CHAPTER 21 Neuroanesthesia
Preoperative Evaluation
The preanesthetic evaluation is defined as the process of clinical assessment that precedes the delivery of anesthesia care for surgical and nonsurgical procedures. The primary aim of preanesthetic evaluation is to minimize the overall patient morbidity associated with surgery and anesthesia. This goal is achieved by assessing the patient’s medical condition and the balance between anesthetic risk and surgical benefit, optimizing the medical condition within the limitations of the surgical circumstances, and formulating the best possible anesthesia plan. Other benefits may include improved safety of perioperative care, optimal resource utilization, improved outcomes, and patient satisfaction.1 Hence, the objectives of preanesthetic evaluation include the following:
The preanesthetic evaluation may be performed well in advance of the planned surgery for most elective procedures during a visit to the preanesthetic evaluation clinic. Otherwise, it may be performed at the bedside in the hospital ward or intensive care unit the “night before” for inpatients or on the day of surgery for morning-admission patients. For urgent and emergency procedures, this evaluation may of necessity take place just before surgery. The consensus of the American Society of Anesthesiologists (ASA) Task Force on preanesthesia evaluation is that an initial record review, patient interview, and physical examination should be performed before the day of surgery for patients with high severity of disease.1 Of patients with low severity of disease, those undergoing procedures with high surgical invasiveness should have the interview and physical examination performed before the day of surgery, whereas those undergoing procedures with medium or low surgical invasiveness may be interviewed and examined on or before the day of surgery.1 Although the task force cautions that the timing of such assessments may not be practical with the limitation of resources, it recommends that at a minimum, a focused preanesthetic examination should include assessment of the airway, lungs, and heart and documentation of vital signs.1
Preanesthesia clinics are ideally run by anesthesiologists with or without the assistance of trained nurses. These clinics have been shown to improve operating room efficiency and minimize unexpected delays and cancellations because of poorly prepared patients.2,3 To be able to run smoothly, however, good organization, concise guidelines and protocols, and adequate medical support are required. Additional staffing issues are also important considerations.
It has been shown that the patient’s preoperative condition predicts postoperative mortality and morbidity.4–8 In one study, preoperative evaluation of patients led to a change in the proposed anesthesia plan in up to 15% of healthy individuals and 20% of ill patients.9 Although these changes in plan do not necessarily reduce patient morbidity, they can lead to delays caused by the need to obtain different drugs and equipment and further specialist consultations and result in increased operating room downtime and cost. Establishment of a preanesthesia assessment clinic streamlines the process and obviates this potential source of delay. In a study from Stanford University, implementation of a preanesthetic evaluation clinic produced an 87.9% reduction in day-of-surgery cancellations.3 It is estimated that $30 to $40 billion is spent annually on preoperative testing and subsequent follow-up in North America alone, 50% of which could be saved by the appropriate and selective ordering of tests.10 In one study, implementation of a preoperative clinic, in which tests were ordered at the anesthesiologist’s request, resulted in a savings of $112.09 per patient. This equated to an annual potential saving of more than $1.01 million at one institution.3
General Preanesthetic Evaluation
The ASA classification of physical status is a universally accepted system used for stratification of a patient’s preexisting health status (Table 21-1). Although it does not take into account surgical risk and is not primarily designed for prediction of outcome, it has been found to correlate with perioperative morbidity and mortality.11–13 In fact, ASA physical status 3 to 5 has been found to independently predict perioperative cardiovascular complications in intracranial surgical patients and is also a risk factor for perioperative mortality.8
| ASA PHYSICAL STATUS | DISEASE STATE |
|---|---|
| 1 | A normal healthy patient |
| 2 | A patient with mild systemic disease |
| 3 | A patient with severe systemic disease |
| 4 | A patient with severe systemic disease that is a constant threat to life |
| 5 | A moribund patient who is not expected to survive without the operation |
| 6 | A patient declared brain-dead whose organs are being removed for donor purposes |
ASA, American Society of Anesthesiologists.
Excerpted from the Relative Value Guide 2008 of the American Society of Anesthesiologists. A copy of the full text can be obtained from ASA, 520 N. Northwest Highway, Park Ridge, IL 60068-2573.
History and Physical Examination
Medical History
General Physical Examination
Before proceeding to examination of individual systems, a general physical examination should be conducted and take into account the patient’s level of consciousness, mental status, build, nutrition, and vital parameters. Patients with malignant tumors and those with high cervical lesions might be emaciated with significantly reduced muscle mass. Conversely, obesity might be coexistent in many patients. Obese individuals have an increased likelihood of associated diabetes, hypertension, coronary artery disease, restrictive lung disease, sleep apnea, and gastroesophageal reflux, which might warrant alteration of the anesthesia plan. Difficulty with tracheal intubation may be encountered more frequently in obese than in lean individuals,14 and the pharmacologic profile of anesthetic agents may also be altered.15 Some neurosurgical patients might be dehydrated because of reduced intake of fluids (as a result of impaired consciousness), vomiting, or the use of diuretics and contrast agents. Correction of significant dehydration before induction of anesthesia can prevent postinduction hypotension in such patients. Significant blood loss is a possibility with surgery for intracranial aneurysms, arteriovenous malformations (AVMs), vascular tumors, craniosynostoses, and extensive spine problems. Preanesthetic evaluation should look for preexisting anemia and attempt to correct it preoperatively or arrange for intraoperative transfusion on a case-by-case basis. Recording of preoperative vital parameters (heart rate, blood pressure) provides baseline values for intraoperative management, which is particularly important in surgeries requiring strict hemodynamic control (e.g., aneurysms and AVMs).
Perhaps the most crucial aspect of the general examination is assessment of the patient’s airway. Although the primary neurosurgical problem may be responsible for potential difficulties in intubation and airway management, inadequate management of the airway may adversely affect the neurological outcome. Routine maneuvers used for airway management may worsen spinal instability in patients with cervical lesions and lead to increased intracranial pressure (ICP) with potentially devastating consequences in patients with decreased intracranial compliance. Hence, the patient’s airway should be assessed carefully for ease of ventilation and difficulty of tracheal intubation, in conjunction with specific surgical needs such as hemodynamic stability and spine immobilization. Mallampati scoring16 thyromental distance, presence of overbite or underbite, and the range of neck flexion-extension collectively provide an estimate of the risk for difficult intubation.17 Some specific situations in which a difficult airway should be anticipated include patients who have recently undergone supratentorial craniotomy, in whom mouth opening might be significantly reduced secondary to ankylosis of the temporomandibular joint,18 acromegalic patients undergoing pituitary surgery,19 and patients with cervical spine lesions. Recognition of potential airway difficulty allows proper planning with the availability of accessory equipment and resources, as well as formulation of a back-up plan, and results in improved patient safety and efficient use of operating time.
Assessment of System Functions
Neurological System
Patients with a depressed level of consciousness preoperatively are likely to have a reduced anesthetic need for induction and more likely to have a slow or delayed emergence postoperatively and need for postoperative mechanical ventilation. Such patients should not receive any sedative or narcotic agents unless they are under continuous supervision, preferably in the operating room itself with vigilance for respiratory depression. Moreover, in patients with previous motor deficits, exacerbation of focal neurological signs may develop after sedative doses of benzodiazepines and narcotics.20 The presence of brainstem lesions or lower cranial nerve dysfunction, or both, predisposes patients to an increased risk for aspiration postoperatively. Finally, life-threatening hyperkalemia secondary to succinylcholine administration may develop in patients with preexisting motor deficits.21 Succinylcholine has also been reported to cause hyperkalemia in patients with ruptured cerebral aneurysms independent of the presence of motor nerve disturbances,22 although this appears to be uncommon. Elevated ICP is often manifested as headache with nausea and vomiting, but it can also lead to olfactory nerve dysfunction with loss of the sense of smell. Unilateral uncal herniation would result in a dilated unresponsive ipsilateral pupil, which should be distinguished from incidental anisocoria, or a unilateral third nerve palsy resulting from compression by a space-occupying lesion. Field of vision might be significantly limited in patients with pituitary and other suprasellar tumors and should be documented for postoperative comparison. Dysfunction of the trigeminal and facial nerves may interfere with mask ventilation and tracheal intubation. A patient with a damaged vagus nerve may have a hoarse voice secondary to vocal cord paralysis and may be at increased risk for airway obstruction.
Respiratory System
Risk for perioperative respiratory complications is increased in patients with preexisting obstructive or restrictive pulmonary disease. Perioperative hypoxemia or hypercapnia is more likely to occur and in turn can further aggravate an already compromised cardiorespiratory status. Patients with a history of pulmonary disease require an assessment of their baseline status, and any element of potential reversibility should be addressed.23–25 Smoking is a common important risk factor for both cardiovascular and pulmonary disease and is associated with a threefold increase in perioperative morbidity. Cessation of smoking for 6 to 8 weeks is recommended for reactivation of mucociliary clearance, but as little cessation as 24 hours can reduce carboxyhemoglobin levels and improve oxygenation.26 The presence of reactive airway disease indicates an increased risk for bronchospasm with airway manipulation and tracheal extubation and an increased risk for coughing and laryngospasm during emergence.
Management of upper respiratory tract infection preoperatively in children is controversial because the effects on the airway last for 2 to 4 weeks after clinical resolution. The patient is at increased risk for perioperative respiratory morbidity during this period.27 Postponement of elective surgery must be balanced against the risk for progressive neurological disability or the occurrence of a potentially catastrophic complication during the waiting period.
Patients with decreased levels of consciousness because of intracranial pathology and those with high spinal lesions or lower cranial nerve paralysis might have preexisting atelectasis preoperatively, which puts them at increased risk for postoperative mechanical ventilation. Aspiration pneumonitis or superimposed pneumonia, or both, can also develop. A restrictive pattern of lung disease often occurs in patients with craniovertebral junction anomalies preoperatively and persists in the postoperative period.28 Some patients, such as those with head injury, spinal cord injury, or subarachnoid hemorrhage (SAH), might be intubated and mechanically ventilated preoperatively and usually remain intubated postoperatively as well.
In their systematic review of preoperative pulmonary risk stratification for noncardiothoracic surgery for the American College of Physicians, Smetana and colleagues found good evidence to support the following patient-related risk factors as being predictive of postoperative pulmonary complications: advanced age, ASA class 2 or greater, functional dependence, chronic obstructive pulmonary disease, and congestive heart failure.29 They also found fair evidence indicating increased risk in patients with impaired sensorium, abnormal findings on chest examination, cigarette use, alcohol use, and weight loss.29 Although asthma is not a risk factor if well controlled, perioperative risk may be increased if it is poorly controlled.29 Important procedure-related risk factors include neurosurgery, emergency surgery, and prolonged surgery.29 The value of preoperative testing in estimating pulmonary risk is controversial. Even though an abnormal chest radiograph does indicate increased risk for postoperative pulmonary complications and spirometry may provide some risk stratification, among potential laboratory tests for stratifying risk, a serum albumin level of less than 35 g/L is the most powerful predictor.29
Cardiovascular System
Anesthesia, surgical positioning, and surgery itself put additional demands on the cardiovascular system. Moreover, intraoperative maintenance of hemodynamic stability is important to avoid adverse neurological effects in neurosurgical patients. The presence of cardiovascular disease significantly increases the risk associated with anesthesia, and optimizing the patient’s condition can significantly improve outcome. The overall risk of cardiac patients undergoing a noncardiac procedure has traditionally been assessed with the Goldman index.4 However, it has now been superseded by the Revised Cardiac Risk Index.5 According to this index,5 the presence of three or more of the following factors is associated with a cardiac morbidity rate of 9%: (1) high-risk surgery, (2) history of ischemic heart disease, (3) history of congestive heart failure, (4) history of cerebrovascular disease, (5) preoperative treatment with insulin, and (6) preoperative serum creatinine level greater than 2.0 mg/dL. Because most patients undergoing intracranial procedures often have two or more of these risk factors, careful evaluation of the other organ systems is important to quantify risk for cardiac morbidity. Preanesthetic evaluation should also be focused on detecting and assessing the physiologic effects of cardiovascular conditions known to be associated with specific neurosurgical conditions, such as hypertension and coarctation of the aorta in patients with aneurysms.
Coronary artery disease is associated with diabetes mellitus, hypertension, smoking, hypercholesterolemia, and peripheral vascular disease. The presence of angina is a significant risk factor—although unstable or resting angina predicts the highest risk for postoperative cardiac complications, the risk with angina on exertion can be minimized with appropriate intraoperative management. Left ventricular dysfunction with symptoms of cardiac failure (dyspnea on mild exertion, orthopnea, peripheral edema) is indicative of significantly reduced cardiac output, which can worsen with general anesthesia. Mannitol must be used carefully and judiciously or not at all in patients with left ventricular failure. Hypertension is a common preexisting condition and is frequently inadequately controlled. These patients often have reduced plasma volume, thus making them more susceptible to the systemic vasodilatory effects of anesthetic agents, which can result in cardiovascular instability and labile blood pressure intraoperatively. Moreover, in patients with chronic hypertension, increased cerebrovascular resistance causes the lower and upper limits of cerebral blood flow (CBF) autoregulation to shift to higher pressure levels, and such patients consequently have poor tolerance of acute hypotension.30,31 However, adaptive hypertensive changes in CBF autoregulation may be reversible with adequate control of blood pressure.30,31 Patients with evidence of myocardial ischemia or myocardial infarction (MI) are at increased risk for postoperative MI, congestive heart failure, malignant arrhythmias, and death.
The American College of Cardiology/American Heart Association (ACC/AHA) 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery grade clinical risk factors as major, intermediate, and minor.32 The presence of one or more of the major risk factors (active cardiac conditions) mandates intensive management and may require delay or cancellation of surgery unless the surgery is being performed on an emergency basis. Major risk factors include
According to the guidelines,32 intermediate-risk factors include
A history of MI or abnormal Q waves on ECG is listed as a clinical risk factor, whereas acute MI (defined as at least one documented MI 7 days or less before the examination) or recent MI (more than 7 days but 1 month or less before the examination) with evidence of important ischemic risk by clinical symptoms or noninvasive study is an active cardiac condition. This definition reflects the consensus of the ACC Cardiovascular Database Committee. If a recent stress test does not indicate residual myocardium at risk, the likelihood of reinfarction after noncardiac surgery is low. Despite the lack of adequate clinical trials on which to base firm recommendations, it appears reasonable to wait 4 to 6 weeks after an MI to perform elective surgery.32
The guidelines recommend the following stepwise approach to perioperative cardiac assessment for noncardiac surgery32:
Step 4 (Patients with Good Functional Capacity and No Symptoms)
Functional status is a reliable predictor of perioperative and long-term cardiac events. In highly functional asymptomatic patients, management will rarely be changed by the results of any further cardiovascular testing. It is therefore appropriate to proceed with the planned surgery. In patients with known cardiovascular disease or at least one clinical risk factor (ischemic heart disease, compensated or previous heart failure, diabetes mellitus, renal insufficiency, and cerebrovascular disease), perioperative heart rate control with beta blockade is considered appropriate. However, results from the Perioperative Ischemic Evaluation Study (POISE) indicate that the reduced cardiac morbidity with perioperative beta-blocker therapy in patients not previously taking beta blockers is achieved at the expense of an increased stroke rate and an overall increase in mortality.33
Hematologic System
Postoperative intracranial hemorrhage is a potentially lethal catastrophe. Thus, any bleeding tendency should be investigated thoroughly and corrected preoperatively. If deemed necessary, appropriate clotting factors and platelets should be made available at the time of surgery.34 Patients taking NSAIDs such as aspirin should have their medications stopped for a week before intracranial surgery.35 This decision may have to be modified in patients suffering from transient ischemic attacks, in whom the risk associated with discontinuation may exceed the benefits.
Endocrine System
Patients with diabetes mellitus who are about to undergo surgery require special attention because hyperglycemia is associated with hyperosmolarity, infection, and poor wound healing. More importantly, it may worsen neurological outcome after an episode of cerebral ischemia. Nonetheless, hypoglycemia is also detrimental because the brain depends on glucose for its energy supply. Close monitoring of glucose perioperatively is therefore essential, and treatment with insulin is often required to maintain euglycemia, but sulfonylureas and metformin should not be used for 24 to 48 hours before surgery because of their long half-lives. The perioperative morbidity of diabetic patients is related to their preoperative end-organ damage. Hence, the pulmonary, cardiovascular, and renal systems should be examined carefully. Diabetics have an increased incidence of ST-segment and T-wave abnormalities on ECG, and myocardial ischemia may be evident despite a negative history (silent myocardial ischemia). Diabetic autonomic neuropathy may predispose patients to cardiovascular instability and even sudden cardiac death. Furthermore, autonomic dysfunction contributes to gastroparesis, which may require treatment with H2 blockers or metoclopramide, or both, preoperatively. Chronic hyperglycemia can lead to glycosylation of tissue proteins and a stiff joint syndrome. Diabetic patients, especially those with type 1 diabetes, should be routinely evaluated preoperatively for adequate temporomandibular joint and cervical spine mobility to help anticipate difficult intubation.36
Laboratory Investigations
The current literature is not sufficiently rigorous to permit an unambiguous assessment of the clinical benefit or harm associated with routine or selected preoperative tests. According to the ASA Task Force on preanesthesia evaluation,1 preoperative tests may be ordered, required, or performed selectively on the basis of clinical characteristics for the purpose of guiding or optimizing perioperative management. ECG may be indicated for patients with known cardiovascular risk factors or for patients with risk factors identified in the course of a preanesthesia evaluation.1 Chest radiography may be required in smokers and patients with recent upper respiratory infection, chronic obstructive pulmonary disease, and cardiac disease.1 Hemoglobin or hematocrit, serum glucose and electrolytes, and coagulation studies are indicated in most neurosurgical patients, whereas blood levels of phenytoin may sometimes be required. For all intracranial procedures, blood should be typed and crossmatched, and for minor neurosurgical procedures, blood should at least be typed and screened. Hormone assays are often ordered for patients with endocrinopathies. Urinalysis is not indicated when urinary tract symptoms are absent. Pregnancy testing may be considered for all female patients of childbearing age, especially when the pregnancy history is not clear or is suggestive of current pregnancy.1 The ASA Task Force suggests that test results obtained from the medical record within 6 months of surgery are generally acceptable if the patient’s medical history has not changed substantially but that more recent test results may be desirable when the medical history has changed or when the test results may play a role in the selection of a specific anesthetic technique.1
General Principles and Normal Cerebrovascular Physiology
The normal brain is a metabolically active organ that receives 14% of the cardiac output while consuming 20% of the oxygen intake. Its blood flow is coupled to metabolic needs, both globally and regionally. The homeostatic mechanism of autoregulation keeps CBF relatively constant at approximately 50 mL/100 g per minute over a wide range of cerebral perfusion pressure (CPP) (50 to 150 mm Hg), although the actual limits of autoregulation vary among healthy individuals.37 CPP is related to both mean arterial pressure and ICP. The latter is dependent on intracranial blood volume (CBV), brain mass, cerebrospinal fluid (CSF) volume, and central venous pressure. When CPP is low, neurological symptoms in an awake patient, such as confusion and dizziness, appear when blood flow and oxygen extraction are inadequate and ischemia occurs. Conversely, an increase in CPP leads to vasoconstriction of the cerebral vasculature, which lowers CBV and reduces ICP. If CPP exceeds the upper limit of autoregulation, CBF increases and may lead to disruption of the blood-brain barrier and increased formation of edema or hemorrhage.
Anesthetic techniques may also affect cerebral physiology. Intravenous anesthetic agents, including thiopental and propofol,38 are indirect cerebral vasoconstrictors that reduce cerebral metabolism coupled with a corresponding reduction in CBF. Both autoregulation39 and CO2 reactivity38 are preserved. Ketamine is a weak noncompetitive N-methyl-D-aspartate (NMDA) antagonist that has sympathomimetic properties. Its cerebral effects are complex and partly dependent on the action of other concurrently administered drugs. For example, concurrent administration of benzodiazapines40 or inhaled anesthetics41 would eliminate any cerebral stimulatory or vasodilatory actions. Etomidate decreases the cerebral metabolic rate (CMR), CBF, and ICP. At the same time, because of minimal cardiovascular effects, CPP is well maintained. Although the changes on electroencephalography resemble those associated with barbiturates, etomidate enhances somatosensory evoked potentials42 and causes less reduction of motor evoked potential amplitudes than thiopental or propofol does.43 However, etomidate may reduce tissue oxygen tension. Dexmedetomidine is a highly selective α2-adrenoreceptor agonist that provides sedation without causing respiratory depression, does not interfere with electrophysiologic mapping, and provides hemodynamic stability. It has been found to be particularly useful for implantation of deep brain stimulators in patients with Parkinson’s disease44 and for awake craniotomies45 when sophisticated neurological testing is required. CMR-CBF coupling has been shown to be preserved during dexmedetomidine administration in human volunteers.46 The cerebral effects of inhaled anesthetics are twofold: they are intrinsic cerebral vasodilators, but their vasodilatory actions are partly opposed by flow-metabolism coupling–mediated vasoconstriction secondary to a reduction in CMR.47 The overall effect is unchanged flow during low-dose inhaled anesthesia but increased flow during high doses. With the exception of sevoflurane, which appears to preserve autoregulation at all clinically relevant doses,48–50 other inhaled agents impair autoregulation in a dose-dependent manner.39 Opioids can precipitate chest wall rigidity and thereby result in increased ICP either directly or indirectly from hypercapnia secondary to respiratory depression if ventilation is not well controlled. In patients with decreased intracranial compliance, systemic hypotension can also lead to a secondary increase in ICP from compensatory vasodilation.51 There is no evidence of direct opiate-mediated cerebral vasodilatory action.52 Muscle relaxants generally have negligible or clinically insignificant effects on ICP, although tracheal intubation itself may cause intracranial hypertension, which may be attenuated by pretreatment with lidocaine or opioids, or both.
Specific Neurosurgical Categories
Intracranial Tumors
In patients with pituitary adenomas, the visual field and endocrine function need careful evaluation. The presence of Cushing’s disease should alert the anesthesiologist to the risk for sleep apnea (which occurs in a third of patients), in addition to the other metabolic effects described previously. Acromegalic patients with growth hormone–producing pituitary adenomas typically undergo transsphenoidal resection. The incidence of difficult laryngoscopy and intubation in acromegalic patients is higher than in normal patients, and the difficulty may not be predictable with conventional methods of airway assessment.53 Diabetes mellitus, hypertension, and cardiomyopathy might be associated with acromegaly, and the presence of carpal tunnel syndrome as a result of hypertrophic ligaments may make radial artery cannulation more hazardous if ulnar flow is already compromised.54
Computed tomography (CT) and magnetic resonance imaging (MRI) scans should be examined for the size, location, and vascularity of tumors and signs of elevated ICP. The degree of midline shift and a diagnosis of glioblastoma multiforme or metastasis are independent predictors of brain swelling,55 as is peritumoral edema.56 Small increases in intracranial volume in patients with signs of elevated ICP may lead to brain swelling and further disproportionate increases in ICP or herniation. Supratentorial meningiomas are vascular tumors that may occur in surgically difficult locations (e.g., near the sagittal sinus, optic nerve sheath) but are optimally treated by complete surgical excision. When dealing with them, anesthesiologists should anticipate blood loss with a need for transfusion and sometimes long, technically difficult surgery requiring maximal brain relaxation.
Although most commonly used anesthetic regimens have been shown to be acceptable in patients undergoing elective supratentorial surgery because short-term outcomes are not affected,57 lower subdural ICP and better brain relaxation have been observed during anesthesia maintained with propofol than with isoflurane or sevoflurane.58 Hence, intravenous anesthetics may be preferable over inhaled agents in patients thought to be disposed to intraoperative brain swelling. In contrast, a recent multicenter trial did not find the anesthetic regimen to affect brain bulk assessment or ICP, whereas hyperventilation was found to decrease the risk for increased brain bulk.59
Vascular Diseases
Ischemic Cerebrovascular Disease
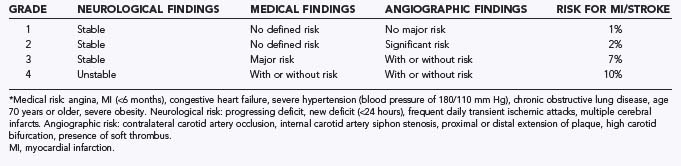
Patients with arteriosclerotic carotid disease are often scheduled for CEA and, less frequently, for extracranial/intracranial revascularization. The cerebral vascular disease is but one manifestation of the underlying disorder of generalized atherosclerosis. Patients who undergo CEA commonly have significant coronary artery disease, arterial hypertension, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes mellitus, or renal insufficiency.60 The predominant symptoms and any neurological deficits should be recorded because neurologically unstable patients are more likely to suffer perioperative stroke. The Mayo Clinic classification of preoperative risk is used widely (Table 21-2).61
Data from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) suggest that increased surgical risk is associated with five baseline variables: (1) hemispheric versus retinal transient ischemic attack as a qualifying event, (2) a left-sided procedure, (3) contralateral carotid occlusion, (4) an ipsilateral ischemic lesion on CT, and (5) irregular or ulcerated ipsilateral plaque.62 A review of medical, non–stroke-related complications in patients enrolled in NASCET reported cardiac complications to be the most common cause of postoperative medical morbidity and responsible for all fatalities.63 The NASCET results indicated that a history of MI or unstable angina and hypertension are independent risk factors for medical complications. Conversely, aggressive blood pressure control in patients undergoing CEA, including preoperative treatment of hypertension, has been associated with improved outcome, thus emphasizing preoperative treatment of high blood pressure.64 Despite the fact that routine coronary angiography before CEA has been suggested by some, there is no evidence to suggest improved cardiac outcome attributable to preoperative coronary angiography. Hence, a prudent approach is to assume that all patients scheduled for CEA have associated atherosclerotic heart disease and base perioperative risk and the need for further investigation and intervention on patients’ functional status. Although diabetes mellitus was not an independent risk factor for medical complications in NASCET patients,63 it does increase the risk for perioperative stroke or death.65 Because hyperglycemia adversely affects outcome after temporary focal or global ischemia, it is best to optimize the blood glucose level preoperatively and manage it carefully in the perioperative period to avoid both hyperglycemia and hypoglycemia.
Aneurysmal Subarachnoid Hemorrhage
The most important aspect of the preoperative evaluation of patients with intracranial aneurysms is assessment of the patient’s neurological status and grading of SAH. Classically, SAH is graded with the Hunt and Hess scale (Table 21-3).66 This grading system has prognostic importance because patients with higher grades of SAH have higher morbidity and mortality. However, higher grades are also more likely to be associated with vasospasm, elevated ICP,67 impaired cerebral autoregulation,68,69 and impaired cerebrovascular reactivity to CO2.69 A worse clinical grade is also associated with a higher incidence of cardiac arrhythmia and myocardial dysfunction,70 hypovolemia, and hyponatremia.71,72 All these observations are crucial for anesthetic management of these patients.
| GRADE | CRITERIA |
|---|---|
| 0 | Unruptured aneurysm |
| I | Asymptomatic or minimal headache and slight nuchal rigidity |
| II | Moderate to severe headache, nuchal rigidity, but no neurologic deficit other than cranial nerve palsy |
| III | Drowsiness, confusion, or mild focal deficit |
| IV | Stupor, mild or severe hemiparesis, possible early decerebrate rigidity, vegetative disturbance |
| V | Deep coma, decerebrate rigidity, moribund appearance |
* Serious systemic diseases such as hypertension, diabetes, severe arteriosclerosis, chronic pulmonary disease, and severe vasospasm seen on arteriography result in placement of the patient in the next less favorable (higher) category.
Modified from Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysm. J Neurosurg. 1968;28:14-20.
An alternative scale for grading the severity of SAH is that of the World Federation of Neurological Surgeons, which is based on the Glasgow Coma Scale and the presence or absence of a motor deficit (Table 21-4).73 The Fisher scale is based on the amount of subarachnoid blood seen on CT, which correlates with risk for the development of vasospasm (Table 21-5).74 While examining the CT scan, the anesthesiologist can also assess the degree of mass effect, midline shift, cerebral edema, and hydrocephalus, which can help anticipate intraoperative brain swelling.
TABLE 21-4 World Federation of Neurological Surgeons Grading Scale
| WFNS GRADE | GCS SCORE | MOTOR DEFICIT |
|---|---|---|
| I | 15 | Absent |
| II | 14-13 | Absent |
| III | 14-13 | Present |
| IV | 12-7 | Absent or present |
| V | 6-3 | Absent or present |
GCS, Glasgow Coma Scale; WFNS, World Federation of Neurological Surgeons.
From Drake C. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985-986.
TABLE 21-5 Fisher Grading of CT Scans in Patients with Subarachnoid Hemorrhage
| GRADE | CT SCAN FINDING |
|---|---|
| 1 | No blood detected |
| 2 | Diffuse thin layer of subarachnoid blood (vertical layers <1 mm thick) |
| 3 | Localized clot or thick layer of subarachnoid blood (vertical layers ≥1 mm thick) |
| 4 | Intracerebral or intraventricular blood with diffuse or no subarachnoid blood |
CT, computed tomography.
From Fisher C, Kistler J, Davis J. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1-9.
A variety of medical conditions are known to be associated with the development of cerebral aneurysms and SAH. The preoperative evaluation should look for any of these associated conditions, which include hypertension, coarctation of the aorta, polycystic kidney disease, and fibromuscular dysplasia, as well as a history of smoking, and take into consideration the specific anesthetic concerns with any of these conditions if they exist in a given patient.75
Patients with SAH frequently have a contracted intravascular volume that may be multifactorial: altered sensorium associated with reduced fluid intake, use of diuretics and radiographic contrast agents (for diagnostic imaging), bed rest, supine diuresis, negative nitrogen balance, decreased erythropoiesis, and iatrogenic blood loss. In some patients, hypovolemia may be paradoxically associated with hyponatremia secondary to increased release of atrial natriuretic peptide (cerebral salt wasting syndrome)76 and may be related to the subsequent development of vasospasm.77 Erroneous attribution of hyponatremia to the syndrome of inappropriate secretion of antidiuretic hormone may lead to treatment by fluid restriction, thereby increasing the risk for delayed cerebral ischemia and MI.78 Hypertonic or isotonic saline should be used judiciously to correct the hyponatremia associated with SAH.79 Other significant electrolyte abnormalities include hypokalemia and hypocalcemia. Preoperative treatment should be aimed at correcting the electrolyte abnormalities while maintaining normal intravascular status.
ECG changes, primarily involving ST-segment changes or T-wave inversion, are common and occur in 40% to 60% of patients suffering from SAH.80 Three causes could account for the ECG abnormalities in these patients: coincidental MI, SAH-induced MI, or ECG changes without infarction. The ECG changes, however, usually correlate with the neurological dysfunction in that they are more prevalent in patients with poor-grade SAH,81,82 but they do not affect surgical morbidity or mortality.83 They do not necessarily correlate with ventricular dysfunction, although the presence of symmetrical inverted T waves and severe QTc-segment prolongation on serial ECG has been shown to be indicative of ventricular dysfunction.84 Echocardiographic ventricular dysfunction can occur in 10% to 20% of patients and is also more prevalent in patients with high-grade SAH.26,85 Acute ST-segment elevation is rare with SAH and should be viewed with suspicion for MI. Cardiac enzymes and echocardiography are required to rule out MI and to ascertain the degree of ventricular dysfunction in these patients,81 and elevated levels of troponin and brain natriuretic protein are associated with a poor prognosis.86 Although anesthetic risk is increased if MI has occurred, this must be balanced against the risk of rebleeding from postponing surgery. Unless the patient is hemodynamically unstable, has poor ventricular function (ejection fraction <30%), or is clinically in heart failure, surgery should proceed with appropriate hemodynamic monitoring.87 In contrast, in patients with poor ventricular function refractory to medical management, it would be prudent to defer surgery until the patient is hemodynamically stable; alternatively, endovascular treatment of aneurysm might have to be considered. Table 21-6 shows the various ECG changes seen in patients with SAH and their possible implications.88
TABLE 21-6 Electrocardiographic and Myocardial Dysfunction Seen in Patients with Subarachnoid Hemorrhage
Arteriovenous Malformations
Treatment options for patients with intracranial AVMs include surgical resection, endovascular embolization, and stereotactic radiosurgery, or combinations thereof. The usual indications for intervention are spontaneous intracranial hemorrhage, intractable seizures, or progressive neurological deficits. The surgical risk associated with resection of AVMs is classically estimated according to the grading system of Spetzler and Martin (Table 21-7).89 In general, patients with a Spetzler-Martin score of 1 to 3 have a lower risk for permanent neurological deficit after surgery than do patients with higher scores.
TABLE 21-7 Grading System of Spetzler and Martin for Arteriovenous Malformations*
| GRADED FEATURE | POINTS ASSIGNED |
|---|---|
| Size of the Arteriovenous Malformation (Diameter) | |
| Small (<3 cm) | 1 |
| Medium (3-6 cm) | 2 |
| Large (>6 cm) | 3 |
| Eloquence of Adjacent Brain | |
| Noneloquent | 0 |
| Eloquent | 1 |
| Pattern of Venous Drainage | |
| Superficial only | 0 |
| Deep | 1 |
* Grade = Size + Eloquence + Venous drainage.
Based on Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
Posterior Fossa Procedures
The posterior cranial fossa is a tight anatomic space that contains vital structures, including the brainstem with the lower cranial nerves, cerebellar hemispheres, and the vertebrobasilar vascular system. A variety of lesions and vascular malformations can occur in the posterior fossa, and surgical exposure in this region is complex and requires any of the variety of surgical positions. The lateral position is usually needed for surgery on cerebellopontine angle tumors, cerebellar hemispheric lesions, and lesions involving the clivus, petrous ridge, and anterior and lateral foramen magnum. Midline and fourth ventricular lesions can be operated on in the prone or sitting positions, and the park bench position allows rapid positioning with quick access to the cerebellar hemispheres. Each position has its advantages and disadvantages. Preoperative evaluation of these patients should highlight the patient’s physical status, especially cardiopulmonary status because positioning can exert extra demands on the cardiorespiratory system. Although the sitting position offers considerable advantages to the neurosurgeon in terms of an optimal operating field (gravity-assisted drainage of blood and CSF) and reduced cerebellar retraction and venous bleeding, it can be associated with serious complications, including venous air embolism and paradoxical air embolism, hypotension (leading to cerebral/cervical ischemia), pneumocephalus, lingual and laryngeal trauma, and rarely, quadriplegia/paraplegia (midcervical flexion myelopathy). Despite several studies substantiating the relative safety of the sitting position,90,91 its use is diminishing largely because of the potential for serious complications and malpractice liability claims. Venous air embolism is the most common complication, and because 20% to 25% of the general population has a probe-patent foramen ovale,92 reversal of the right-left atrial pressure gradient can lead to paradoxical air embolism with devastating complications. Hence, a probe-patent foramen ovale is considered a relative contraindication to the sitting position, and preoperative or intraoperative echocardiography should be performed before proceeding to this position.93 Other contraindications to the sitting position include a patent ventriculoatrial shunt, severe hypovolemia, cachexia, severe cardiovascular disease, and postural hypotension, all of which should be ruled out preoperatively.
Head-Injured Patients
Head-injured patients usually undergo emergency surgery. Hence, a full preoperative history and examination are often difficult, if not impossible to obtain. A brief, quick history pertaining to the time and mode of injury and associated extracranial injuries may be obtained. The anesthesiologist’s goal in this setting is to prevent secondary injury to the already injured brain (Table 21-8) and facilitate early surgery by providing optimal operating conditions while continuing cerebral protection.
TABLE 21-8 Causes of Secondary Insults to an Already Injured Brain
The preoperative assessment often involves ongoing resuscitation and management of other injuries. In line with the guidelines of advanced trauma life support, care should be continued from the preoperative period. As in all resuscitation situations, the priorities are establishment of the airway, breathing, and circulation, and the anesthesiologist should ensure the adequacy of ventilation and oxygenation first of all. If the patient is already intubated and mechanically ventilated, the anesthesiologist should reconfirm correct position of the endotracheal tube and its patency and note the ventilator settings to ensure good ventilation so that hypoxemia and hypercapnia can be avoided. If the patient is not intubated, the anesthesiologist should quickly decide whether intubation is required immediately. Tracheal intubation can be challenging in patients with an uncleared cervical spine. Decision making regarding the technique of airway management is based on the urgency of the situation and the anesthesiologist’s personal expertise. Fiberoptic intubation may not be possible because of the urgency of the situation or bleeding in the airway. In such situations, direct laryngoscopy with manual in-line stabilization should be performed. Pharmacologic measures (opioids, lidocaine, beta blockers, or intravenous anesthetics) may be used to prevent increases in ICP in response to laryngoscopy and intubation. After securing the airway, ventilation should be adjusted to avoid both hypercapnia and profound hypocapnia. During the preoperative evaluation and continuing into anesthetic care, maintenance of adequate systemic blood pressure and CPP is vital. Repeated studies have shown that even transient hypotension is associated with a poor outcome.94 In adults, systemic hypotension as a manifestation of isolated head trauma is rare in the absence of brainstem injury. When present, it is often associated with an extracranial source of bleeding from thoracic, abdominal, long-bone, or spinal cord injuries, and all these injuries should be quickly ruled out. Aggressive fluid resuscitation with or without vasopressors should be continued. To avoid any delay in surgery, quick examination should focus on the Glasgow Coma Scale score, gross motor deficits, and pupillary abnormalities, as well as assessment of the extracranial injuries. If ICP is being monitored, it should be noted and any therapeutic modalities being used to control elevated ICP (e.g., mannitol) should be continued. CPP should be maintained above 60 mm Hg.95 Head injury may have multisystem sequelae (Table 21-9), in addition to the coexisting injuries, which the anesthesiologist has to carefully observe and manage during anesthesia. As part of the preoperative assessment, only the most important laboratory tests are required, including hemoglobin, blood glucose, coagulation profile, and toxicology screens. Blood must be crossmatched and blood products (fresh frozen plasma, platelets) should be available if needed.
TABLE 21-9 Major Multisystem Sequelae of Acute Head Injury
Spine Surgery
Methylprednisolone therapy is usually started in patients with spinal cord injury and should be continued through the intraoperative period.96 The prone position is most commonly used and necessitates a firmly secured airway and placement of adequate intravenous and arterial lines before turning the patient. The preoperative interview should include providing the patient information about possible complications of the prone position, including orbital edema, facial swelling, and airway swelling, which may warrant elective postoperative ventilation, as well as the potential for postoperative visual loss.97 Patients with a history of coronary artery bypass may have an increased risk for myocardial ischemia because of compression of the graft against the chest wall.
Adequate ventilatory effort depends on the integrity of the phrenic nerve (C3-5) and the innervation of the intercostal muscles. A spinal cord injury above C3 will result in a ventilator-dependent patient.98 An inability to cough and clear secretions increases the risk for respiratory insufficiency and occurs with loss of intercostal muscle action and chest wall excursion. Elective tracheal intubation and ventilation may be required preoperatively, as indicated by the parameters listed in Table 21-10.
TABLE 21-10 Indications for Tracheal Intubation in Spinal Cord–Injured Patients
Cardiovascular collapse is common after acute cervical cord trauma. Immediately at the time of trauma, activation of the sympathetic nervous system leads to a slight increase in cardiac contractility, as well as mean arterial pressure and systemic vascular resistance. In some patients this intense sympathetic discharge can result in neurogenic pulmonary edema, followed by the onset of spinal shock characterized by bradycardia and hypotension with reduced contractility.99 The spinal shock can last from 1 to 4 weeks. During this period there is complete vasoparalysis. Vasoconstriction for maintenance of cardiac filling pressure cannot take place, and fluid resuscitation is required to correct the relative hypovolemia and restore cardiac output. Because contractility is also reduced, inotropic support may be required. Invasive hemodynamic monitoring with placement of a central venous or pulmonary artery catheter will facilitate management of these patients during the acute period.100
Epilepsy Disorders
Epileptic patients can be encountered by the anesthesiologist for a number of reasons, including resection of the epileptic focus, electrocorticographic recording, diagnostic radiologic procedures, nonepilepsy surgery, and management of status epilepticus. Surgery for epilepsy can be performed with the patient under general anesthesia or under local anesthesia with minimal intravenous sedation. A sleep-awake-sleep technique is frequently used, with or without an artificial airway.101,102 Awake craniotomy allows the surgeon to communicate with a sedated, yet cooperative patient and precisely map the location and extent of resection. An important part of the preanesthetic evaluation is counseling of patients regarding the procedure to allay their fear and anxiety. With the introduction of propofol, it is now possible to induce short periods of “deep anesthesia” during the painful period to maximize patient comfort without sacrificing the subsequent need for a lucid and cooperative patient.102 Intraoperative electrocorticography has shown that high-dose propofol does not cause seizures103 and that it does not interfere with mapping, provided that the infusion is suspended for 15 minutes before recording.104 Good preoperative rapport with the patient nevertheless facilitates this process. In some centers, patient-controlled sedation has been used with some success.105
The newer anesthetic drugs have minimal effects on the electrocorticogram and do not interfere with mapping—hence patients with increased ICP or impaired cerebral autoregulation can safely undergo general anesthesia with controlled ventilation to prevent intraoperative brain swelling from retention of carbon dioxide—and provide spontaneous breathing under sedation alone. For all epileptic patients, it is important to note that the use of phenytoin makes them more resistant to nondepolarizing neuromuscular blocking drugs,106 and these patients may also have an increased requirement for opioids.107 Placement of a stereotactic frame is a potential airway concern for the anesthesiologist. Ventilation by mask can be difficult, and direct laryngoscopy could be impossible. If tracheal intubation is required, fiberoptic laryngoscopy or intubation with a laryngeal mask airway may be required and must be readily available. Laryngeal mask airways are useful under these conditions and should always be considered.108 Any sedation technique carries a potential risk for overmedication with the associated loss of protective airway reflexes or the onset of severe respiratory depression. Thus, during the preoperative evaluation, special attention should be paid to airway assessment and the need for airway adjuncts.
Neuroradiology
With the exception of very young children, most neuroradiologic procedures are performed on sedated patients under local anesthesia. Anesthetic assistance is usually requested for children, uncooperative adults, and debilitated patients at high risk. Monitored sedation may be adequate even in children.109 The aim is to provide a calm, comfortable, and cooperative patient. The technique of patient-controlled sedation with propofol has also been used successfully for interventional radiologic procedures.110 However, patients with mass lesions and intracranial hypertension who would benefit from avoidance of sedation-induced hypercapnia need general anesthesia with controlled ventilation. General anesthesia and complete immobility are also desirable for embolization of AVMs, endovascular coiling of aneurysms in many centers, and intraluminal balloon angioplasty for cerebral vasospasm.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular SurgeryFleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg. 2008;106:685-712.
American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2002;96:485-496.
Dernbach PD, Little JR, Jones SC, et al. Altered cerebral autoregulation and CO2 reactivity after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1988;22:822-826.
Diringer MN, Lim JS, Kirsch JR, et al. Suprasellar and intraventricular blood predict elevated plasma atrial natriuretic factor in subarachnoid hemorrhage. Stroke. 1991;22:577-581.
Drummond JC, Dao AV, Roth DM, et al. Effect of dexmedetomidine on cerebral blood flow velocity, cerebral metabolic rate, and carbon dioxide response in normal humans. Anesthesiology. 2008;108:225-232.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1-9.
Gelb AW, Craen RA, Rao GS, et al. Does hyperventilation improve operating condition during supratentorial craniotomy? A multicenter randomized crossover trial. Anesth Analg. 2008;106:585-594.
Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
Gronert GA, Theye RA. Pathophysiology of hyperkalemia induced by succinylcholine. Anesthesiology. 1975;43:89-99.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14-20.
Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652-659.
Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429-434.
, 1991 North American Symptomatic Carotid Endarterectomy Trial Steering Committee: North American Symptomatic Carotid Endarterectomy Trial: Methods, patient characteristics, and progress. Stroke. 1991;22:711-720.
Paciaroni M, Eliasziw M, Kappelle LJ, et al. Medical complications associated with carotid endarterectomy. North American Symptomatic Carotid Endarterectomy Trial (NASCET). 1999;30:1759-1763.
Petersen KD, Landsfeldt U, Cold GE, et al. Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: a randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329-336.
POISE Study GroupDevereauz PJ, Yang H, Ysusf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839-1847.
Rasmussen M, Bundgaard H, Cold GE. Craniotomy for supratentorial brain tumors: risk factors for brain swelling after opening the dura mater. J Neurosurg. 2004;101:621-626.
, 1988 Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985-986.
Rozet I, Vavilala MS, Lindley AM, et al. Cerebral autoregulation and CO2 reactivity in anterior and posterior cerebral circulation during sevoflurane anesthesia. Anesth Analg. 2006;102:560-564.
Skucas AP, Artru AA. Anesthetic complications of awake craniotomies for epilepsy surgery. Anesth Analg. 2006;102:882-887.
Smetana GW, Lawrence VA, Cornell JE, American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
Thal GD, Szabo MD, Lopez-Bresnahan M, et al. Exacerbation or unmasking of focal neurologic deficits by sedatives. Anesthesiology. 1996;85:21-25.
Van der Bilt IA, Hasan D, Vandertop WP, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology. 2009;72:635-642.
Wolters U, Wolf T, Stützer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217-222.
1 American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2002;96:485-496.
2 Conway JB, Goldberg J, Chung F. Preadmission anaesthesia consultation clinic. Can J Anaesth. 1992;39:1051-1057.
3 Fischer SP. Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital. Anesthesiology. 1996;85:196-206.
4 Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845-850.
5 Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
6 Radcliff TA, Henderson WG, Stoner TJ, et al. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90:34-42.
7 Skaga NO, Eken T, Søvik S, et al. Pre-injury ASA physical status classification is an independent predictor of mortality after trauma. J Trauma. 2007;63:972-978.
8 Akavipat P, Ittichaikulthol W, Tuchinda L, et al. The Thai Anesthesia Incidents (THAI Study) of anesthetic risk factors related to perioperative death and perioperative cardiovascular complications in intracranial surgery. J Med Assoc Thai. 2007;90:1565-1572.
9 Gibby GL, Gravenstein SJ, Leton AJ, et al. How often does the preoperative interview change anesthetic management. Anesthesiology. 1992;77:A1134.
10 Narr BJ, Hansen TR, Warner MA. Preoperative laboratory screening in healthy Mayo patients: cost-effective elimination of tests and unchanged outcomes. Mayo Clin Proc. 1991;66:155-159.
11 Menke H, Klein A, John KD, et al. Predictive value of ASA classification for the assessment of the perioperative risk. Int Surg. 1993;78:266-270.
12 Wolters U, Wolf T, Stützer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217-222.
13 Wolters U, Wolf T, Stützer H, et al. Risk factors, complications, and outcome in surgery: a multivariate analysis. Eur J Surg. 1997;63:563-568.
14 Gonzalez H, Minville V, Delanoue K, et al. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth Analg. 2008;106:1132-1136.
15 La Colla L, Albertin A, La Colla G, et al. Faster wash-out and recovery for desflurane vs sevoflurane in morbidly obese patients when no premedication is used. Br J Anaesth. 2007;99:353-358.
16 Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429-434.
17 Arné J, Descoins P, Fusciardi J, et al. Preoperative assessment for difficult intubation in general and ENT surgery: predictive value of a clinical multivariate risk index. Br J Anaesth. 1998;80:140-146.
18 Kawaguchi M, Sakamoto T, Furuya H, et al. Pseudoankylosis of the mandible after supratentorial craniotomy. Anesth Analg. 1996;83:731-734.
19 Schmitt H, Buchfelder M, Radespiel-Tröger M, et al. Difficult intubation in acromegalic patients: incidence and predictability. Anesthesiology. 2000;93:110-114.
20 Thal GD, Szabo MD, Lopez-Bresnahan M, et al. Exacerbation or unmasking of focal neurologic deficits by sedatives. Anesthesiology. 1996;85:21-25.
21 Gronert GA, Theye RA. Pathophysiology of hyperkalemia induced by succinylcholine. Anesthesiology. 1975;43:89-99.
22 Iwatsuki N, Kuroda N, Amaha K, et al. Succinylcholine-induced hyperkalemia in patients with ruptured cerebral aneurysms. Anesthesiology. 1980;53:64-67.
23 Mohr DN, Lavender RC. Preoperative pulmonary evaluation. Identifying patients at increased risk for complications. Postgrad Med. 1996;100:241-252.
24 Williams-Russo P, Charlson ME, MacKenzie CR, et al. Predicting postoperative pulmonary complications. Is it a real problem? Arch Intern Med. 1992;152:1209-1213.
25 Doyle RL. Assessing and modifying the risk of postoperative pulmonary complications. Chest. 1999;115(5 suppl):S77-S81.
26 Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg. 1986;65:1186-1188.
27 Cohen MM, Cameron CB. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72:282-288.
28 Rath GP, Bithal PK, Guleria R, et al. A comparative study between preoperative and postoperative pulmonary functions and diaphragmatic movements in congenital craniovertebral junction anomalies. J Neurosurg Anesthesiol. 2006;18:256-261.
29 Smetana GW, Lawrence VA, Cornell, JE, American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
30 Barry DI. Cerebral blood flow in hypertension. J Cardiovasc Pharmacol. 1985;7(suppl 2):S94-S98.
31 Strandgaard S, Paulson OB. Cerebral blood flow and its pathophysiology in hypertension. Am J Hypertens. 1989;2:486-492.
32 American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular SurgeryFleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg. 2008;106:685-712.
33 POISE Study GroupDevereauz PJ, Yang H, Ysusf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839-1847.
34 Powner DJ, Hartwell EA, Hoots WK. Counteracting the effects of anticoagulants and antiplatelet agents during neurosurgical emergencies. Neurosurgery. 2005;57:823-831.
35 Korinth MC. Low-dose aspirin before intracranial surgery—results of a survey among neurosurgeons in Germany. Acta Neurochir (Wien). 2006;148:1189-1196.
36 Salzarulo HH, Taylor LA. Diabetic “stiff joint syndrome” as a cause of difficult endotracheal intubation. Anesthesiology. 1986;64:366-368.
37 Drummond JC. The lower limit of autoregulation: time to revise our thinking? Anesthesiology. 1997;86:1431-1433.
38 Eng C, Lam AM, Mayberg TS, et al. The influence of propofol with and without nitrous oxide on cerebral blood flow velocity and CO2 reactivity in humans. Anesthesiology. 1992;77:872-879.
39 Strebel S, Lam AM, Matta B, et al. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995;83:66-76.
40 Strebel S, Kaufmann M, Maître L, et al. Effects of ketamine on cerebral blood flow velocity in humans. Influence of pretreatment with midazolam or esmolol. Anaesthesia. 1995;50:223-228.
41 Mayberg TS, Lam AM, Matta BF, et al. Ketamine does not increase cerebral blood flow velocity or intracranial pressure during isoflurane/nitrous oxide anesthesia in patients undergoing craniotomy. Anesth Analg. 1995;81:84-89.
42 Koht A, Schütz W, Schmidt G, et al. Effects of etomidate, midazolam, and thiopental on median nerve somatosensory evoked potentials and the additive effects of fentanyl and nitrous oxide. Anesth Analg. 1988;67:435-441.
43 Taniguchi M, Nadstawek J, Langenbach U, et al. Effects of four intravenous anesthetic agents on motor evoked potentials elicited by magnetic transcranial stimulation. Neurosurgery. 1993;33:407-415.
44 Rozet I, Muangman S, Vavilala MS, et al. Clinical experience with dexmedetomidine for implantation of deep brain stimulators in Parkinson’s disease. Anesth Analg. 2006;103:1224-1228.
45 Mack PF, Perrine K, Kobylarz E, et al. Dexmedetomidine and neurocognitive testing in awake craniotomy. J Neurosurg Anesthesiol. 2004;16:20-25.
46 Drummond JC, Dao AV, Roth DM, et al. Effect of dexmedetomidine on cerebral blood flow velocity, cerebral metabolic rate, and carbon dioxide response in normal humans. Anesthesiology. 2008;108:225-232.
47 Matta BF, Mayberg TS, Lam AM. Direct cerebrovasodilatory effects of halothane, isoflurane, and desflurane during propofol-induced isoelectric electroencephalogram in humans. Anesthesiology. 1995;83:980-985.
48 Summors AC, Gupta AK, Matta BF. Dynamic cerebral autoregulation during sevoflurane anesthesia: a comparison with isoflurane. Anesth Analg. 1999;88:341-345.
49 Cho S, Fujigaki T, Uchiyama Y, et al. Effects of sevoflurane with and without nitrous oxide on human cerebral circulation. Transcranial Doppler study. Anesthesiology. 1996;85:755-760.
50 Rozet I, Vavilala MS, Lindley AM, et al. Cerebral autoregulation and CO2 reactivity in anterior and posterior cerebral circulation during sevoflurane anesthesia. Anesth Analg. 2006;102:560-564.
51 Werner C, Kochs E, Bause H, et al. Effects of sufentanil on cerebral hemodynamics and intracranial pressure in patients with brain injury. Anesthesiology. 1995;83:721-726.
52 Hänel F, Werner C, von Knobelsdorff G, et al. The effects of fentanyl and sufentanil on cerebral hemodynamics. J Neurosurg Anesthesiol. 1997;9:223-227.
53 Schmitt H, Buchfelder M, Radespiel-Tröger M, et al. Difficult intubation in acromegalic patients: incidence and predictability. Anesthesiology. 2000;93:110-114.
54 Campkin TV. Radial artery cannulation. Potential hazard in patients with acromegaly. Anaesthesia. 1980;35:1008-1009.
55 Rasmussen M, Bundgaard H, Cold GE. Craniotomy for supratentorial brain tumors: risk factors for brain swelling after opening the dura mater. J Neurosurg. 2004;101:621-626.
56 Bedford RF, Morris L, Jane JA. Intracranial hypertension during surgery for supratentorial tumor: correlation with preoperative computed tomography scans. Anesth Analg. 1982;61:430-433.
57 Todd MM, Warner DS, Sokoll MD, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy. Propofol/fentanyl, isoflurane/nitrous oxide, and fentanyl/nitrous oxide. Anesthesiology. 1993;78:1005-1020.
58 Petersen KD, Landsfeldt U, Cold GE, et al. Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: a randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329-336.
59 Gelb AW, Craen RA, Rao GS, et al. Does hyperventilation improve operating condition during supratentorial craniotomy? A multicenter randomized crossover trial. Anesth Analg. 2008;106:585-594.
60 North American Symptomatic Carotid Endarterectomy Trial Steering Committee: North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke. 1991;22:711-720.
61 Sundt TM, Sandok BA, Whisnant JP. Carotid endarterectomy. Complications and preoperative assessment of risk. Mayo Clin Proc. 1975;50:301-306.
62 Ferguson GG, Eliasziw M, Barr HW, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751-1758.
63 Paciaroni M, Eliasziw M, Kappelle LJ, et al. Medical complications associated with carotid endarterectomy. North American Symptomatic Carotid Endarterectomy Trial (NASCET). Stroke. 1999;30:1759-1763.
64 Skudlarick JL, Mooring SL. Systolic hypertension and complications of carotid endarterectomy. South Med J. 1982;75:1563-1565.
65 Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415-1425.
66 Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14-20.
67 Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62:59-67.
68 Dernbach PD, Little JR, Jones SC, et al. Altered cerebral autoregulation and CO2 reactivity after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1988;22:822-826.
69 Tenjin H, Hirakawa K, Mizukawa N, et al. Dysautoregulation in patients with ruptured aneurysms: cerebral blood flow measurements obtained during surgery by a temperature-controlled thermoelectrical method. Neurosurgery. 1988;23:705-709.
70 Davies KR, Gelb AW, Manninen PH, et al. Cardiac function in aneurysmal subarachnoid haemorrhage: a study of electrocardiographic and echocardiographic abnormalities. Br J Anaesth. 1991;67:58-63.
71 Diringer MN, Lim JS, Kirsch JR, et al. Suprasellar and intraventricular blood predict elevated plasma atrial natriuretic factor in subarachnoid hemorrhage. Stroke. 1991;22:577-581.
72 Nelson RJ, Roberts J, Rubin C, et al. Association of hypovolemia after subarachnoid hemorrhage with computed tomographic scan evidence of raised intracranial pressure. Neurosurgery. 1991;29:178-182.
73 Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985-986.
74 Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1-9.
75 Kotapka MJ, Flamm ES. Cerebral aneurysms: Surgical considerations. In: Cottrell JE, Smith DS, editors. Anesthesia and Neurosurgery. St. Louis: CV Mosby; 2001:353-365.
76 Harrigan MR. Cerebral salt wasting syndrome: a review. Neurosurgery. 1996;38:152-160.
77 Sviri GE, Feinsod M, Soustiel JF. Brain natriuretic peptide and cerebral vasospasm in subarachnoid hemorrhage. Clinical and TCD correlations. Stroke. 2000;31:118-122.
78 Diringer MN. Management of sodium abnormalities in patients with CNS disease. Clin Neuropharmacol. 1992;15:427-447.
79 Suarez JI, Qureshi AI, Parekh PD, et al. Administration of hypertonic (3%) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:178-184.
80 Andreoli A, di Pasquale G, Pinelli G, et al. Subarachnoid hemorrhage: frequency and severity of cardiac arrhythmias. A survey of 70 cases studied in the acute phase. Stroke. 1987;18:558-564.
81 Di Pasquale G, Andreoli A, Lusa AM, et al. Cardiologic complications of subarachnoid hemorrhage. J Neurosurg Sci. 1998;42:33-36.
82 Brouwers PJ, Wijdicks EF, Hasan D, et al. Serial electrocardiographic recording in aneurysmal subarachnoid hemorrhage. Stroke. 1989;20:1162-1167.
83 Zaroff JG, Rordorf GA, Newell JB, et al. Cardiac outcome in patients with subarachnoid hemorrhage and electrocardiographic abnormalities. Neurosurgery. 1999;44:34-39.
84 Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg. 1995;83:889-896.
85 Pollick C, Cujec B, Parker S, et al. Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol. 1988;12:600-605.
86 Van der Bilt IA, Hasan D, Vandertop WP, et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology. 2009;72:635-642.
87 Sakka SG, Huettemann E, Reinhart K. Acute left ventricular dysfunction and subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:209-213.
88 Lam AM. Cerebral aneurysms: Anesthetic considerations. In: Cottrell JE, Smith DS, editors. Anesthesia and Neurosurgery. St. Louis: CV Mosby; 2001:367-397.
89 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
90 Black S, Ockert DB, Oliver WCJr, et al. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988;69:49-56.
91 Porter JM, Pidgeon C, Cunningham AJ. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999;82:117-128.
92 Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20.
93 Konstadt SN, Louie EK, Black S, et al. Intraoperative detection of patent foramen ovale by transesophageal echocardiography. Anesthesiology. 1991;74:212-216.
94 Winchell RJ, Simons RK, Hoyt DB. Transient systolic hypotension. A serious problem in the management of head injury. Arch Surg. 1996;131:533-539.
95 Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSBratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(suppl 1):S59-S64.
96 Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1988;89:699-706.
97 Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652-659.
98 Oo T, Watt JW, Soni BM, et al. Delayed diaphragm recovery in 12 patients after high cervical spinal cord injury. A retrospective review of the diaphragm status of 107 patients ventilated after acute spinal cord injury. Spinal Cord. 1999;37:117-122.
99 Lam AM. Acute spinal cord injury: monitoring and anaesthetic implications. Can J Anaesth. 1991;38:R60-R73.
100 Mackenzie CF, Shin B, Krishnaprasad D, et al. Assessment of cardiac and respiratory function during surgery on patients with acute quadriplegia. J Neurosurg. 1985;62:843-849.
101 Sarang A, Dinsmore J. Anaesthesia for awake craniotomy—evolution of a technique that facilitates awake neurological testing. Br J Anaesth. 2003;90:161-165.
102 Skucas AP, Artru AA. Anesthetic complications of awake craniotomies for epilepsy surgery. Anesth Analg. 2006;102:882-887.
103 Cheng MA, Tempelhoff R, Silbergeld DL, et al. Large-dose propofol alone in adult epileptic patients: electrocorticographic results. Anesth Analg. 1996;83:169-174.
104 Herrick IA, Craen RA, Gelb AW, et al. Propofol sedation during awake craniotomy for seizures: electrocorticographic and epileptogenic effects. Anesth Analg. 1997;84:1280-1284.
105 Herrick IA, Craen RA, Gelb AW, et al. Propofol sedation during awake craniotomy for seizures: patient-controlled administration versus neurolept analgesia. Anesth Analg. 1997;84:1285-1291.
106 Ornstein E, Matteo RS, Weinstein JA, et al. Accelerated recovery from doxacurium-induced neuromuscular blockade in patients receiving chronic anticonvulsant therapy. J Clin Anesth. 1991;3:108-111.
107 Tempelhoff R, Modica PA, Spitznagel ELJr. Anticonvulsant therapy increases fentanyl requirements during anaesthesia for craniotomy. Can J Anesth. 1990;37:327-332.
108 Brimacombe JR, Ferson D, Osborn I, et al. Specialized uses of the LMA. Int Anesthesiol Clin. 1998;36:123-138.
109 Osborn IP. Intravenous conscious sedation for pediatric patients. Int Anesthesiol Clin. 1999;37:99-111.
110 Herrick IA, Gelb AW, Tseng PS, et al. Patient-controlled sedation using propofol during interventional neuroradiologic procedures. J Neurosurg Anesthesiol. 1997;9:237-241.