39 Neuraxial Block Anatomy
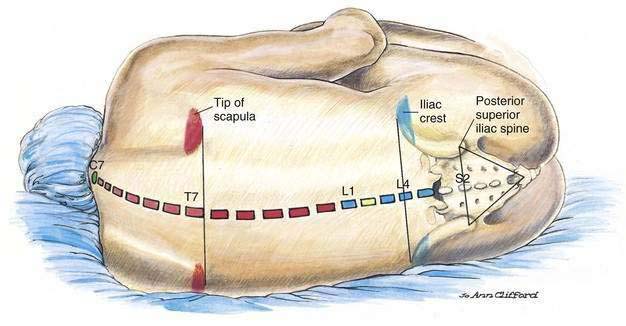
To understand the neuraxial anatomy it is necessary to develop a concept of the relationship between surface and bony anatomy pertinent to the neuraxial structures (Fig. 39-1). Beginning cephalad, the spinous process of the seventh cervical vertebra, the vertebral prominence, is the most prominent midline structure at the base of the neck. A line drawn between the lower borders of the scapula crosses the vertebral axis at approximately the spinous process of T7. The lower extent of the spinal cord, the conus medullaris, ends in the adult at approximately L1. (In the infant the conus medullaris may extend to L3.) The line between the iliac crests (Tuffier’s or the intercrestal line) most often crosses through the spinous process of L4. A line drawn between the posterior superior iliac spines identifies the level of the second sacral vertebra and the caudal extent of the dural sac containing cerebrospinal fluid (CSF).
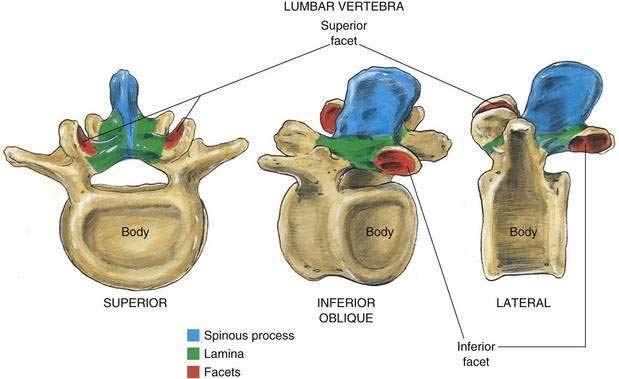
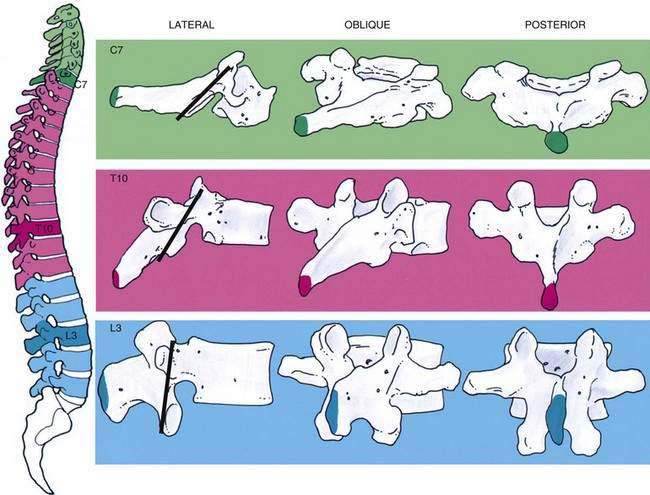
The 33 vertebrae from C1 to the tip of the coccyx have a number of common features as well as differences that should be highlighted. Each vertebra contains a spinous process joined to the lamina, from which a transverse process extends laterally into both lamina and pedicle. The pedicle joins this posterior assembly to the vertebral body, which relates to the neighboring vertebral bodies through both superior and inferior facet joints (Fig. 39-2). Figure 39-3 outlines the general relationship of these structures at levels that correspond to common sites for cervical, thoracic, and lumbar punctures of the neuraxis.
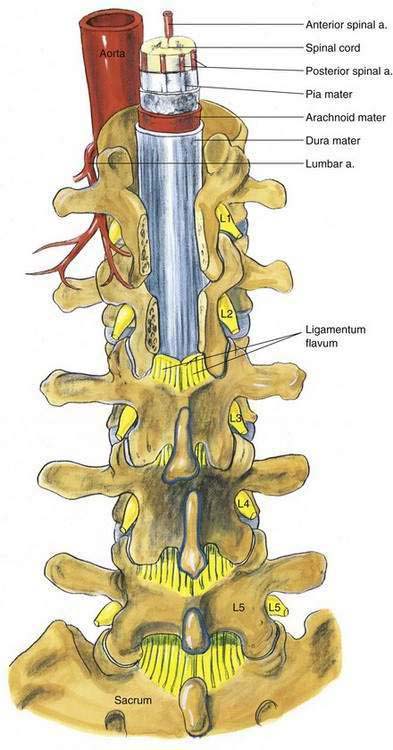
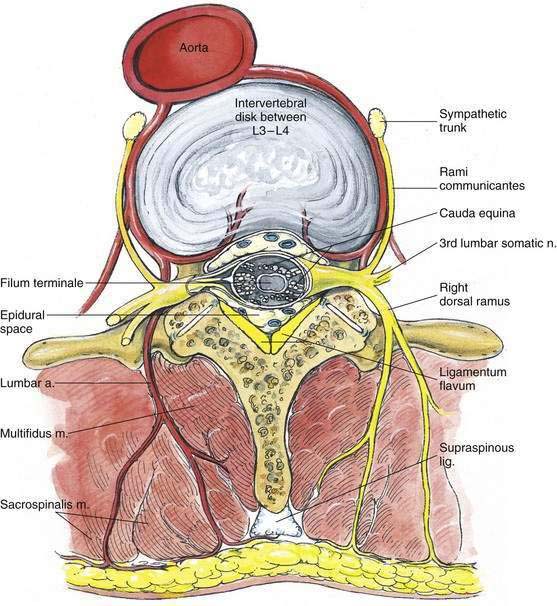
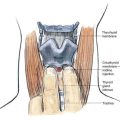
In addition to the bony relationships of the vertebral bodies, there are important ligamentous relationships. As illustrated in Figure 39-4, defining the posterior limit of the epidural space is the ligamentum flavum, or “yellow ligament.” This ligament extends from the foramen magnum to the sacral hiatus. Although classically portrayed as a single ligament, it is really composed of two ligamenta flava, the right and the left, which join in the midline. The ligamentum flavum is not uniform from skull to sacrum or even within an intervertebral space. Within an individual intervertebral space, the ligamentum flavum is thicker caudally than cephalad and thicker in the midline than on its lateral borders. Immediately posterior to the ligamentum flavum are either the lamina and spinous processes of the vertebral body, or the interspinous ligament. Extending from the external occipital protuberance to the coccyx posterior to these structures is the supraspinous ligament, which joins the vertebral spinous processes.
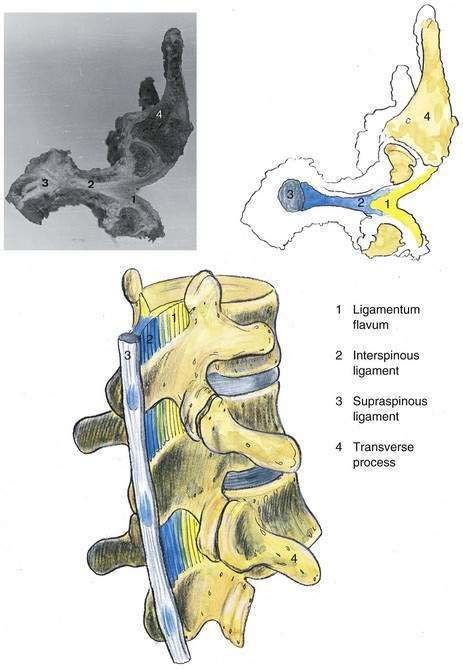
Figure 39-4. Neuraxial anatomy: lumbar vertebral ligaments.
(From Zarzur E. Anatomic studies of the human lumbar ligamentum flavum. Anesth Analg. 1984;63:499-502, with permission.)
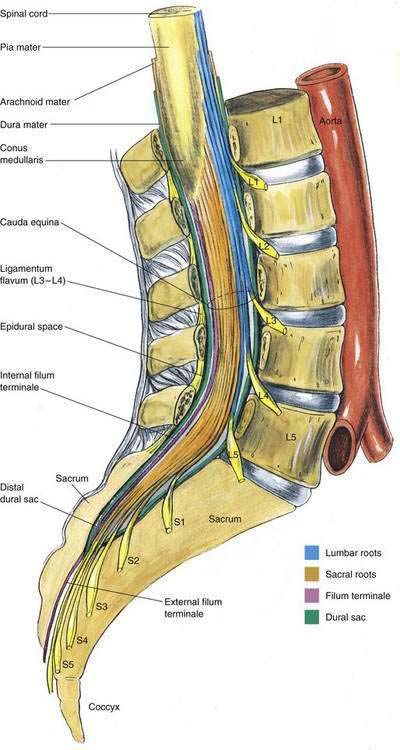
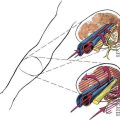
Most neuraxial blocks are performed in the lumbar region. Figures 39-5, 39-6, and 39-7 illustrate the lumbar anatomy in the posterior, lateral, and horizontal planes, respectively. Surrounding the spinal cord in the bony vertebral column are three membranes. From the immediate overlay of the cord to the periphery, these are the pia mater, the arachnoid mater, and the dura mater. The pia mater is a highly vascular membrane that closely invests the spinal cord. The arachnoid mater is a delicate, nonvascular membrane that is closely attached to the outermost layer, the dura mater. Between the pia mater and the arachnoid mater is the space of interest in spinal anesthesia, the subarachnoid space. In this space are the CSF, spinal nerves, a trabecular network between the two membranes, blood vessels that supply the spinal cord, and the lateral extensions of the pia mater, the dentate ligaments. These dentate ligaments supply lateral support from the spinal cord to the dura mater and may become important conceptually when unilateral or patchy spinal anesthesia results from what appears to be a technically adequate block. The third and outermost membrane in the spinal canal is the longitudinally organized fibroelastic membrane called the dura mater (or theca). This layer is the direct extension of the cranial dura mater and extends as spinal dura mater from the foramen magnum to S2, where the filum terminale (an extension of the pia mater beginning at the conus medullaris) blends with the periosteum on the coccyx (see Fig. 39-6). There is a potential space between the dura mater and the arachnoid, the subdural space, which contains only small amounts of serous fluid to allow the dura and arachnoid to move over each other. This space is not intentionally used by anesthesiologists, although injection into it during spinal anesthesia may explain the occasional failed spinal anesthetic and the rare “total spinal” after epidural anesthesia, when there was no indication of errant injection of the local anesthetic into the CSF.
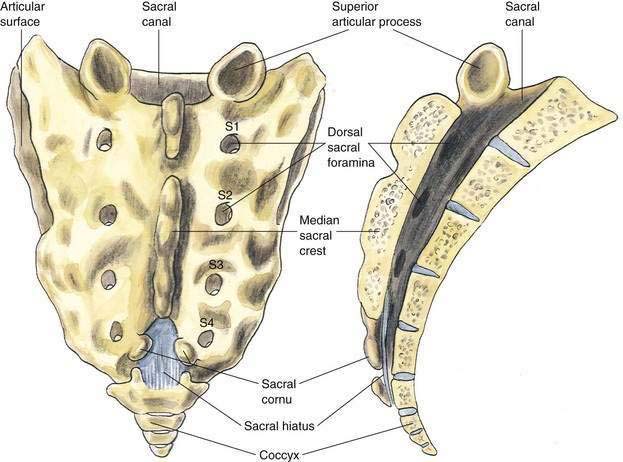
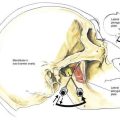
The anatomy important during caudal anesthesia is an extension of the epidural anatomy, although the frequent variations in sacral anatomy deserve emphasis. The sacrum results from the fusion of the five sacral vertebrae, whereas the sacral hiatus results from the failure of the laminae of S5 and usually part of S4 to fuse in the midline. The sacral hiatus results in a variably shaped and sized, inverted “V”-shaped bony defect, covered by the posterior sacrococcygeal ligament, which is a functional counterpart to the ligamentum flavum (Fig. 39-8). The hiatus may be identified by locating the sacral cornu (remnants of the S5 articular processes). This bony defect allows percutaneous access to the sacral canal, although the frequent anatomic variation of the sacral hiatus can make caudal block confusing. The sacral canal is functionally the distal extent of the epidural space, and from this canal the pelvic sacral foramina open ventrally toward the ischial rectal fossa, whereas the dorsal sacral foramina open in a posterior direction (see Fig. 39-8). In the sacral canal, the nerves of the cauda equina continue until they exit through their respective vertebral foramina. Once again, the dural sac continues to the level of S2, or the line joining the posterior superior iliac spines.