Nephrolithiasis
1. Define hypercalciuria, kidney (renal) stones, renal calculi, nephrolithiasis, urolithiasis, renal lithiasis, and nephrocalcinosis.
Hypercalciuria is urinary calcium excretion greater than 300 mg/day in men and greater than 250 mg/day in women. A more accurate definition is urinary calcium excretion greater than 4 mg per kg of ideal body weight per day in either sex. A good estimate of the 24-hour urine calcium excretion is 1.1 times the calcium-to-creatinine ratio (Ca/Cr) on a random urine specimen. For example, if urine calcium is 20 mg/dL and urine creatinine is 70 mg/dL, then the Ca/Cr would be 20:70 or 0.286 g (286 mg/day). The estimated 24-hour urinary calcium excretion would be 1.1 × 286 = 315 mg/day. Kidney stones, renal calculi, nephrolithiasis, urolithiasis, and renal lithiasis are synonymous terms that define the clinical syndrome of formation and movement of stones in the urinary collecting system. Renal calculi are abnormally hard, crystalline, insoluble substances that form in the renal collecting system. Nephrocalcinosis is deposition of calcium salts in the renal parenchyma.
2. Who is at risk for the development of kidney stones?
The average prevalence of kidney stones in the United States is approximately 5%, with the lifetime risk for a stone being 13% in men and 7% in women. The yearly cost of kidney stone disease in the United States is more than $5 billion. Fifty percent of patients with kidney stones have a recurrence within 5 to 10 years. Stones occur most often between ages 20 and 60 years and occur in Caucasians more than other ethnicities. Women have had more stones in recent years, possibly because of increased calcium and protein intake and greater exercise with the potential for dehydration. Review of nephrolithiasis in the Women’s Health Initiative suggests that hormone replacement therapy is a risk for renal stones. Other risks for stones include a family history of stones, obesity, diabetes mellitus, hypertension, autosomal dominant polycystic kidney disease, medullary sponge kidney, renal tubular acidosis, urine volume less than 2 L/day, dietary sodium greater than 2 g/day, low water intake, and high protein intake (see question 4).
3. What are the compositions and approximate frequencies of kidney stones in the United States?
There are six major types of stones, as outlined in Figure 17-1, which also shows the approximate frequency of occurrence of each type of stone.
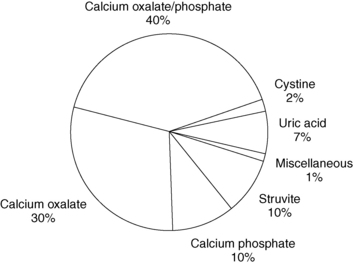
Figure 17-1. Frequency of the kinds of kidney stones.
4. What are the main causes of nephrolithiasis?
The most common causes of nephrolithiasis are the various types of idiopathic hypercalciuria (IH): absorptive hypercalciuria (AH) types AH-I to AH-III (renal phosphate leak) and renal hypercalciuria (RH). Other causes are primary hyperparathyroidism, hyperoxaluria, hyperuricosuria, hyperphosphaturia, hypocitraturia, hypomagnesuria, infection stones, gouty diathesis, renal tubular acidosis, cystinuria, and, possibly, nanobacteria. Rarely, kidney stones may form from xanthine, triamterene, monosodium urate, ephedrine, guaifenesin, and indinavir (protease inhibitor). Patients with idiopathic nephrolithiasis make up 10% to 20% of “stone formers” in whom routine workup yields no identifiable cause.
5. Describe the conditions associated with both renal stone disease and hypercalciuria.
Calcium stones account for 80% of all kidney stones. Approximately 40% to 50% of calcium stone formers have hypercalciuria. Of those with hypercalciuria, 40% have IH, 5% have primary hyperparathyroidism, and 3% have renal tubular acidosis. Other causes of hypercalciuria include excessive dietary vitamin D, excessive calcium and alkali intake, sarcoidosis, Cushing’s syndrome, hyperthyroidism, Paget’s disease of bone, and immobilization. Nephrolithiasis is also associated with infection, acute and chronic kidney injury, coronary artery disease, type 2 diabetes mellitus, hypertension, and the metabolic syndrome.
6. What are the most important causes of normocalciuric calcium nephrolithiasis?
The most important and most common causes of normocalciuric calcium nephrolithiasis are hypocitraturia (50%), hyperuricosuria (25%), hyperoxaluria (10%), and urinary stasis (5%).
7. Describe the process of renal stone formation.
Initially, urinary crystallization or precipitation of sparingly soluble salts and acids occurs. Nucleation follows as the initial crystals and urinary matrix ions form a stable framework for crystal enlargement through growth and aggregation. After they are sufficiently large, crystals become trapped in a narrow portion of the urinary collecting system (often at the end of collecting ducts), forming a nidus for further stone growth. Alternatively, crystals form in the medullary interstitium, are extruded, and adhere to the renal papilla and form a Randall’s plaque nidus for further crystal accumulation and stone growth. Once stone growth occurs, the stone may detach from the renal papilla, move distally, and cause obstruction. Common sites for obstruction are the ureteropelvic junction, midureter, and ureterovesical junction.
8. Discuss the pathophysiologic factors that influence the formation of renal stones.
Renal stones result from hereditary or acquired disorders causing supersaturation of stone precursors, deficiency of stone inhibitors, and possibly excess promoters. Supersaturation causes crystallization with mineral precursors, such as calcium and oxalate. Calcium oxalate crystals bind to anionic, sialic acid–containing glycoproteins on the apical surfaces of renal tubular epithelial cells, allowing further growth. Other factors that increase stone formation include urinary stasis (medullary sponge kidney), decreased flow (obstruction), increased urine ammonium (infection), dehydration (concentrated urine), and increased urinary alkalinity (renal tubular acidosis [RTA]). Type I RTA promotes stone formation through the increased release of calcium and phosphorus from bone to buffer the acidemia, with resulting hypercalciuria and hyperphosphaturia. The acidemia enhances proximal tubule reabsorption of citrate with resulting hypocitraturia. The alkaline urine of RTA promotes precipitation of calcium phosphate stones. Acidemia with a positive urine anion gap (UNa + UK − UCl) is a clue to the presence of RTA.
9. What are the main chemical precursors of renal stones?
Relatively high concentrations of salt and acid solutes are the main determinants of crystalluria and stone formation. Calcium oxalate is most common and is supersaturated to four to five times its solubility in normal urine. Other precursors are calcium phosphate (hydroxyapatite) and calcium phosphate monohydrate (brushite). Uric acid, cystine, struvite (magnesium ammonium phosphate), and mucoprotein are undersaturated stone precursors. Drugs such as ascorbic acid (conversion to oxalate) and triamterene (nidus for stone formation) also may promote renal stone formation.
10. What are the main inhibitors of renal stone formation? How do they work?
Inhibitors include urinary citrate, pyrophosphate, magnesium, nephrocalcin, uropontin, glycosaminoglycans, and Tamm-Horsfall protein. Most of them bind crystal precursors; for example, citrate binds calcium, making it less available to bind to oxalate. Inhibitors improve solubility and impair precipitation, nucleation, crystal growth, or aggregation. They also compete with stone precursor minerals, such as calcium oxalate, for binding to the apical surfaces of epithelial cells and inhibit epithelial cell adhesion and internalization of calcium oxalate crystals. Finally, inhibitors impair stone precursor transformation to a focus for crystallization and stone growth.
11. What is nephrocalcin? What role does it play in the formation of renal stones?
Nephrocalcin is an anionic protein produced by the proximal renal tubule and the loop of Henle. It normally inhibits the nucleation, crystal growth, and aggregation phases of stone formation. However, nephrocalcin isolated from some stone formers has defective structure and function and is found in the matrix of many calcium stones. Thus nephrocalcin may have a dual role in stone formation. When normal, it acts as an inhibitor of stone formation. When abnormal, it may act as a promoter by binding calcium and forming a nidus for crystallization.
12. What are the promoters of renal stone formation?
Promoters of renal stone formation are poorly characterized but are believed to be primarily urinary mucoproteins and glycosaminoglycans. Under certain conditions, promoters enhance the formation of renal stones.
13. How does the kidney handle calcium?
Approximately 60% of the serum calcium is ionized or complexed and freely filtered by the glomerulus. The kidney reabsorbs 98% of the filtered calcium passively throughout the nephron. Sixty percent of the reabsorption occurs in the proximal convoluted tubule, 30% in the loop of Henle, and 10% in the distal tubule. Furosemide impairs calcium reabsorption in the loop of Henle and increases urinary calcium excretion. Thiazide diuretics impair distal tubule reabsorption of sodium, thereby increasing intracellular negativity and calcium reabsorption. PTH increases distal tubular calcium reabsorption by enhancing calcium channel activity.
14. Calculate the normal filtered and excreted load of calcium per day.
The serum calcium concentration is normally 10 mg/dL. The kidney filters complexed and free calcium, which makes up 60% of the total, or 6 mg/dL. The normal glomerular filtration rate (GFR) is 120 mL/min. Thus the filtered load of calcium is 6 mg/100 mL × 120 mL/min × 1440 min/day = 10,368 mg/day. Because the kidney reabsorbs 98% of the filtered calcium, only 2% is excreted. Thus normally the kidney excretes about 200 mg of calcium/day (10,368 mg/day × 0.02 = 207 mg/day). If the excreted calcium level increases to 5%, the urinary calcium level increases to 500 mg/day.
15. How do the serum calcium level and dietary sodium intake affect hypercalciuria?
To help prevent hypercalcemia, nonrenal elevation in serum calcium causes increase in both filtered calcium and urinary calcium. Increased sodium delivery to the loop of Henle and the distal tubule also raises urinary calcium. In non–stone formers, urinary calcium excretion increases about 40 mg for each 100 mEq of sodium excretion. In hypercalciuric stone formers, calcium excretion increases up to 80 mg per each 100 mEq of sodium. Because urinary sodium excretion equilibrates to dietary sodium intake, restricting dietary sodium reduces urinary calcium excretion. In patients with stones, recommended daily dietary sodium is no more than 100 mEq (2300 mg).
16. Describe the etiology and pathophysiology of IH.
IH affects 10% of the general population and 40% of stone formers. The four types of IH are AH-I to AH-III and RH. AH-I and AH-II result from increased intestinal sensitivity to calcitriol with intestinal calcium hyperabsorption and higher numbers of vitamin D receptors in osteoblasts, causing greater bone resorption and resorptive hypercalciuria. The latter accounts for decreased bone mass seen in many patients with AH-I and some of those with AH-II. AH-III, an unusual disorder, is due to a renal phosphate leak with urinary loss of phosphate, decreased serum phosphate, and increases in renal calcitriol production and intestinal calcium absorption. The level of the phosphaturic factor, fibroblast growth factor-23, is increased in some patients with calcium nephrolithiasis, hypophosphatemia, and renal phosphate leak. RH is characterized by impaired tubular reabsorption of calcium, which causes a decrease in serum calcium, elevations in parathyroid hormone (PTH) and calcitriol, and increases in bone resorption and intestinal calcium absorption.
17. Distinguish among the various forms of IH.
18. When is it necessary to distinguish among the various forms of IH?
Only complicated nephrolithiasis unresponsive to usual therapy requires differentiation (see Website reference on hypercalciuria review at the end of the chapter).
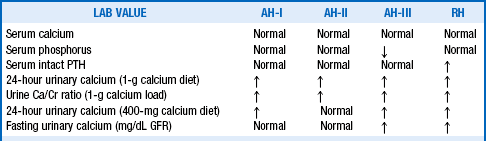
19. Explain the differences in serum levels of phosphorus and PTH in AH-III and RH.
Serum phosphorus is low in AH-III because of a primary renal phosphate leak. The level of intact PTH is high in RH because the primary defect is decreased renal tubular calcium reabsorption, causing relative hypocalcemia that stimulates PTH.
20. How do changes in calcium intake help distinguish the different types of absorptive hypercalciuria and renal leak hypercalciuria (see Table 17-1)?
In AH-II, the 24-hour urine calcium normalizes with a restricted calcium diet (400 mg/day) because the absorptive excess is not as severe. However, the 24-hour urine calcium during calcium restriction remains high in AH-I because of marked calcium hyperabsorption, in AH-III because hypophosphatemia decreases renal tubular calcium reabsorption, and in RH because decreased renal tubular calcium reabsorption is the primary defect.
High 24-hour urinary calcium is more than 4 mg per kg ideal body weight. Normal 24-hour urinary calcium with a 400-mg/day calcium restriction is less than 200 mg/day. For improved accuracy, urinary calcium measurements are at times expressed as mg/100 mL GFR to account for changes related to altered kidney function. Normal fasting urine calcium level is less than 0.11 mg per 100 mL glomerular filtration rate. Normal urine Ca/Cr is less than 0.20 after a 1-g oral load of calcium.
21. Define a low serum phosphorus level on an 800-mg/day phosphorus-restricted diet.
22. What causes hyperoxaluria?
Approximately 14% of urinary oxalate comes from dietary absorption and the remainder from metabolism of glyoxylate and ascorbic acid. Increased oxidation of glyoxylate to oxalate occurs in the rare autosomal recessive hereditary hyperoxaluria. The clinically more important enteric hyperoxaluria occurs with small bowel resection, bypass, or inflammation. Small bowel disease may cause bile salt and fat malabsorption, resulting in increased delivery of bile salts and fats to the colon. Bile salts damage colonic mucosa, increasing colonic permeability and oxalate absorption. Intestinal fatty acids are negatively charged and bind calcium and magnesium, decreasing the amounts of calcium and magnesium available for binding intestinal oxalate and leaving more oxalate free for intestinal absorption. Low-calcium diets do the same. Excess oxalate is primarily absorbed in the bile salt–damaged colon. Thus, patients with small bowel disease and an ileostomy do not hyperabsorb oxalate. Excessive dietary oxalate or ascorbic acid (> 2 g/day) also leads to hyperoxaluria.
23. Why is hyperoxaluria important in nephrolithiasis?
Oxalate is a major component of the most commonly formed stones (calcium oxalate) and contributes to supersaturation. Previously it was believed to be a much stronger stimulus to calcium oxalate stone formation than calcium. Newer data suggest that calcium may be just as potent, however, and a high urinary concentration of either calcium or oxalate is a powerful stimulus for calcium oxalate stone formation.
24. How does hyperuricosuria contribute to renal stones?
Uric acid stones develop in approximately 25% of patients with symptomatic tophaceous gout. Excessive urinary uric acid (> 600 mg/day) supersaturates the urine, crystallizes, and forms uric acid stones. However, most uric acid stone formers do not have gout, hyperuricemia, or hyperuricosuria. All do have a urinary pH lower than 5.5, which promotes uric acid stone formation. Approximately 25% of calcium stone formers have hyperuricosuria. Hyperuricosuria decreases the solubility of calcium oxalate. Monosodium urate may interfere with inhibitors, resulting in increased calcium oxalate stone formation. This disorder, called hyperuricosuric calcium nephrolithiasis, is characterized by normal serum calcium, urinary uric acid greater than 600 mg/day, urine pH greater than 5.5, and recurrent calcium stones.
25. How does urinary pH relate to renal stones?
Because uric acid has a pKa of 5.5, acid urine shifts the equilibrium so that the concentration of uric acid is greater than the concentration of sodium urate. At urine pH 6.5, only 10% is in the form of uric acid, and approximately 90% in the form of sodium urate. Because uric acid is 100 times less soluble than urate, uric acid stones are more likely to form in acid urine. This equilibrium is so important that uric acid stones virtually never develop unless the urinary pH is less than 5.5. Because of low urinary pH, uric acid stones occur more frequently in obesity and diabetes. Obesity and type 2 diabetes are associated with insulin resistance, renal steatosis, and renal lipotoxicity. This association results in decreased insulin-dependent renal production of ammonia, decreased urinary ammonium excretion, a lower urinary pH, and a propensity for uric acid stones. Additionally, obesity and type 2 diabetes are associated with hyperinsulinemia, which decreases distal nephron calcium reabsorption and increases net calcium excretion and the risk for calcium stones. Cystine stones are also more likely in acid urine, whereas calcium phosphate (brushite) stones usually form primarily in alkaline urine (pH > 7.0). Calcium oxalate stones may develop in either acid or alkaline urine.
26. What conditions cause low levels of urinary citrate?
Patients with hypocitraturia excrete less than 320 mg/day. Idiopathic hypocitraturia occurs in less than 5% of patients with calcium stones, and secondary hypocitraturia may occur in 30%. Citrate is freely filtered by the glomerulus, 75% is reabsorbed by the proximal renal tubule, and little citrate is secreted. Most secondary causes of hypocitraturia decrease urinary citrate by increasing proximal renal tubular reabsorption. Secondary causes of low citrate include dehydration, metabolic acidosis, hypokalemia, thiazide diuretics, carbonic anhydrase inhibitors, magnesium depletion, renal tubular acidosis, and diarrhea. Diarrhea also causes direct gastrointestinal loss of citrate and magnesium.
27. What is the role of diet in the formation of kidney stones?
The high animal protein (beef, poultry, pork, and fish) intake of many Americans (> 1.5-2 g/kg/day) acidifies the urine with phosphoric, sulfuric, and uric acids; decreases urinary citrate; increases urinary calcium; and raises the risk for nephrolithiasis. Higher-protein diets, such as the Atkins diet, worsen these effects. Increased sulfates and uric acid may act as cofactors in the formation of calcium oxalate and uric acid stones. High sodium intake increases urinary calcium (see question 15). High calcium intake (> 1200 mg) contributes to hypercalciuria. However, low calcium intake (< 600 mg) without low oxalate intake decreases oxalate binding in the gut, increases oxalate absorption, and increases urinary oxalate. High dietary oxalate (Table 17-2) increases calcium oxalate crystalluria. Orange juice may help prevent kidney stones by increasing urinary potassium and citrate. Potassium citrate as Urocit-K is commonly prescribed to increase urinary citrate. From Micromedex, Urocit-K at 60 mEq/day raises urinary citrate by approximately 400 mg/day and increases urinary pH by approximately 0.7 units. An 8-oz glass of orange juice supplies 12 mEq potassium and 38 mEq citrate (more than a 10-mEq/1080-mg tablet of Urocit-K). Cranberry juice has mixed reviews, but data now suggest that it should not be used in excess in stone disease because it may increase urinary oxalate. Citric acid juices (lemon and lime) supply little potassium and only one third as much citrate as orange juice. Although potassium citrate juices are more powerful at stone inhibition, nearly all citrus drinks are useful. An exception is grapefruit juice, which may increase stone formation by 30% to 50%. The clinician should be flexible with the patient’s choice of fluid, because the importance of the fluid intake may outweigh some of the theoretical negatives of the particular drink.
TABLE 17-2.
| Fruits | Rhubarb Raspberries Blueberries Blackberries Gooseberries Strawberries Fruit cocktail Tangerines Purple grapes Citrus peel |
| Vegetables | Leafy dark greens Spinach Mustard greens Collard greens Cucumbers Green beans Beets Sweet potatoes Summer squash Celery |
| Others | Roasted coffee Ovaltine Tea Cocoa Chocolate Nuts Peanuts Wheat germ Baked beans Tofu |
Adapted from Nelson JK, Moxness KE, Jensen MD, Gastineau CF, editors: Mayo Clinic Diet Manual, ed 7, St. Louis, 1994, Mosby, pp 315-362.
28. Summarize the presenting symptoms and signs of renal stones.
Approximately 30% of renal stones are asymptomatic and are found incidentally on radiographic studies. Seventy percent of renal stones are symptomatic. The patient may present with a dull ache in the posterior flank. However, the classic presenting symptom of renal stones is excruciating unilateral flank pain that waxes and wanes, and most patients have associated hematuria. The pain starts in the posterior lumbar area and then radiates anteroinferiorly into the abdomen, groin, genital region, and medial thigh. Intense pain may last several hours and may be followed by dull flank pain. Nausea, vomiting, sweating, fever, and chills may occur. Patients with renal colic appear acutely ill and restless and move from side to side in an attempt to relieve the pain. Physical examination shows tenderness and guarding of the respective lumbar area. Deep palpation worsens discomfort, but rebound tenderness is absent. Urinary tract infection may be present. Obstruction, if present, is usually unilateral. Clinical evidence of renal failure is usually absent.
29. What elements of the history and physical examination are important in patients with kidney stones?
Obtain present, past, and family histories and ask about previous stone disease. Because all of the following may be associated with stones, ask about use of guaifenesin, ephedrine, indinavir, triamterene, sulfonamides, acyclovir, and vitamins A, C, and D. Determine fluid intake and sources of excess calcium, salt, oxalate, uric acid, and protein. Physical examination is generally not helpful except during acute disease (see question 28).
30. What lab tests are appropriate in the diagnosis of kidney stones?
Perform a urinalysis with focus on pH, hematuria, pyuria, bacteriuria, and crystalluria. If pH is high or bacteriuria is seen, perform urine culture. Perform appropriate radiographic studies (see question 34). Have the patient strain all urine and save the stone, if passed, for stone analysis. If this is the patient’s first stone, the pain subsides, and the stone is less than 5 mm, conservative management with follow-up for several months is acceptable. More than 50% of stones in the proximal ureter and 75% of stones in the distal ureter less than 5 mm pass spontaneously. Order a blood chemistry panel that includes serum sodium, potassium, chloride, carbon dioxide, creatinine, calcium, albumin, phosphorus, magnesium, and uric acid. Consider measurements of serum PTH and random urine for determination of the Ca/Cr ratio. If the patient has continued symptoms, if the stone is larger than 5 mm, or if obvious obstruction is present, consult a urologist and plan for a more extensive evaluation. Include a 24-hour urine test for creatinine, sodium, calcium, phosphorus, magnesium, oxalate, citrate, and uric acid. Consider repeating the 24-hour urine test to focus on abnormalities 6 weeks after medical intervention.
31. Summarize the therapeutic approach to patients with kidney stones.
Kidney stones do not require procedural intervention unless they are associated with pain, obstruction, infection, or significant bleeding. Ureteral stones may also be treated conservatively (monitored) if there is no renal failure, fever, infection, or pain. Pain can be controlled with nonsteroidal antiinflammatory drugs, but opioid analgesics may be necessary to treat acute pain exacerbations. Stones smaller than 5 mm usually pass but those larger than 10 mm do not. Passage of stones ranging from 5 mm to 10 mm in diameter is variable. Calcium channel blockers such as extended-release nifedipine and alpha-blockers such as tamsulosin, doxazosin, and terazosin may facilitate stone passage by reducing ureteral spasm and improving peristalsis during acute colic episodes. Patients with symptomatic stones, stones larger than 5 mm, or multiple stones should be referred for nephrologic or urologic evaluation. Unless contraindicated, preventing stone recurrence requires 2 to 3 L/day of fluid to increase urine output to more than 2 L/day; no more than 2 g/day of sodium; 0.8 to 1.0 g/kg ideal body weight of protein with more plant protein (2/3 of total) and less animal protein (1/3); 1000 mg/day of dietary calcium; and avoidance of grapefruit juice, calcium supplements, oxalate, and vitamin C.
32. Describe the clinical significance of a urinalysis in patients with renal stones.
Most stone formers have macroscopic or microscopic hematuria and may have some crystalluria. The remainder of the urinalysis findings are usually normal. Crystals are normally absent in warm, freshly voided urine but, if present, suggest a diagnosis. However, most urine specimens cool before examination, and crystals may form in normal urine with time and cooling. Thus, by the time urine is usually examined, the finding of crystalluria may have little clinical significance. An exception is the presence of cystine crystals, which are diagnostic of cystinuria. Persistently acidic urine (pH < 5.5) suggests uric acid or cystine stones. More alkaline urine (pH > 6.5-7.0) suggests calcium phosphate stones. Persistently alkaline urine (pH > 7.0-7.5) and recurrent urinary tract infections strongly suggest struvite stones. Struvite stones never form unless the urine pH is alkaline.
33. What are the characteristics of urinary crystals in patients with renal stones?
Calcium oxalate monohydrate crystals may be dumbbell-shaped, needle-shaped, or oval, with the last resembling red blood cells. Calcium oxalate dihydrate crystals are pyramid-shaped and have an envelope appearance. Calcium phosphate and uric acid crystals are too small for standard light microscopic resolution and look like amorphous debris. Uric acid crystals are characteristically yellow-brown. Less commonly, uric acid dihydrate crystals may be rhomboid-shaped or resemble the four-sided diamonds on a deck of cards. Because all of these crystals may be found in normal urine, they are not necessarily diagnostic of disease. However, the presence of cystine crystals, which are flat, hexagonal plates resembling benzene rings, always means cystinuria. Struvite (magnesium ammonium phosphate) crystals are rectangular prisms that resemble coffin lids.
34. How do radiographic tests help to evaluate patients with renal stones?
A plain radiograph of the abdomen (kidney-ureters-bladder [KUB]) should be obtained in all stone formers and shows stones with the following features: calcium (small, dense, and circumscribed); cystine (faint, soft, and waxy); struvite (irregular and dense). Uric acid stones are radiolucent and not seen. Intravenous pyelography (IVP) localizes stones in the urinary tract and shows the degree of obstruction. Radiolucent obstruction on IVP suggests a uric acid stone. Ultrasonography reveals the size and location of larger stones, is sensitive for diagnosing obstruction, and may be best when radiation should be avoided, as in pregnancy. However, the initial radiographic procedure of choice for stone evaluation requires no patient preparation and is easy, sensitive, specific, and accurate. It should be ordered as follows: helical noncontrast computed tomography (CT) scan renal-stone protocol using 3- to 5-mm cuts. A new technique that is not widely available is dual-energy CT with advanced post-acquisition processing that can discriminate among several subtypes of urinary calculi without formal stone analysis. Indinavir stones are not seen on KUB or CT scan and may be missed on IVP. Indinavir stones, which are diagnosed after suspicion is raised by history, physical examination, and signs of obstruction, may require contrast-enhanced CT scanning.
35. Which medications are useful for treating the various stone-forming conditions?
TABLE 17-3.
ORAL DRUG THERAPY FOR RENAL STONES
| DISORDER | DRUG | DOSAGE |
| Absorptive type I | Hydrochlorothiazide | 12.5-25 mg b.i.d. |
| Potassium citrate | 10-30 mEq t.i.d. | |
| Cellulose sodium phosphate | 5 g 1-3 times/day c.c. | |
| Magnesium gluconate | 1-1.5 g b.i.d. | |
| Magnesium oxide | 400 mg b.i.d. | |
| Absorptive type II | Hydrochlorothiazide | 12.5-25 mg b.i.d. |
| Renal phosphate leak | Neutral sodium phosphate | 500 mg t.i.d. |
| RH | Hydrochlorothiazide | 12.5-25 mg b.i.d. |
| Hypocitraturia | Potassium citrate | 10-30 mEq b.i.d.-t.i.d. |
| Hyperuricosuria | Potassium citrate | 10-30 mEq b.i.d.-t.i.d. |
| Allopurinol | 100-300 mg/day | |
| Enteric hyperoxaluria | Potassium citrate | 10-30 mEq t.i.d. |
| Magnesium gluconate | 1-1.5 g b.i.d. | |
| Calcium citrate | 950 mg q.i.d. | |
| Calcium carbonate | 250-500 mg q.i.d. | |
| Cholestyramine | 4 g t.i.d. | |
| Pyridoxine | 100 mg/day | |
| Cystinuria | Potassium citrate | 10-30 mEq t.i.d. |
| α-Mercaptopropionylglycine | 200-400 mg t.i.d. | |
| d-Penicillamine | 250-500 mg q.i.d. | |
| Pyridoxine | 50 mg/day | |
| Struvite stones | Acetohydroxamic acid | 250 mg 2-4 times/day |
| Antispasmodic therapy | Tamsulosin | 0.4 mg once daily |
b.i.d., twice daily; q.i.d., four times daily; t.i.d., three times daily; c.c., with meals.
Note: All medications are given orally. Dosages are estimated ranges and not absolute recommendations. Each drug must be adjusted according to the patient’s tolerance. Use the lowest dosage necessary to attain the desired effect and avoid side effects. Always use drug therapy in addition to appropriate dietary changes and fluid input. Potassium citrate is better tolerated in lower dosages taken three times a day. However, twice-daily dosing may improve compliance. Potassium citrate is often required to correct thiazide-induced hypokalemia and hypocitraturia (see question 36). Chlorthalidone or indapamide may be substituted for hydrochlorothiazide for more convenient once-daily dosing (see question 37).
36. What are special considerations in the drug therapy of nephrolithiasis?
Potassium citrate, and not sodium citrate, for urine alkalinization to a pH > 7.0 is recommended for uric acid and cystine stones. Sodium citrate increases urinary sodium and calcium, and in alkaline urine, sodium urate may increase calcium stone formation. Cystine stone formers require higher fluid intake to reduce urinary cystine below its solubility limit of 200 to 250 mg/L. Fluid and potassium citrate often are the only therapy necessary for uric acid stones if uricosuria is less than 800 mg/day. Use allopurinol with potassium citrate if uric acid stones continue or hyperuricemia is more severe. Use cellulose sodium phosphate (CSP) only for refractory stone disease in AH-I. CSP binds calcium and magnesium in the gut, decreases absorption of both, and may worsen osteopenia and increase urinary oxalate. Replace magnesium as required. Monitor bone mass, and treat osteopenia as necessary.
37. Why are thiazide diuretics the first-line drug therapy for hypercalciuria-induced nephrolithiasis?
Thiazides are first-line therapy because they increase proximal (indirectly) and distal (directly) tubular reabsorption of calcium. However, thiazides can cause depletion of potassium and citrate, which should be replaced with potassium citrate. Avoid triamterene, which can cause kidney stones. If potassium supplementation is added, use amiloride with caution to avoid hyperkalemia. The thiazide-like diuretics, chlorthalidone (12.5-50.0 mg daily) or indapamide (1.25-2.5 mg daily), may be preferred to hydrochlorothiazide for the convenience of once-daily dosing. Additionally, indapamide is less likely to cause lipid disturbances associated with the higher thiazide dosages needed to reduce urinary calcium.
38. How should you treat a symptomatic patient with a renal stone 1 to 2 cm in size?
Apply the therapeutic options in question 31. About 10% to 20% of all kidney stones require surgical removal because of size and symptoms. Many urologists treat symptomatic patients with calcium stones 1 to 2 cm in size in the renal pelvis or a significant proximally obstructing stone (0.5-2.0 cm) with extracorporeal shock wave lithotripsy (ESWL). If the stone is too large or too hard, as estimated from CT scan, or is not in a good location for ESWL, percutaneous stone removal or a ureteroscopic approach may be indicated (see question 39). Additionally, because of the achievement of higher stone-free rates, many urologists choose percutaneous nephrolithotomy (PCNL) for 1- to 2-cm stones. Distal ureteral stones are best managed with ureteroscopic stone extraction or in situ ESWL.
39. How should you treat an asymptomatic patient with a renal stone of the same size?
Treatment of the asymptomatic patient with a 1- to 2-cm renal stone is a toss-up. Each expert has an opinion based on the experience of the local medical community. Many asymptomatic stones can be monitored without intervention other than that noted in question 31. Specifics of stone location, duration, and overall patient health are important in the decision. Recurrent, enlarging, or multiple asymptomatic stones probably should be treated. Nephrology and urology consultations are appropriate. Other forms of lithotripsy include percutaneous ultrasonic lithotripsy and endoscopic ultrasonic lithotripsy. Intracorporeal lithotripsy uses the holmium:yttrium-aluminum-garnet laser and electrohydraulic lithotripsy.
40. What treatment should be used if the stone is larger than 3 cm?
If the stone is larger than 3 cm, lithotripsy usually fails. The initial approach to patients with stones of this size is PCNL. However, many urologists choose this therapy for stones larger than 1.5 cm. Open lithotomy is now unusual. Therapy for stones larger than 2 cm depends on the patient’s overall status, wishes, and experiences and the experiences of the patient’s physician and urologist.
Alan, G, Wasserstein, AG. Nephrolithiasis. Am J Kidney Dis. 2005;45:422–428.
Cameron, MA, Khashayar Sakhaee, K. Uric acid nephrolithiasis. Urol Clin North Am. 2007;34:335–346.
Graham, A, Luber, S, Wolfson, AB. Urolithiasis in the emergency department. Emerg Med Clin N Am. 2011;29:519–538.
Hollingsworth, JM, Rogers, MA, Kaufman, SR, et al, Medical therapy to facilitate urinary stone passage. a meta-analysis. Lancet 2006;368:1171–1179.
Lotan, Y, Pearls, MS. Economics of stone management. Urol Clin North Am. 2007;34:443–453.
Maalouf, NM, Sato, AH, Welch, BJ, et al. Postmenopausal hormone use and the risk of nephrolithiasis. Arch Intern Med. 2010;170(18):1678–1685.
Meschi, T, Nouvenne, A, Borghi, L. Lifestyle recommendations to reduce the risk of kidney stones. Urol Clin N Am. 2011;38:313–320.
Monk, RD, Bushinsky, DA, et al. Kidney stones. In: Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. ed 11. Philadelphia: Saunders Elsevier; 2011:1311.
Park, S, Pearle, MS. Pathophysiology and management of calcium stones. Urol Clin North Am. 2007;34:323–334.
Rendina, D, Mossetti, G, De Filippo, G, et al. Fibroblast growth factor 23 is increased in calcium nephrolithiasis with hypophosphatemia and renal phosphate leak. J Clin Endocrinol Metab. 2006;91:959–963.
Sakhaee, K, Maalouf, NM, Sinnott, B. Clinical review—kidney stones 2011. Pathogenesis, diagnosis and management. J Clin Endocrinol Metab. 2012;97:1847–1860.
Taylor, EN, Fung, TT, Curhan, GC. DASH-style diet associated with reduced risk for kidney stones. J Am Soc Nephrol. 2009;20:2253–2259.
Wimpissinger, F, Türk, C, Kheyfets, O, et al, The silence of the stones. asymptomatic ureteral calculi. J Urol 2007;178:1341–1344.
Worcester, EM, Coe, FL. Calcium kidney stones. N Engl J Med. 2010;363:954–963.
Ziberman, DE, Ferrandino, MN, Preminger, GM, et al. In vivo determination of urinary stone composition using dual energy computerized tomography with advanced post-acquisition processing. J Urol. 2010;184:2354–2359.