CHAPTER 191 Neuroendoscopy
History
Endoscopic neurosurgery began in the early 20th century as an effort to diagnose and treat hydrocephalus. Surgeons realized that the same instruments used in urologic procedures could be inserted into the cerebral ventricles. In 1910, Victor Darwin Lespinasse, a urologist in Chicago, cauterized the choroid plexus of two infants with hydrocephalus using a rigid cystoscope. The results were not published but were presented locally. One child died immediately; the other lived for 5 years.1 Lespinasse subsequently abandoned the procedure, calling it an “intern’s stunt.”
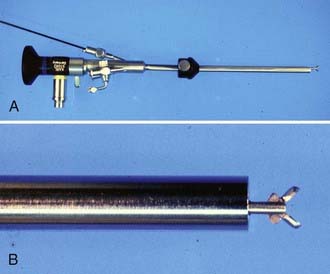
Walter Dandy is considered to be the father of neuroendoscopy. In 1918, he attempted to treat hydrocephalus in four infants by using a thin-bladed nasal speculum to gain access to the ventricles.2 He extirpated the choroid plexus by avulsing it. Only one infant survived. Later in 1922, in a one paragraph landmark article in the Johns Hopkins Hospital Bulletin, Dandy coined the term ventriculoscope and described his use of a rigid cystoscope to gain access to the ventricles and fulgurate the choroid plexus in two hydrocephalic infants.3 Using electrocautery and long-handled scissors, Dandy was able to extirpate the choroid plexus under endoscopic guidance (Fig. 191-1). Subsequently, using similar techniques, he successfully removed choroid plexus tumors in three patients.4
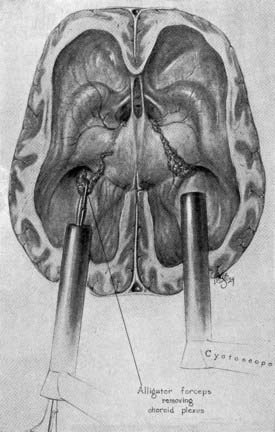
FIGURE 191-1 Dandy’s ventriculoscope. The choroid plexus of the lateral ventricles was removed using two Kelly cystoscopes.
(From Dandy WE. Surgery of the Brain, Hagerstown, MD: WF Prior, 1945:245.)
Although Dandy performed the first third ventriculostomy through an open procedure, it was in 1923 when W. Jason Mixter reported the first successful endoscopic third ventriculostomy (ETV) using a flexible urethroscope.5 The operation was considered a success in that postoperatively indigo carmine dye instilled into the lateral ventricle could be recovered by a needle in the lumbar subarachnoid space. This recovery could not be demonstrated preoperatively, purportedly because of the patient’s noncommunicating hydrocephalus.
For years, the main surgical treatment of hydrocephalus was either ETV or endoscopic coagulation of the choroid plexus. Ultimately, neuroendoscopy fell out of favor because of the high rate of complications related to the primitive nature of the instruments and the advent of successful extracranial ventricular shunting. The first successful ventricular shunt procedure was performed by Frank Nulsen and Eugene Spitz in 1919 and reported in Surgical Forum in 1951.6 Extracranial ventricular shunting revolutionized the treatment of hydrocephalus and quickly became the procedure of choice for treating hydrocephalus.
Instrumentation
There are two classes of neuroendoscopes: rigid and flexible. The modern endoscope would not have been possible without the innovations of a British optical physicist, Harold Hopkins.7 Rigid endoscopes have optics that are superior to flexible fiberoptic endoscopes. Karl Storz adopted the solid rod lens developed by Hopkins and used it in his rigid endoscope systems. Hopkins and Storz developed the SELFOC lens, which is still used in modern rigid endoscopes and is more efficient than earlier lens models.8 The SELFOC lens has a refractive index that varies with the radial dimension of the lens, whereas the conventional lens has a uniform refractive index.8,9 The lens expands the field of vision and eliminates the need for a relay lens while preserving light transmission.8,10 The Hopkins lens system could also be made with a significantly smaller diameter than the earlier, more primitive lenses designed by Nitze for urologic use. Adding a series of angled rod lenses to the 0-degree straightforward lens system enhanced the maneuverability of the instrument.
There are devices designed for cutting, grasping, aspirating, and sampling lesions (Fig. 191-2). Balloon catheters and rigid and flexible probes are available for fenestration of cystic lesions, the septum pellucidum, and the floor of the third ventricle. Small catheters introduced through working channels in the endoscope sheath can provide irrigation and suction. It is important to keep the operative field clear by irrigation because even a small amount of blood can impair visualization. It is imperative that there be an escape route for irrigating fluid to prevent ventricular dilation and increased intracranial pressure (ICP) in a closed system.
The energy sources for neuroendoscopic dissection include monopolar and bipolar electrocoagulators and a number of fiberoptic lasers. Two of the most commonly used lasers are the neodymium-doped yttrium-aluminum-garnet (Nd : YAG) laser and the potassium titanyl phosphate (KTP) laser. Nd : YAG lasers emit light that is invisible and require a visible helium neon pilot beam. Pigmented tissues have a preferential absorption of the light emitted by the Nd : YAG laser. Thus, ventricular cyst walls and the whitish septum pellucidum require higher power settings for fenestration. KTP lasers emit a green light and do not require a pilot beam. Other, less commonly used lasers include argon and holmium sources. We have used endoscopic laser dissection for various lesions such as third ventricular colloid cysts, but prefer not to use the laser during ETV for fear of injuring the basilar artery. Oertel and colleagues have shown promising results in selected patients with a water jet system for dissection with preservation of nearby vessels.11
Stereotactic Endoscopy
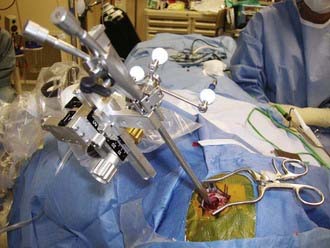
Both rigid and flexible endoscopes can be used with stereotactic guidance.12–17 Standard frame-based stereotaxy is useful to guide an endoscope to the proximity of a lesion with small ventricles. Visual anatomic clues, once the endoscopist has accessed the ventricle, can further guide the operator in dissecting closer to the lesion. In addition, the frame itself can serve as a mount for the endoscope. However, frame-based endoscopy is not applicable to infants and young children. Frameless stereotaxy defines a three-dimensional coordinate space for a preoperative imaging modality and translates it to the three-dimensional coordinate space of the operative field. Articulated mechanical arms, sonic or optical digitizers, and electromagnetic systems are substituted for the frame (Fig. 191-3).18–21
Endoscopic Anatomic Considerations
After the orientation of the image on the monitor is concordant with the position of the patient, standard intraventricular landmarks are identified.22,23 The foramen of Monro is usually identified first,24 given that it is in line with the trajectory of the ventriculoscope from a coronal approach. The septum pellucidum is located medially, and the head of the caudate nucleus is situated laterally. The choroid plexus is always projected posterior to the foramen of Monro. The posterolateral thalamostriate vein joins the anteromedial septal vein to form the internal cerebral vein. The caliber of these veins increases as they approach the foramen of Monro. A pair of white C-shaped structures, the fornices, are seen as they curve ventrally and inferiorly to define the medial and anterior borders of the foramen of Monro.
Endoscopic Procedures
Endoscopic Third Ventriculostomy
Despite Mixter’s first successful ETV in 1923,5 the procedure did not gain early popularity because of poor illumination, primitive and bulky instruments, inconsistent results, and high complication rates. After the introduction of valve-regulated shunts in the early 1950s, ETV fell out of favor for some time. However, ventricular shunts are troublesome devices and pose lifelong problems for shunted patients. The significant improvement in endoscopic technology in recent decades has led to a renewed interest in ETV.
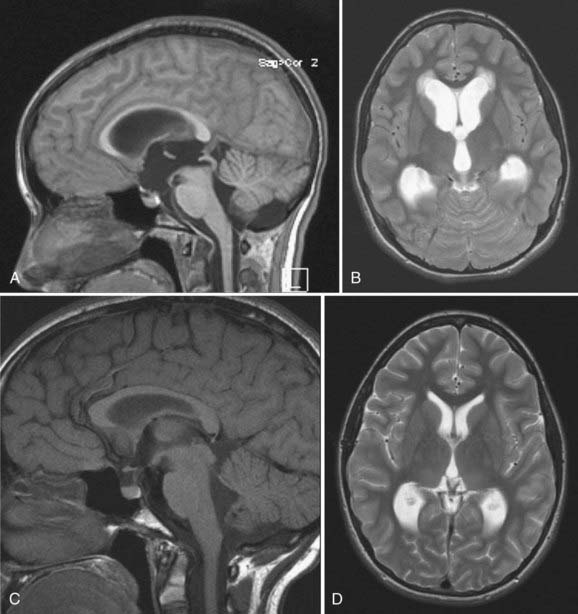
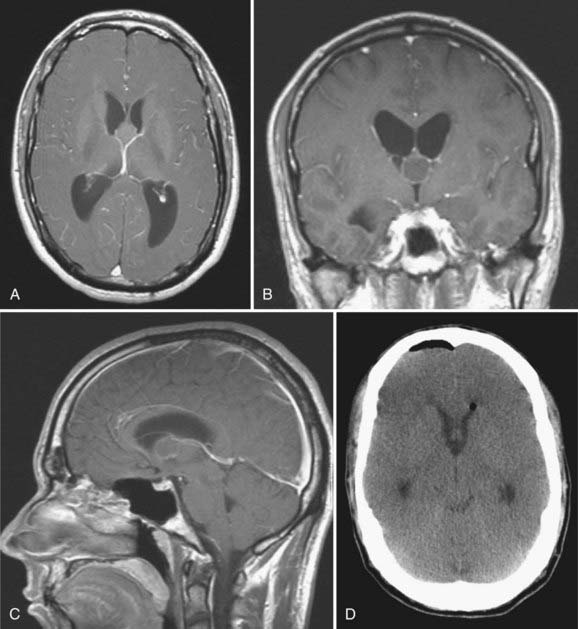
Candidates for ETV should have symptomatic noncommunicating hydrocephalus with a patent subarachnoid space. A classic example is acquired aqueductal stenosis with resulting proximal dilation of lateral and third ventricles. Neuroimaging, in particular magnetic resonance imaging (MRI), is useful in diagnosing noncommunicating hydrocephalus (Fig. 191-4). The ideal candidate for ETV has enlarged lateral and third ventricles, including a third ventricular floor that is thinned and bowed inferiorly. Preoperative planning with MRI delineates the anatomy of the third ventricle and the aqueduct of Sylvius and the location of the basilar artery on the sagittal view.
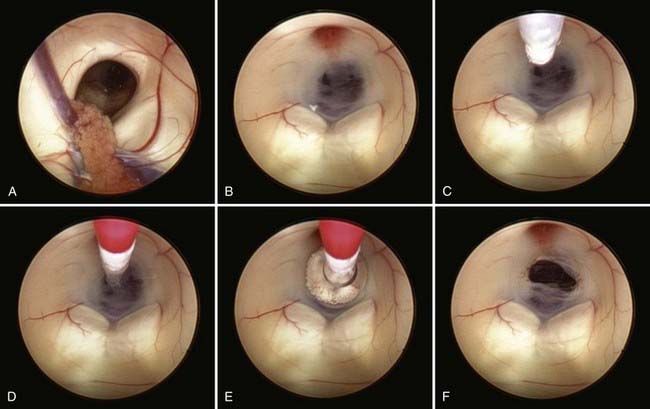
Safe fenestration of the floor of the third ventricle requires the procedure to be performed in the midline and anterior to the mamillary bodies and the underlying basilar artery apex (Fig. 191-5). Fenestration of the floor of the third ventricle can be performed by blunt penetration with the endoscope or a rigid probe, electrocoagulation, balloon catheterization, water jet fenestration, or laser coagulation. Our preference is to use a rigid probe introduced through a working channel in the endoscope sheath to puncture the floor of the third ventricle. A Fogarty balloon catheter with its stylet in place is also an effective means of puncturing the third ventricular floor. The balloon catheter is then repetitively inflated and deflated to widen the fenestration (Fig. 191-6). Both the ependyma and underlying arachnoid are opened. Bleeding from the edges of the opening can be tamponaded by keeping the balloon inflated for a slightly longer period. Fluctuation of the margins of the fenestration indicates cerebrospinal fluid (CSF) flow. The basilar artery complex can be visualized through the fenestration without inserting the endoscope into the basal cisterns (Fig. 191-7).
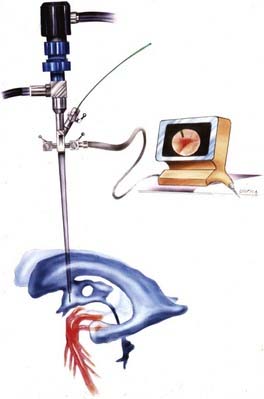
FIGURE 191-5 Endoscopic third ventriculostomy.
(From Cohen AR. Images in clinical medicine. N Engl J Med. 1993;328:552.)
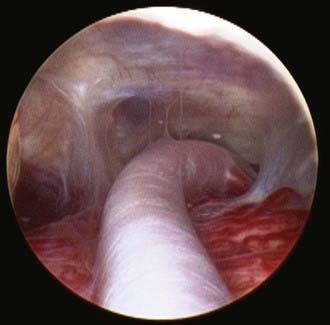
FIGURE 191-7 Endoscopic view of basilar artery in the prepontine cistern after third ventriculostomy.
The reported success rate of ETV ranges from 50% to 95%.25–33 There are conflicting studies that have described the outcome after ETV to be dependent on age,25,26,34–36 independent of age,37,38 or dependant mainly on etiology.28,35,39,40 In a multivariate analysis, Drake and associates reported a higher failure rate for younger patients, particularly neonates and infants.26 Similarly, Kadrian and coworkers have shown a higher failure rate for patients younger than 6 months.25 The procedure is less likely to be successful if there is any history of communicating hydrocephalus.24,41 For example, patients with aqueductal stenosis who have prior evidence of ventricular hemorrhage, shunt infection, or meningitis are less likely to have a good result.36,42,43 However, this is not uniformly the case because ETV has been reported to be successful in some patients with hydrocephalus and associated ventricular shunt infections.44,45 The success of an ETV procedure is determined mainly by clinical evaluation, not postoperative imaging, because the ventricular system may not change significantly in size.
Although ETV is an effective treatment for selected cases of noncommunicating hydrocephalus, it is not without risks. The reported complication rates range from 0% to 20% in different series,46–48 with a mortality rate of less than 1%.30,47,49 Complications can be categorized as intraoperative, early postoperative, and late postoperative. Intraoperative complications include neurovascular injury and bradycardia with cardiac arrest.42,50–54 Massive subarachnoid bleeding from perforation of the basilar artery or its branches has been reported.55–59 The Canadian cooperative study reported a 1.4% bleeding rate in 368 patients who underwent endoscopic ETV.26 Other authors have reported both arterial and venous bleeding during their procedures.28,60–62
Intraoperative hemodynamic changes have been documented in different series. El-Dawlatly and coworkers experienced a 41% rate of intraoperative bradycardia during perforation of the third ventricular floor in their patients.63 A proposed mechanism for intraoperative hemodynamic changes suggests Cushing’s response from elevated ICP from aggressive irrigation and stimulation of the preoptic area or posterior hypothalamus resulting in bradycardia or tachycardia, respectively.64 Handler and associates documented an intraoperative cardiac arrest during ETV in a patient with aqueductal stenosis.52 The patient required cardioversion. This case demonstrates the danger of infusing a high-flow fluid irrigation in a closed system.
Early postoperative complications include subdural hematoma, CSF leak, infection, and endocrinologic disorders. A large corticotomy draining into the subdural space or sudden excessive CSF drainage during ETV is a possible risk factor for the development of subdural hematomas.61,65,66 Restoration of CSF absorption by the arachnoid granulations may not be immediate in the early postoperative period. The increased ICP can lead to the development of a CSF collection under the incision or leakage of CSF resulting in meningitis or ventriculitis. Several investigators have reported electrolyte and endocrinologic abnormalities following ETV.51,67–69 Abnormalities include the syndrome of inappropriate antidiuretic hormone secretion (SIADH), diabetes insipidus, and secondary amenorrhea.51,68,69 Other early postoperative complications include transient memory loss and personality disorders from injury to the fornices as well as cranial nerve palsies and seizures.26,33,46,47,54,61,70,71
Late postoperative complications consist mainly of delayed failure from closure of the ETV stoma. The reported rate of delayed failure ranges from 2% to 15% in different series.26,28,72–74 Late failure leading to rapid clinical deterioration and death are rare but real occurrences.48,72,75 The mechanism of ETV failure may be multifactorial. Causes include inadequate size of the initial fenestration, underappreciated secondary membranes, reduced or no CSF flow through the fenestration, narrowing or closure of the stoma due to hemorrhagic obstruction, elevated CSF protein and fibrinogen, postoperative infection with CSF obstruction at the fenestration site or inadequate CSF absorption by the arachnoid granulations, and tumor progression resulting in blockage.
Endoscopic Aqueductoplasty
The first aqueductal reconstruction was performed by Dandy in 1920.76 In cases of hydrocephalus caused by membranous occlusion or short segment stenosis of the aqueduct of Sylvius, endoscopic aqueductoplasty (EA) with and without stenting has been reported.77–81 The bur hole for EA is placed more anteriorly than the one for standard ETV. The tip of the Fogarty balloon catheter may be shaped with a gentle curve given that the aqueduct is not straight. The catheter is then gently slid into the lumen of the aqueduct and the balloon is carefully dilated. A flexible endoscope may be useful for perforating membranous obstruction, especially if the obstruction is in the distal aqueduct, which is not well visualized by a rigid endoscope. Stenting of the aqueduct may be performed for patients at high risk for aqueductal restenosis or patients with a trapped fourth ventricle.80,82–85 The stent is usually a ventricular catheter with additional holes.79–8186
Shunted patients with a trapped fourth ventricle often have slit-like lateral ventricles, making them poor candidates for the standard EA.82 A suboccipital approach for retrograde aqueductoplasty and stenting can be performed.82 The entry point is usually just lateral to midline. These patients are at some risk for aqueductal restenosis; thus, aqueductal stenting has been advocated.82
Success rates of EA have been reported in the 69% to 76% range in the largest two series.80,81 Other, smaller groups have also reported high rates of success.78,79 Most of the failures in these studies were restenoses, and reoperation with stenting was performed to correct this problem. Proponents of EA have illustrated the advantages of the procedure compared with ETV.81 EA restores the physiologic CSF pathways and eliminates the risk for basilar artery injury. There are no arachnoidal adhesions around the aqueduct to interfere with CSF flow. The risk for injuring the hypothalamus is avoided, especially during cases when the floor of the third ventricle is thickened and a considerable amount of force is required to perforate the floor. Strictures at the aqueduct are usually not as tough to penetrate; thus, less force is required for fenestration.
A major risk of EA is injuring the periaqueductal gray matter and the floor of the fourth ventricle.77 Other complications reported, especially in long stenoses, include midbrain injury causing transient or permanent dysconjugate eye movements, Parinaud-syndrome, and cranial nerve palsies.77–7981 In cases with long stenoses, ETV may be a more appropriate procedure.
Septostomy
In 1991, Heilman and Cohen87 reported neuroendoscopic septostomies that were performed using a “saline torch.” In the largest published series of endoscopic septostomy by Aldana and associates, 53% of the procedures were thought to be successful.88 Repeat septostomy was performed in 10 patients, with improvement noted in 81% at follow-up.88
Foraminoplasty
Although rare, membranous obstructions of the foramen of Monro causing unilateral hydrocephalus have been reported in the literature.86,89–91 Endoscopic foraminoplasty can be carried out in such patients. When solid brain parenchyma is blocking the foramen of Monro, the risk for closure from foraminoplasty may be high. Schroeder and colleagues have suggested inserting a 10-cm ventricular stent from the lateral ventricle through the foraminoplasty and into the aqueduct to prevent closure.82
Ventricular Tumors and Cysts
Neuroendoscopy has improved the diagnosis and treatment of selected third ventricular tumors and cysts. Endoscopic biopsy of third ventricular tumors was first reported by Fukushima.92 Both rigid and flexible endoscopes have been used to sample the tumors.12,93 In the setting of associated hydrocephalus, placement of a ventricular catheter, ETV, or septostomy may be performed simultaneously, if indicated.
Pineal Tumors and Cysts
Optimal management of pineal tumors remains controversial. Patients often present with acute symptoms of hydrocephalus and require immediate treatment to relieve increased ICP. Traditionally, hydrocephalus is controlled by placement of an external ventricular drain (EVD) or a ventricular shunt. The disadvantages of an EVD include infection and malfunction. Additionally, shunts have been implicated in extraneural dissemination of some pineal tumors.94–99
Standard methods to obtain tumor diagnosis include stereotactic biopsy and open microsurgical procedures. Stereotactic biopsy of pineal tumors is limited by the sampling errors secondary to frequent tumor heterogeneity and by the risk for intraoperative bleeding due to the presence of large midline veins. Microsurgical resection is often the treatment of choice but carries a reported postoperative mortality and morbidity rate of 5% to 15%.100–102 The management of selected pineal tumors, including germ cell tumors and pineal parenchymal tumors, often includes chemotherapy and radiotherapy.103–105 In patients who present with pineal tumors and hydrocephalus, ETV and tumor biopsy are a useful first-line treatment.
ETV can be performed to control increased ICP and establish a tissue diagnosis. Cytologic samples and CSF markers such as α-fetoprotein and β-human chorionic gonadotropin can be collected simultaneously. An endoscopic biopsy is performed unless the tumor appears hypervascular. The surrounding neural structures also can be examined to identify malignant dissemination that may not be seen on neuroimaging. A diagnostic yield of 75% to 100% has been reported.106–109
Colloid Cysts
Colloid cysts are benign lesions constituting 0.5% to 2% of all brain tumors.110,111 They arise from the anterior aspect of velum interpositum or the choroid plexus of the third ventricle adjacent to the foramen of Monro. These lesions can lead to rapid clinical deterioration and even death from increased ICP. The natural history of the lesions is variable, and patients with small cysts are often asymptomatic. When symptomatic, colloid cysts usually cause hydrocephalus secondary to obstruction of both foramina of Monro (Fig. 191-8).
The numerous existing surgical approaches reflect the technical difficulty inherent in removing these lesions with minimal morbidity. Previous studies of stereotactic aspiration of colloid cysts showed a high recurrence rate of 30% to 80%.112–114 It is imperative that colloid cyst patients be followed over the long term because many recurrences can occur in a delayed fashion, sometimes after many years. Other problems associated with stereotactic aspiration include neural and vascular injury; difficulty puncturing small, thick-walled lesions; and difficulty aspirating viscous cystic contents (Fig. 191-9). Open microsurgical approaches may be associated with hemiparesis from venous infarction, seizures, and even death.115–120 Memory deficits have been reported in up to 26% of microsurgical cases.115,121–123
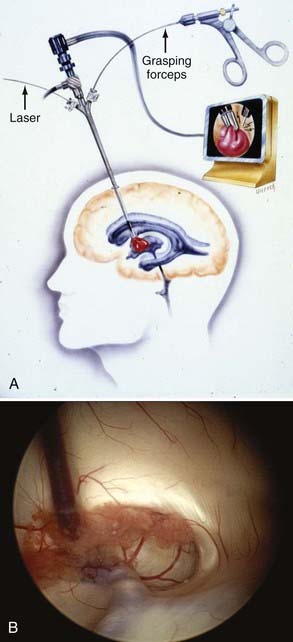
FIGURE 191-9 A, Ventriculoscopic resection of colloid cyst. B, Cyst in foramen of Monro approached from right lateral ventricle.
Neuroendoscopy provides a useful modality for the management of colloid cysts. Image guidance is useful in helping to place the bur hole more lateral and anterior than the standard shunt bur hole. This permits a face-on view of the surface of the cyst in the foramen of Monro. The operator can open the cyst wall with a fiberoptic laser and empty the cystic contents. The cyst wall can be shrunk by the laser or by bipolar coagulation, and the residual cyst can be excised. Horn and associates compared their patient groups in the transcallosal versus endoscopic approach to colloid cysts.119 In the transcallosal group, they found a higher rate of infection, longer operative times, longer hospital stays, and more patients who required ventricular shunts. The endoscopic group had a higher rate of residual cysts on follow-up neuroimaging studies. There was no significant difference in neurologic outcome between the two groups. Other published data show similar results.120 In the largest series with the longest follow-up, with median of 88 months, Greenlee and associates reported only one recurrence in 34 patients treated purely endoscopically.124 Thus, endoscopic resection of colloid cysts represents a safe and effective modality and may be considered a first-line treatment of symptomatic colloid cysts.
Endoscopic colloid cyst resection is not without risks. Some patients experience persistent low-grade headaches and fever that can last for a few weeks.41,125 This may be related to aseptic ventriculitis provoked by spilled cystic contents. The spilled contents can cause obstructive hydrocephalus in the aqueduct, even after the colloid cyst has been removed.116,126,127 Other complications include mechanical injury to neural or vascular structures.
Conclusion
Endoscopic neurosurgery has evolved and revolutionized treatments of certain intracranial pathologies, including hydrocephalus and tumors. Skull base lesions, once considered major surgical challenges, are now enhanced with endoscope-assisted microsurgery. Difficult angles inherent to the skull base can be visualized with the endoscope. Its applications in microsurgery have expanded to include endonasal tumor resection, cerebral aneurysm clipping,128–130 basal encephalocele repair,131–134 and diskectomy and other spinal procedures.135–143 The role of endoscopy in minimally invasive neurosurgery will continue to expand as technologic advances improve the quality of the optics and instrumentation.
Cohen A. Endoscopic ventricular anatomy. In: Cohen A, Haines S, editors. Concepts in Neurosurgery: Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:14-24.
Kadrian D, van Gelder J, Florida D, et al. Long-term reliability of endoscopic third ventriculostomy. Neurosurgery. 2005;56:1271-1278.
Rhoton AJ. Microsurgical anatomy of the lateral ventricles. In: Wilkins R, Rengachary S, editors. Neurosurgery Update I: Diagnosis, Operative Technique, and Neuro-oncology. New York: McGraw-Hill; 1990:354-368.
Rhoton AJ. Microsurgical anatomy of the third ventricular region. In: Apuzzo M, editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:92-166.
Roberts D. Frameless stereotaxy. In: Cohen A, Haines S, editors. Concepts in Neurosurgery: Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:78-84.
Roberts DW, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545-549.
1 Davis L. Neurological Surgery. Philadelphia: Lea & Febiger; 1936.
2 Dandy WE. Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg. 1918;68:569-579.
3 Dandy W. Cerebral ventriculoscopy. Johns Hopkins Hosp Bull. 1922;33:189.
4 Dandy WE. Benign Tumors in the Third Ventricle of the Brain: Diagnosis and Treatment. Springfield, Ill., Baltimore, Md.: C.C. Thomas; 1933.
5 Mixter W. Ventriculoscopy and puncture of the floor of the third ventricle. Boston Med Surg J. 1923;188:277-278.
6 Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vain. Surg Forum. 1951:399-403.
7 Griffith HB. Advances and technical standards in neurosurgery. In: Symon L, editor. Endoneurosurgery: Endoscopic Intracranial Surgery. New York: Springer-Verlag; 1986:2-24.
8 Li KW, Nelson C, Suk I, et al. Neuroendoscopy: past, present, and future. Neurosurg Focus. 2005;19:E1.
9 Liu CY, Wang MY, Apuzzo ML. The physics of image formation in the neuroendoscope. Childs Nerv Syst. 2004;20:777-782.
10 Apuzzo ML, Heifetz MD, Weiss MH, et al. Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg. 1977;46:398-400.
11 Oertel J, Gen M, Krauss JK, et al. The use of waterjet dissection in endoscopic neurosurgery. Technical note. J Neurosurg. 2006;105:928-931.
12 Apuzzo ML, Chandrasoma PT, Zelman V, et al. Computed tomographic guidance stereotaxis in the management of lesions of the third ventricular region. Neurosurgery. 1984;15:502-508.
13 Hellwig D, Bauer BL. Minimally invasive neurosurgery by means of ultrathin endoscopes. Acta Neurochir Suppl (Wien). 1992;54:63-68.
14 Hellwig D, Bauer BL, List-Hellwig E, et al. Stereotactic-endoscopic procedures on processes of the cranial midline. Acta Neurochir Suppl (Wien). 1991;53:23-32.
15 Hellwig D, Bauer B, Dauch W. Stereotactic endoscopic treatment of brain abscesses. In: Cohen A, Haines S, editors. Concepts in Neurosugery: Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:66-72.
16 Zamorano L, Chavantes C, Jiang Z, Kadi M. Stereotactic neuroendoscopy. In: Cohen A, Haines S, editors. Concepts in Neurosurgery: Minimally Invasive Neurosurgery. Baltimore: Williams & Wilkins; 1995:49-66.
17 Zamorano L, Nolte L, Jiang Z, Kadi M. Image-guided neurosurgery: frame-based and frameless approaches. In: Rengachary S, Wilkins R, editors. Neurosurgery Operative Atlas. Baltimore: Williams & Wilkins; 1993:403-422.
18 Heilbrun MP, McDonald P, Wiker C, et al. Stereotactic localization and guidance using a machine vision technique. Stereotact Funct Neurosurg. 1992;58:94-98.
19 Kato A, Yoshimine T, Hayakawa T, et al. A frameless, armless navigational system for computer-assisted neurosurgery. Technical note. J Neurosurg. 1991;74:845-849.
20 Roberts DW, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545-549.
21 Roberts D. Frameless stereotaxy. In: Cohen A, Haines S, editors. Concepts in Neurosurgery: Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:78-84.
22 Rhoton AJ. Microsurgical anatomy of the third ventricular region. In: Apuzzo M, editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:92-166.
23 Rhoton AJ. Microsurgical anatomy of the lateral ventricles. In: Wilkins R, Rengachary S, editors. Neurosurgery Update I: Diagnosis, Operative Technique, and Neuro-oncology. New York: McGraw-Hill; 1990:354-368.
24 Cohen A. Endoscopic ventricular anatomy. In: Cohen A, Haines S, editors. Concepts in Neurosurgery: Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:14-24.
25 Kadrian D, van Gelder J, Florida D, et al. Long-term reliability of endoscopic third ventriculostomy. Neurosurgery. 2005;56:1271-1278.
26 Drake JM. Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery. 2007;60:881-886.
27 Boschert J, Hellwig D, Krauss JK. Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg. 2003;98:1032-1039.
28 Feng H, Huang G, Liao X, et al. Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. J Neurosurg. 2004;100:626-633.
29 Gangemi M, Donati P, Maiuri F, et al. Endoscopic third ventriculostomy for hydrocephalus. Minim Invasive Neurosurg. 1999;42:128-132.
30 Hopf NJ, Grunert P, Fries G, et al. Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery. 1999;44:795-804.
31 Li KW, Roonprapunt C, Lawson HC, et al. Endoscopic third ventriculostomy for hydrocephalus associated with tectal gliomas. Neurosurg Focus. 2005;18:E2.
32 Santamarta D, Diaz Alvarez A, Goncalves JM, et al. Outcome of endoscopic third ventriculostomy. Results from an unselected series with noncommunicating hydrocephalus. Acta Neurochir (Wien). 2005;147:377-382.
33 Choi JU, Kim DS, Kim SH. Endoscopic surgery for obstructive hydrocephalus. Yonsei Med J. 1999;40:600-607.
34 Koch D, Wagner W. Endoscopic third ventriculostomy in infants of less than 1 year of age: which factors influence the outcome? Childs Nerv Syst. 2004;20:405-411.
35 Baldauf J, Oertel J, Gaab MR, et al. Endoscopic third ventriculostomy in children younger than 2 years of age. Childs Nerv Syst. 2007;23:623-626.
36 Scarrow AM, Levy EI, Pascucci L, et al. Outcome analysis of endoscopic III ventriculostomy. Childs Nerv Syst. 2000;16:442-444.
37 Gorayeb RP, Cavalheiro S, Zymberg ST. Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg. 2004;100:427-429.
38 Fritsch MJ, Kienke S, Ankermann T, et al. Endoscopic third ventriculostomy in infants. J Neurosurg. 2005;103:50-53.
39 Beems T, Grotenhuis JA. Is the success rate of endoscopic third ventriculostomy age-dependent? An analysis of the results of endoscopic third ventriculostomy in young children. Childs Nerv Syst. 2002;18:605-608.
40 Etus V, Ceylan S. Success of endoscopic third ventriculostomy in children less than 2 years of age. Neurosurg Rev. 2005;28:284-288.
41 Cohen AR. Ventriculoscopic surgery. Clin Neurosurg. 1994;41:546-562.
42 Jones R, Brazier D, Kwok B, et al. Neuroendoscpic third ventriculostomy. In: Cohen A, Haines S, editors. Concepts in Neurosurgery: Minimally Invasive Techniques in Neurosurgery. Baltimore: Williams & Wilkins; 1995:33-48.
43 Fukuhara T, Vorster SJ, Luciano MG. Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery. 2000;46:1100-1109.
44 Warf BC. Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg. 2005;102:1-15.
45 O’Brien DF, Javadpour M, Collins DR, et al. Endoscopic third ventriculostomy: an outcome analysis of primary cases and procedures performed after ventriculoperitoneal shunt malfunction. J Neurosurg. 2005;103:393-400.
46 Di Rocco C, Cinalli G, Massimi L, et al. Endoscopic third ventriculostomy in the treatment of hydrocephalus in pediatric patients. Adv Tech Stand Neurosurg. 2006;31:119-219.
47 Schroeder HW, Niendorf WR, Gaab MR. Complications of endoscopic third ventriculostomy. J Neurosurg. 2002;96:1032-1040.
48 Cinalli G, Spennato P, Ruggiero C, et al. Complications following endoscopic intracranial procedures in children. Childs Nerv Syst. 2007;23:633-644.
49 Walker ML. Complications of third ventriculostomy. Neurosurg Clin N Am. 2004;15:61-66.
50 Ersahin Y, Arslan D. Complications of endoscopic third ventriculostomy. Childs Nerv Syst. 2008;24:943-948.
51 Anandh B, Madhusudan Reddy KR, Mohanty A, et al. Intraoperative bradycardia and postoperative hyperkalemia in patients undergoing endoscopic third ventriculostomy. Minim Invasive Neurosurg. 2002;45:154-157.
52 Handler MH, Abbott R, Lee M. A near-fatal complication of endoscopic third ventriculostomy: case report. Neurosurgery. 1994;35:525-527.
53 Jones RF, Stening WA, Brydon M. Endoscopic third ventriculostomy. Neurosurgery. 1990;26:86-91.
54 Bonanni R, Carlesimo GA, Caltagirone C. Amnesia following endoscopic third ventriculostomy: a single case study. Eur Neurol. 2004;51:118-120.
55 Abtin K, Thompson BG, Walker ML. Basilar artery perforation as a complication of endoscopic third ventriculostomy. Pediatr Neurosurg. 1998;28:35-41.
56 Horowitz M, Albright AL, Jungreis C, et al. Endovascular management of a basilar artery false aneurysm secondary to endoscopic third ventriculostomy: case report. Neurosurgery. 2001;49:1461-1464.
57 McLaughlin MR, Wahlig JB, Kaufmann AM, et al. Traumatic basilar aneurysm after endoscopic third ventriculostomy: case report. Neurosurgery. 1997;41:1400-1403.
58 Schroeder HW, Warzok RW, Assaf JA, et al. Fatal subarachnoid hemorrhage after endoscopic third ventriculostomy. Case report. J Neurosurg. 1999;90:153-155.
59 Vandertop PW. Traumatic basilar aneurysm after endoscopic third ventriculostomy: case report. Neurosurgery. 1998;43:647-648.
60 Teo C. Complications of endoscopic third ventriculostomy. In: Cinalli G, Maixner WJ, Sainte-Rose C, editors. Pediatric Hydrocephalus. Milan: Springer; 2004:411-420.
61 Beems T, Grotenhuis JA. Long-term complications and definition of failure of neuroendoscopic procedures. Childs Nerv Syst. 2004;20:868-877.
62 Navarro R, Gil-Parra R, Reitman AJ, et al. Endoscopic third ventriculostomy in children: early and late complications and their avoidance. Childs Nerv Syst. 2006;22:506-513.
63 El-Dawlatly AA, Murshid WR, Elshimy A, et al. The incidence of bradycardia during endoscopic third ventriculostomy. Anesth Analg. 2000;91:1142-1144.
64 van Aken J, Struys M, Verplancke T, et al. Cardiovascular changes during endoscopic third ventriculostomy. Minim Invasive Neurosurg. 2003;46:198-201.
65 Kamel MH, Murphy M, Aquilina K, et al. Subdural haemorrhage following endoscopic third ventriculostomy. A rare complication. Acta Neurochir (Wien). 2006;148:591-593.
66 Sgaramella E, Castelli G, Sotgiu S. Chronic subdural collection after endoscopic third ventriculostomy. Acta Neurochir (Wien). 2004;146:529-530.
67 Fritsch MJ, Bauer M, Partsch CJ, et al. Endocrine evaluation after endoscopic third ventriculostomy (ETV) in children. Childs Nerv Syst. 2007;23:627-631.
68 Teo C, Rahman S, Boop FA, et al. Complications of endoscopic neurosurgery. Childs Nerv Syst. 1996;12:248-253.
69 Vaicys C, Fried A. Transient hyponatremia complicated by seizures after endoscopic third ventriculostomy. Minim Invasive Neurosurg. 2000;43:190-191.
70 Ferrer E, Santamarta D, Garcia-Fructuoso G, et al. Neuroendoscopic management of pineal region tumours. Acta Neurochir (Wien). 1997;139:12-20.
71 Peretta P, Ragazzi P, Galarza M, et al. Complications and pitfalls of neuroendoscopic surgery in children. J Neurosurg. 2006;105:187-193.
72 Drake J, Chumas P, Kestle J, et al. Late rapid deterioration after endoscopic third ventriculostomy: additional cases and review of the literature. J Neurosurg. 2006;105:118-126.
73 Hader WJ, Drake J, Cochrane D, et al. Death after late failure of third ventriculostomy in children. Report of three cases. J Neurosurg. 2002;97:211-215.
74 Tuli S, Alshail E, Drake J. Third ventriculostomy versus cerebrospinal fluid shunt as a first procedure in pediatric hydrocephalus. Pediatr Neurosurg. 1999;30:11-15.
75 Mobbs RJ, Vonau M, Davies MA. Death after late failure of endoscopic third ventriculostomy: a potential solution. Neurosurgery. 2003;53:384-385.
76 Dandy W. The diagnosis and treatment of hydrocephalus resulting from strictures of the aqueduct of Sylvius. Surg Gynecol Obstet. 1920;31:340-358.
77 Enchev Y, Oi S. Historical trends of neuroendoscopic surgical techniques in the treatment of hydrocephalus. Neurosurg Rev. 2008;31:249-262.
78 Cinalli G, Spennato P, Savarese L, et al. Endoscopic aqueductoplasty and placement of a stent in the cerebral aqueduct in the management of isolated fourth ventricle in children. J Neurosurg. 2006;104:21-27.
79 da Silva LR, Cavalheiro S, Zymberg ST. Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Childs Nerv Syst. 2007;23:1263-1268.
80 Ersahin Y. Endoscopic aqueductoplasty. Childs Nerv Syst. 2007;23:143-150.
81 Schroeder HW, Oertel J, Gaab MR. Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Childs Nerv Syst. 2004;20:821-827.
82 Schroeder HW, Joachim O, Gaab MR. Endoscopic treatment of cerebrospinal fluid pathway obstructions. Neurosurgery. 2007;60(ONS suppl 1):ONS-44-52.
83 Teo C, Burson T, Misra S. Endoscopic treatment of the trapped fourth ventricle. Neurosurgery. 1999;44:1257-1261.
84 Fritsch MJ, Kienke S, Mehdorn HM. Endoscopic aqueductoplasty: stent or not to stent? Childs Nerv Syst. 2004;20:137-142.
85 Manwaring KH, Fritsch MJ. Endoscopic aqueductal stenting as an option for obstructive hydrocephalus. Neurosurgery. 1998;43:712-713.
86 Oi S, Hidaka M, Honda Y, et al. Neuroendoscopic surgery for specific forms of hydrocephalus. Childs Nerv Syst. 1999;15:56-68.
87 Heilman CB, Cohen AR. Endoscopic ventricular fenestration using a saline torch. J Neurosurg. 1991;74:224-229.
88 Aldana PR, Kestle JR, Brockmeyer DL, et al. Results of endoscopic septal fenestration in the treatment of isolated ventricular hydrocephalus. Pediatr Neurosurg. 2003;38:286-294.
89 Mohanty A, Das BS, Sastry Kolluri VR, et al. Neuro-endoscopic fenestration of occluded foramen of Monro causing unilateral hydrocephalus. Pediatr Neurosurg. 1996;25:248-251.
90 Wong TT, Lee LS. Membranous occlusion of the foramen of Monro following ventriculoperitoneal shunt insertion: a role for endoscopic foraminoplasty. Childs Nerv Syst. 2000;16:213-217.
91 Javier-Fernandez J, Garcia-Cosamalon PJ, Vinuela J, et al. [Endoscopic fenestration as a treatment for asymmetrical hydrocephalus due to obstruction of the foramen of Monro]. Neurocirugia (Astur). 2001;12:513-515.
92 Fukushima T. Endoscopic biopsy of intraventricular tumors with the use of a ventriculofiberscope. Neurosurgery. 1978;2:110-113.
93 Abdullah J, Caemaert J. Endoscopic management of craniopharyngiomas: a review of 3 cases. Minim Invasive Neurosurg. 1995;38:79-84.
94 Gururangan S, Heideman RL, Kovnar EH, et al. Peritoneal metastases in two patients with pineoblastoma and ventriculo-peritoneal shunts. Med Pediatr Oncol. 1994;22:417-420.
95 Pallini R, Bozzini V, Scerrati M, et al. Bone metastasis associated with shunt-related peritoneal deposits from a pineal germinoma. Case report and review of the literature. Acta Neurochir (Wien). 1991;109:78-83.
96 Ung AO, Triscott JA, Leditschke JF, et al. Metastasis of pineal germinoma via ventriculoperitoneal shunt. Aust N Z J Surg. 1993;63:409-412.
97 Galassi E, Tognetti F, Frank F, et al. Extraneural metastases from primary pineal tumors. Review of the literature. Surg Neurol. 1984;21:497-504.
98 Shibasaki T, Takeda F, Kawafuchi J. [Extraneural metastases of malignant brain tumors through ventriculoperitoneal shunt—report of two autopsy cases and a review of the literature (author’s transl)]. No Shinkei Geka. 1977;5:71-79.
99 Uchino M, Nemoto M, Ohtsuka T, et al. [Extraneural metastasis of pineal germinoma through a ventriculoperitoneal shunt, following histological change]. No Shinkei Geka. 1999;27:269-274.
100 Chernov MF, Kamikawa S, Yamane F, et al. Neurofiberscopic biopsy of tumors of the pineal region and posterior third ventricle: indications, technique, complications, and results. Neurosurgery. 2006;59:267-277.
101 Konovalov AN, Pitskhelauri DI. Principles of treatment of the pineal region tumors. Surg Neurol. 2003;59:250-268.
102 Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446-455.
103 Abay EO2nd, Laws ERJr, Grado GL, et al. Pineal tumors in children and adolescents. Treatment by CSF shunting and radiotherapy. J Neurosurg. 1981;55:889-895.
104 Lutterbach J, Fauchon F, Schild SE, et al. Malignant pineal parenchymal tumors in adult patients: patterns of care and prognostic factors. Neurosurgery. 2002;51:44-55.
105 Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13:690-699.
106 Gangemi M, Maiuri F, Colella G, et al. Endoscopic surgery for pineal region tumors. Minim Invasive Neurosurg. 2001;44:70-73.
107 Oi S, Shibata M, Tominaga J, et al. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: a prospective study. J Neurosurg. 2000;93:245-253.
108 Pople IK, Athanasiou TC, Sandeman DR, et al. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg. 2001;15:305-311.
109 Al-Tamimi YZ, Bhargava D, Surash S, et al. Endoscopic biopsy during third ventriculostomy in paediatric pineal region tumours. Childs Nerv Syst. 2008;24:1323-1326.
110 Hwang DH, Townsend JC, Ilsen PF, et al. Colloid cyst of the third ventricle. J Am Optom Assoc. 1996;67:227-234.
111 Jeffree RL, Besser M. Colloid cyst of the third ventricle: a clinical review of 39 cases. J Clin Neurosci. 2001;8:328-331.
112 Kondziolka D, Lunsford LD. Stereotactic techniques for colloid cysts: roles of aspiration, endoscopy, and microsurgery. Acta Neurochir Suppl. 1994;61:76-78.
113 Mathiesen T, Grane P, Lindquist C, et al. High recurrence rate following aspiration of colloid cysts in the third ventricle. J Neurosurg. 1993;78:748-752.
114 Skirving DJ, Pell MF. Early recurrence from stereotactic aspiration of a colloid cyst of the third ventricle. J Clin Neurosci. 2001;8:570-571.
115 Camacho A, Abernathey CD, Kelly PJ, et al. Colloid cysts: experience with the management of 84 cases since the introduction of computed tomography. Neurosurgery. 1989;24:693-700.
116 Antunes JL, Louis KM, Ganti SR. Colloid cysts of the third ventricle. Neurosurgery. 1980;7:450-455.
117 Morita A, Kelly PJ. Resection of intraventricular tumors via a computer-assisted volumetric stereotactic approach. Neurosurgery. 1993;32:920-926.
118 Jeeves MA, Simpson DA, Geffen G. Functional consequences of the transcallosal removal of intraventricular tumours. J Neurol Neurosurg Psychiatry. 1979;42:134-142.
119 Horn EM, Feiz-Erfan I, Bristol RE, et al. Treatment options for third ventricular colloid cysts: comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60:613-618.
120 Grondin RT, Hader W, MacRae ME, et al. Endoscopic versus microsurgical resection of third ventricle colloid cysts. Can J Neurol Sci. 2007;34:197-207.
121 Desai KI, Nadkarni TD, Muzumdar DP, et al. Surgical management of colloid cyst of the third ventricle—a study of 105 cases. Surg Neurol. 2002;57:295-302.
122 Friedman MA, Meyers CA, Sawaya R. Neuropsychological effects of third ventricle tumor surgery. Neurosurgery. 2003;52:791-798.
123 Mathiesen T, Grane P, Lindgren L, et al. Third ventricle colloid cysts: a consecutive 12-year series. J Neurosurg. 1997;86:5-12.
124 Greenlee JD, Teo C, Ghahreman A, et al. Purely endoscopic resection of colloid cysts. Neurosurgery. 2008;62:51-55.
125 Cohen A, Shucart W. Ventriculoscopic management of colloid cysts of the third ventricle. In: Manwaring K, Crone K, editors. Neuroendoscopy. New York: Mary Ann Liebert; 1992:109-117.
126 Brun A, Egund N. The pathogenesis of cerebral symptoms in colloid cysts of the third ventricle: a clinical and pathoanatomical study. Acta Neurol Scand. 1973;49:525-535.
127 Stookey B, Scarff J. Occlusion of the aqueduct of Sylvius by neoplastic and nonneoplastic processes with rational surgical treatment for relief of the resultant hydrocephalus. Bull Neurol Inst NY. 1936;5:348-377.
128 Zhao J, Wang Y, Zhao Y, et al. Neuroendoscope-assisted minimally invasive microsurgery for clipping intracranial aneurysms. Minim Invasive Neurosurg. 2006;49:335-341.
129 Profeta G, De Falco R, Ambrosio G, et al. Endoscope-assisted microneurosurgery for anterior circulation aneurysms using the angle-type rigid endoscope over a 3-year period. Childs Nerv Syst. 2004;20:811-815.
130 Taniguchi M, Takimoto H, Yoshimine T, et al. Application of a rigid endoscope to the microsurgical management of 54 cerebral aneurysms: results in 48 patients. J Neurosurg. 1999;91:231-237.
131 Lee TJ, Chang PH, Huang CC, et al. Endoscopic treatment of traumatic basal encephaloceles: a report of 8 cases. J Neurosurg. 2008;108:729-735.
132 Boseley ME, Tami TA. Endoscopic management of anterior skull base encephaloceles. Ann Otol Rhinol Laryngol. 2004;113:30-33.
133 Lanza DC, O’Brien DA, Kennedy DW. Endoscopic repair of cerebrospinal fluid fistulae and encephaloceles. Laryngoscope. 1996;106:1119-1125.
134 Marshall AH, Jones NS, Robertson IJ. Endoscopic management of basal encephaloceles. J Laryngol Otol. 2001;115:545-547.
135 Magrassi L, Chiaranda I, Minelli M, et al. Total endoscopic approach to the cauda in a patient with a tight filum. Minim Invasive Neurosurg. 2008;51:350-353.
136 Hiraizumi Y. Spinal cord anterior decompression for delayed spinal cord paralysis after osteoporotic vertebral compression fracture: application of thoracoscopic approach. Chir Narzadow Ruchu Ortop Pol. 2008;73:67-73.
137 Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine. 2008;33:E508-E515.
138 Ruetten S, Komp M, Merk H, et al. Full-endoscopic cervical posterior foraminotomy for the operation of lateral disc herniations using 5.9-mm endoscopes: a prospective, randomized, controlled study. Spine. 2008;33:940-948.
139 Sairyo K, Sakai T, Higashino K, et al. Minimally invasive excision of lumbar epidural lipomatosis using a spinal endoscope. Minim Invasive Neurosurg. 2008;51:43-46.
140 Barami K, Dagnew E. Endoscope-assisted posterior approach for the resection of ventral intradural spinal cord tumors: report of two cases. Minim Invasive Neurosurg. 2007;50:370-373.
141 Higashino K, Sairyo K, Katoh S, et al. Minimally invasive technique for direct repair of the pars defects in young adults using a spinal endoscope: a technical note. Minim Invasive Neurosurg. 2007;50:182-186.
142 Le Huec JC, Lesprit E, Guibaud JP, et al. Minimally invasive endoscopic approach to the cervicothoracic junction for vertebral metastases: report of two cases. Eur Spine J. 2001;10:421-426.
143 Burtscher J, Felber S, Twerdy K, et al. Endoscope-assisted interlaminar removal of an ependymoma of the cauda equina. Minim Invasive Neurosurg. 2002;45:41-44.