Chapter 36 Neuro-ophthalmology
Afferent Visual System
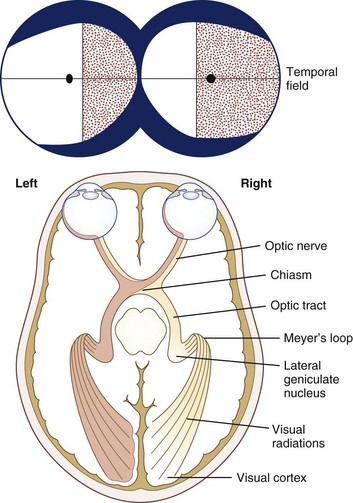
From a conceptual standpoint, it is useful to consider vision as having two components: central or macular vision (high acuity, color perception, light adapted) and peripheral or ambulatory vision (low acuity, poor color perception, dark-adapted). Light, refracted by the cornea and lens, then focuses on the retina. For the best possible vision, the object of regard must focus on the fovea, which is the most sensitive part of the macula. The cone photoreceptors, which mediate central and color vision, are greatest in density at the fovea. The cone system functions optimally in conditions of light adaptation. Visual acuity and cone density fall off rapidly as eccentricity from the fovea increases. For example, the retina 20 degrees eccentric to the fovea can only resolve objects equal to Snellen 20/200 (6/60 metric) optotypes or larger. Rod photoreceptors are present in highest numbers approximately 20 degrees from the fovea and are more abundant than cones in the more peripheral retina; rods function best in dim illumination. The total extent of the normal peripheral visual field in each eye is approximately 60 degrees superior, 60 degrees nasal, 70 to 75 degrees inferior, and 100 degrees temporal to fixation (Fig. 36.1) (see Chapter 14).
Each eye sends visual information, transduced by the retina, to both hemispheres of the brain by the optic nerves, each of which contains over 1 million axons. Axons that arise from the ganglion cells of the nasal retina of each eye cross in the optic chiasm to the contralateral optic tract. Axons from the temporal retina do not decussate at the chiasm. The percentages of crossed and uncrossed axons in the human optic chiasm are approximately 53% and 47%, respectively. Because of the optical properties of the eye, the nasal retina receives visual information from the temporal visual field, while the temporal retina receives visual information from the nasal visual field (see Fig. 36.1). Similarly, the superior retina receives information from the inferior visual field, and vice versa. These points are clinically important in evaluating visual loss (see Chapter 14).
Visual information stratifies further in the lateral geniculate nucleus (LGN), which is the only way station between the retinal ganglion cells and the primary visual cortex. The LGN, a portion of the thalamus, has six layers. Axons from ipsilateral retinal ganglion cells synapse in layers 2, 3, and 5; contralateral axons synapse in layers 1, 4, and 6. Layers 1 and 2 of the LGN are the magnocellular layers and receive input from M retinal ganglion cells. The magnocellular pathway is concerned mainly with movement detection, detection of low contrast, and dynamic form perception. After projecting to the primary visual cortex (visual area 1, V1, or Brodmann area 17), information from the M pathway is distributed to V2 (part of area 18) and V5 (junction of areas 19 and 37). Layers 3 to 6 of the LGN are the parvocellular layers and receive input from retinal P cells, which are color selective and responsive to high contrast. Information from the P pathway is distributed to V2 and V4 (fusiform gyrus) (Trobe, 2001). Superior fibers that leave the LGN go straight back to the primary visual cortex; inferior fibers loop anteriorly around the temporal horn of the lateral ventricles (Meyer loop). Since these fibers pass close to the tip of the temporal lobe, temporal lobectomy sometimes damages these fibers causing a “pie in the sky” homonymous visual field defect.
The primary visual cortex (striate cortex, area V1, or Brodmann area 17) is in the occipital lobe. Fibers from the macula project to the visual cortex closest to the occipital poles (each fovea appears to project to both occipital lobes), while fibers from the peripheral retina project to the visual cortex lying more anteriorly. The nonoverlapping part of the most peripheral temporal visual field (monocular temporal crescent) arises from unpaired crossed axons from the nasal retina that project to the most anteromedial part of the visual cortex. The primary visual cortex has interconnections with visual association areas concerned with color, motion, and object recognition (Trobe, 2001).
Neuro-ophthalmological Examination of the Afferent Visual System
Examination of Visual Acuity
Visual acuity is the spatial resolution of vision. Visual acuity should always be measured with each eye individually and with the best possible optical correction (i.e., with the patient’s glasses); other optical means such as the pinhole device or a refraction may be needed (Wall and Johnson, 2005). The resultant measure, called best-corrected visual acuity, is the only universally interpretable measurement of central visual function. Ideally, always measure vision both at a standard distance (usually 20 feet or 6 m) and at near (usually 0.33 m). The notation 20/20 (6/6 metric) means the patient (numerator) is able to see the optotypes seen by a normal subject at 20 feet (denominator). A vision of 20/60 (6/18 metric) means that the patient sees an optotype at 20 feet that a normal person would see at 60 feet.
When measuring near vision, the reading card should be held at the specified distance of 14 inches (or 0.33 m) to control for variation in image size on the retina. The medical record should clearly specify if a nonstandard distance is used. Two types of near cards are readily available; one has numbers and the other has written text (Fig. 36.2). Both are useful, but in neurological practice, a near card with text measures not only visual acuity but also reading ability to some degree. A disparity between the measurements from the two types of near card might suggest a disturbance of some other cortical function such as language function (see Chapter 11).
Light-Stress Test
In some disorders of the macula, abnormalities are undetectable with the direct ophthalmoscope. The light-stress (or photo-stress) test is a useful method for determining whether reduced visual acuity is a consequence of macular dysfunction (Wall and Johnson, 2005). Prior to the test, the best-corrected visual acuity is measured in each eye. Then, with the eye with decreased vision occluded, the other eye is exposed to a bright light for 10 seconds. Immediately thereafter, the patient is instructed to read the next larger line on the eye chart, and the recovery period is timed. The same procedure is followed for the eye with decreased vision, and the results are compared. Fifty seconds is the upper limit of normal for visual recovery, although most normal subjects recover within several seconds. In patients with macular disease, the recovery period often takes several minutes.
Color Vision Testing
Dyschromatopsia, especially if asymmetrical between the eyes, is a good indication of optic nerve dysfunction, but it can also occur with retinal disease (Almog and Nemet, 2010). Symmetrical acquired dyschromatopsia might indicate a retinal degeneration, such as a cone-rod dystrophy. Congenital dyschromatopsia occurs in about 8% of men and 0.5% of women.
Examination of the Pupils
Examination of the pupils involves assessing pupil size and shape, direct and consensual reactions to light, near reaction, and the presence or absence of a relative afferent pupillary defect (RAPD). If a difference in pupil size (anisocoria) is noted, look for ptosis and ophthalmoplegia, keeping in mind the possibility of a Horner syndrome or third cranial nerve palsy. Record findings in an easily understood format (Table 36.1).
Measurements of pupil size and light reaction are made in dim illumination with the patient fixating on an immobile distant target. If there is anisocoria, it is useful to measure pupil size in darkness and bright ambient light. Anisocoria due to oculosympathetic paresis (Horner syndrome) is often greater in the dark, because the affected pupil does not dilate well. Conversely, anisocoria due to parasympathetic denervation (e.g., Adie tonic pupil) is often more evident in bright light, because the affected pupil does not constrict well (see Chapter 16).
The observation of an RAPD (formerly called a Marcus Gunn pupil) is an invaluable indication of a conduction defect in the optic nerve. A difference in pupillary reaction is best elicited by alternately illuminating the pupils by swinging a flashlight between them at a frequency of about once per second—hence the name, swinging flashlight test. The swinging flashlight test compares the direct and consensual reactions in the same eye. Normally these reactions are equal. However, in patients with an optic neuropathy, because of reduction in the direct reaction as compared with the consensual reaction, the pupil dilates when re-illuminated. Box 36.1 and Fig. 36.3 describe the method for testing for an RAPD. Two caveats exist. The test brings out an asymmetry of optic nerve conduction, so with both optic nerves injured to the same extent, an RAPD is not present. In addition, a macular or retinal lesion can sometimes produce an RAPD, but abnormalities are usually evident on funduscopic examination. In contrast, an optic neuropathy with minimal loss of visual acuity often gives an obvious RAPD. See Chapter 16 for further discussion of pupillary abnormalities.
Box 36.1 Testing for a Relative Afferent Pupillary Defect
1. The patient should fixate on an immobile distant target to minimize fluctuation in pupillary size and accommodative miosis.
2. A light bright enough to cause maximum pupillary constriction should be used.
3. Each pupil should be checked individually for its direct light response, which can be graded on a scale of 1 to 4 (see Table 36.1).
4. The light should be moved quickly to illuminate each eye alternately every 1 second (the swinging flashlight test).
5. The pupil should be observed for initial constriction and the presence or absence of pupillary escape.
6. Only 3 or 4 swings of the light should be made; this minimizes bleaching of the retina, with subsequent slowing of the pupillary reaction.
Visual Field Testing
1. The clinician has the patient occlude one eye and maintain fixation on the clinician’s nose.
2. Finger counting in the quadrants: The clinician holds up fingers sequentially in each of the four quadrants of the visual field and asks the patient to count the number seen.
3. Simultaneous finger counting using both hands: If step 2 is completed normally, the clinician asks the patient to count the number of fingers displayed with both hands, first in both of the upper quadrants of the patient’s visual field and then in the lower quadrants. Then ask the patient to add the total number of fingers shown with both hands. Visual inattention is often identifiable during this step of confrontation testing.
4. Simultaneous hand comparison: Finally, the clinician holds both hands open, first in both upper quadrants and then both lower quadrants, and the patient is asked to compare the quality of the images. For example, a patient with a subtle bitemporal hemianopia may be able to “pass” steps 1 through 3, but when shown hands on either side of the midline may state that the hands held in the temporal hemifields are not seen as clearly as those held in the nasal hemifields.
A major advantage of the finger counting method over kinetic (wiggling finger) methods is that it minimizes the potential for confounding by the Riddoch phenomenon, which refers to a dissociation between the visual perception of form and movement such that the patient can perceive moving but not stationary targets in half of the visual field (Zeki and Ffytche, 1998). The Riddoch phenomenon can occur when homonymous hemianopia results from occipital cortex lesions. Accordingly, the clinician may miss a hemianopia when using only a moving target, such as wiggling fingers, in the far periphery.
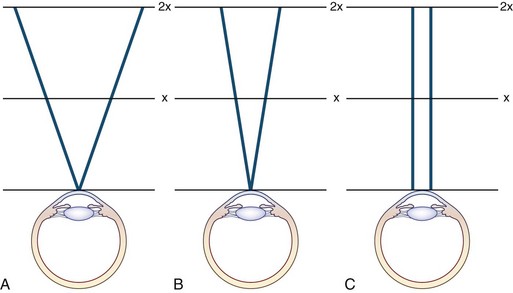
Confrontation methods using colored objects can be effective in detecting subtle defects. Confrontation testing is also useful for assessing patients with constricted visual fields. As the distance between the clinician and patient increases, the visual field should expand, producing a funnel. However, with nonorganic visual field constriction, the visual field often does not expand as the distance between the clinician and patient increases, thereby producing a tunnel (Fig. 36.4).
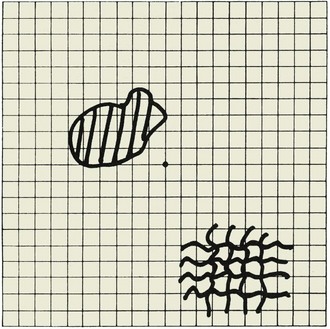
The central 20 degrees of the visual field (in each eye separately) can be assessed using the Amsler grid (Fig. 36.5). With the chart held in good light at a distance of 30 cm from the eye, the patient wearing their reading glasses if needed, the following questions are asked:
1. Can you see the spot in the center of the grid?
2. While looking at the center spot, can you see the entire grid or are any sides or corners missing?
3. While you are looking at the center spot, are any of the lines in the grid missing, blurred, or distorted?
If the patient indicates an abnormality, the clinician should ask the patient to draw the abnormal areas on the chart. The chart can then be kept in the patient’s medical record. Patients with a central scotoma often report that the center of the grid is missing or blurred; those with hemianopic defects say that half of the grid is missing; and those with macular disease might report that the lines are wavy or distorted (i.e., metamorphopsia) (see Chapter 14).
Numerous other methods for examining the visual fields are available (Wall and Johnson, 2005), but these are beyond the scope of this chapter and the expertise of most neurologists. Examination of the entire visual field requires a perimeter; the tangent screen measures only the central 30 degrees of visual field at a distance of 1 m. Perimeters can be divided into those that use a moving (kinetic) stimulus and those that use a static stimulus. Most static perimeters available today are automated and driven by computer. Static perimeters can determine the actual visual threshold at defined points in the visual field (threshold static perimetry) or may test these points using stimuli of set luminance (suprathreshold static perimetry). The Goldmann perimeter is the most commonly used kinetic perimeter, while the Humphrey and Octopus perimeters are the most commonly used static perimeters. On theoretical grounds, threshold static perimeters are the most sensitive and quantitative, but the examination is more time consuming and tiring for the patient. To gain useful information from static perimetry, intense concentration and alertness are required. Many patients with neurological disorders are unable to sit and concentrate for an examination that can take as long as 15 minutes per eye, so static perimetry findings can be unreliable in these patients. Recent refinements in testing strategy have made it possible to reduce testing time and increase reliability, but many neuro-ophthalmologists continue to use Goldmann perimetry to assess the visual fields in selected patients.
Interpretation of Visual Field Defects
Eight general rules for visual field interpretation are summarized in Box 36.2. Comments relating to six of these general rules are presented here.
Box 36.2 General Rules of Visual Field Interpretation
1. Lesions of the retina and optic nerve produce visual field defects in the ipsilateral eye, only unless the lesions are bilateral.
2. True bitemporal hemianopia is caused only by a lesion at the optic chiasm.
3. Retrochiasmal lesions produce homonymous visual field defects.
4. Anterior retrochiasmal lesions produce incongruent homonymous visual field defects.
5. Posterior retrochiasmal lesions produce congruent visual field defects.
6. Temporal lobe lesions give slightly incongruent homonymous hemianopias involving the upper quadrant.
7. No localizing value can be assigned to a complete homonymous hemianopia, except that the lesion is retrochiasmal and contralateral to the visual field defect.
8. A unilateral homonymous hemianopia does not reduce visual acuity.
Rule 1
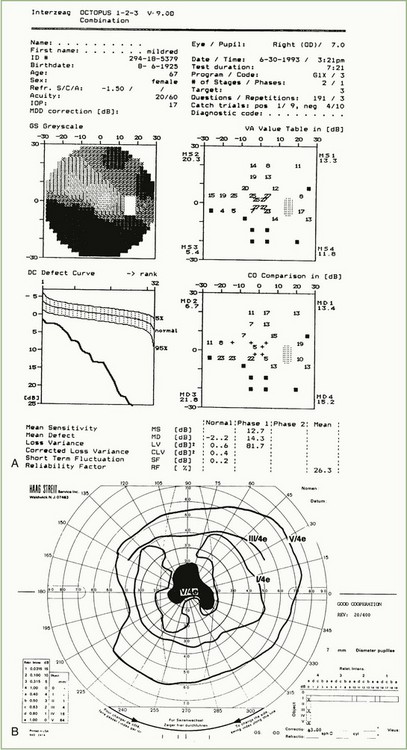
Optic nerve lesions can produce prechiasmal visual field abnormalities that are characteristic. Nonarteritic anterior ischemic optic neuropathy often produces an inferior altitudinal defect (Fig. 36.6, A), optic neuritis often produces a central or cecocentral scotoma (see Fig. 36.6, B), and compressive optic neuropathies often produce abnormalities in both the peripheral and central portions of the visual field (Fig. 36.7).
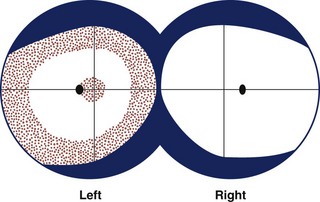
A lesion at the junction of the optic nerve and chiasm produces a junctional scotoma (i.e., ipsilateral cecocentral scotoma and contralateral temporal defect) due to involvement of both ipsilateral fibers and crossing fibers from the contralateral nasal retina. Binasal hemianopias (Fig. 36.8) can result from papilledema, ischemic optic neuropathy, glaucoma, optic nerve head drusen, optic nerve pits, optic nerve hypoplasia, and sectoral retinitis pigmentosa. Less often, hydrocephalus, ectatic parasellar arteries, and basal tumors can produce binasal field defects. Binasal field defects that are organic in etiology do not respect the vertical meridian, whereas nonorganic binasal defects may.
Rule 2
True bitemporal hemianopias are the hallmark of chiasmal disease; common causes are discussed in Chapter 14. Less commonly, ischemia, radiotherapy, and demyelination can cause a chiasmal syndrome. Bitemporal defects that do not respect the vertical meridian (pseudobitemporal hemianopias) are almost always due to congenital rotation or tilting of the optic discs (Fig. 36.9) (see Chapter 15). Bilateral cecocentral scotomas can masquerade as bitemporal field defects, and the defining difference is whether or not the defect respects the vertical meridian of visual field.
Rule 4
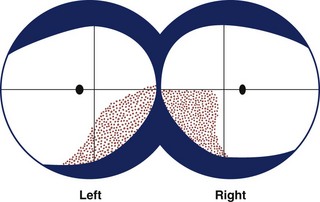
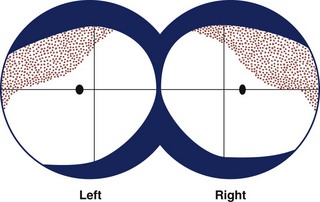
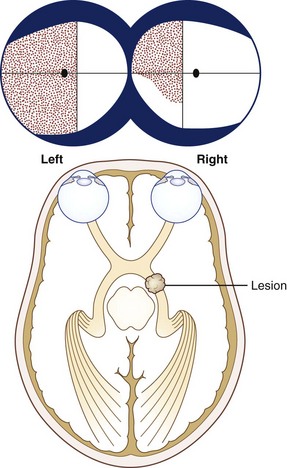
Incongruous hemianopias tend to result from more anterior retrochiasmal lesions (e.g., those affecting the optic tract or temporal lobe) (Fig. 36.10) (Kedar et al., 2007). Optic tract lesions often produce a contralateral relative afferent pupillary defect, which is helpful for clinical localization.
Rule 5
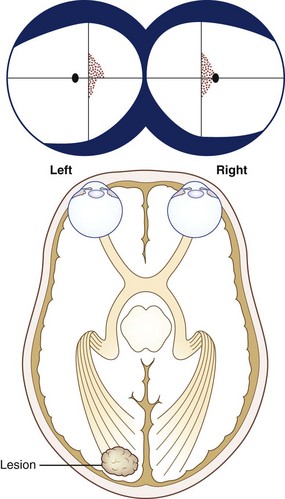
Congruous homonymous hemianopias have patterns that are very similar or identical in the two eyes. Congruous hemianopias usually result from occipital lobe infarcts (Fig. 36.11) but can sometimes occur with more anterior retrochiasmal lesions (Kedar et al., 2007).
Nonorganic (Functional) Visual Disturbances
Nonorganic (functional) visual disturbance can occupy up to 5% of an ophthalmologist’s practice. Nonorganic visual disturbances can have a variety of manifestations (Box 36.3) and, like other nonorganic conditions, can be a challenge to diagnose and manage (Friedman et al., 2010).
Diagnostic Techniques
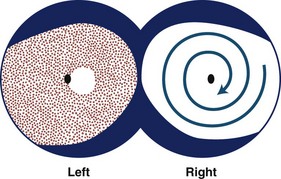
Testing of visual fields is extremely important in patients with suspected nonorganic visual loss and may reveal one of several patterns including tubular constriction, cloverleaf constriction, spiraling isopters, crossing isopters, or inverted isopters (Fig. 36.12). Confrontation or tangent screen testing done at different distances can be useful in evaluating a patient with visual field constriction, since the visual field area should increase as the distance between the patient and clinician increases. Lack of expansion of the visual field area with an increasing distance from the clinician suggests nonorganic visual field constriction (see Fig. 36.4). Cloverleaf constriction is best detected on automated static perimetry and cannot be produced by organic disease. Spiraling, crossing, and inversion of isopters can be detected using kinetic perimetry and cannot be produced by organic disease. Kinetic perimetry with both eyes opened in a patient with suspected nonorganic monocular visual loss may demonstrate nonphysiologic visual field constriction on the side of the reported visual loss.
Pediatric Afferent Neuro-ophthalmology
Examination techniques for children with visual loss often differ from those for adults, and the reader is referred to a more comprehensive discussion of the pediatric neuro-ophthalmological examination (Brodsky et al., 1996). Many of the same diseases that affect vision in adults can also affect children, but some are specific to children or present during growth and development (Box 36.4). Optic nerve hypoplasia, Leber congenital amaurosis, and albinism are discussed here in more detail.
Optic Nerve Hypoplasia
Optic nerve hypoplasia is a congenital, nonprogressive condition that may result from a defect in differentiation of the retinal ganglion cell axons. The optic disc and scleral canal are of subnormal diameter, and a peripapillary ring of pigmentation is often present (the double-ring sign) (see Chapter 15). The hypoplasia can be diffuse or segmental, as in superior segmental hypoplasia, which is occasionally seen in children born to insulin-dependent diabetic mothers.
Patients with bilateral optic nerve hypoplasia often have strabismus, nystagmus, endocrine disturbances, and developmental delay. The common endocrine disturbances include hypothyroidism, growth hormone deficiency, diabetes insipidus, and neonatal hypoglycemia. Bilateral optic nerve hypoplasia in association with absence of the septum pellucidum and hypopituitarism is called septo-optic dysplasia or de Morsier syndrome; these patients often have associated nystagmus. Optic nerve hypoplasia is usually sporadic but may be associated with fetal alcohol syndrome, maternal diabetes, or maternal anticonvulsant ingestion during pregnancy. Mutations in the HESX1 homeobox gene may play a role in some cases of septo-optic dysplasia (Dattani et al., 2000).
Leber Congenital Amaurosis
Leber congenital amaurosis (LCA) is most broadly defined as a syndrome of bilaterally poor vision beginning in early childhood, which is associated with depressed or absent signal on the electroretinogram. It is estimated to be the cause of 10% to 18% of cases of childhood blindness and is not related to Leber hereditary optic neuropathy (see Chapters 14 and 15). Visual acuity in LCA is usually less than 20/200 (6/60 metric). The retinal pigment epithelium has a salt-and-pepper appearance, and retinal vascular attenuation is sometimes marked. Optic disc swelling may be present. Pathological changes in the retina are variable and can affect all layers or just the ganglion cell layer. Nystagmus occurs in approximately 75% of patients, and ocular motor apraxia or other eye movement problems may be present. Approximately 30% of patients have associated neurological abnormalities that include hyperkinetic behavior, poor coordination, spastic paraparesis, psychomotor retardation, and hydrocephalus. Some patients can exhibit the oculodigital phenomenon (eye gouging), which is thought to be an attempt to produce phosphenes from mechanical stimulation of the retina. The condition is usually sporadic but is inherited as an autosomal recessive trait in approximately a third of cases. Mutations in the retinal RPE65 gene appear to be the cause in some cases (Pang et al., 2005). Gene therapy shows promise for improving vision and alleviating nystagmus in patients with this syndrome (Bennicelli et al., 2008).
Albinism
In albinos, approximately 20% of axons from the temporal retina cross at the optic chiasm, so the ratio of crossed axons to uncrossed axons is higher than in normal persons (Brodsky et al., 1996). This crossing asymmetry has been confirmed by VEP studies that demonstrate significant hemispheric asymmetry to monocular stimulation. Not surprisingly, many albinos have abnormal stereopsis. Albinism is also associated with Hermansky-Pudlak syndrome, in which there is associated hemorrhagic diathesis, and Chédiak-Higashi syndrome, in which there is associated immune system dysfunction with recurrent pyogenic infections.
Almog Y., Nemet A. The correlation between visual acuity and color vision as an indicator of the cause of visual loss. Am J Ophthalmol. 2010;149:1000-1004.
Bennicelli J., Wright J.F., Komaromy A., et al. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458-465.
Brodsky M.C., Baker R.S., Hamed L.M. Pediatric Neuro-ophthalmology. New York: Springer; 1996.
Dattani M.L., Martinez-Barbera J., Thomas P.Q., et al. Molecular genetics of septo-optic dysplasia. Horm Res. 2000;53(Suppl 1):26-33.
Friedman J.H., LaFrance W.C. Psychogenic disorders: the need to speak plainly. Arch Neurol. 2010;67:753-755.
Kedar S., Zhang X., Lynn M.J., et al. Congruency in homonymous hemianopia. Am J Ophthalmol. 2007;143:772-780.
Pang J.J., Chang B., Hawes N.L. Retinal degeneration 12 (RD12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis. 2005;11:152-162.
Trobe J.D. The Neurology of Vision. New York: Oxford University Press; 2001.
Wall M., Johnson C.A. Principles and techniques of the examination of the visual sensory system. In: Miller N.R., Newman N.J., Biousse V., et al. Walsh and Hoyt’s Clinical Neuro-ophthalmology. sixth ed. Philadelphia: Lippincott Williams and Wilkins; 2005:83-149.
Zeki S., Ffytche D.H. The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain. 1998;121:25-45.