classification of the cortical dysplasias. Neurology. 2004;62(6 suppl 3):S2–S8.
23. Kuzniecky, RI. MRI in cerebral developmental malformations and epilepsy. Magn Reson Imaging. 1995;13(8):1137–1145.
24. Blumcke, I, Thom, M, Aronica, E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52(1):158–174.
25. Urbach, H, Scheffler, B, Heinrichsmeier, T, et al. Focal cortical dysplasia of Taylor’s balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features, and favorable postsurgical outcome. Epilepsia. 2002;43(1):33–40.
26. Colombo, N, Citterio, A, Galli, C, et al. Neuroimaging of focal cortical dysplasia: neuropathological correlations. Epileptic Disord. 2003;5(suppl 2):S67–S72.
27. Colombo, N, Salamon, N, Raybaud, C, et al. Imaging of malformations of cortical development. Epileptic Disord. 2009;11(3):194–205.
28. Yagishita, A, Arai, N, Maehara, T, et al. Focal cortical dysplasia: appearance on MR images. Radiology. 1997;203(2):553–559.
29. Demerens, C, Stankoff, B, Logak, M, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93(18):9887–9892.
30. Widjaja, E, Li, B, Schinkel, CD, et al. Cost-effectiveness of pediatric epilepsy surgery compared to medical treatment in children with intractable epilepsy. Epilepsy Res. 2011;94(1-2):61–68.
31. Kral, T, Clusmann, H, Blumcke, I, et al. Outcome of epilepsy surgery in focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2003;74(2):183–188.
32. Keene, DL, Ventureyra, EC. Epilepsy surgery for 5- to 18-year old patients with medically refractory epilepsy—is it cost efficient? Childs Nerv Syst. 1999;15(9):433.
33. Sperling, MR, O’Connor, MJ, Saykin, AJ, et al. Temporal lobectomy for refractory epilepsy. JAMA. 1996;276(6):470–475.
34. Britton, JW, Cascino, GD, Sharbrough, FW, et al. Low-grade glial neoplasms and intractable partial epilepsy: efficacy of surgical treatment. Epilepsia. 1994;35(6):1130–1135.
35. Widdess-Walsh, P, Kellinghaus, C, Jeha, L, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: correlation with pathological subtypes. Epilepsy Res. 2005;67(1-2):25–33.
36. Chassoux, F, Devaux, B, Landre, E, et al. Stereoelectroencephalography in focal cortical dysplasia: a 3D approach to delineating the dysplastic cortex. Brain. 2000;123(pt 8):1733–1751.
37. Fauser, S, Schulze-Bonhage, A, Honegger, J, et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004;127(pt 11):2406–2418.
38. Palmini, A, Gambardella, A, Andermann, F, et al. Operative strategies for patients with cortical dysplastic lesions and intractable epilepsy. Epilepsia. 1994;35(suppl 6):S57–S71.
39. Robain, O, Gelot, A. Neuropathology of hemimegalencephaly. In: Guerrini R, Andermann F, Canapicchi R, et al, eds. Dysplasias of cerebral cortex and epilepsy. Philadelphia: Lippincott-Raven, 1996.
40. Barkovich, AJ, Chuang, SH. Unilateral megalencephaly: correlation of MR imaging and pathologic characteristics. AJNR Am J Neuroradiol. 1990;11(3):523–531.
41. Broumandi, DD, Hayward, UM, Benzian, JM, et al. Best cases from the AFIP: hemimegalencephaly. Radiographics. 2004;24(3):843–848.
42. Yagishita, A, Arai, N, Tamagawa, K, et al. Hemimegalencephaly: signal changes suggesting abnormal myelination on MRI. Neuroradiology. 1998;40(11):734–738.
43. Wolpert, SM, Cohen, A, Libenson, MH. Hemimegalencephaly: a longitudinal MR study. AJNR Am J Neuroradiol. 1994;15(8):1479–1482.
44. Roach, ES, Gomez, MR, Northrup, H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13(12):624–628.
45. Jansen, FE, Huiskamp, G, van Huffelen, AC, et al. Identification of the epileptogenic tuber in patients with tuberous sclerosis: a comparison of high-resolution EEG and MEG. Epilepsia. 2006;47(1):108–114.
46. Jozwiak, S, Schwartz, RA, Janniger, CK, et al. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol. 2000;15(10):652–659.
47. Wu, JY, Salamon, N, Kirsch, HE, et al. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology. 2010;74(5):392–398.
48. Juhasz, C, Chugani, HT. Imaging the epileptic brain with positron emission tomography. Neuroimaging Clin N Am. 2003;13(4):705–716. [viii].
49. Bourgeois, M, Crimmins, DW, de Oliveira, RS, et al. Surgical treatment of epilepsy in Sturge-Weber syndrome in children. J Neurosurg. 2007;106(1 suppl):20–28.
50. Griffiths, PD. Sturge-Weber syndrome revisited: the role of neuroradiology. Neuropediatrics. 1996;27(6):284–294.
51. Di Rocco, C, Tamburrini, G. Sturge-Weber syndrome. Childs Nerv Syst. 2006;22(8):909–921.
52. Puttgen, KB, Lin, DD. Neurocutaneous vascular syndromes. Childs Nerv Syst. 2010;26(10):1407–1415.
53. Harvey, AS, Berkovic, SF, Wrennall, JA, et al. Temporal lobe epilepsy in childhood: clinical, EEG, and neuroimaging findings and syndrome classification in a cohort with new-onset seizures. Neurology. 1997;49(4):960–968.
54. Harvey, AS, Grattan-Smith, JD, Desmond, PM, et al. Febrile seizures and hippocampal sclerosis: frequent and related findings in intractable temporal lobe epilepsy of childhood. Pediatr Neurol. 1995;12(3):201–206.
55. Grattan-Smith, JD, Harvey, AS, Desmond, PM, et al. Hippocampal sclerosis in children with intractable temporal lobe epilepsy: detection with MR imaging. AJR Am J Roentgenol. 1993;161(5):1045–1048.
56. Mohamed, A, Wyllie, E, Ruggieri, P, et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56(12):1643–1649.
57. Cendes, F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol. 2004;17(2):161–164.
58. Scott, RC, King, MD, Gadian, DG, et al. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126(pt 11):2551–2557.
59. Bronen, RA. Epilepsy: the role of MR imaging. AJR Am J Roentgenol. 1992;159(6):1165–1174.
60. Jack, CR, Jr., Sharbrough, FW, Cascino, GD, et al. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol. 1992;31(2):138–146.
61. Cascino, GD, Jack, CR, Jr., Parisi, JE, et al. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol. 1991;30(1):31–36.
62. Meiners, LC, van Gils, AD, De Kort, G, et al. Fast fluid-attenuated inversion recovery (FLAIR) compared with T2-weighted spin-echo in the magnetic resonance diagnosis of mesial temporal sclerosis. Invest Radiol. 1999;34(2):134–142.
63. Oppenheim, C, Dormont, D, Biondi, A, et al. Loss of digitations of the hippocampal head on high-resolution fast spin-echo MR: a sign of mesial temporal sclerosis. AJNR Am J Neuroradiol. 1998;19(3):457–463.
64. Baldwin, GN, Tsuruda, JS, Maravilla, KR, et al. The fornix in patients with seizures caused by unilateral hippocampal sclerosis: detection of unilateral volume loss on MR images. AJR Am J Roentgenol. 1994;162(5):1185–1189.
65. Knowlton, RC. Multimodality imaging in partial epilepsies. Curr Opin Neurol. 2004;17(2):165–172.
66. Knowlton, RC, Laxer, KD, Ende, G, et al. Presurgical multimodality neuroimaging in electroencephalographic lateralized temporal lobe epilepsy. Ann Neurol. 1997;42(6):829–837.
67. Hogan, RE, Carne, RP, Kilpatrick, CJ, et al. Hippocampal deformation mapping in MRI negative PET positive temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2008;79(6):636–640.
68. Engel, J, Jr., McDermott, MP, Wiebe, S, et al. Design considerations for a multicenter randomized controlled trial of early surgery for mesial temporal lobe epilepsy. Epilepsia. 2010;51(10):1978–1986.
69. Ozkara, C, Uzan, M, Benbir, G, et al. Surgical outcome of patients with mesial temporal lobe epilepsy related to hippocampal sclerosis. Epilepsia. 2008;49(4):696–699.
70. Spencer, SS. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002;1(6):375–382.
71. Smyth, MD, Limbrick, DD, Jr., Ojemann, JG, et al. Outcome following surgery for temporal lobe epilepsy with hippocampal involvement in preadolescent children: emphasis on mesial temporal sclerosis. J Neurosurg. 2007;106(3 suppl):205–210.
72. Benifla, M, Otsubo, H, Ochi, A, et al. Multiple subpial transections in pediatric epilepsy: indications and outcomes. Childs Nerv Syst. 2006;22(8):992–998.
73. Mittal, S, Montes, JL, Farmer, JP, et al. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg. 2005;103(5 suppl):401–412.
74. Luyken, C, Blumcke, I, Fimmers, R, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44(6):822–830.
75. Clusmann, H, Kral, T, Gleissner, U, et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004;54(4):847–859. [discussion 859-860].
76. Koeller, KK, Henry, JM. From the archives of the AFIP: superficial gliomas: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics. 2001;21(6):1533–1556.
77. Raybaud, C, Shroff, M, Rutka, JT, et al. Imaging surgical epilepsy in children. Childs Nerv Syst. 2006;22(8):786–809.
78. Matsumoto, K, Tamiya, T, Ono, Y, et al. Cerebral gangliogliomas: clinical characteristics, CT and MRI. Acta Neurochir (Wien). 1999;141(2):135–141.
79. Provenzale, JM, Ali, U, Barboriak, DP, et al. Comparison of patient age with MR imaging features of gangliogliomas. AJR Am J Roentgenol. 2000;174(3):859–862.
80. Luyken, C, Blumcke, I, Fimmers, R, et al. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101(1):146–155.
81. Castillo, M, Davis, PC, Takei, Y, et al. Intracranial ganglioglioma: MR, CT, and clinical findings in 18 patients. AJNR Am J Neuroradiol. 1990;11(1):109–114.
82. Tampieri, D, Moumdjian, R, Melanson, D, et al. Intracerebral gangliogliomas in patients with partial complex seizures: CT and MR imaging findings. AJNR Am J Neuroradiol. 1991;12(4):749–755.
83. Peretti-Viton, P, Perez-Castillo, AM, Raybaud, C, et al. Magnetic resonance imaging in gangliogliomas and gangliocytomas of the nervous system. J Neuroradiol. 1991;18(2):189–199.
84. Altman, NR. MR and CT characteristics of gangliocytoma: a rare cause of epilepsy in children. AJNR Am J Neuroradiol. 1988;9(5):917–921.
85. Ostertun, B, Wolf, HK, Campos, MG, et al. Dysembryoplastic neuroepithelial tumors: MR and CT evaluation. AJNR Am J Neuroradiol. 1996;17(3):419–430.
86. Stanescu Cosson, R, Varlet, P, Beuvon, F, et al. Dysembryoplastic neuroepithelial tumors: CT, MR findings and imaging follow-up: a study of 53 cases. J Neuroradiol. 2001;28(4):230–240.
87. Shin, JH, Lee, HK, Khang, SK, et al. Neuronal tumors of the central nervous system: radiologic findings and pathologic correlation. Radiographics. 2002;22(5):1177–1189.
88. Fernandez, C, Girard, N, Paz Paredes, A, et al. The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol. 2003;24(5):829–834.
89. Parmar, HA, Hawkins, C, Ozelame, R, et al. Fluid-attenuated inversion recovery ring sign as a marker of dysembryoplastic neuroepithelial tumors. J Comput Assist Tomogr. 2007;31(3):348–353.
90. Thom, M, Gomez-Anson, B, Revesz, T, et al. Spontaneous intralesional haemorrhage in dysembryoplastic neuroepithelial tumours: a series of five cases. J Neurol Neurosurg Psychiatry. 1999;67(1):97–101.
91. Petropoulou, K, Whiteman, ML, Altman, NR, et al. CT and MRI of pleomorphic xanthoastrocytoma: unusual biologic behavior. J Comput Assist Tomogr. 1995;19(6):860–865.
92. Giannini, C, Scheithauer, BW, Burger, PC, et al. Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer. 1999;85(9):2033–2045.
93. Tien, RD, Cardenas, CA, Rajagopalan, S. Pleomorphic xanthoastrocytoma of the brain: MR findings in six patients. AJR Am J Roentgenol. 1992;159(6):1287–1290.
94. Lipper, MH, Eberhard, DA, Phillips, CD, et al. Pleomorphic xanthoastrocytoma, a distinctive astroglial tumor: neuroradiologic and pathologic features. AJNR Am J Neuroradiol. 1993;14(6):1397–1404.
95. Im, SH, Chung, CK, Kim, SK, et al. Pleomorphic xanthoastrocytoma: a developmental glioneuronal tumor with prominent glioproliferative changes. J Neurooncol. 2004;66(1-2):17–27.
96. Lach, B, Duggal, N, DaSilva, VF, et al. Association of pleomorphic xanthoastrocytoma with cortical dysplasia and neuronal tumors. A report of three cases. Cancer. 1996;78(12):2551–2563.
97. Benifla, M, Otsubo, H, Ochi, A, et al. Temporal lobe surgery for intractable epilepsy in children: an analysis of outcomes in 126 children. Neurosurgery. 2006;59(6):1203–1213. [discussion 1213-1214].
98. Nolan, MA, Sakuta, R, Chuang, N, et al. Dysembryoplastic neuroepithelial tumors in childhood: long-term outcome and prognostic features. Neurology. 2004;62(12):2270–2276.
99. Sakuta, R, Otsubo, H, Nolan, MA, et al. Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol. 2005;20(4):377–384.
100. Marton, E, Feletti, A, Orvieto, E, et al. Malignant progression in pleomorphic xanthoastrocytoma: personal experience and review of the literature. J Neurol Sci. 2007;252(2):144–153.
101. Harvey, AS, Freeman, JL. Epilepsy in hypothalamic hamartoma: clinical and EEG features. Semin Pediatr Neurol. 2007;14(2):60–64.
102. Arita, K, Kurisu, K, Kiura, Y, et al. Hypothalamic hamartoma. Neurol Med Chir (Tokyo). 2005;45(5):221–231.
103. Freeman, JL, Coleman, LT, Wellard, RM, et al. MR imaging and spectroscopic study of epileptogenic hypothalamic hamartomas: analysis of 72 cases. AJNR Am J Neuroradiol. 2004;25(3):450–462.
104. Frazier, JL, Goodwin, CR, Ahn, ES, et al. A review on the management of epilepsy associated with hypothalamic hamartomas. Childs Nerv Syst. 2009;25(4):423–432.
105. Rasmussen, T, Olszewski, J, Lloydsmith, D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8(6):435–445.
106. Rogers, SW, Andrews, PI, Gahring, LC, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen’s encephalitis. Science. 1994;265(5172):648–651.
107. McNamara, JO, Whitney, KD, Andrews, PI, et al. Evidence for glutamate receptor autoimmunity in the pathogenesis of Rasmussen encephalitis. Adv Neurol. 1999;79:543–550.
108. He, XP, Patel, M, Whitney, KD, et al. Glutamate receptor GluR3 antibodies and death of cortical cells. Neuron. 1998;20(1):153–163.
109. Aguilar, MJ, Rasmussen, T. Role of encephalitis in pathogenesis of epilepsy. Arch Neurol. 1960;2:663–676.
110. Fukuda, T, Oguni, H, Yanagaki, S, et al. Chronic localized encephalitis (Rasmussen’s syndrome) preceded by ipsilateral uveitis: a case report. Epilepsia. 1994;35(6):1328–1331.
111. Geller, E, Faerber, EN, Legido, A, et al. Rasmussen encephalitis: complementary role of multitechnique neuroimaging. AJNR Am J Neuroradiol. 1998;19(3):445–449.
112. Chiapparini, L, Granata, T, Farina, L, et al. Diagnostic imaging in 13 cases of Rasmussen’s encephalitis: can early MRI suggest the diagnosis? Neuroradiology. 2003;45(3):171–183.
113. Giulioni, M, Acciarri, N, Padovani, R, et al. Results of surgery in children with cerebral cavernous angiomas causing epilepsy. Br J Neurosurg. 1995;9(2):135–141.
114. Pozzati, E, Padovani, R, Morrone, B, et al. Cerebral cavernous angiomas in children. J Neurosurg. 1980;53(6):826–832.
115. Lee, JW, Kim, DS, Shim, KW, et al. Management of intracranial cavernous malformation in pediatric patients. Childs Nerv Syst. 2008;24(3):321–327.
116. Kim, DS, Park, YG, Choi, JU, et al. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48(1):9–17. [discussion 17-18].
117. Cohen, DS, Zubay, GP, Goodman, RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. 1995;83(2):237–242.
118. Acciarri, N, Galassi, E, Giulioni, M, et al. Cavernous malformations of the central nervous system in the pediatric age group. Pediatr Neurosurg. 2009;45(2):81–104.
119. Jallo, GI, Kothbauer, K, Mehta, V, et al. Meningioangiomatosis without neurofibromatosis: a clinical analysis. J Neurosurg. 2005;103(4 suppl):319–324.
120. Arcos, A, Serramito, R, Santin, JM, et al. Meningioangiomatosis: clinical-radiological features and surgical outcome. Neurocirugia (Astur). 2010;21(6):461–466.
121. Wiebe, S, Munoz, DG, Smith, S, et al. Meningioangiomatosis. A comprehensive analysis of clinical and laboratory features. Brain. 1999;122(pt 4):709–726.