14 Nerve roots
Development of the Spinal Cord
Cellular differentiation
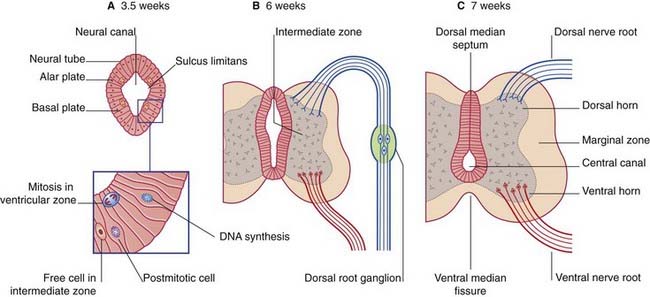
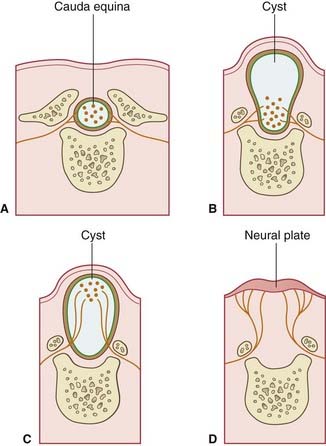
The neural tube of the embryo consists of a pseudostratified epithelium surrounding the neural canal (Figure 14.1A). Dorsal to the sulcus limitans, the epithelium forms the alar plate; ventral to the sulcus it forms the basal plate.
The microglial cells of the CNS are derived from basophil cells of the blood.
Enlargement of the intermediate zone of the alar plate creates the dorsal horn of gray matter. The dorsal horn receives central processes of dorsal root ganglion cells (Figure 14.1B). As explained in Chapter 1, the ganglion cells derive from the neural crest.
Partial occlusion of the neural canal by the developing dorsal gray horn gives rise to the dorsal median septum and to the definitive central canal of the cord (Figure 14.1C).
Enlargement of the intermediate zone of the basal plate creates the ventral gray horn and the ventral median fissure (Figure 14.1C). Axons emerge from the ventral horn and form the ventral nerve roots.
In the outermost, marginal zone of the cord, axons run to and from spinal cord and brain.
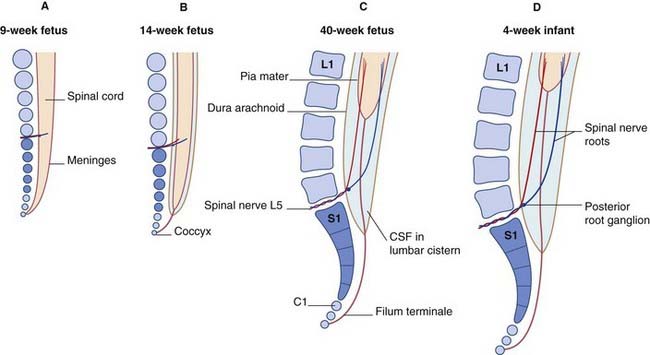
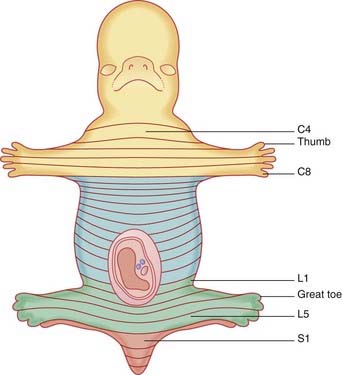
Ascent of the spinal cord (Figure 14.2)
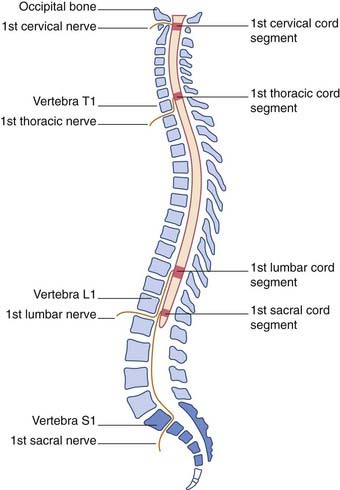
In consequence of greater ascent of the lower part of the cord compared to the upper part, the spinal nerve roots show an increasing disparity between their segmental levels of attachment to the cord and the corresponding vertebral levels (Figure 14.3).
Neural arches
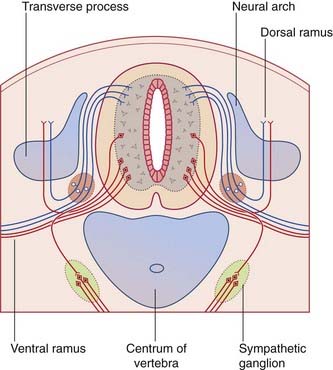
During the fifth week, the mesenchymal vertebrae surrounding the notochord give rise to neural arches for protection of the spinal cord (Figure 14.4). The arches are initially bifid (split). Later, they fuse in the midline and form the vertebral spines.
Conditions where the two halves of the neural arches have failed to unite are collectively known as spina bifida (Clinical Panel 14.1).
Clinical Panel 14.1 Spina bifida
Among the more common congenital malformations of the CNS are several conditions included under the general heading spina bifida. The ‘bifid’ effect is produced by failure of union of the two halves of the neural arches, usually in the lumbosacral region (Figure CP 14.1.1)
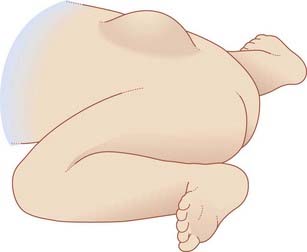
In spina bifida cystica, a meningeal cyst protrudes through the vertebral defect. In 10% of these cases, the cyst is a meningocele containing no nervous elements (B). In 90%, unfortunately, the cyst is a meningomyelocele, containing either spinal cord or cauda equina (C); the lower limbs, bladder, and rectum are paralyzed, as in the case illustrated in Figure CP 14.1.2, and meningitis is likely to supervene sooner or later. To make matters worse, an Arnold–Chiari malformation (Ch. 4) is almost always present as well.
Adult Anatomy
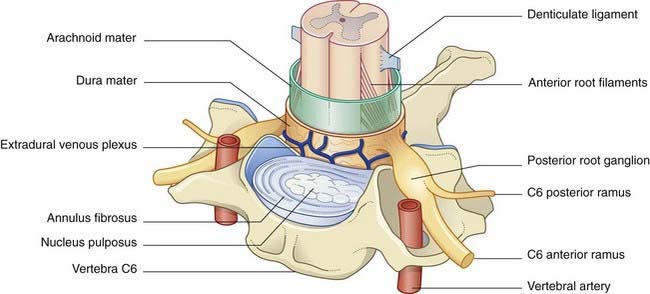
The spinal cord and nerve roots are sheathed by pia mater and float in cerebrospinal fluid contained in the subarachnoid space. The pial denticulate ligament pierces the arachnoid and anchors the cord to the dura mater on each side. Outside the dura is the extradural (epidural) venous plexus (Figure 14.5), which harvests the vertebral red marrow and empties into the segmental veins (deep cervical, intercostal, lumbar, sacral). These veins are without valves, and reflux of blood from the territory of segmental veins is a notorious cause of cancer spread from prostate, lung, breast, and thyroid gland. For example, nerve root compression from collapse of an invaded vertebra may be the presenting sign of cancer in one of these organs.
The respective anterior and posterior nerve roots join at the intervertebral foramina, where the posterior root ganglia are located (Figure 14.5). The arachnoid mater blends with the perineurium of the spinal nerve, and the dura mater blends with the epineurium. The nerve roots carry extensions of the subarachnoid space into the intervertebral foramina.
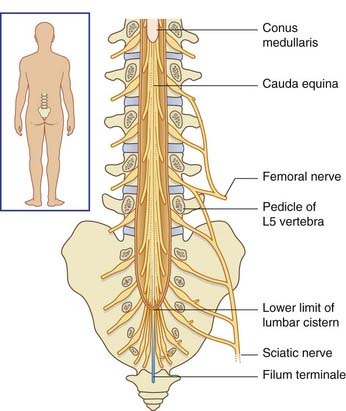
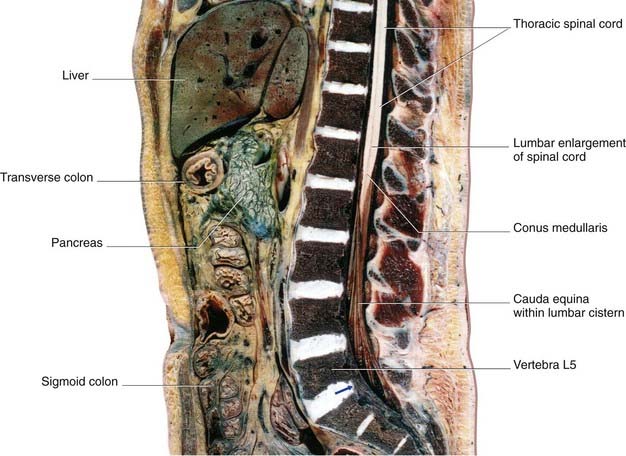
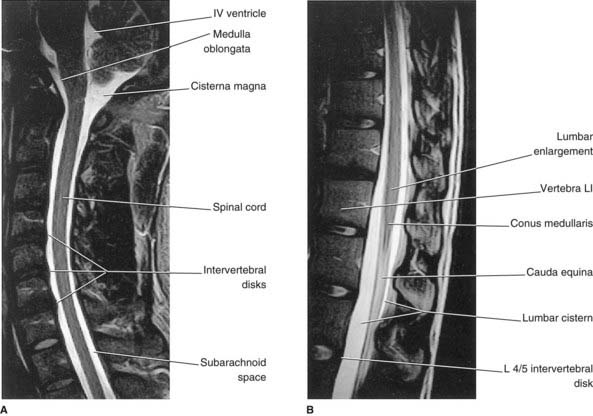
Below cord level, nerve roots seeking the lower lumbar and sacral intervertebral foramina constitute the cauda equina (‘horse’s tail’). The cauda equina floats in the lumbar subarachnoid cistern (Figures 14.6–14.8), which reaches to the level of the second sacral vertebra. At its upper end, the cauda comprises nerve roots L3–S5 of both sides – a total of 32 roots (excluding the insignificant coccygeal roots).
Distribution of Spinal Nerves
Each spinal nerve gives off a recurrent branch which provides mechanoreceptors and pain receptors for the dura mater, posterior longitudinal ligament, and intervertebral disc. The synovial facet joints between successive articular processes are each supplied by the nearest three spinal nerves. Pain caused by injury or disease of any of the above structures is referred to the cutaneous territory of the corresponding posterior rami (Figure 14.9).
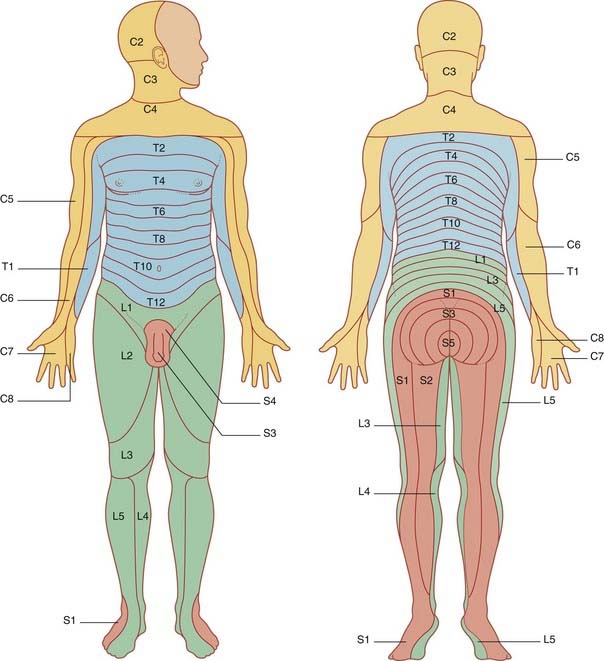
Segmental sensory distribution: the dermatomes
A dermatome is the strip of skin supplied by an individual spinal nerve. The dermatomes are orderly in the embryo (Figure 14.10) but they are distorted by outgrowth of the limbs (Figure 14.11). Spinal nerves C5–T1 are drawn into the upper limb, so that C4 dermatome abuts T2 at the level of the sternal angle. Nerves L2–S2 are drawn into the lower limb, so that L2 abuts S3 dermatome over the buttock. Maps like those in Figure 14.11 fail to portray overlap in the cutaneous distribution of successive dorsal nerve roots. On the trunk, e.g., the skin over an intercostal space is supplied by the nerves immediately above and below in addition to the proper nerve.
Segmental motor distribution
In the limbs, the individual muscles are supplied by more than one spinal nerve because of interchange in the brachial and lumbosacral plexuses. The segmental supply of the limbs is expressed in terms of movements in Figure 14.12.
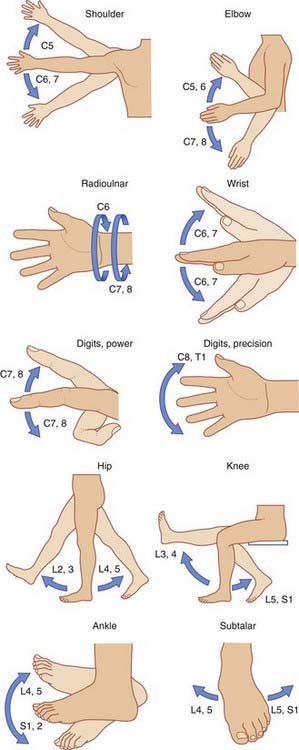
Figure 14.12 Segmental control of limb movements.
(Adapted from Last, R.J. (1973) Anatomy: Regional and Applied, 5th edn. Edinburgh: Churchill Livingstone; and Rosse, C. and Clawson, D.K. (1980) The Musculoskeletal System in Health and Disease. Hagerstown: Harper & Row.)
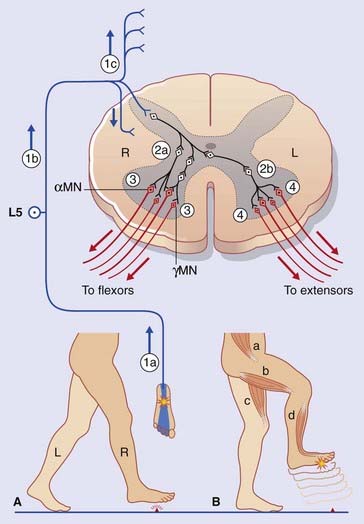
Segmental sensory inputs and segmental motor outputs are combined during execution of withdrawal or avoidance reflexes (Box 14.1). (The prevalent term, flexor reflex, is too limited; e.g. a stimulus applied to the lateral surface of a limb may elicit adduction.)
Box 14.1 Lower limb withdrawal reflex
Figure Box 14.1.1 depicts a lower limb withdrawal reflex with crossed extensor thrust. (A) The right foot is about to enter the stance phase of locomotion. (B) Contact with a sharp object initiates a withdrawal reflex, together with the crossed extensor response required to support the entire body weight.
Sequence of events
Note: Not shown in the figure are lateral spinothalamic tract relay neurons (see Ch. 15). These neurons receive inputs from nociceptive afferent fibers in Lissauer’s tract and they relay impulses to brain sites able to decode the location and nature of the initial stimulus.
Nerve root compression syndromes
Nerve root compression within the vertebral canal is most frequent where the spine is most mobile, namely at lower cervical and lower lumbar levels (Clinical Panel 14.2). The effects of root compression may be expressed in five different ways:
Clinical Panel 14.2 Nerve root compression
Cervical roots
The intervertebral disks and synovial joints of the neck are subject to degenerative disease (cervical spondylosis) in 50% of 50-year-olds and in 70% of 70-year-olds. Although any or all of the joints may deteriorate, problems are most frequent in relation to vertebra C6, which provides the fulcrum for flexion/extension movements of the neck. Spinal nerve C6 (above) or C7 (below) may be pinched by extruded disk material or by bony outgrowths (osteophytes) beside the synovial joints (Figure CP 14.2.1). Sensory, motor, and reflex disturbances may result in accordance with the data in Figures 14.10 and 14.11 and Table 14.1.
Lumbosacral roots
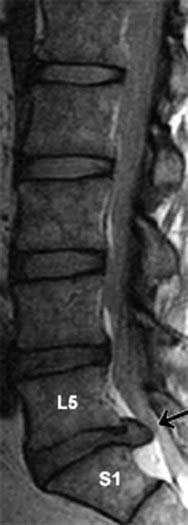
The term lumbar spinal stenosis signifies narrowing of the lumbar vertebral canal due to encroachment by a prolapsing intervertebral disk or by bony osteophytes. Fully 95% of all disk prolapses occur immediately above or below the last lumbar vertebra. The typical herniation is posterolateral, with compression of the nerve roots passing to the next intervertebral foramen (Figure CP 14.2.2).
With an L5–S1 prolapse (the commonest of all) (Figure CP 14.2.3), symptoms are felt in the back of the leg/sole of foot (S1 dermatome). Plantar flexion may be weak and the ankle jerk reduced or absent.
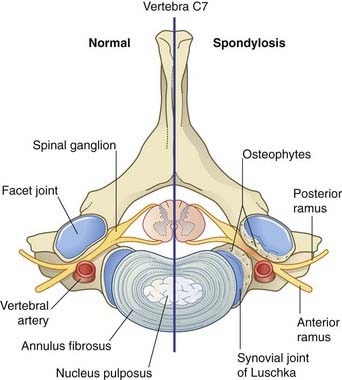
Figure CP 14.2.1 Spondylosis on the right side of vertebra C7. Osteophytes are pinching C7 spinal nerve trunk.
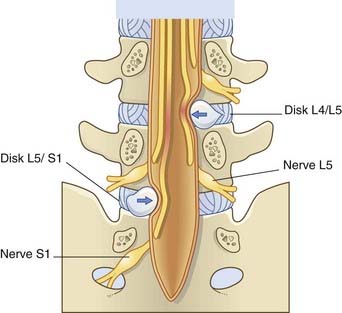
Figure CP 14.2.2 Nerves compressed (arrows) by posterolateral prolapse of the two lowest intervertebral disks.
| Segmental level | Reflex |
|---|---|
| C5, 6 | Biceps |
| Brachioradialis (‘supinator reflex’) | |
| C7 | Triceps |
| L3, 4 | Knee jerk |
| S1 | Ankle jerk |
Note: Peripheral nerve entrapment syndromes are considered in Chapter 12.
Lumbar puncture (spinal tap)
The procedure involved in removing a sample of cerebrospinal fluid from the lumbar cistern is described in Clinical Panel 4.2. This procedure should not be performed if there is any reason to suspect the presence of raised intracranial pressure. (It is performed occasionally when there is some uncertainty, but only under immediate neurosurgical cover.)
Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418.
Polston DW. Cervical radiculopathy. Neurol Clin. 2007;25:373-385.
Postacchini F, Rauschning W. Anatomy. In: Postacchini F, editor. Lumbar disc herniation. Berlin: Springer-Verlag; 1999:18-58.
Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794-810.