Chapter 93 Nervous System Disorders
93.1 The Cranium
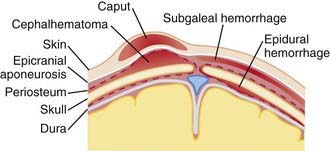
Erythema, abrasions, ecchymoses, and subcutaneous fat necrosis of facial or scalp soft tissues may be noted after a normal delivery or after forceps or vacuum-assisted deliveries. Their location depends on the area of contact with the pelvic bones or of application of the forceps. Traumatic hemorrhage may involve any layer of the scalp as well as intracranial contents (Fig. 93-1).
Caput succedaneum is a diffuse, sometimes ecchymotic, edematous swelling of the soft tissues of the scalp involving the area presenting during vertex delivery (see Fig. 93-1). It may extend across the midline and across suture lines. The edema disappears within the 1st few days of life. Molding of the head and overriding of the parietal bones are frequently associated with caput succedaneum and become more evident after the caput has receded; they disappear during the 1st weeks of life. Rarely, a hemorrhagic caput may result in shock and require blood transfusion. Analogous swelling, discoloration, and distortion of the face are seen in face presentations. No specific treatment is needed, but if extensive ecchymoses are present, hyperbilirubinemia may develop.
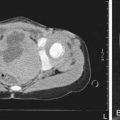
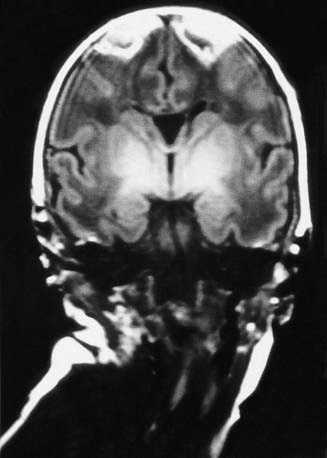
Cephalohematoma (Fig. 93-2) is a subperiosteal hemorrhage, hence always limited to the surface of one cranial bone. Cephalohematomas occur in 1-2% of live births. No discoloration of the overlying scalp occurs, and swelling is not usually visible for several hours after birth because subperiosteal bleeding is a slow process. The lesion becomes a firm tense mass with a palpable rim localized over one area of the skull. Most cephalohematomas are resorbed within 2 wk-3 mo, depending on their size. They may begin to calcify by the end of the 2nd week. A few remain for years as bony protuberances and are detectable on radiographs as widening of the diploic space; cystlike defects may persist for months or years. An underlying skull fracture, usually linear and not depressed, may be associated with 10-25% of cases. A sensation of central depression suggesting but not indicative of an underlying fracture or bony defect is usually encountered on palpation of the organized rim of a cephalohematoma. Cephalohematomas require no treatment, although phototherapy may be necessary to treat hyperbilirubinemia. Infection of the hematoma is a very rare complication.
93.3 Intracranial-Intraventricular Hemorrhage and Periventricular Leukomalacia
Clinical Manifestations
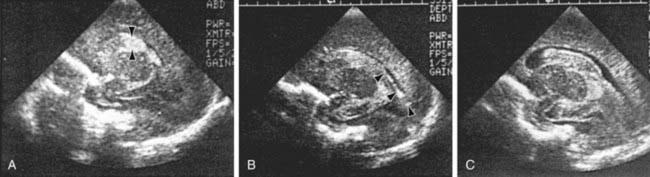
The severity of hemorrhage may be defined on CT scans by the location and degree of ventricular dilatation. In a grade I hemorrhage, bleeding is isolated to the subependymal area. In Grade II hemorrhage, there is bleeding within the ventricle but without evidence of ventricular dilatation. Grade III hemorrhage consists of IVH with ventricular dilatation. In Grade IV hemorrhage, there is intraventricular and parenchymal hemorrhage. Another grading system describes 3 levels of increasing severity of IVH detected on ultrasound: In grade I, bleeding is confined to the germinal matrix–subependymal region or to <10% of the ventricle (≈35% of IVH cases); grade II is defined as intraventricular bleeding with 10-50% filling of the ventricle (≈40% of IVH cases) and in grade III, more than 50% of the ventricle is involved, with dilated ventricles (Fig. 93-3). Ventriculomegaly is defined as mild (0.5-1 cm), moderate (1.0-1.5 cm), or severe (>1.5 cm).
Prognosis
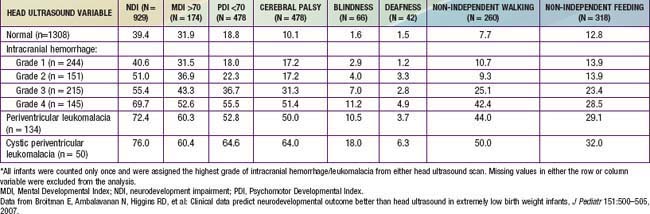
The degree of IVH and the presence of PVL are strongly linked to neurodevelopmental impairment. For infants with birthweight <1,000 g, the incidences of severe neurologic impairment (defined as mental developmental index <70, psychomotor development index <70, cerebral palsy, blindness, or deafness) are about 50%, 55%, and 70% for infants with grade II, grade III, and grade IV IVH, respectively (Table 93-1). In contrast, the rate of neurodevelopmental impairment is approximately 40% in infants without IVH and those with grade I IVH. PVL, cystic PVL, and progressive hydrocephalus requiring shunt insertion are each independently associated with a poorer prognosis.
Accardo J, Kammann H, Hoon AHJr. Neuroimaging in cerebral palsy. J Pediatr. 2004;145:S19-S27.
Adams-Chapman I, Hansen NI, Stoll BJ, et al. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:167-177.
Armstrong-Wells J, Johnston SC, Wu YW, et al. Prevalence and predictors of perinatal hemorrhagic stroke: results from the Kaiser pediatric stroke study. Pediatrics. 2009;123:823-828.
Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1-8.
Bassan H, Limperopoulos C, Visconti K, et al. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120:785-792.
Broitman E, Ambalavanan N, Higgins RD, et al. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr. 2007;151:500-505.
Brouwer A, Groenendaal F, Van Haastert IL, et al. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648-654.
Fowlie PW, Davis PG: Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants, Cochrane Database of Systematic Reviews (3):CD000174, 2002.
Heuchan AM, Evans N, Henderson DJ, et al. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand neonatal network, 1995–1997. Arch Dis Child. 2002;86:F86-F90.
Maunu J, Ekholm E, Parkkola R, et al. Antenatal Doppler measurements and early brain injury in very low birthweight infants. J Pediatr. 2007;150:51-56.
Roze E, Van Braeckel KNJA, van der Veere CH, et al. Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2009;123:1493-1500.
Saigal S, Stoskopf BL, Streiner DL, et al. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108:407-415.
Schmidt B, Davis P, Moddemann PD, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966-1972.
Soul JS, Eichenwald E, Walter G, et al. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvements in cerebral hemodynamics. Pediatr Res. 2004;55:872-876.
Vincer MJ, Allen AC, Joseph KS, et al. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics. 2006;118:e1621-e1626.
Whitelaw A: Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage, Cochrane Database Syst Rev (1):CD000216, 2002.
93.4 Brain Injury from Inflammation, Infection, and Medications
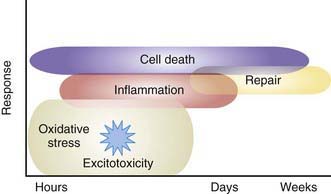
Severe IVH and PVL are the most commonly associated risk factors for adverse outcome in the VLBW infant. Other factors are also involved in the etiology of perinatal brain injury. Cytokines and prenatal or postnatal infection or inflammation may contribute to brain injury. A systemic inflammatory response syndrome in the mother, fetus, or infant may induce the production of various inflammatory mediators that are directly cytotoxic or cause decreased CNS perfusion (Fig. 93-4). Preterm infants with evidence (often subclinical) of intrauterine or postnatal infection or maternal chorioamnionitis are more likely than uninfected infants to have adverse neurodevelopmental outcome including cerebral palsy.
In utero infections may involve the developing CNS and directly impair cell growth or produce cell neurosis, resulting in microcephaly, developmental delay, mental retardation, or cerebral palsy. These specific congenital or perinatal acquired infections include those due to cytomegalovirus (Chapter 247), toxoplasmosis (Chapter 282), herpes simplex (Chapter 244), syphilis (Chapter 210), rubella (Chapter 239), and human immunodeficiency virus (Chapter 268). Postnatal acquired bacterial meningitis in the 1st year, but even more so in the 1st month of life, is another major risk factor for CNS injury and associated adverse neurodevelopmental outcome (Chapter 595).
Necrotizing enterocolitis (NEC) affects approximately 9-14% of LBW infants and is associated with significant morbidity and mortality (Chapter 96.2). Patients with NEC requiring surgery are more likely to have Mental Developmental Index (MDI) scores <70, Psychomotor Developmental Index (PDI) scores <70, and evidence of overall neurodevelopmental impairment. Infants with severe NEC are reported to have a higher incidence of PVL, postnatal infections, and poor growth.
AAP Committee of the Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330-338.
Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985-1995.
Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453-460.
Pierrat V, Haiuari N, Liska A, et al. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child. 2005;90:F257-F261.
Stark AR, Carlo WA, Tyson JE, et al. Adverse effects of early dexamethasone treatment in extremely low birth weight infants. N Engl J Med. 2001;344:95-101.
Wood NS, Costeloe K, Gibson AT, et al. The EPICure Study: Associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134-F140.
Wu YW, Colford JMJr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417-1424.
Yang SH, Choi SJ, Roh CR, et al. Multiple courses of antenatal corticosteroid therapy in patients with preterm premature rupture of membranes. J Perinat Med. 2004;32:42-48.
Yeh TF, Lin YJ, Hung C, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304-1313.
93.5 Hypoxic-Ischemic Encephalopathy
Anoxia is a term used to indicate the consequences of complete lack of oxygen as a result of a number of primary causes. Hypoxemia refers to decreased arterial concentration of oxygen. Hypoxia refers to a decreased oxygenation to cells or organs. Ischemia refers to blood flow to cells or organs that is insufficient to maintain their normal function. Hypoxic-ischemic encephalopathy (HIE) is an important cause of permanent damage to CNS tissues that may result in neonatal death or manifest later as cerebral palsy or developmental delay. About 20-30% of infants with HIE die in the neonatal period, and ≈ 33-50% of survivors are left with permanent neurodevelopmental abnormalities (cerebral palsy, mental retardation). The greatest risk of adverse outcome is seen in infants with severe fetal acidosis (pH <6.7) (90% death/impairment) and a base deficit >25 mmol/L (72% mortality). Multiorgan failure and insult can occur (Table 93-2).
| SYSTEM | EFFECT(S) |
|---|---|
| Central nervous system | Hypoxic-ischemic encephalopathy, infarction, intracranial hemorrhage, seizures, cerebral edema, hypotonia, hypertonia |
| Cardiovascular | Myocardial ischemia, poor contractility, cardiac stunning, tricuspid insufficiency, hypotension |
| Pulmonary | Pulmonary hypertension, pulmonary hemorrhage, respiratory distress syndrome |
| Renal | Acute tubular or cortical necrosis |
| Adrenal | Adrenal hemorrhage |
| Gastrointestinal | Perforation, ulceration with hemorrhage, necrosis |
| Metabolic | Inappropriate secretion of antidiuretic hormone, hyponatremia, hypoglycemia, hypocalcemia, myoglobinuria |
| Integument | Subcutaneous fat necrosis |
| Hematology | Disseminated intravascular coagulation |
Etiology
Placental insufficiency often remains undetected on clinical assessment. Intrauterine growth restriction may develop in chronically hypoxic fetuses without the traditional signs of fetal distress. Doppler umbilical waveform velocimetry (demonstrating increased fetal vascular resistance) and cordocentesis (demonstrating fetal hypoxia and lactic acidosis) identify a chronically hypoxic infant (Chapter 90). Uterine contractions may further reduce umbilical oxygenation, depressing the fetal cardiovascular system and CNS and resulting in low Apgar scores and respiratory depression at birth.
Pathophysiology and Pathology
The pathology of hypoxia-ischemia depends on the affected organ and the severity of the injury. Early congestion, fluid leak from increased capillary permeability, and endothelial cell swelling may then lead to signs of coagulation necrosis and cell death. Congestion and petechiae are seen in the pericardium, pleura, thymus, heart, adrenals, and meninges. Prolonged intrauterine hypoxia may result in inadequate perfusion of the periventricular white matter, resulting, in turn, in PVL. Pulmonary arteriole smooth muscle hyperplasia may develop, which predisposes the infant to pulmonary hypertension (Chapter 95.7). If fetal distress produces gasping, the amniotic fluid contents (meconium, squames, lanugo) are aspirated into the trachea or lungs.
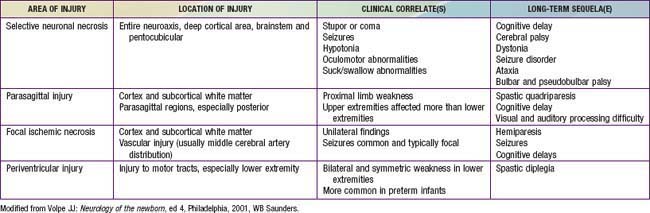
The combination of chronic fetal hypoxia and acute hypoxic-ischemic injury around the time of birth results in gestational age–specific neuropathology (Table 93-3). Term infants demonstrate neuronal necrosis of the cortex (later, cortical atrophy) and parasagittal ischemic injury. Preterm infants demonstrate PVL (later, spastic diplegia), status marmoratus of the basal ganglia, and IVH. Term more often than preterm infants have focal or multifocal cortical infarcts that manifest clinically as focal seizures and hemiplegia.
Clinical Manifestations
Intrauterine growth restriction with increased vascular resistance may be the 1st indication of fetal hypoxia. During labor, the fetal heart rate slows and beat-to-beat variability declines. Continuous heart rate recording may reveal a variable or late deceleration pattern (see Fig. 90-4). Particularly in infants near term, these signs should lead to the administration of high concentrations of oxygen to the mother and consideration of immediate delivery to avoid fetal death and CNS damage.
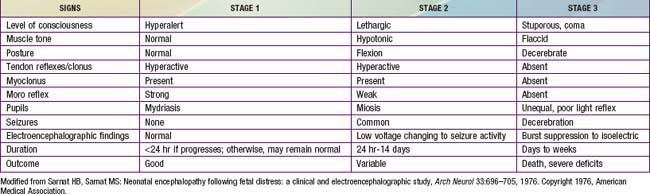
At delivery, the presence of meconium-stained amniotic fluid is evidence that fetal distress has occurred. At birth, affected infants may be depressed and may fail to breathe spontaneously. During the ensuing hours, they may remain hypotonic or change from a hypotonic to a hypertonic state, or their tone may appear normal (Tables 93-4 and 93-5). Pallor, cyanosis, apnea, a slow heart rate, and unresponsiveness to stimulation are also signs of HIE. Cerebral edema may develop during the next 24 hr and result in profound brainstem depression. During this time, seizure activity may occur; it may be severe and refractory to the usual doses of anticonvulsants. Though most often a result of the HIE, seizures in asphyxiated newborns may also be due to hypocalcemia, hypoglycemia, or infection.
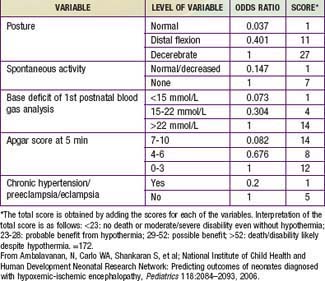
Table 93-4 PREDICTOR VARIABLES, ODDS RATIOS, AND SCORES ASSIGNED TO EACH VARIABLE FOR DEATH/DISABILITY SCORING IN INFANTS WITH HYPOXIC-ISCHEMIC ENCEPHALOPATHY
In addition to CNS dysfunction, heart failure and cardiogenic shock, persistent pulmonary hypertension, respiratory distress syndrome, gastrointestinal perforation, hematuria, and acute tubular necrosis are associated with perinatal asphyxia secondary to inadequate perfusion (see Table 93-2).
The severity of neonatal encephalopathy depends on the duration and timing of injury. Symptoms develop over a series of days, making it important to perform serial neurologic examinations (see Tables 93-4 and 93-5). During the initial hours after an insult, infants have a depressed level of consciousness. Periodic breathing with apnea or bradycardia is present, but cranial nerve functions are often spared with intact pupillary responses and spontaneous eye movement. Seizures are common with extensive injury. Hypotonia is also common as an early manifestation.
Diagnosis
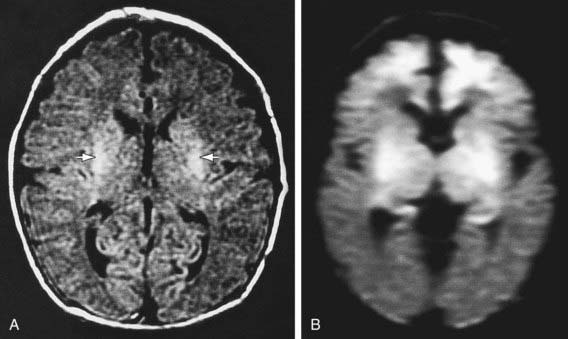
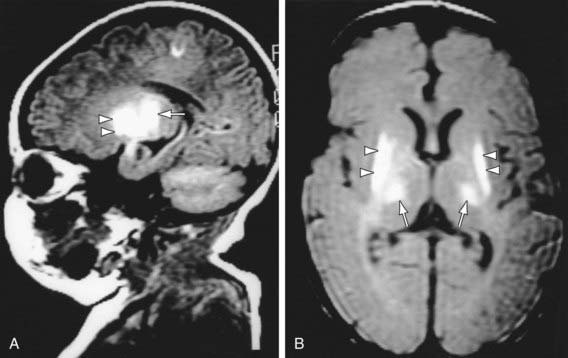
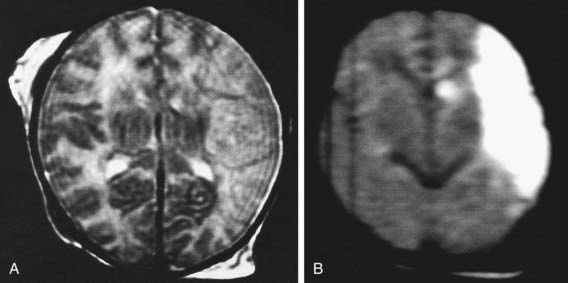
Diffusion-weighted MRI is the preferred imaging modality in neonates with HIE because of its increased sensitivity and specificity early in the process and its ability to outline the topography of the lesion (Figs. 93-5 to 93-8). CT scans are helpful in identifying focal hemorrhagic lesions, diffuse cortical injury, and damage to the basal ganglia; CT has limited ability to identify cortical injury during the 1st few days of life. Ultrasonography has limited utility in evaluation of hypoxic injury in the term infant; it is the preferred modality in evaluation of the preterm infant.
Prognosis
The outcome of HIE, which correlates with the timing and severity of the insult, ranges from complete recovery to death. The prognosis varies depending on the severity of the insult and the treatment. Infants with initial cord or initial blood pH <6.7 have a 90% risk for death or severe neurodevelopmental impairment at 18 mo of age. In addition, infants with Apgar scores of 0-3 at 5 min, high base deficit (>20-25 mmol/L), decerebrate posture, and lack of spontaneous activity are also at increased risk for death or impairment. These predictor variables can be combined to determine a score that helps with prognosis (see Table 93-4). Infants with the highest risk are likely to die or have severe disability despite aggressive treatment including hypothermia. Those with intermediate scores are likely to benefit from treatment. In general, severe encephalopathy, characterized by flaccid coma, apnea, absence of oculocephalic reflexes, and refractory seizures, is associated with a poor prognosis (see Table 93-5). A low Apgar score at 20 min, absence of spontaneous respirations at 20 min of age, and persistence of abnormal neurologic signs at 2 wk of age also predict death or severe cognitive and motor deficits. The combined use of early EEG and MRI is useful in predicting outcome in term infants with HIE. Normal MRI and EEG findings are associated with a good recovery, whereas severe MRI and EEG abnormalities predict a poor outcome. Microcephaly and poor head growth during the 1st year of life also correlate with injury to the basal ganglia and white matter and adverse developmental outcome at 12 mo. All survivors of moderate to severe encephalopathy require comprehensive high-risk medical and developmental follow-up. Early identification of neurodevelopmental problems allows prompt referral for developmental, rehabilitative, neurologic care, and early intervention services so that the best possible outcome can be achieved.
Brain death after neonatal HIE is diagnosed from the clinical findings of coma unresponsive to pain, auditory, or visual stimulation; apnea with PCO2 rising from 40 to >60 mm Hg without ventilatory support; and absence of brainstem reflexes (pupillary, oculocephalic, oculovestibular, corneal, gag, sucking) (Chapter 63.1). These findings must occur in the absence of hypothermia, hypotension, and elevations of depressant drugs (phenobarbital). An absence of cerebral blood flow on radionuclide scans and of electrical activity on EEG (electrocerebral silence) is inconsistently observed in clinically brain-dead neonatal infants. Persistence of the clinical criteria for 2 days in term infants and 3 days in preterm infants predicts brain death in most asphyxiated newborns. Nonetheless, no universal agreement has been reached regarding the definition of neonatal brain death. Consideration of withdrawal of life support should include discussions with the family, the health care team, and, if there is disagreement, an ethics committee. The best interest of the infant involves judgments about the benefits and harm of continuing therapy or avoiding ongoing futile therapy.
Ambalavanan N, Carlo WA, Shankaran S, et al. Predicting outcomes of neonates diagnosed with hypoxic-ischemic encephalopathy. Pediatrics. 2006;118:2084-2093.
Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349-1358.
Bonifacio SI, Glass HC, Vanderpluym J, et al. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011;158:360-365.
Chau V, Poskitt KJ, Sargent MA, et al. Comparison of computer tomography and magnetic resonance imaging scans on the third day of life in term newborns with neonatal encephalopathy. Pediatrics. 2009;123:319-326.
Davis AS, Hintz SR, Van Meurs KP, et al. Seizures in extremely low birth weight infants are associated with adverse outcome. J Pediatr. 2010;157:720-725.
De Vries LS, Hellstrom-Westas. Role of cerebral function monitoring in the newborn. Arch Dis Child Fetal Neonatal Ed. 2005;90:F201-F207.
Edwards AD. Hypothermic neural rescue: work continues. J Pediatr. 2010;157(3):351-352.
Glass HC, Glidden D, Jeremy RJ, et al. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318-323.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling and mild systemic hypothermia after neonatal encephalopathy: multicenter randomized trial. Lancet. 2005;365:663-670.
Gray J, Geva A, Zheng Z, et al. CoolSim: Using industrial modeling techniques to examine the impact of selective head cooling in a model of perinatal regionalization. Pediatrics. 2008;121:28-36.
Groenendaal F, De Vooght KMD, van Bel F. Blood gas values during hypothermia in asphyxiated term neonates. Pediatrics. 2009;123:170-172.
Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55-58.
Hall RT, Hall FK, Daily DK. High-dose phenobarbital therapy in term newborn infants with severe perinatal asphyxia: a randomized, prospective study with three-year follow-up. J Pediatr. 1998;132:345-348.
Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:34F-38F.
Jacobs SE, Hunt R, Tarnow-Mordi WO, et al: Cooling for newborns with hypoxic ischaemic encephalopathy, Cochrane Database Syst Rev (17):CD003311, 2007.
Laptook AR, Shankaran S, Ambalavanan N, et al. Outcome of term infants using Apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619-1626.
Levene MI. Cool treatment for birth asphyxia, but what’s next? Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F154-F156.
Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res. 2009;66:222-229.
Murray DM, Boylan GB, Ryan CA, et al. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459-e467.
Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574-1584.
Shankaran S, Pappas A, Laptook AR, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791-e798.
Toet MC, Hellstrom-Westas L, Broenendaal F, et al. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 1999;81:19F-23F.
93.6 Spine and Spinal Cord
MacKinnon JA, Perlman M, Kirpalani H, et al. Spinal cord injury at birth: diagnostic and prognostic data in 22 patients. J Pediatr. 1993;122:431-437.
Mills JF, Dargaville PA, Coleman LT, et al. Upper cervical spinal cord injury in neonates: the use of magnetic resonance imaging. J Pediatr. 2001;138:105-108.
93.7 Peripheral Nerve Injuries
Brachial Palsy
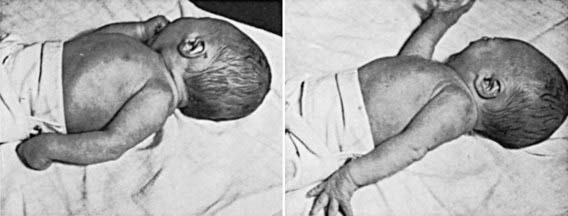
Brachial plexus injury is a common problem, with an incidence of 0.6-4.6/1,000 live births. Injury to the brachial plexus may cause paralysis of the upper part of the arm with or without paralysis of the forearm or hand or, more commonly, paralysis of the entire arm. These injuries occur in macrosomic infants and when lateral traction is exerted on the head and neck during delivery of the shoulder in a vertex presentation, when the arms are extended over the head in a breech presentation, or when excessive traction is placed on the shoulders. Approximately 45% of brachial plexus injuries are associated with shoulder dystocia. In Erb-Duchenne paralysis, the injury is limited to the 5th and 6th cervical nerves. The infant loses the power to abduct the arm from the shoulder, rotate the arm externally, and supinate the forearm. The characteristic position consists of adduction and internal rotation of the arm with pronation of the forearm. Power to extend the forearm is retained, but the biceps reflex is absent; the Moro reflex is absent on the affected side (Fig. 93-9). The outer aspect of the arm may have some sensory impairment. Power in the forearm and hand grasps is preserved unless the lower part of the plexus is also injured; the presence of hand grasp is a favorable prognostic sign. When the injury includes the phrenic nerve, alteration in diaphragmatic excursion may be observed with ultrasonography or fluoroscopy. Klumpke paralysis is a rare form of brachial palsy, in which injury to the 7th and 8th cervical nerves and the 1st thoracic nerve produces a paralyzed hand and ipsilateral ptosis and miosis (Horner syndrome) if the sympathetic fibers of the 1st thoracic root are also injured. Mild cases may not be detected immediately after birth. Differentiation must be made from cerebral injury; from fracture, dislocation, or epiphyseal separation of the humerus; and from fracture of the clavicle. MRI demonstrates nerve root rupture or avulsion.
Brown T, Cupido C, Scarfone H, et al. Developmental apraxia arising from neonatal brachial plexus palsy. Neurology. 2000;55:24-30.
Hoeksma AF, Wolf H, Oei SL. Obstetrical brachial plexus injuries: incidence, natural course and shoulder contracture. Clin Rehabil. 2000;14:523-526.
Noetzel MJ, Wolpaw JR. Emerging concepts in the pathophysiology of recovery from neonatal brachial plexus injury. Neurology. 2000;55:5-6.
Strombeck C, Krumlinde-Sundholm L, Forrsberg H. Functional outcome at 5 years in children with obstetrical brachial plexus palsy with and without microsurgical reconstruction. Dev Med Child Neurol. 2000;42:148-157.

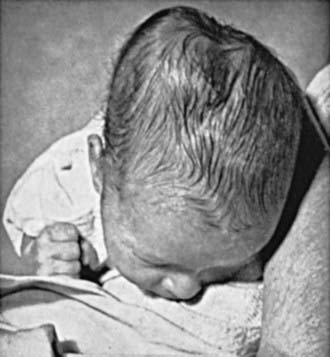
 of the face is involved. The infant also usually has other manifestations of intracranial injury, most commonly 6th nerve palsy. The prognosis depends on whether the nerve was injured by pressure or the nerve fibers were torn. Improvement occurs within a few weeks in the former instance. Care of the exposed eye is essential. Neuroplasty may be indicated when the paralysis is persistent. Facial palsy may be confused with absence of the depressor muscles of the mouth, which is a benign problem.
of the face is involved. The infant also usually has other manifestations of intracranial injury, most commonly 6th nerve palsy. The prognosis depends on whether the nerve was injured by pressure or the nerve fibers were torn. Improvement occurs within a few weeks in the former instance. Care of the exposed eye is essential. Neuroplasty may be indicated when the paralysis is persistent. Facial palsy may be confused with absence of the depressor muscles of the mouth, which is a benign problem.