CHAPTER 173 Neuroanesthesia in Children
Preoperative Evaluation and Preparation
Preparation of pediatric patients for anesthesia and surgery is essential to minimize perioperative morbidity. Given the systemic effects of general anesthesia and the physiologic stress of surgery, an organ system review should be performed to anticipate potential physiologic derangements and coexisting disease states that may increase the risk for perioperative complications.1 Because some children are preverbal or do not fully understand their medical condition, their parents or primary caretakers should be carefully interviewed to obtain information regarding coexisting medical problems. A thorough review of the patient’s history can reveal conditions that may increase the risk for adverse reactions and identify patients who need more extensive evaluation or whose medical condition needs to be optimized before surgery. Certain medical problems may require that the anesthetic be modified (Table 173-1). There are also special perioperative concerns regarding children with neurological abnormalities (Table 173-2). Preoperative fasting is necessary to minimize aspiration of gastric contents during the operative procedure, guidelines for which are listed in Table 173-3.
TABLE 173-1 Common Perioperative Concerns in Infants and Children
| CONDITION | ANESTHETIC IMPLICATIONS |
|---|---|
| Congenital heart disease | Hypoxia and cardiovascular collapse |
| Prematurity | Postoperative apnea |
| Gastrointestinal reflux | Aspiration pneumonia |
| Upper respiratory tract infection | Laryngospasm and postoperative hypoxia or pneumonia |
| Craniofacial abnormality | Difficulty with airway management |
TABLE 173-2 Common Perioperative Concerns in Infants and Children with Neurologic Problems
| CONDITION | ANESTHETIC IMPLICATIONS |
|---|---|
| Denervation injuries | Hyperkalemia after succinylcholine |
| Resistance to nondepolarizing muscle relaxants | |
| Chronic anticonvulsant therapy for epilepsy | Hepatic and hematologic abnormalities |
| Increased metabolism of anesthetic agents | |
| Arteriovenous malformation | Potential congestive heart failure |
| Neuromuscular disease | Malignant hyperthermia |
| Respiratory failure | |
| Sudden cardiac death | |
| Chiari’s malformation | Apnea |
| Aspiration pneumonitis | |
| Hypothalamic/pituitary lesions | Diabetes insipidus |
| Hypothyroidism | |
| Adrenal insufficiency |
TABLE 173-3 Guidelines for Fasting before Surgery or Anesthesia
Closed-claim analysis studies have revealed that neonates and infants are at higher risk than any other age group for morbidity and mortality.2,3 Respiratory and cardiac-related events account for a majority of these complications. Given the urgent nature of neonatal neurosurgical procedures, a thorough preoperative evaluation may be difficult. Preoperative evaluation and laboratory tests should be tailored to the proposed neurosurgical procedure. Patients with suprasellar pathology should have thyroid function tests performed because hypothyroidism can lead to bradycardia and hypotension and prolong emergence from general anesthesia. The latter can mimic an adverse neurological event such as stroke or cerebral edema. Diabetes insipidus can be diagnosed by history and abnormal serum electrolytes. A complete airway examination is essential because some craniofacial anomalies may require specialized techniques to secure the airway.4 Congenital heart disease may not be detected immediately after birth. Therefore, echocardiography can be helpful in assessment of the heart, especially in a neonate, and a pediatric cardiologist should evaluate patients with suspected problems to help optimize cardiac function before surgery. Table 173-2 matches special concerns in pediatric patients with neurological problems. Subspecialists may be helpful to optimize the patient’s condition before surgery and assist in postoperative management.
The infant or child may not have the cognitive ability to rationalize the gravity of the situation. Patients and family members are usually frightened by the strange surroundings of the hospital and operating room, exposure to unfamiliar hospital workers, the possibility of painful stimuli, and the thought of separation from each other and treasured comfort objects. Therefore, the approach to a pediatric patient should take into account the patient’s developmental age. Table 173-4 lists the cognitive stages of pediatric patients and age-appropriate concerns.
TABLE 173-4 Preoperative Concerns in Pediatric Patients
| AGE GROUP | CONCERNS |
|---|---|
| Infants (0-9 mo) | None; separate easily from parents |
| Preschoolers (9 mo-5 yr) | Stranger anxiety, difficulty with parental separation |
| Grade school children (6-12 yr) | Fear of needles and pain |
| Adolescents (>12 yr) | Anxious about surgery and self-image |
Preoperative administration of sedatives before induction of anesthesia can ease the transition from the preoperative holding area to the operating room.5 Midazolam given orally is particularly effective in relieving anxiety and producing amnesia. Preoperative sedation should be withheld or administered only with close observation in patients with deteriorating findings on neurological examination or lethargy because it can induce respiratory depression and interfere with serial neurological examinations.
Intraoperative Management
Induction of Anesthesia
The patient’s neurological status and coexisting abnormalities dictate the most appropriate technique and drugs for induction of anesthesia. Generally, alert patients should be able to tolerate any type of induction technique. If intravenous (IV) access has not been established, general anesthesia can be induced by inhalation of sevoflurane and nitrous oxide with oxygen.6,7 This can be facilitated by having one parent present in the operating room during induction to help calm small children. Once the patient is unresponsive, the parent is escorted out of the operating room and IV access is rapidly established. A nondepolarizing muscle relaxant is then administered to facilitate intubation of the trachea. Despite the widespread use of inhaled induction in pediatric anesthesia, complications such as laryngospasm or vomiting may occur and lead to airway obstruction and hypoxia. Alternatively, if the patient has IV access, anesthesia can be induced rapidly with sedative-hypnotic drugs such as thiopental or propofol.
Airway Management
Anatomic differences between the pediatric and adult airway are primarily due to the size and orientation of components of the upper airway, larynx, and trachea. Neonates and infants have the greatest differences from adults in this respect. However, the configuration of the larynx becomes similar to that of adults after the second year of life. Table 173-5 highlights the major differences between pediatric and adult airway anatomy. An infant’s larynx is also funnel shaped and narrowest at the level of the cricoid, thus making this region the smallest cross-sectional area in the infant airway. This places the infant at risk for life-threatening subglottic obstruction secondary to mucosal swelling after prolonged intubation with a tight-fitting endotracheal tube. An endotracheal tube can also migrate into a mainstem bronchus if the infant’s head is flexed for a suboccipital approach to the posterior fossa or the cervical spine. Therefore, the anesthesiologist should auscultate both lung fields to rule out inadvertent intubation of a mainstem bronchus after the patient is positioned for the surgical procedure.
TABLE 173-5 Differences between Pediatric and Adult Airway Anatomy
| INFANT | ADULT | |
|---|---|---|
| Tongue | Relatively large | Normal |
| Epiglottis | Floppy, angled posteriorly | Firm, less posterior angle |
| Vocal cord angle | Inclined | Flat |
| Glottis | C3-4 level | C5 level |
| Cricothyroid membrane | Small | Large |
| Trachea | Mobile, posterior displacement into the thorax | Stationary, vertical descent into the thorax |
Because an immediate neurological examination is essential for assessment of the patient, the timing of tracheal extubation may be challenging after neurosurgical procedures. Infants, particularly those with a Chiari malformation,8 or older children after procedures in the posterior fossa9 may exhibit intermittent apnea, vocal cord paralysis, or other irregularities before resuming a stable respiratory pattern. Significant airway edema and postoperative obstruction can complicate prolonged prone procedures or those involving significant blood loss and large-volume replacement. Finally, preexisting pulmonary dysfunction, as in infants with bronchopulmonary dysplasia or older children with neuromuscular disease, may force delays in extubation because of respiratory insufficiency. In these cases, objective criteria and the presence of an air leak around the endotracheal tube with airway pressures less than 20 to 25 cm H2O will determine the appropriate timing of postoperative extubation.10 Lingual or supraglottic swelling may require direct laryngoscopy to assess the airway. Head-up positioning and gentle forced diuresis usually improve airway edema within 24 hours.
Maintenance of Anesthesia
The choice of anesthetic agents for maintenance of anesthesia has been shown to not affect the outcome of neurosurgical procedures.11 Table 173-6 lists commonly used anesthetic drugs. The most frequently used technique for neurosurgery consists of IV administration of the opioid fentanyl or sufentanil, along with inhaled nitrous oxide and low-dose isoflurane or sevoflurane. Remifentanil is a unique opioid that is rapidly metabolized by plasma esterases and characterized by rapid emergence from anesthesia.12,13 However, it is frequently accompanied by delirium and inadequate analgesia.14,15 Deep neuromuscular blockade is maintained during most neurosurgical procedures to avoid any patient movement. Patients receiving chronic anticonvulsant therapy require larger doses of muscle relaxants and narcotics because of induced enzymatic metabolism of these agents.16 Muscle relaxation should be withheld or not maintained when assessment of motor function during seizure or spinal cord surgery is planned.
| Inhaled Anesthetics |
|
Nitrous oxide |
Hemodynamic stability during intracranial surgery requires careful maintenance of the patient’s fluids and electrolytes. Given the risk for significant blood loss associated with many neurosurgical procedures, a hematocrit and prothrombin time/partial thromboplastin time should be obtained to uncover any insidious hematologic disorders. Typed and cross-matched blood should be ordered before all craniotomies. Small patients have a greater percentage (up to 25%) of their cardiac output directed toward the head.17 Fluid restriction and diuretic therapy may lead to hemodynamic instability and even cardiovascular collapse if sudden blood loss occurs during surgery. Therefore, normovolemia should be maintained throughout the procedure. Normal saline is commonly used as the maintenance fluid during neurosurgery because it is mildly hyperosmolar (308 mOsm/kg) and it theoretically attenuates brain edema. However, rapid infusion of a large amount of normal saline (30 mL/kg per hour) is associated with hyperchloremic acidosis.18 Table 173-7 provides guidelines for IV fluid administration in pediatric patients. Hyperventilation and careful patient positioning to maximize cerebral venous drainage can minimize brain swelling. Should these maneuvers fail, mannitol can be given at a dose of 0.25 to 1 g/kg intravenously. This transiently alters cerebral hemodynamics and raises serum osmolality by 10 to 20 mOsm/kg.19 However, repeated dosing can lead to extreme hyperosmolality, renal failure, and further brain edema. Furosemide is a useful adjunct to mannitol for decreasing acute cerebral edema and has been shown in vitro to prevent rebound swelling caused by mannitol.20 All diuretics interfere with the ability to use urine output as a guide to intravascular volume status.
TABLE 173-7 Guidelines for Maintenance of Intravenous Fluids in Infants and Children
| WEIGHT (kg) | MAINTENANCE FLUIDS PER HOUR |
|---|---|
| <10 | 4 mL/kg |
| 10-20 | 40 mL + 2 mL/kg for every kg between 10 and 20 kg |
| >20 | 60 mL + 1 mL/kg for every kg >20 |
Monitoring
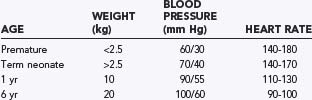
Standard monitoring for all neurosurgical anesthetics includes a stethoscope (precordial or esophageal), electrocardiogram, pulse oximetry, blood pressure, end-tidal carbon dioxide, temperature, and an indwelling bladder catheter. Given the potential for sudden hemodynamic instability as a result of venous air embolism (VAE), hemorrhage, herniation syndromes, or manipulation of cranial nerves, placement of an intra-arterial catheter for continuous blood pressure monitoring is appropriate for most neurosurgical procedures. An arterial catheter also provides access for serial sampling of blood for analysis of blood gases, electrolytes, and hematocrit. Normal age-dependent vital signs are listed in Table 173-8. Central venous pressure may not accurately reflect vascular volume, especially in a child in the prone position. Therefore, the risks associated with a central venous catheter may outweigh its benefits.
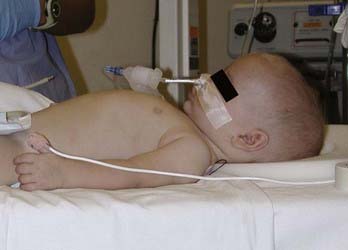
VAE is a common occurrence during craniotomies in infants because the head of a small child is large in relation to the rest of the body and rests above the heart, even in the supine position (Fig. 173-1). Standard neurosurgical techniques may elevate the head of the table to improve venous drainage and are conducive to air entrainment into the venous system through open venous channels in bone and sinuses. Patients with cardiac defects, such as a patent foramen ovale or patent ductus arteriosus, are at risk for arterial air emboli through these defects and should be monitored carefully. Precordial Doppler ultrasonography is extremely sensitive but not specific for VAE and can be used in conjunction with an end-tidal carbon dioxide analyzer and arterial catheter in all craniotomies. The Doppler probe is best positioned on the anterior aspect of the chest, usually just to the right of the sternum at the fourth intercostal space. An alternative site on the posterior of the thorax can be used in the prone position for infants weighting approximately 6 kg or less.21
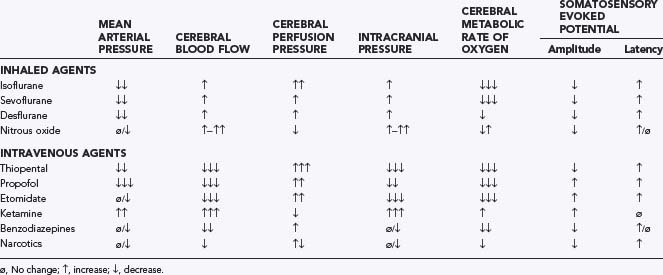
Recent advances in neurophysiologic monitoring have enhanced our ability to safely perform more definitive neurosurgical resections in functional areas of the brain and spinal cord. However, the depressant effects of many anesthetic agents limit the utility of these monitors. A major part of preoperative planning should include a thorough discussion of the modality and type of neurophysiologic monitoring to be used during any surgical procedure. In general, electrocorticography and electroencephalography require low levels of volatile anesthetics and barbiturates. Somatosensory evoked potentials used during spinal and brainstem surgery can be depressed by volatile agents and, to a lesser extent, by nitrous oxide. An opioid-based anesthetic is the most appropriate agent for this type of monitoring. Baseline function should be obtained before surgical manipulation to detect significant deviations during the procedure. Spinal cord and peripheral nerve surgery may require electromyography and detection of muscle movement as an end point. Therefore, muscle relaxation should be avoided or not maintained during the monitoring period. Table 173-9 lists common anesthetic agents and their effects on various neurophysiologic monitors.
Positioning
Patient positioning for surgery requires careful preoperative planning to allow adequate access to the patient for both the neurosurgeon and the anesthesiologist. Table 173-10 describes common surgical positions and their physiologic sequelae. These issues should be considered because the duration of most neurosurgical procedures can lead to significant physiologic impairment or injury if positioning problems occur. Before placement of the sterile drapes, all pressure points should be padded and peripheral pulses checked to prevent compression or pressure injury. It is also important to avoid stretching peripheral nerves and prevent skin and soft tissue injury because of improper contact with surgical accessories such as instrument stands and grounding wires. Given the limited access to the patient’s airway once surgery begins, the endotracheal tube must be secured carefully.
| POSITION | PHYSIOLOGIC EFFECT |
|---|---|
| Head elevated | Enhanced cerebral venous drainage |
| Decreased cerebral blood flow | |
| Increased venous pooling in the lower extremities | |
| Postural hypotension | |
| Head down | Increased cerebral venous and intracranial pressure |
| Decreased functional residual capacity (lung function) | |
| Decreased lung compliance | |
| Prone | Venous congestion of face, tongue, and neck |
| Decreased lung compliance | |
| Increased abdominal pressure can lead to venocaval compression | |
| Lateral decubitus | Decreased compliance of the down-side lung |
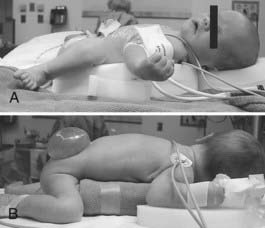
The prone position is commonly used for posterior fossa and spinal cord surgery. In addition to the physiologic sequelae of this position, a whole spectrum of compression and stretch injuries have been reported. Padding under the chest and pelvis can support the torso. It is important to ensure free abdominal wall motion because increased intra-abdominal pressure can impair ventilation, cause venocaval compression, and increase epidural venous pressure and bleeding. Figure 173-2 demonstrates proper positioning for these patients. Soft rolls are used to elevate and support the lateral chest wall and hips to minimize any increase in abdominal and thoracic pressure. In addition, this allows a Doppler probe to be placed on the chest without pressure. The head must be carefully flexed to avoid kinking of the endotracheal tube, inadvertently advancing the tube into an endobronchial position, or compressing the chin on the chest. Too much flexion for an extended time can cause head and tongue swelling from blockage of venous or lymphatic drainage. This can lead to postextubation airway obstruction or croup.
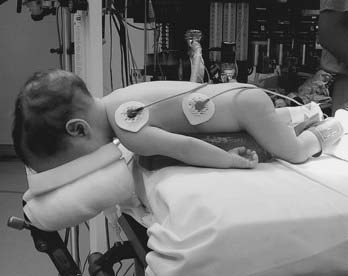
FIGURE 173-2 Prone infant. Lateral rolls are used to elevate the infant and minimize thoracic and abdominal pressure.
Positioning of the patient’s head can be a contentious issue between the anesthesiologist and neurosurgeon. Many neurosurgical procedures are performed with the head slightly elevated to facilitate venous and cerebrospinal fluid drainage from the surgical site. However, superior sagittal sinus pressure decreases with greater head elevation, and this increases the likelihood of VAE.22 Extreme head flexion can cause brainstem compression in patients with pathologic conditions of the posterior fossa, such as mass lesions or Arnold-Chiari malformations. It can also cause endotracheal tube problems, including obstruction from kinking or displacement to the carina or right mainstem bronchus. Extreme rotation of the head can impede venous return through the jugular veins and lead to impaired cerebral perfusion, increased intracranial pressure, and cerebral venous bleeding.
Emergence from Anesthesia
Prompt emergence from general anesthesia is important so that neurological function can be assessed immediately after neurosurgical procedures. Therefore, one of the goals of neurosurgical anesthesia is to time the duration of anesthesia to allow smooth awakening with spontaneous respiration and hemodynamic stability. A drawback of rapid emergence from anesthesia is coughing on the endotracheal tube, which can lead to arterial and intracranial hypertension. Low-dose infusion of dexmedetomidine may facilitate smooth emergence from anesthesia.23 Premature extubation of the trachea can lead to laryngospasm or apnea, which can progress to hypoxia and cardiac arrest if not treated promptly. Hypertension during emergence from anesthesia can be controlled with vasodilator drugs such as labetalol. Neuromuscular blockade should be fully antagonized, and all anesthetic agents should be discontinued. Once the patient exhibits spontaneous ventilation and appropriate responses to verbal commands are demonstrated, the trachea can be extubated. Failure to achieve these two criteria should prompt a search for additional problems (Table 173-11). If anesthetic causes of delayed awakening are not apparent, neurological conditions should be strongly considered and evaluated with a computed tomographic (CT) scan of the head. Transportation to the CT suite requires maintenance of general anesthesia and continuous hemodynamic monitoring. In these circumstances it is often safer for the patient’s trachea to remain intubated. This is also the case for procedures in which several blood volumes have been lost and replaced with crystalloid and blood, which frequently results in airway and facial edema and may lead to postextubation airway obstruction. In addition, operations that result in disruption of cranial nerve nuclei or brainstem may lead to impairment of airway reflexes and respiratory drive. However, residual anesthetic and neuromuscular blockade should always be ruled out before making the diagnosis of neurological injury. Antagonism of residual narcotic by naloxone can result in uncontrolled hypertension and coughing on the endotracheal tube and should be avoided.
TABLE 173-11 Conditions Leading to Delayed Emergence from Anesthesia
| Residual Anesthetic Drugs |
Postoperative Management
Close observation in an intensive care unit with serial neurological examinations and invasive hemodynamic monitoring is helpful for the prevention and early detection of postoperative problems. Respiratory dysfunction is the leading complication after posterior fossa craniotomies.24 Airway obstruction after a craniotomy can be due to airway edema or cranial nerve dysfunction after surgical manipulation of the cranial nerves or nuclei. Airway edema is usually self-limited but may require endotracheal intubation as a stent. Occasionally, ischemia or edema of the respiratory centers of the brainstem interferes with respiratory control and leads to postoperative apnea. Residual narcotics can also produce transient apnea. Naloxone can antagonize the residual narcosis but may result in pain and hypertension. Acute changes in the neurological examination may be due to a mass effect secondary to intracranial bleeding, hydrocephalus, or cerebral infarction. An emergency CT scan of the head may be helpful to confirm the diagnosis and guide management of the inciting event. Derangements in sodium concentration in the postoperative period are typically due to overproduction or underproduction of antidiuretic hormone and result in the syndrome of inappropriate antidiuretic hormone secretion or diabetes insipidus, respectively. Diabetes insipidus commonly occurs after operations in the region of the hypothalamus and pituitary gland and can be managed acutely by infusion of vasopressin.25 Postoperative seizures may be a clinical manifestation of intracranial hemorrhage or acute hyponatremia and should be treated rapidly with anticonvulsants and correction of the initiating process. Analgesia with morphine is often required in the postoperative period to minimize stress and discomfort. However, postcraniotomy patients are frequently lethargic, and excessive administration of narcotics and sedatives may interfere with serial neurological examinations. Postoperative nausea and vomiting can cause sudden increases in intracranial pressure and should be treated with nonsedating antiemetics such as ondansetron and dexamethasone.
Special Issues
Neonatal Emergencies
Most neonatal surgery is performed on an emergency basis,26 and in such cases neonates have more than a 10-fold increase in perioperative morbidity and mortality than do other pediatric age groups.2 The major concern in managing neonates is uncovering congenital anomalies, primarily of the cardiovascular and respiratory systems. Depending on the lesion, congenital heart disease may not be apparent immediately after birth, but it is frequently associated with intraoperative hypoxia and hemodynamic instability. Echocardiography can be helpful in assessment of the heart, and a pediatric cardiologist should evaluate patients with suspected problems to help optimize cardiac function before surgery. Perinatal cardiovascular physiology is an evolving process; the fetal circulation is primarily a parallel circuit that converts to a serial one after birth. Hypoxia, hypercapnia, hypothermia, acidosis, and stress can precipitate regression to a fetal circulation in neonates during the intraoperative period. Congestive heart failure can occur in neonates with large cerebral arteriovenous malformations, and this condition requires aggressive hemodynamic support. Management of the neonatal respiratory system may be difficult because of the diminutive size of the airway, craniofacial anomalies, laryngotracheal lesions, and acute (hyaline membrane disease, retained amniotic fluid) or chronic (bronchopulmonary dysplasia) pulmonary disease.
The neonatal central nervous system is capable of sensing pain and mounting a stress response after a surgical stimulus.27 Therefore, it is imperative that neonates receive adequate anesthesia for surgical procedures. Yet, immature neonatal organ systems are highly sensitive to anesthetic agents. Neonatal myocardial function is particularly sensitive to both inhaled and IV anesthetics, and these agents must be used judiciously to block surgical stress without causing myocardial depression. Use of an opioid-based anesthetic is generally the most stable hemodynamic technique for neonates. However, the hepatic and renal systems are not fully developed, and neonates anesthetized with a narcotic technique often have delayed emergence and may require postoperative mechanical ventilation.
Closure of a myelomeningocele or encephalocele presents special problems. Positioning the patient for tracheal intubation can lead to rupture of the membranes covering the spinal cord or brain. Therefore, careful padding of the lesion (Fig. 173-3) and, in some cases, intubation of the neonate’s trachea in the left lateral decubitus position may be necessary.
Tumors
A majority of intracranial tumors in children occur in the posterior fossa and are associated with obstructed cerebrospinal fluid flow, intracranial hypertension, and hydrocephalus. Most neurosurgeons approach this region with children in the prone position. The patient’s head is often secured with a Mayfield head frame. Use of pins in small children can cause skull fractures, dural tears, and intracranial hematomas. During elevation of the bone flap, the venous sinuses can be inadvertently lacerated, and massive blood loss or VAE, or both, can occur. Therefore, both the anesthesiologist and the neurosurgeon should be alert for sudden changes in monitors for VAE and arterial blood pressure. Surgical resection of tumors in the posterior fossa can lead to brainstem or cranial nerve damage. Table 173-12 lists some of the signs of encroachment on these structures. Damage to the cranial nerves innervating the vocal cords and soft tissues of the upper airway can result in airway obstruction after extubation of the patient’s trachea. Furthermore, edema or damage to the respiratory centers can cause apnea in the postoperative period. Seizures frequently occur in patients with supratentorial lesions. Anticonvulsants can be administered intravenously in the perioperative period until oral intake is resumed. Tumors in the hypothalamic-pituitary region present special problems. Craniopharyngiomas are the most common lesions in this area in children and are often accompanied by derangements in the neuroendocrine axis. These patients should therefore receive a thorough endocrine evaluation. Steroids should be administered if there is potential impairment in the hypothalamic-pituitary-adrenal axis. Thyroid function should also be evaluated to rule out hypothyroidism. Preoperative diabetes insipidus may lead to severe hypovolemia and electrolyte imbalances and should be corrected before surgery. Mannitol should not be administered routinely because it may mask the diagnosis of diabetes insipidus. Diabetes insipidus can be managed intraoperatively with an IV infusion of aqueous vasopressin and strict control of IV fluids. Diabetes insipidus rarely occurs in the intraoperative period but frequently develops in the postoperative period because the posterior pituitary gland’s store of antidiuretic hormone lasts several hours.
| BRAINSTEM AREA | SIGNS | CHANGES IN MONITOR |
|---|---|---|
| CN V | Hypertension, bradycardia | Arterial pressure, ECG |
| CN VII | Facial muscle movement | EMG |
| CN X | Hypotension, bradycardia | Arterial pressure, ECG |
| Pons, medulla | Arrhythmias, hypotension, hypertension, tachycardia or bradycardia, irregular breathing pattern | ECG, arterial pressure, end-tidal carbon dioxide |
CN, cranial nerve; ECG, electrocardiogram; EMG, electromyogram.
Epilepsy
Surgical treatment has become a viable option for many patients with medically intractable epilepsy. Two major considerations should be kept in mind. First, chronic administration of anticonvulsant drugs such as phenytoin and carbamazepine induces rapid metabolism and clearance of several classes of anesthetic agents, including neuromuscular blockers and opioids. Therefore, the anesthetic requirements for patients taking these drugs are increased; close monitoring of the anesthetic’s effect is required, along with frequent redosing. Second, various neurophysiologic monitors can be used to guide actual resection of the epileptogenic focus, and general anesthetics can compromise the sensitivity of these devices.28 Table 173-9 lists the effects of anesthetic agents on the electroencephalogram and electrocorticogram.
All patients scheduled for epilepsy surgery have undergone drug treatment of their seizures. Each drug class has side effects that can affect the conduct of anesthesia. The traditional anticonvulsant drugs, phenobarbital, phenytoin, and carbamazepine, are potent inducers of hepatic microsomal P-450 enzymes. Fosphenytoin and carbatrol/oxcarbazepine are recent reformulations of the latter two drugs. The hepatic P-450 enzymes mediate biotransformation and enhanced elimination of many drugs. Long-term administration of these specific anticonvulsant drugs results in drug resistance and increases requirements for both nondepolarizing muscle relaxants and opioids during general anesthesia.16,29 Patients receiving chronic anticonvulsant drug therapy with phenobarbital, phenytoin, or carbamazepine need to be monitored closely for drug effect and the anesthetic dosage increased accordingly. In general, the newer classes of anticonvulsant drugs do not appear to alter the metabolism of anesthetic drugs. However, other side effects have been reported with chronic administration of these new drugs. Topiramate has been shown to cause an asymptomatic non–ion gap metabolic acidosis because of inhibition of carbonic anhydrase.30 This can exaggerate the metabolic acidosis that often occurs as a result of hypoperfusion secondary to massive blood loss. Sodium valproate is associated with platelet abnormalities and can cause bleeding disorders. Sodium valproate and felbamate can induce liver failure, and therefore patients receiving these drugs should have the appropriate laboratory tests to determine baseline platelet and liver function before surgery. The ketogenic diet is a high-fat, low-carbohydrate, low-protein regimen that promotes a chronic metabolic state of ketosis and acidosis, which is an adjunct in the treatment of many children with intractable epilepsy.30 Because the target metabolic state can be disrupted by the administration of carbohydrate-containing intravenous solutions or by ingestion of the sweetened syrups contained in some premedications, they should be avoided by the anesthesiologist. Although both normal saline and lactated Ringer’s solution are acceptable fluid choices, there is limited margin for additional acidosis, and acid-base status must be monitored closely during surgery. Bicarbonate, plasma glucose, and serum ketone levels should be measured preoperatively and then tested regularly to avoid hypoglycemia or excessive ketosis (serum or urine ketones >160 mg/dL).
Because epileptogenic foci may be in close proximity to the cortical areas controlling speech, memory, and motor or sensory function, monitoring of the patient’s electrophysiologic responses is frequently used to minimize iatrogenic injury to these areas.31 Cortical stimulation of the motor strip in a child under general anesthesia requires either electromyography or direct visualization of muscle movement. Neuromuscular blockade should not be used in this situation. Neural function is best assessed in an awake and cooperative patient. We reported a series of children undergoing awake craniotomy for resections in eloquent areas of the brain with local anesthesia and propofol and fentanyl for sedation and analgesia, respectively.28 Positioning of the patient was critical for success of this technique. The patient should be in a semilateral position to allow both patient comfort and surgical and airway access (Fig. 173-4). It is imperative that candidates for awake craniotomy be mature and psychologically prepared to participate in this procedure. Therefore, patients who are developmentally delayed or have a history of severe anxiety or psychiatric disorders are not appropriate candidates. Very young patients cannot be expected to cooperate for these procedures and usually require general anesthesia with extensive neurophysiologic monitoring to minimize inadvertent resection of the motor strip and eloquent cortex. Occasionally, repeat craniotomy must be performed in a child shortly after the primary procedure. This can be done on an emergency basis for evacuation of intracranial hemorrhage. Indications for elective repeat craniotomy include removal of the electrocorticographic leads and depth electrodes used for chronic invasive electroencephalographic monitoring and subsequent resection of the seizure focus. It is important to avoid nitrous oxide until the dura is opened because intracranial air can persist up to 3 weeks after a craniotomy and lead to tension pneumocephalus.32
Vascular Anomalies
Vascular anomalies are uncommon in infants and children. Most of these conditions are congenital anomalies that are recognized early in life. Large arteriovenous malformations in neonates may be associated with high-output congestive heart failure and require hemodynamic support. Initial treatment of large arteriovenous malformations often consists of intravascular embolization in the radiology suite.33 Massive blood loss may still ensue during surgery. These patients require several IV access sites and invasive hemodynamic monitoring to gauge fluid and blood resuscitation. Ligation of an arteriovenous malformation can lead to sudden hypertension with hyperemic cerebral edema.34 Vasodilators such as labetalol or nitroprusside can be used to control a hypertensive crisis.
Moyamoya syndrome is a rare, chronic vaso-occlusive disorder of the internal carotid arteries characterized by transient ischemic attacks or recurrent strokes in childhood.35 The cause is unknown, but the syndrome can be associated with previous intracranial radiation therapy, neurofibromatosis, Down syndrome, and a variety of hematologic disorders, including sickle cell anemia. Anesthetic management of these patients is directed at optimizing cerebral perfusion,36 which includes ensuring generous preoperative hydration and maintaining blood pressure within the patient’s preoperative range. Maintenance of normocapnia is essential in patients with moyamoya syndrome because both hypercapnia and hypocapnia can lead to a steal phenomenon from the ischemic region and further aggravate the cerebral ischemia.37 An opioid-based anesthetic provides a stable level of anesthesia for these patients and is compatible with intraoperative electroencephalographic monitoring. Once the patient emerges from anesthesia, the same maneuvers that optimize cerebral perfusion should be extended into the postoperative period. These patients should receive IV fluids to maintain sufficient cerebral perfusion and adequate narcotics to avoid hyperventilation induced by pain and crying.
Trauma
Pediatric head trauma requires a multiorgan approach to minimize morbidity and mortality.38 A small child’s head is often the point of impact in injuries, but other organs can also be damaged. Basic life support algorithms should be applied immediately to ensure a patent airway and adequate respiration and circulation. Immobilization of the cervical spine is essential to avoid secondary injury caused by manipulation of the patient’s airway until radiologic clearance is confirmed. Blunt abdominal trauma and long-bone fractures frequently occur with head injury and can be major sources of blood loss. To ensure tissue perfusion during the operative period, the patient’s blood volume should be restored with crystalloid solutions or blood products, or both. Ongoing blood loss can lead to coagulopathies and should be treated with specific blood components.
Spine Surgery
Spinal dysraphism is the primary indication for laminectomy in pediatric patients. Many of these patients have undergone meningomyelocele closure followed by several corrective surgical procedures. These patients have been exposed to latex products, and hypersensitivity to latex may develop. Latex allergy can be manifested as a severe anaphylactic reaction heralded by hypotension and wheezing and should be treated rapidly by removal of the source of latex and administration of fluid and vasopressors.39 Patients at risk for latex allergy should have a latex-free environment.
Neuroradiology
Advances in imaging technology have provided less invasive procedures to diagnose and treat lesions in the central nervous system. The major issues in providing anesthesia in radiology suites are (1) the availability of anesthetic equipment outside the operating room and compatibility in a magnetic environment, (2) training of ancillary staff in the radiology suite to appreciate anesthetic issues and concerns, and (3) transport of the patient to and from the radiology suite.40 These issues require thorough planning by the radiologist, neurosurgeon, and anesthesiologist and an established set of guidelines to provide a safe environment for the patient. Most neuroradiologic studies such as computed tomography and magnetic resonance imaging can be accomplished with light sedation. Recommendations have been published by consensus groups of anesthesiologists and pediatricians and can serve as guidelines for managing these patients.41,42 General anesthesia is typically used for uncooperative patients, patients with coexisting medical problems, and patients undergoing potentially painful procedures such as embolization of vascular lesions or tumors. Some patients with neurovascular problems are at risk for hemorrhage and hemodynamic instability and require invasive monitoring. Children requiring application of a head frame for stereotactic-guided radiosurgery or a craniotomy need general anesthesia to tolerate the procedure. Special head frames that allow access to the airway should be used in these patients.43
Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321-1329.
Cohen MM, Cameron CB, Duncan PG. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70:160-167.
Grady MS, Bedford RF, Park TS. Changes in superior sagittal sinus pressure in children with head elevation, jugular venous compression, and PEEP. J Neurosurgy. 1986;65:199-202.
Lam WH, MacKersie A. Paediatric head injury: incidence, aetiology and management. Paediatr Anaesth. 1999;9:377-385.
McCann ME, Kain ZN. Management of perioperative anxiety in children. Anesth Analg. 2001;93:98-105.
Meridy HW, Creighton RE, Humphreys RB. Complications during neurosurgical procedures in the prone position. Can J Anaesth. 1974;21:445-452.
Morray JP, Geiduschek JM, Ramamoorthy C, et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology. 2000;93:6-14.
Nargozian CD. The difficult airway in the pediatric patient with craniofacial anomaly. Anesthesiol Clin North Am. 1999;16:839-852.
Reasoner DK, Todd MM, Scamman FL, et al. The incidence of pneumocephalus after supratentorial craniotomy. Observations on the disappearance of intracranial air. Anesthesiology. 1994;80:1008-1012.
Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265-1270.
Scott Scott RM, Smith ER. Moyamoya disease and Moyamoya Syndrome. N Engl J Med. 2009;60:1226-1237.
Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100:142-149.
Soriano SG, Martyn JA. Antiepileptic-induced resistance to neuromuscular blockers: mechanisms and clinical significance. Clin Pharmacokinet. 2004;43:71-81.
Soriano SG, Sethna NF, Scott RM. Anesthetic management of children with moyamoya syndrome. Anesth Analg. 1993;77:1066-1070.
Todd MM, Warner DS, Sokoll MD, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Anesthesiology. 1993;78:1005-1020.
Wintermark M, Lepori D, Cotting J, et al. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113:1642-1652.
Wise-Faberowski L, Soriano SG, Ferrari L, et al. Perioperative management of diabetes insipidus in children. J Neurosurg Anesthesiol. 2004;16:220-225.
1 Ferrari LR. Anesthesia and Pain Management for the Pediatrician. Baltimore: Johns Hopkins University Press; 1999.
2 Cohen MM, Cameron CB, Duncan PG. Pediatric anesthesia morbidity and mortality in the perioperative period. Anesth Analg. 1990;70:160-167.
3 Morray JP, Geiduschek JM, Ramamoorthy C, et al. Anesthesia-related cardiac arrest in children: initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology. 2000;93:6-14.
4 Nargozian CD. The difficult airway in the pediatric patient with craniofacial anomaly. Anesthesiol Clin North Am. 1999;16:839-852.
5 McCann ME, Kain ZN. Management of perioperative anxiety in children. Anesth Analg. 2001;93:98-105.
6 Holzman RS, van der Velde ME, Kaus SJ, et al. Sevoflurane depresses myocardial contractility less than halothane during induction of anesthesia in children. Anesthesiology. 1996;85:1260-1267.
7 Sarner JB, Levine M, Davis PJ, et al. Clinical characteristics of sevoflurane in children. A comparison with halothane. Anesthesiology. 1995;82:38-46.
8 Cochrane DD, Adderley R, White CP, et al. Apnea in patients with myelomeningocele. Pediatr Neurosurg. 1990;16:232-239.
9 Cochrane DD, Gustavsson B, Poskitt KP, et al. The surgical and natural morbidity of aggressive resection for posterior fossa tumors in childhood. Pediatr Neurosurg. 1994;20:19-29.
10 Foland JA, Super DM, Dahdah NS, et al. The use of the air leak test and corticosteroids in intubated children: a survey of pediatric critical care fellowship directors. Respir Care. 2002;47:662-666.
11 Todd MM, Warner DS, Sokoll MD, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Anesthesiology. 1993;78:1005-1020.
12 German JW, Aneja R, Heard C, et al. Continuous remifentanil for pediatric neurosurgery patients. Pediatr Neurosurg. 2000;33:227-229.
13 Chiaretti A, Pietrini D, Piastra M, et al. Safety and efficacy of remifentanil in craniosynostosis repair in children less than 1 year old. Pediatr Neurosurg. 2000;33:83-88.
14 Guy J, Hindman BJ, Baker KZ, et al. Comparison of remifentanil and fentanyl in patients undergoing craniotomy for supratentorial space-occupying lesions. Anesthesiology. 1997;86:514-524.
15 Gelb AW, Salevsky F, Chung F, et al. Remifentanil with morphine transitional analgesia shortens neurological recovery compared to fentanyl for supratentorial craniotomy. Can J Anaesth. 2003;50:946-952.
16 Soriano SG, Martyn JA. Antiepileptic-induced resistance to neuromuscular blockers: mechanisms and clinical significance. Clin Pharmacokinet. 2004;43:71-81.
17 Wintermark M, Lepori D, Cotting J, et al. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113:1642-1652.
18 Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265-1270.
19 Soriano SG, McManus ML, Sullivan LJ, et al. Cerebral blood flow velocity after mannitol infusion in children. Can J Anaesth. 1996;43:461-466.
20 McManus ML, Soriano SG. Rebound swelling of astroglial cells exposed to hypertonic mannitol. Anesthesiology. 1998;88:1586-1591.
21 Soriano SG, McManus ML, Sullivan LJ, et al. Doppler sensor placement during neurosurgical procedures for children in the prone position. J Neurosurg Anesthesiol. 1994;6:153-155.
22 Grady MS, Bedford RF, Park TS. Changes in superior sagittal sinus pressure in children with head elevation, jugular venous compression, and PEEP. J Neurosurg. 1986;65:199-202.
23 Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115-131.
24 Meridy HW, Creighton RE, Humphreys RB. Complications during neurosurgical procedures in the prone position. Can J Anaesth. 1974;21:445-452.
25 Wise-Faberowski L, Soriano SG, Ferrari L, et al. Perioperative management of diabetes insipidus in children. J Neurosurg Anesthesiol. 2004;16:220-225.
26 Koka BV, Soriano SG. Anesthesia for neonatal surgical emergencies. Semin Anesth. 1992;9:309-316.
27 Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321-1329.
28 Soriano SG, Eldredge EA, Wang FK, et al. The effect of propofol on intraoperative electrocorticography and cortical stimulation during awake craniotomies in children. Paediat Anaesth. 2000;10:29-34.
29 Soriano SG, Sullivan LJ, Venkatakrishnan K, et al. Pharmacokinetics and pharmacodynamics of vecuronium in children receiving phenytoin or carbamazepine for chronic anticonvulsant therapy. Br J Anaesth. 2001;86:223-229.
30 Groeper K, McCann ME. Topiramate and metabolic acidosis: a case series and review of the literature. Paediatr Anaesth. 2005;15:167-170.
31 Black PM, Ronner SF. Cortical mapping for defining the limits of tumor resection. Neurosurgery. 1987;20:914-919.
32 Reasoner DK, Todd MM, Scamman FL, et al. The incidence of pneumocephalus after supratentorial craniotomy. Observations on the disappearance of intracranial air. Anesthesiology. 1994;80:1008-1012.
33 Burrows PE, Robertson RL. Neonatal central nervous system vascular disorders. Neurosurg Clin N Am. 1998;9:155-180.
34 Morgan MK, Sekhon LH, Finfer S, et al. Delayed neurological deterioration following resection of arteriovenous malformations of the brain. J Neurosurg. 1999;90:695-701.
35 Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100:142-149.
36 Soriano SG, Sethna NF, Scott RM. Anesthetic management of children with moyamoya syndrome. Anesth Analg. 1993;77:1066-1070.
37 Kuwabara Y, Ichiya Y, Sasaki M, et al. Response to hypercapnia in moyamoya disease. Cerebrovascular response to hypercapnia in pediatric and adult patients with moyamoya disease. Stroke. 1997;28:701-707.
38 Lam WH, MacKersie A. Paediatric head injury: incidence, aetiology and management. Paediatr Anaesth. 1999;9:377-385.
39 Holzman RS. Clinical management of latex-allergic children. Anesth Analg. 1997;85:529-533.
40 Soriano SG, Eldredge EA, Rockoff MA. Pediatric neuroanesthesia. Neuroimaging Clin N Am. 2007;17:259-267.
41 Cote CJ, Wilson S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Paediatr Anaesth. 2008;18:9-10.
42 Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017.
43 Stokes MA, Soriano SG, Tarbell NJ, et al. Anesthesia for stereotactic radiosurgery in children. J Neurosurg Anesthesiol. 1995;7:100-108.