CHAPTER 142 Neoplasms of the Paranasal Sinuses
Sinonasal neoplasms constitute a rare group of tumors in Western society. They account for only 0.2% to 0.8% of all malignancies and 2% to 3% of all head and neck cancers.1,2 The incidence rate, adjusted for age and sex, is 0.3 to 1 case per 100,000 people. These tumors are extremely rare in childhood; the incidence increases with age beginning around the fourth decade. The median age at diagnosis is 62 years in men and 72 years in women, and the male-to-female ratio is 3 : 2.3 The intimate relationships between the nasal passages and sinuses allow easy extension of tumor from one cavity to another. When initially seen, most patients have advanced disease, which is correlated with a less favorable outcome.4,5 This problem is compounded by the proximity of the orbit, brain, and cranial nerves. The techniques of cranial base surgery can extend the anatomic margins of resection and, as part of an aggressive multimodal therapeutic approach, can result in improved oncologic control of these tumors.
Pathogenesis
Epidemiologic studies have shown associations between a variety of environmental hazards and the development of paranasal sinus tumors. Although previously debated,6 a significant association between paranasal sinus tumors and cigarette smoking was demonstrated in a large case-control analysis of white American males.7 The risk doubles in heavy or long-term smokers, and there is a reduction in risk in long-term quitters. A significantly elevated risk for paranasal sinus carcinoma was also found in nonsmokers whose spouses smoked. Other less pronounced associations include increased alcohol intake, high consumption of salted or smoked foods, and decreased intake of vegetables.
The association between exposure to various occupational hazards and the development of paranasal sinus tumors has been the subject of many careful epidemiologic studies (Table 142-1). In an analysis of European case-control studies, occupation was associated with 39% of all sinonasal cancers in men and 11% in women.8 One of the earliest associations was made with nickel refining, where workers’ relative risk for the development of paranasal sinus malignancy was more than 100 times that of the general population.9 With proper management of the workplace environment, one Canadian plant achieved a zero incidence of paranasal sinus carcinoma over a 40-year period.10,11 Adenocarcinoma of the ethmoidal sinus is highly associated with exposure to hardwood dust.12,13 Co-exposure to pigment stains can exert additional risk for adenocarcinoma.12 Furniture makers and wood machinists in England were shown to have a 1000-fold increased incidence of adenocarcinoma of the ethmoid.14–16 In a recent study by Holmila and coworkers, increased expression of cyclooxygenase-2 in sinonasal adenocarcinoma was found in people exposed to wood dust, thus suggesting a role for inflammatory components in the carcinogenesis process.17 The development of paranasal sinus tumors has been linked with exposure to various other agents, including chromium compounds, radium used in watch dial painting, dichlorodiethyl sulfide, isopropyl oil, dust arising during the machining of shoes, textile dust, polyaromatic hydrocarbons in gasoline manufacturing, flour dust, and asbestos.18–21
TABLE 142-1 Occupational Hazards Associated with Paranasal Sinus Tumors
| TUMOR TYPE | OCCUPATIONAL SETTING | SUSPECTED CARCINOGEN |
|---|---|---|
| Squamous cell carcinoma | Nickel refining | Nickel compounds |
| Mustard gas manufacturing | Dichlorodiethyl sulfide | |
| Isopropyl alcohol manufacturing | Isopropyl alcohol | |
| Watch painting | Radium | |
| Adenocarcinoma | Woodworking | Hardwood dust |
| Chrome pigment manufacturing | Chromium compounds | |
| Isopropyl alcohol manufacturing | Isopropyl oil |
Asia and Africa have a relatively high incidence of paranasal sinus tumors. The Bantu of South Africa have the highest incidence worldwide of carcinoma of the upper jaw, which is probably related to the carcinogenic effects of their homemade snuff.22 In Japan, rates are between 2 and 3.6 cases per 100,000 annually, approximately four times that in the American population.18 This higher incidence is mostly due to an increased rate of squamous cell carcinoma of the maxillary sinus, which is thought to be caused by the high prevalence of chronic sinusitis and cigarette smoking in the Japanese.1,23 Fortunately, the incidence of paranasal sinus tumors in Japan seems to be decreasing.24 Adenocarcinoma of the paranasal sinuses is quite rare in Japan, possibly because of the extensive use of softwood rather than hardwood in the Japanese furniture industry.23
There is a well-known association between paranasal sinus tumors and Epstein-Barr virus (EBV), especially with T-cell non-Hodgkin’s lymphoma.25 The high incidence of sinonasal lymphoma in Uganda is largely due to cases of Burkitt’s lymphoma occurring in that region.18 With the use of in situ hybridization techniques, EBV RNA was found in 7 of 11 cases of sinonasal undifferentiated carcinoma (SNUC) in Asian patients, with no EBV RNA found in tumors of the 11 Western patients evaluated.26 However, the Asian patients with EBV-positive SNUC had tumor features designated “lymphoepithelioma-like.” SNUCs of the “Western” type showed morphology typical for this tumor and were negative for EBV. This suggests the presence of separate pathologic entities and a negative association between EBV and SNUC.27
Pathologic Features
Epithelial tumors include squamous cell carcinoma, transitional (schneiderian) carcinoma, and schneiderian papilloma.28 Squamous cell carcinoma accounts for more than half of all paranasal sinus tumors in most series.1,3,29 It most commonly arises from the maxillary antrum, with the ethmoidal sinuses being the second most common site. Most paranasal sinus tumors (80%29) include areas of keratin formation, either as sheets or as epithelial pearls. Less well differentiated nonkeratinizing and anaplastic carcinomas make up the remainder of the lesions. A well-differentiated squamous cell lesion with exophytic growth and little stromal invasion located in the nasal cavity may mimic a papilloma.
Transitional (schneiderian) carcinoma is a special type of nonkeratinizing squamous cell carcinoma that has no particular site of predilection. It is transitional only in that it tends toward squamous cell differentiation.28 Microscopically, it has the appearance of irregular and thickened columnar metaplastic epithelium with closely packed nuclei and multiple mitotic figures. The tumor invades the surrounding stroma at the base. A benign form, transitional cell (schneiderian) papilloma, may be locally recurrent and progresses to carcinoma in approximately 10% of cases.30 Schneiderian papillomas may be exophytic or inverted. The exophytic forms favor a septal location, whereas the inverted types are most often found on the lateral wall of the nasal cavity or in the sinuses.31 An association with carcinoma is strongest for inverted papillomas.28 Human papillomavirus has been associated with the development of schneiderian papillomas.32,33
Glandular tumors include adenocarcinoma and adenoid cystic carcinoma. Adenocarcinoma occurs most frequently in the upper nasal cavity or in the ethmoidal sinuses. It arises directly from surface respiratory epithelium or from the submucosal glands, which are direct epithelial invaginations and thus not true minor salivary glands.34 There are three basic growth forms: papillary, sessile, and alveolar-mucinous.28 Mucin production is variable in the first two forms and abundant in the third. Enteric and nonenteric phenotypes are recognized, both histopathologically and immunocytochemically. Of the two types, the enteric forms are associated with increased recurrence rates independent of margin status or delivery of adjuvant radiotherapy.34 Adenocarcinomas may be well or poorly differentiated, and this high or low grade is related to prognosis.35 The papillary form is the type most often seen in woodworkers and tends to have a relatively better prognosis than the other two.
Another glandular tumor is adenoid cystic carcinoma, which is an uncommon tumor that arises from the minor salivary glands of the mucosa. This tumor occurs mainly in the lower aspect of the nasal cavity. Adenoid cystic carcinoma characteristically infiltrates diffusely, especially along perineural pathways, which contributes to its high rate of recurrence and late metastasis.36 Perineural propagation is usually evident along the maxillary and mandibular divisions of the trigeminal nerve. At times, the site of secondary perineural extension may become apparent before diagnosis of the primary tumor. Careful evaluation usually identifies the primary tumor.37,38 Adenoid cystic carcinoma also has a propensity for bony invasion, which can lead to significant skull base involvement and intracranial extension, thereby making this tumor difficult to treat.39 Microscopically, these tumors have a characteristic appearance consisting of a mixture of microcystic pseudoluminal spaces and tubular epithelium-lined structures, with many lesions also including solid areas. They are typically divided into tubular, cribriform, and solid subtypes. Survival has been found to be best for patients with the cribriform subtype and worst for those with the solid form. Other factors associated with decreased survival time are tumor site, skull base invasion, and tumor stage.38 Tumors arising from the olfactory epithelium are rare neoplasms found in the upper nasal cavity and have been described with many confusing terms, including olfactory neurocytoma, olfactory neuroepithelioma, neuroendocrine carcinoma, esthesioneuroepithelioma, esthesioneurocytoma, neuroblastoma, and esthesioneuroblastoma.40
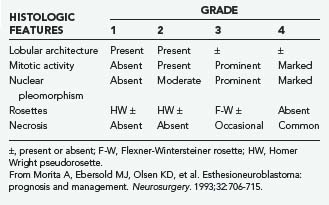
In 1982, Silva and coworkers divided these tumors into two types: ON and NEC.40 Subsequent investigations have identified a number of tumor types that have been grouped under the term sinonasal neuroendocrine tumors, including ON, NEC, small cell carcinoma, and SNUC. ON is typically composed of sheets of uniform small cells with round nuclei, scanty cytoplasm, and prominent fibrillary material between the cells. Homer Wright rosettes (a ring of neuroblastoma cells encircling a small space filled with neurofibrillary material) may be seen. Electron microscopic findings include characteristic neurosecretory (dense-core) granules. NEC is thought to arise from glandular epithelium of the exocrine glands found in the normal olfactory mucosa.40 It thus has a unique admixture with the glandular architecture. There is no neurofibrillary component, and the tumor is composed of solid nests of cells without rosette formation. ON is frequently misdiagnosed41 because tumors such as NEC, pituitary adenoma, melanoma, and SNUC may have a similar histopathologic appearance. Careful analysis of the histopathologic and electron microscopic findings and appropriate use of immunohistochemical analysis should point to the correct diagnosis (Table 142-2).
ON and NEC should be considered separate diseases.40,42 NEC arises from a different cell type than ON does, is found more inferiorly in the nasal cavity, and seldom involves the cribriform plate. Furthermore, NEC is predominantly a disease of older patients (mean age, 50 years), is rarely manifested as regional disease, is more prone to distant metastases, and causes death earlier than ON does. Conversely, ON develops more superiorly in the nasal cavity and is more common in younger patients (mean age, 20 years). Finally, NEC has responded well to chemotherapy combined with radiotherapy, whereas the best results with ON have been obtained with surgery and radiotherapy.42 Grading35 and staging43,44 systems for ON have been proposed, with some reports suggesting that pathologic grade is the more reliable predictor of recurrence and survival (Tables 142-3 and 142-4).45,46
TABLE 142-4 Kadish and UCLA Staging Systems for Esthesioneuroblastoma
| Kadish Staging | |
| Group A | Tumor confined to the nasal cavity |
| Group B | Tumor extending into the paranasal sinuses |
| Group C | Tumor spreading beyond the nasal and paranasal cavities |
| UCLA Staging | |
| T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding the sphenoidal sinus), with sparing of the most superior ethmoidal cells |
| T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoidal sinus), with extension to or erosion of the cribriform plate |
| T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa |
| T4 | Tumor involving the brain |
UCLA, University of California at Los Angeles.
From Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102:843-849.
SNUC and small cell carcinoma are rare aggressive tumors that histologically may be confused with ON, NEC, lymphoma, or melanoma. Features of these tumors include a brisk mitotic rate, prominent cellular pleomorphism and hyperchromatic nuclei, and regions of tumor necrosis and vascular invasion.47 There is a high rate of early metastatic dissemination, especially in the small cell variety, and patients rarely live beyond 2 years.48–50 Rosenthal and colleagues found 5-year overall survival rates of 93.1%, 64.2%, 62.5%, and 28.6% for ON, NEC, SNUC, and small cell carcinoma, respectively.51
Other less common malignant tumors of the nasal cavity and paranasal sinuses include mucoepidermoid carcinoma, melanoma, plasmacytoma, lymphoma, germ cell tumors, and various sarcomas.52 Although malignant tumors are more common overall, benign lesions such as schneiderian papillomas (discussed earlier), osteomas, and chondromas may occur. Other tumors involve the region by direct extension from adjacent sites, such as juvenile nasopharyngeal angiofibroma, chordoma, meningioma, nerve sheath tumors, and pituitary tumors.53,54 Metastases to the sinonasal region are rare. Renal cell carcinoma is by far the most common infraclavicular source of metastases to this area, followed by lung and breast cancer.55 Table 142-5 demonstrates the histologic distribution of paranasal sinus tumors treated by the senior author in the Department of Neurosurgery at The University of Texas M. D. Anderson Cancer Center (M. D. Anderson) between 1992 and 2008. Because this is a neurosurgical series, there is a referral bias for advanced disease with intracranial or orbital extension, which has resulted in a somewhat different distribution of histologic types than noted in other reports.
TABLE 142-5 Histologic Distribution of Paranasal Sinus Tumors in 209 Patients Treated by Craniofacial Resection at The University of Texas M. D. Anderson Cancer Center, 1992 to 2008
| TUMOR TYPE | NO. OF PATIENTS |
|---|---|
| Squamous cell carcinoma | 35 |
| Olfactory neuroblastoma | 28 |
| Adenocarcinoma | 23 |
| Adenoid cystic carcinoma | 21 |
| Sinonasal undifferentiated carcinoma | 13 |
| Osteosarcoma | 11 |
| Neuroendocrine carcinoma | 7 |
| Angiofibroma | 7 |
| Melanoma | 6 |
| Chondrosarcoma | 6 |
| Fibrosarcoma | 5 |
| Low-grade unclassified sarcoma | 4 |
| Inverting papilloma | 4 |
| Rhabdomyosarcoma | 4 |
| Nerve sheath tumor | 3 |
| Meningioma | 3 |
| Malignant fibrous histiocytoma | 3 |
| Malignant peripheral nerve sheath tumor | 2 |
| Mucopyocele | 2 |
| Benign fibrous tumor | 2 |
| Metastases | 5 |
| Singular pathologies* | 15 |
* Includes mucoepidermoid carcinoma, mesenchymal chondrosarcoma, teratocarcinosarcoma, angiosarcoma, osteoma, leiomyosarcoma, fibrovascular polyp, ameloblastic carcinoma, primitive neuroectodermal tumor/Ewing’s sarcoma, high-grade unclassified sarcoma, malignant solitary fibrous tumor, germ cell tumor, myoepithelial carcinoma, liposarcoma, and hemangioma.
It is often difficult to determine the site of origin of these paranasal sinus neoplasms because more than 90% have invaded at least one sinus wall55 and disease may extend well beyond the original sinus.56 The most common location is the maxillary sinus, where 55% of tumors originate. The rest of the paranasal sinuses account for about 10% of cases, with 9% arising from the ethmoidal sinus and only 1% from the sphenoidal and frontal sinuses. In 35% of cases, the tumor originates from within the nasal cavity.18
Diagnostic Evaluation
Table 142-6 summarizes the common initial features of paranasal sinus malignancies. Most patients exhibit many of these symptoms and signs.28 The signs and symptoms of maxillary sinus tumors have been grouped by regional anatomy into nasal, oral, ocular, facial, and neurological findings.57 The most common initial symptom is nasal airway obstruction, frequently unilateral, followed by chronic nasal discharge and epistaxis.29,58 Extension of the neoplasm into the nasal cavity may be seen on anterior rhinoscopy (Fig. 142-1). Oral findings include unexplained pain in the maxillary teeth because of involvement of the posterior superior alveolar nerve. Further expansion may cause loosening of teeth, malocclusion, and trismus. Fullness of the palate or alveolar ridge may lead to ill-fitting dentures in an edentulous patient.59 Tumor bulging into the oral cavity from an adjacent sinus may be visible. Ocular symptoms occur with upward extension into the orbit and include unilateral tearing, diplopia, proptosis, epiphora, and fullness of the lids. Facial findings most often result from involvement of the anterior antral wall. There may be facial asymmetry with cheek swelling; in advanced cases, ulceration and fistulas may develop on the face. Numbness, paresthesia, and pain may result from involvement of the infraorbital nerve or, in more advanced disease, from posterior extension of the tumor into the pterygopalatine fossa with involvement of the entire maxillary division of the trigeminal nerve.59
TABLE 142-6 Common Symptoms in 200 Patients with Malignant Tumors
| SYMPTOM | NO. OF PATIENTS (%) |
|---|---|
| Nasal airway obstruction | 89 (44.5) |
| Nasal discharge | 78 (39) |
| Bloody or blood-tinged discharge | 51 (25.5) |
| Mucus | 27 (13.5) |
| Facial pain | 77 (38.5) |
| Mass in the nasal cavity | 72 (36) |
| Exophthalmos | 47 (23.5) |
| Swelling in the cheek | 43 (21.5) |
| Paresthesia | 39 (19.5) |
| Epiphora | 30 (15) |
| Diplopia | 27 (13.5) |
| Decreased vision | 21 (10.5) |
Modified from Weber AL, Stanton AC. Malignant tumors of the paranasal sinuses: radiologic, clinical and histopathologic evaluation of 200 cases. Head Neck Surg. 1984;6:761-776.
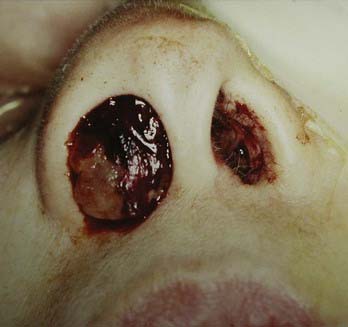
FIGURE 142-1 Tumor tissue and old blood are visible at the nasal vestibule in a patient with a massive sinonasal adenocarcinoma.
(Courtesy of The University of Texas M. D. Anderson Cancer Center, Department of Neurosurgery, Houston, TX.)
Tumors of the upper nasal cavity and ethmoid may extend through the cribriform plate into the anterior cranial fossa and are associated with anosmia and headache. Carcinoma of the frontal sinus may be manifested as acute frontal sinusitis with pain, swelling over the sinus, and evidence of early bone erosion.60 Unlike most paranasal sinus tumors, nasal obstruction, epistaxis, and nasal discharge may be absent when the tumor is located in the sphenoidal sinus. Patients with sphenoidal sinus tumors most commonly suffer from headache, diplopia, and cranial neuropathies.61
Lymph node involvement or metastatic disease is present in less than 10% of patients at initial evaluation,58,62 although the incidence increases throughout the course of the disease. Lymphatic metastasis may occur more frequently than recognized because of inaccessibility of the retropharyngeal nodes for examination.60
Advanced disease is the most accurate predictor of poor outcome.4 Unfortunately, the early symptoms of malignant paranasal sinus tumors are identical to those of benign nasal and paranasal sinus disease.5,63 Combined physician and patient delay ranges from 3 to 14 months.64 With heightened physician awareness, the liberal use of imaging studies, and early biopsy, the median patient-physician delay is reduced from 8 to 4 months, with 33% of tumors being diagnosed at an early (T1 or T2) stage.
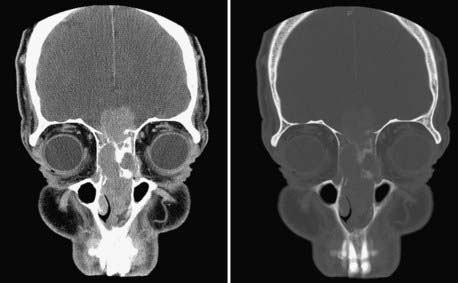
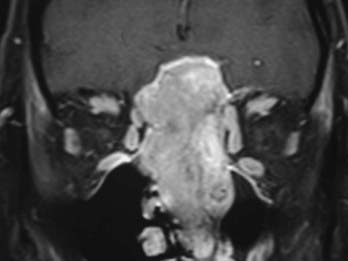
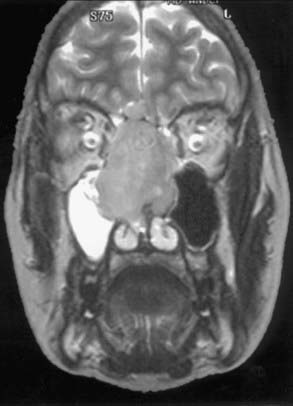
Evaluation of these patients involves a thorough examination of the head and neck, including endoscopic evaluation of the sinonasal and nasopharyngeal regions. The cranial nerves must be evaluated, and all patients should have a baseline neuro-ophthalmologic review. Computed tomography (CT) and magnetic resonance imaging (MRI) are complementary studies and the imaging methods of choice for assessing these tumors.65,66 CT is particularly useful for assessing bony changes, especially erosion. Direct coronal CT is best for assessing the integrity of the anterior skull base, including the orbital roof, cribriform plate, and planum sphenoidale (Fig. 142-2). The extent of tumor is best seen with MRI (Fig. 142-3), which can also differentiate tumor from inflamed mucosa, blood, or inspissated mucus in most cases (Fig. 142-4).67 Signal voids within the tumor identified by MRI or proximity of the neoplasm to the internal carotid artery may be an indication for preoperative angiography to assess tumor vascularity and plan surgical treatment. Postoperative use of positron emission tomography/CT can help distinguish between postoperative changes and recurrence or residual tumor.66
The key to diagnosis and management is biopsy. Flexible endoscopes permit access to most tumors of the paranasal sinuses. In the case of deep-seated lesions, CT-guided needle biopsy may be performed. The importance of evaluation of the biopsy specimen by an experienced pathologist cannot be overemphasized.68
Classification
Many classification systems are currently in use to stage maxillary sinus tumors, all based on the TNM (tumor, node, metastasis) system.69 Because nodal and distant propagation is uncommon and occurs very late in the disease, the T stage is of paramount importance. There is no single widely accepted staging system for ethmoid carcinoma.69–72 The 2007 American Joint Committee on Cancer classification of maxillary and ethmoidal sinus tumors is presented in Table 142-7.
TABLE 142-7 American Joint Committee on Cancer Staging of Carcinoma of the Maxillary and Ethmoid Sinuses
From American Joint Committee on Cancer. AJCC Staging Manual, 7th ed. New York: Springer; 2007.
Principles of Treatment
Tumor pathology and extent of disease, the availability and potential success rates of adjuvant therapies, and the potential for functional impairment and aesthetic deformity are all important parameters to consider when planning the best management options for a patient with a paranasal sinus tumor, which also makes it difficult to generalize and study various treatment options. In most cases, surgery and radiation therapy are used as a combined treatment modality, but other adjuvant therapies such as radiosurgery and chemotherapy may be indicated. It is important to note that management of paranasal sinus tumors is a multidisciplinary endeavor. Assistance and consultation from a team of specialists are required (Table 142-8). The prime parameters that drive management decisions are tumor pathology, the ability to achieve gross total resection, and the availability of adjunctive therapies.
TABLE 142-8 Multidisciplinary Team Members Involved in the Management of Paranasal Sinus Tumors
| Neurosurgery |
| Head and neck surgery |
| Plastic surgery |
| Ophthalmology |
| Dental oncology |
| Diagnostic imaging |
| Pathology |
| Medical oncology |
| Radiation oncology |
Initially, consideration is given to whether gross total excision can be accomplished. For adenocarcinoma and adenoid cystic carcinoma, it is our preference to begin with surgical excision, followed by external beam irradiation. If the patient has previously received radiotherapy, induction chemotherapy should precede surgical excision and be continued postoperatively if a response is obtained. Induction chemotherapy is also used for squamous cell carcinoma in the context of an “organ-sparing” (usually orbit-sparing) approach.73
Surgical Management
The goal of surgical management is to achieve complete tumor resection with a margin of normal tissue. Early reports of treatment of paranasal sinus tumors by local resection (e.g., maxillectomy) combined with radiotherapy were disappointing, with an overall 5-year survival rate of less than 30%.64 Lesions were incompletely excised because of technical difficulties in carrying out thorough en bloc resections.
Smith and coauthors reported the first craniofacial resection for malignant disease of the ethmoidal sinus involving the cribriform plate 74 Ketcham and associates first reported combined frontal craniotomy and maxillectomy to treat malignant tumors75 and later updated their experience with much improved survival rates.76 Terz and colleagues further extended the limits of resection to include the middle cranial fossa so that the pterygoid plates and their attachments to the sphenoid bone could be removed.77
Craniofacial Resection
Patients are selected for craniofacial resection because of involvement of the ethmoidal sinus or cribriform plate by tumor or because of suspicion of dural invasion based on preoperative imaging studies. Most purely ethmoidal tumors can be excised transcranially without the need for facial incisions.78,79 An open transfacial or endoscopic approach is needed if the tumor extends to the anterior nasal cavity or laterally beyond the medial third of the maxillary sinus. In most patients the transcranial approach is performed first, followed, if necessary, by transfacial entry into the paranasal sinuses. This sequence minimizes contact between the sinuses and epidural space until after repair of the frontobasal dura. Broad-spectrum intravenous antibiotics (vancomycin, ceftazidime, and metronidazole) are administered in the perioperative period.68
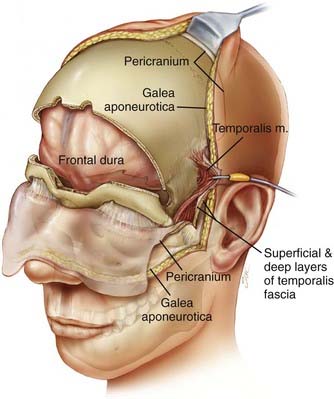
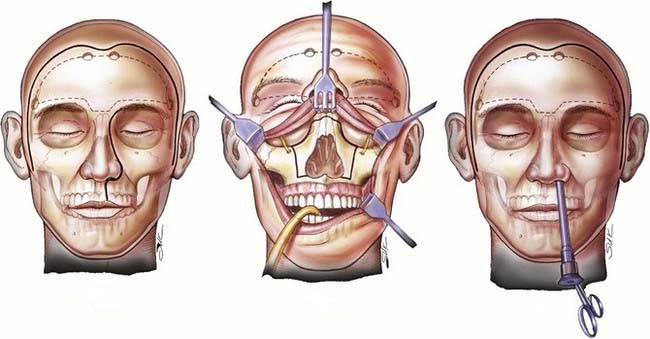
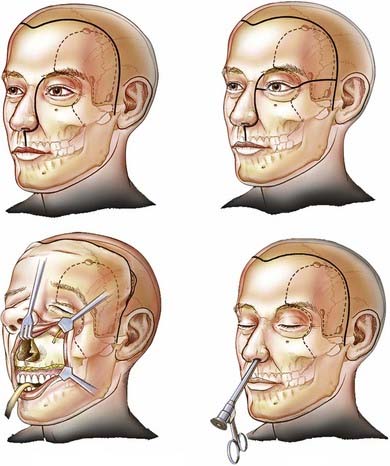
The patient is placed in the supine position with the head supported by a horseshoe headrest or affixed in a rigid cranial clamp. A bicoronal incision is carefully opened to expose the galea. The galea is opened sharply and dissected from the underlying loose connective tissue. Care is taken to preserve the anterior branches of the superficial temporal arteries. The scalp flap is reflected anteriorly by dissecting immediately below the galea, thus maximizing the thickness of the loose connective tissue layer overlying the periosteum. The posterior aspect of the scalp flap is undermined to maximize the length of the pericranial flap. Incisions are made bilaterally through both the superficial and deep layers of the temporalis fascia approximately 1.5 cm above the zygomatic process of the frontal bone and carried posteriorly for approximately 2 to 3 cm. Incisions in the periosteum are made at the level of the superior temporal line bilaterally and extended posteriorly from the previously described fascial incisions. These incisions are carried under the posterior scalp flap and then joined across the top of the head to circumscribe the pericranial flap. The pericranial flap is then elevated and reflected anteriorly. With a sharp periosteal elevator, the pericranial tissue is dissected from the orbital rims and from the zygomatic process of the frontal bone. The anterior portion of the temporalis muscle is dissected from the temporal fossa and reflected posteriorly. This gives access to the “keyhole” regions bilaterally. Entry holes are placed in both keyhole regions and at the superior sagittal sinus. A free bifrontal craniotomy is elevated. Because most of the work will be in the midline, the bone flap is extended inferiorly to just above the frontonasal suture (Fig. 142-5).
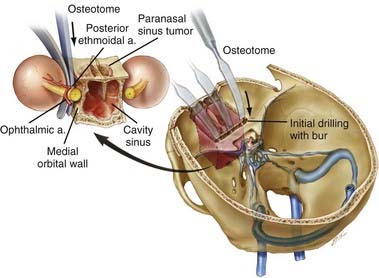
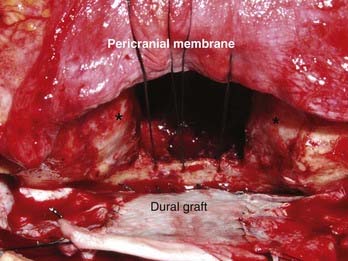
With the high-speed drill, osteotomies are made in the floor of the anterior cranial fossa. If the tumor involves the entire ethmoidal sinus complex, these osteotomies should be placed through the medial part of the orbital roof to enter the orbits bilaterally, lateral to the lamina papyracea. The posterior osteotomy should be at the level of the frontosphenoid suture. This suture overlies the posterior ethmoidal air cells in most people, although it may overlie the anterior part of a large sphenoidal sinus. As mentioned, the posterior ethmoidal foramen is in the posterolateral aspect of this osteotomy. The exit of the optic nerve from the optic canal can be found approximately 5 to 7 mm posteromedial to this artery. From above, the periorbital membrane is dissected from the lamina papyracea bilaterally, and a small curved osteotome is placed medial to the orbital tissues. An osteotomy is made at the inferior aspect of the lamina papyracea just where the bone turns to form the floor of the orbit (Fig. 142-6). This osteotomy is placed bilaterally. An osteotomy is then fashioned across the lamina papyracea posteriorly, with half of the osteotome being intraorbital and half within the posterior ethmoidal sinus. The posterior bony septum is then divided with a curved osteotome. The anterior osteotomy is performed across the base of the frontal sinuses and enters the nasal cavity. The anterior part of the nasal septum can be exposed here and is divided with heavy scissors, which allows removal of the entire specimen transcranially (Fig. 142-7). If the tumor extends lateral to the medial third of the maxilla or into the anterior nasal cavity, a transfacial or endoscopic approach is used to free the specimen for removal (Fig. 142-8). Residual air cells are removed, and any involved or questionable periorbital tissue is resected. Small periorbital defects are well tolerated, but large defects should be repaired with temporalis fascia. The pericranial flap is then cut to the appropriate length and sutured to drill holes placed in the planum sphenoidale and medial orbital roofs. Fibrin glue may be used to reinforce the dural repair and the edges of the pericranial graft. The bone plate is placed back in its anatomic position and rigidly fixated. It is vital to ascertain that no compression of the pericranial graft is being caused by the bone flap. The pericranium should be able to move freely between the bone edges.
Transmaxillary Maxillary Neurectomy
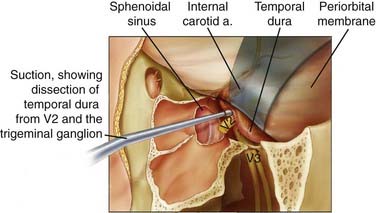
Achieving microscopically tumor-free margins during resection of skull base malignancies has consistently been identified as a positive prognostic factor for patient survival. Traditionally, when malignancies have extended perineurally into the intracranial portion of the maxillary nerve and anterolateral cavernous sinus, a temporal craniotomy was used to access this area for resection. At M. D. Anderson we have pioneered the transmaxillary approach to resect malignancies extending to this intracranial location and have been able to do away with the need for transcranial exposure of the anterolateral cavernous sinus for these tumors.80 Surgical access to the maxilla is obtained through a lateral rhinotomy or sublabial approach, or both. The amount of bone resected depends on the extent of the tumor and may involve a medial, inferior, or complete maxillectomy. The infraorbital nerve is identified and followed to the pterygomaxillary fissure. The nerve is dissected free of adipose tissue and followed to the foramen rotundum, located at the top of the pterygoid plate. The foramen is widened with a high-speed drill to expose the dura of the temporal fossa floor laterally and the cavernous sinus medially. The medial temporal dura is dissected laterally from the maxillary nerve in its course through the lateral wall of the cavernous sinus (Fig. 142-9). The extradural dissection continues posteriorly until the anterior portion of the gasserian ganglion is reached. The neoplastically involved nerve is then resected.80
Orbitectomy
Involvement of the orbit by malignancy is associated with a significant reduction in the survival time of patients with either ethmoid or maxillary sinus tumors. Invasion of the orbital wall occurs in 60% to 80% of patients with ethmoid or maxillary sinus malignancies. The periorbital membrane (orbital periosteum) is involved in 30% to 50% of cases.81 Orbital exenteration was previously the standard treatment of paranasal sinus cancers that approached the eye. However, the periorbital membrane, like the dura, presents an interface between the sinonasal cavities and the globe that is resistant to infiltration by tumor, and this barrier allows eye-sparing protocols that may involve preoperative radiotherapy or chemotherapy.82 A major concern with these techniques is local control. McCary and colleagues reported that only 4 of 36 patients (11%) whose eyes were spared had recurrence involving the orbit.83 The most common tumor type, however, was esthesioneuroblastoma (13 patients), which is a relatively rare tumor in most series, thus making it difficult to compare results. In a clinical series with a predominance of advanced squamous cell carcinoma, there was only a 30% survival rate (10 of 34 patients) in those who had preservation of the orbital contents as opposed to a 50% survival rate (28 of 55 patients) in those undergoing resection.84 Ganly and coauthors reported a disease-specific survival rate of 41% in patients with orbital involvement versus 75% in those without.85 Another concern is a poor functional result when the eye is preserved, with some authors reporting high rates of keratitis, epiphora, diplopia, cataract, and a dysfunctional globe.86 In the series by McCary and associates, of 31 patients with orbital preservation who underwent evaluation of function in the preserved eye, 14 (45%) suffered a variety of ophthalmic morbidities, most commonly exposure keratitis and motility disturbances.83 Stern and colleagues reported outcomes in 28 patients with squamous cell carcinoma of the maxillary sinus who underwent maxillectomy with orbital preservation.87 Only 3 of 18 patients (17%) who had all or part of the orbital floor resected retained significant function in the ipsilateral eye. There was local recurrence in 8 of these patients (44%). Few eye problems occurred in the 10 patients in whom the orbital floor was preserved, especially if the eye was not included in the radiation field. Unfortunately, the local recurrence rate was higher (70%). These authors concluded that when the orbital floor is resected and the radiation field will include the eye, exenteration should be performed. Adverse orbital outcomes have been shown to be strongly associated with resection of the orbital floor and resection of two thirds or more of two or more orbital walls. In patients with orbital floor defects, when they are isolated or part of a multiple wall resection, primary bony and soft tissue reconstruction is recommended.81,88
The decision whether to spare the orbit may be made preoperatively or at the time of surgery. Transgression of the periorbita by tumor cells with invasion of periorbital fat indicates the need for exenteration unless there is bilateral invasion or other involvement that precludes complete resection. Sparing of the soft tissues of the orbit when the periorbita has not been deeply transgressed by tumor does not typically adversely affect cure or local control.81
The transfacial approach is then used to perform either partial or total maxillectomy. If only partial maxillectomy is required, it can be performed completely through the circumorbital incision. If total maxillectomy is required, a lateral rhinotomy and lip-split incision may be needed. Reconstruction can typically be performed by using the pericranial flap previously harvested and the temporalis muscle with a skin graft. At times, a free rectus abdominis tissue graft is necessary. In our experience, there is no significant difference between these two reconstructive methods. Free rectus transfer is preferred if there is a large defect with significant dead space or if the blood supply to the temporalis muscle is deemed tenuous.89
Lateral Approaches
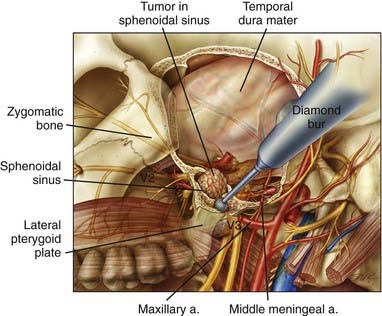
Occasionally, sinonasal tumors may require the addition of a lateral skull base exposure to achieve surgical extirpation. Most commonly, lateral exposure is required for tumors of the maxillary sinus that invade through the posterior wall and enter the infratemporal fossa (Fig. 142-10), tumors with significant perineural extension along the second and third divisions of the trigeminal nerve, and tumors of the sphenoidal sinus.
Access to this region is relatively straightforward. A preauricular incision is used, which may be extended across the scalp into a full bicoronal or three-quarter bicoronal incision, depending on the amount of anterior exposure needed. The incision may also be extended down into the neck to allow parotid and neck dissection or mobilization of the mandible. Once the scalp has been reflected, an incision is made through both the superficial and deep layers of the temporalis fascia from approximately 1.5 cm above the frontozygomatic suture down to the root of the zygoma. Dissecting subfascially protects the branches of the facial nerve and exposes the entire zygomatic arch and lateral orbital rim. The tripod of the zygoma is freed by osteotomies through the root of the zygoma, the lateral orbit, and the body of the zygoma. It can be pedicled inferiorly on the masseter muscle or removed entirely as a free piece of bone. If more extensive exposure of the lateral skull base, including exposure of the carotid artery, is necessary, the posterior osteotomy may be made through the glenoid fossa rather than the root of the zygoma. The temporalis muscle is dissected from the calvaria and reflected inferiorly. Mobilization of the zygoma allows the muscle to be reflected inferiorly for a greater distance to achieve direct exposure of the base of the middle cranial fossa and the infratemporal fossa. A subtemporal craniectomy is then performed to open the superior and inferior orbital fissures, the foramen rotundum, and the foramen ovale. If further medial exposure is required, the middle meningeal artery is coagulated and divided. In most patients, removal of bone between the first and second and the second and third divisions of the trigeminal nerve exposes the lateral sphenoidal sinus (Fig. 142-11). The pterygoid plates are well exposed and can be removed in their entirety. Such exposure now allows resection of tumors with infratemporal fossa or lateral sphenoidal sinus extension. This approach also allows elevation of the medial temporal dura for exposure and resection of the trigeminal nerve, should it be involved by perineural tumor extension. This is generally combined with an anterior or anterolateral approach to resect the primary tumor (Fig. 142-12). Reconstruction is straightforward, with subcutaneous fat being used to obliterate the opened sphenoidal and maxillary sinuses. The muscles and bone are returned to their anatomic positions and rigidly fixated. Should a large resection be necessary, a free tissue transfer procedure is performed.
Complications
Table 142-9 summarizes the postoperative complications identified in our cohort of 209 patients who underwent craniofacial resection for paranasal sinus malignancy between 1992 and 2008. The tension pneumocephalus and hematoma occurred in patients with a spinal drain who were undergoing cribriform plate resection rather than orbitectomy or lateral approaches. The relatively smaller degree of skull base resection needed for cribriform plate resection may contribute to a “ball-valve” mechanism that predisposes patients to the accumulation of air under tension. Only one case of tension pneumocephalus has occurred since 1996, which can be attributed to the fact that very few patients now undergo postoperative CSF drainage. Treatment of tension pneumocephalus has included cessation of CSF drainage, percutaneous aspiration of air, and in some cases, intubation. We do not advocate prophylactic tracheostomy for this condition.
TABLE 142-9 Complications after Craniofacial Resection in 209 Patients with Paranasal Sinus Tumors Treated at The University of Texas M. D. Anderson Cancer Center, 1992 to 2008
| ICHTS | 9 |
| ICHTS/EDH | 4 |
| ICHTS/radiation necrosis | 1 |
| ICHTS/EDH/vasospasm | 1 |
| Subtotal | 15 |
| EDH | 5 |
| CSF leak | 2 |
| Orbital entrapment | 2 |
| Dystopia | 2 |
| Radiation-induced optic neuritis | 1 |
| ICH | 1 |
| Radiation necrosis of the brain | 1 |
| Diverticular perforation | 1 |
| SIADH | 1 |
| MI | 1 |
| Free flap failure | 1 |
| Pneumocephalus/PE | 1 |
| Swollen pericranial graft | 1 |
| Wound seroma | 1 |
| Stroke during preoperative embolization | 1 |
| Temporal lobe cerebritis | 1 |
| Wound infection | 1 |
| Suicide | 2 |
| Total | 41 (20%) |
CSF, cerebrospinal fluid; EDH, epidural hemorrhage; ICH, intracerebral hemorrhage; ICHTS, intracranial hypotension syndrome; SIADH, syndrome of inappropriate antidiuretic hormone secretion; MI, myocardial infarction; PE, pulmonary embolism.
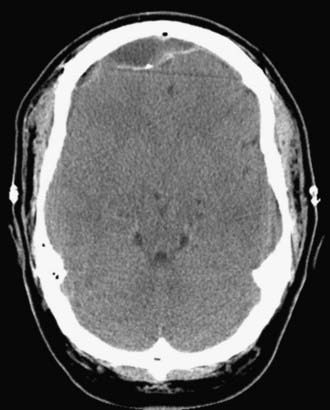
A much more common consequence of lumbar spinal drainage of CSF has been the syndrome of intracranial hypotension. We have identified this complication in 15 of our patients (15 of 41 patients with complications, 37% of all complications) (see Table 142-9). In its mildest form it consists of severe and, at times, incapacitating postural headaches. Findings on CT are typically unremarkable but usually include a mix of air and fluid in the epidural space accompanied by a concave appearance of the dura (as though it is being sucked in) (Fig. 142-13). Management consists of fluid hydration and slow mobilization. Intravenous caffeine may also help. In more advanced cases, large epidural collections of air and fluid may necessitate operative intervention. The key maneuver, however, is placement of a high-volume (at least 30 mL) lumbar spinal epidural blood patch. This may need to be repeated to achieve resolution of the presumed CSF leakage at the site of dural puncture created by placement of the spinal drain. We have not seen this complication in this group of patients since we have discontinued routine spinal CSF drainage.
The incidence of complications reported in the literature is difficult to evaluate because of a lack of uniformity in reporting. Most series report complications in 25% to 40% of patients undergoing craniofacial resection,71,84,85,90–96 with postoperative mortality rates ranging from 0% to 7%.95,97 The most commonly identified complications include wound infections (especially osteomyelitis), meningitis, CSF leakage, delayed return of neurological function, and pneumocephalus. Most of these series have spanned many years. In our modern series, where aggressive use of antibiotics is routine, there have been no cases of osteomyelitis and only one wound infection and one case of temporal lobe cerebritis after a transmaxillary approach to the middle fossa. In no patients did meningitis develop. Similarly, consistent attention to watertight dural closure, the use of vascularized grafts, and liberal use of free tissue transfer have resulted in a mere 1% incidence of CSF leakage in this group of patients. Note should be taken of the two suicides in this group of patients. Both patients were socially isolated men of late middle age. Particular attention to this subgroup of patients is warranted.
Endoscopic Surgery
The role of endoscopic resection in the management of malignant paranasal sinus tumors has been widely debated because historically it has carried a poor prognosis. Theoretical advantages of endoscopic resection include improved visualization and illumination, avoidance of facial or scalp incisions, functional and structural preservation of uninvolved structures, minimal trauma to surrounding structures, shorter hospital stays, and lower cost.2,68,98–100 The primary disadvantages of endoscopic surgery include an inability to provide a watertight dural closure and skull base reconstruction, the piecemeal nature of tumor removal, limited depth perception because of lack of stereoscopic visualization, and ergonomic restraints. Potential complications include intraorbital or intracranial perforation, CSF leaks, epistaxis, and cranial nerve V and VI palsy.98,99 Yet in experienced hands and in well-selected patient populations, endoscopic resection of sinonasal malignancies is safe, and survival and recurrence data comparable to those of open techniques are emerging. Lund and associates reported a 5-year overall survival rate of 88% and a disease-free survival rate of 68% in a cohort of 49 patients with sinonasal malignancies managed by endoscopic resection with intent to cure. However, it should be noted that there were no T4 tumors and that the pathologies tended to be of lower grade (adenocarcinoma and ON). Of particular interest is the excellent outcome of patients in their series with mucosal melanoma. Most patients (76%) received radiation therapy with or without chemotherapy. Fourteen patients underwent removal of involved bone of the skull base. The dura was resected in 1 patient. There were no postoperative complications. The authors do not counsel endoscopic resection in isolation if there is transdural tumor extension, for which craniofacial resection is typically recommended. Similarly, given the more extensive invasiveness and perineural propagation associated with adenoid cystic carcinomas, open techniques are typically recommended by this group.101 Bogaerts and coworkers, in a very recent report, achieved a 5-year overall survival rate of 53%, a disease-specific survival rate of 83%, and a recurrence-free survival rate of 62% in 44 patients with sinonasal adenocarcinoma managed by endoscopic resection and postoperative irradiation. Preoperative identification of dural, brain, or intraperiorbital tumor extension was a contraindication to endoscopic resection in isolation, and craniofacial resection was offered. Of the early recurrences noted in this series, the roof of the ethmoid sinus, the sphenoidal sinus, and the nasal septum were the most likely sites of recurrent disease.102 Similar to Lund and associates, these authors continue to recommend transcranial resection for tumors involving the dura to best achieve a margin-negative resection. Kassam and colleagues have shown that resection of tumoral skull base extensions is feasible with expanded endoscopic endonasal approaches, although the incidence of CSF leakage remains high and insufficient data are available to allow comparison with standard management recommendations.103 In an effort to overcome the limitations of current endoscopic techniques, Hanna and coworkers in a recent cadaveric study performed robot-assisted endoscopic surgery, which allowed three-dimensional, two-handed, tremor-free dissection and precise closure of dural defects.104 This use of three-dimensional visualization and robotic assistance is likely to expand the use of endoscopic surgery in sinonasal disease involving the skull base.
Adjuvant Therapies
Radiotherapy is an important treatment modality for malignant paranasal sinus tumors. The most widely accepted view is that radiotherapy should be given after surgical resection of the tumor, especially if complete resection is not achieved. Several studies comparing patients treated by surgery alone with a similar group given additional postoperative radiotherapy showed that the addition of radiotherapy improved local control.105,106 Radiotherapy is thought to augment surgical 5-year cure rates by 10% to 15% overall.107 Some centers routinely use preoperative radiotherapy,108 which has the theoretical advantage of a more homogeneous dose distribution, but recurrence and survival benefits are not clear. There are also some concerns regarding wound healing.109 Sisson and coworkers found no differences in outcome or morbidity when patients who received preoperative or postoperative radiotherapy were compared.64 The most frequent serious complication from radiotherapy is damage to the optic nerve or retina, but other significant complications may include bone necrosis, necrosis of the brain, pituitary insufficiency, and soft tissue necrosis.
A proposed alternative to surgical excision and postoperative radiotherapy is radiotherapy alone with a curative intent. In a series of 48 patients with malignant paranasal sinus disease, Parsons and colleagues achieved an overall 5-year survival rate of 52% with radiotherapy alone.70 If the 22 patients in this series with intracranial extension are considered separately, the 5-year survival rate falls to 30%. There was a 33% (16 of 48) incidence of unilateral blindness in this series and an 8% (4 of 48) incidence of bilateral blindness. Of concern is that none of the 4 patients who were left totally blind had orbital invasion. Similarly, not all the patients with treatment-induced unilateral blindness had orbital invasion. Other complications of high-dose radiation therapy encountered in this series were CSF leaks, oral-antral fistulas, acute sinusitis requiring drainage, and meningitis. For advanced, unresectable tumors, high-dose radiotherapy alone results in 5-year survival rates of 10% to 15%.110 The case series evidence available on the use of surgery and radiation therapy suggests a local recurrence and overall survival benefit from the combined use of surgery and radiotherapy, most often performed postoperatively.111–115
Our practice has been to reserve external beam radiotherapy for postoperative treatment or palliation. Treatment generally consists of 60 Gy delivered to the tumor bed, with adjustments as needed to limit the dose to the optic chiasm to 54 Gy. If orbital exenteration has not been performed, it is important to construct the treatment fields so that they spare the lacrimal gland to avoid exposure keratitis. The cervical lymphatics are also treated in patients with advanced squamous cell carcinoma of the maxillary sinus because a high incidence of regional treatment failure (38%) occurs when the lymphatics are not treated.116
There is still much controversy in the literature with respect to prophylactically irradiating the cervical lymphatics of patients with N0 status because metastases are rare and are frequently salvaged by neck dissection.62 The series by Cantu and coworkers showed a low rate of nodal metastasis (1.6% at initial evaluation and 4.3% at follow-up) from ethmoid malignant tumors, most often occurring with the primary recurrence.62 Only patients with undifferentiated carcinoma had high regional recurrence (25%) and thus probably needed prophylactic radiotherapy.111 The problem lies with maxillary sinus malignant tumors. Nodal metastases were rare in patients with non–squamous cell carcinoma, except for those with undifferentiated carcinoma (13%) and adenocarcinoma (22.2%). Neck metastasis from squamous cell carcinoma occurred in 10.2% of patients and was higher for stage T2 tumors than for stage T3 or T4 tumors. Because the prognosis of tumors of higher stage (T3/T4) is related more to primary disease recurrence than to the presence of nodal metastases, prophylactic irradiation is not required.27,114 The recommendation of prophylactic irradiation for patients who have T2N0 squamous cell carcinoma remains questionable.
Three-dimensional radiotherapy planning has been developed to deliver higher doses of radiation to the target volume while avoiding damage to surrounding structures.117,118 Radiosurgical techniques using the Gamma Knife or linear accelerator–based systems are proving to be helpful adjuncts for small-volume areas with residual tumor near critical structures such as the cavernous sinus or optic chiasm.119 Radiosurgery combined with endoscopic surgery would provide a minimally invasive treatment scheme for malignant paranasal sinus tumors.120 Intensity-modulated radiotherapy has been shown to be as effective as conventional radiotherapy,115 with diminished rates of ocular toxicity.121 The precise extent to which these newer techniques will reduce morbidity and diminish tumor recurrence is still to be determined.
The use of chemotherapy to treat paranasal sinus tumors was previously limited to the treatment of patients with systemic disease and for palliation in patients with massive recurrent tumors who had few other therapeutic options.122 For squamous cell carcinoma of the head and neck, cisplatin and 5-fluorouracil have been the standard drugs used for induction.123 Two recent phase III studies have confirmed the benefit of adding docetaxel to this regimen. A 27% to 30% reduction in risk for death was reported.124,125 In one study of unresectable disease, use of radiotherapy after this treatment regimen extended survival time from a median of 14.5 months to 18.8 months.125 In the second study of patients with stage III and stage IV cancer, the 3-year survival rate in the group receiving cisplatin, fluorouracil, and docetaxel was 62% as opposed to 48% in the group receiving cisplatin plus fluorouracil.124 Both groups in this later study received chemoradiation with carboplatin. Ongoing phase II studies are evaluating the benefits of other induction regimens such as cetuximab, paclitaxel, and carboplatin on advanced locoregional squamous cell carcinoma of the head and neck.126
Intra-arterial delivery of chemotherapeutic agents was developed in an effort to deliver higher concentrations to the tumor site while minimizing toxic reactions.127,128 Neoplasms of the paranasal sinuses are particularly suited to this approach because they tend to be encompassed within the territory of the internal maxillary artery, which can be selectively catheterized. Using this method for induction chemotherapy, Lee and associates127 achieved an immediate tumor response in 91% of patients.
Some advocate a preoperative trial of cisplatin in all patients who have large paranasal sinus tumors with intracranial extension129 because substantial regression of the tumor may allow easier and more complete excision. Induction chemotherapy is a key aspect of orbit-sparing protocols.73,83,130 Chemotherapeutic agents may also be used as radiosensitizers to enhance the effects of radiation on tumor cells. In one series, patients with locally advanced or massive recurrent disease received cisplatin infusions in conjunction with hyperfractionated radiation administered over a period of 8 weeks and achieved a 92% response rate and a 58% survival rate after 3 to 6 years.131 In a small series of just 11 patients, preoperative cisplatin administered concurrently with radiotherapy followed by conservative craniofacial resection produced an overall 5-year survival rate of 81%.130 Certain tumor pathologies are best treated by chemotherapy. The prime example is NEC, for which we recommend induction chemotherapy with etoposide and cisplatinum, followed by radiotherapy if a complete response is obtained or by surgical excision followed by radiotherapy if a partial response is obtained.45,132 The best long-term outcome for patients with advanced sinonasal malignancy will probably be achieved with multimodality therapy, including induction chemotherapy, surgery, and chemoradiotherapy.126,130,133–135
Outcome
Because of the variety of tumor types occurring in the paranasal sinuses and their consequent great range of biologic behavior, analysis of outcomes is difficult. Early reports failed to demonstrate a preferred treatment modality, and overall 5-year survival rates did not exceed 30%.64 A substantial improvement in long-term disease control has been attributed to craniofacial resection of these tumors. Recent large surgical series using craniofacial resection have reported survival rates of 47% to 70% at 5 years and 25% to 48% at 10 years for all types of malignant tumors.71,84,90,91,93–95,136–138 Five-year survival rates are 32% to 73% for squamous cell carcinoma and 43% to 82% for adenocarcinoma.90,93,94,96 The largest published series is that of Ganly and associates, who reported the results of an international collaborative study with data from 17 institutions on 334 patients with paranasal sinus tumors who underwent craniofacial resection (excluding those with ON).85 The overall survival rate at 5 years was 48.3%, the 5-year disease-specific survival rate was 53.3%, and the 5-year recurrence-free survival rate was 45.8%.
Another large series is that of Lund and coauthors, who reported their 17-year experience with craniofacial resection for paranasal sinus tumors in 209 patients.94 The overall survival rates were 51% at 5 years and 41% at 10 years. Five- and 10-year survival rates for all malignant tumors were 44% and 32%, respectively. Survival rates at 5 and 10 years, respectively, for individual histologies were as follows: squamous cell carcinoma, 32% and 32%; adenocarcinoma, 43% and 38%; adenoid cystic carcinoma, 51% and 36%; and ON, 62% and 47%. Reporting specifically on the management outcomes of patients with ON, Diaz and coworkers, who primarily used craniofacial resection and postoperative radiation therapy, reported 5- and 10-year overall survival rates of 89% and 81%, respectively.139 The mean time to recurrence in these patients was just less than 5 years.
McCutcheon and colleagues reviewed 76 patients who underwent craniofacial resection (47 patients) or anterior transcranial resection (29 patients) for tumors of the paranasal sinuses between 1984 and 1993.79 Most patients had ethmoidal sinus involvement, with adenocarcinoma (13 patients) being the most common tumor type, followed by squamous cell carcinoma and ON (11 patients each). The mean follow-up interval for the 50 patients who were still alive at the end of the study period was 34 months (range, 1 month to 10.4 years). Forty-two (84%) of these patients were without evidence of disease. Calculation of survival times in the 26 patients who died revealed that most (74%) did so in the first 20 months after surgery. The median survival time was 20 months for patients with squamous cell carcinoma, 26 months for those with adenocarcinoma, and 40 months for those with ON. Twenty-seven patients (36%) experienced complications, and 1 died (1%) on the second postoperative day as a result of cardiac dysrhythmia.
In various reports, factors that had a significant effect on patient outcome were a tumor that had malignant histology, involved the brain, or involved the orbit.5,94,95 The presence of resection margins not free of tumor and brain invasion by the tumor has also been shown to be a predictor of local recurrence and shorter survival.5,95,136–138,140–142 Yet even in those with transdural extension of malignancy, well-selected patients can have outcomes similar to those without intradural tumoral extension. In this group of patients, the most important factors affecting overall and progression-free survival were the ability to achieve resection margins that were negative for tumor, when viewed microscopically, and the lack of direct brain invasion. Patients with malignant tumors showing primary involvement of the sphenoidal sinus, although constituting only 1% to 2% of all patients with paranasal sinus tumors, are an especially difficult subgroup to manage effectively. However, even in this group of patients, aggressive multimodality therapy can result in a 2-year survival rate of 44% for patients with squamous cell carcinoma.61
Hentschel and associates found no difference in oncologic outcome in a population of patients who were at least 70 years old and a younger cohort.143 The elderly cohort did, however, have a significantly greater incidence of systemic complications.
There are many treatment strategies for recurrent disease. Dissemination to the lymph nodes, which occurs in 5% to 10% of patients in long-term follow-up, can be managed with radical neck dissection and radiotherapy. Distant metastases to the bone, lung, and liver, seen in a similar proportion of patients, can be treated by local excision or radiotherapy, or both, as well as by systemic chemotherapy.144 Intracranial metastasis is very rare.145
Blacklock JB, Weber RS, Lee YY, et al. Transcranial resection of tumors of the paranasal sinuses and nasal cavity. J Neurosurg. 1989;71:10-15.
Cantu G, Solero CL, Mariani L, et al. Anterior craniofacial resection for malignant ethmoid tumors—a series of 91 patients. Head Neck. 1999;21:185-191.
Chang DW, Langstein HN, Gupta A, et al. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107:1346-1355.
Cohen ZR, Marmor E, Fuller GN, et al. Misdiagnosis of olfactory neuroblastoma. Neurosurg Focus. 2002;12(5):E3.
DeMonte F, Ginsberg LE, Clayman GL. Primary malignant tumors of the sphenoidal sinus. Neurosurgery. 2000;46:1084-1091.
DeMonte F, Hanna EY. Transmaxillary exploration of the intracranial portion of the maxillary nerve in malignant perineural disease. J Neurosurg. 2007;107:672-677.
DeMonte F, Tabrizi P, Culpepper SA, et al. Ophthalmological outcome after orbital entry during anterior and anterolateral skull base surgery. J Neurosurg. 2002;97:851-856.
Diaz EM, Johnigan RH, Pero E, et al. Olfactory neuroblastoma. The 22-year experience at one comprehensive cancer center. Head Neck. 2005;27(2):138-149.
Dirix P, Nuyts S, Vanstraelen B, et al. Post-operative intensity-modulated radiotherapy for malignancies of the nasal cavity and paranasal sinuses. Radiother Oncol. 2007;85:385-391.
Feiz-Erfan I, Suki D, Hanna E, et al. Prognostic significance of transdural invasion of cranial base malignancies in patients undergoing craniofacial resection. Neurosurgery. 2007;61:1178-1185.
Fukuda S, Sakai N, Kamata SE, et al. Surgical results of skull base surgery for the treatment of head and neck malignancies involving skull base: multi-institutional studies on 143 cases in Japan. Auris Nasus Larynx. 2001;28(suppl):S71-S75.
Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: report of an International Collaborative Study. Head Neck. 2005;27:575-584.
Hentschel SJ, Nader R, Suki D, et al. Craniofacial resections in the elderly: an outcome study. J Neurosurg. 2004;101:935-943.
Howard DJ, Lund VJ, Wei WI. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses: a 25-year experience. Head Neck. 2006;28:867-873.
Kies MS. Induction chemotherapy for head and neck squamous cell carcinomas (SCCHN). Curr Treat Options Oncol. 2007;8:252-260.
Lund V, Howard DJ, Wei WI. Endoscopic resection of malignant tumors of the nose and sinuses. Am J Rhinol. 2007;21:89-94.
Lupinetti AD, Roberts DB, Williams MD, et al. Sinonasal adenoid cystic carcinoma: the M. D. Anderson Cancer experience. Cancer. 2007;110:2726-2731.
Madison Michael L2nd, Sorenson JM, Samant S, et al. The treatment of advanced sinonasal malignancies with pre-operative intra-arterial cisplatin and concurrent radiation. J Neurooncol. 2005;72:67-75.
McCutcheon IE, Blacklock JB, Weber RS, et al. Anterior transcranial (craniofacial) resection of tumors of the paranasal sinuses: surgical technique and results. Neurosurgery. 1996;38:471-479.
Pesch B, Pierl CB, Gebel M, et al. Occupational risks for adenocarcinoma of the nasal cavity and paranasal sinuses in the German wood industry. Occup Environ Med. 2008;65:191-196.
Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705-1715.
Roh HJ, Batra PS, Citardi MJ, et al. Endoscopic resection of sinonasal malignancies: a preliminary report. Am J Rhinol. 2004;18:239-246.
Rosenthal DI, Barker JL, El-Naggar AK, et al. Sinonasal malignancies with neuroendocrine differentiation. Patterns of failure according to histologic phenotype. Cancer. 2004;101:2567-2573.
Shah JP, Kraus DH, Bilsky MH, et al. Craniofacial resection for malignant tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1997;123:1312-1317.
Suarez C, Ferlito A, Lund VJ, et al. Management of the orbit in malignant sinonasal tumors. Head Neck. 2008;30:242-250.
Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695-1704.
1 Muir CS, Nectoux J. Descriptive epidemiology of malignant neoplasms of nose, nasal cavities, middle ear and accessory sinuses. Clin Otolaryngol Allied Sci. 1980;5:195-211.
2 Ali S, McGarry GW. Endoscopic minimal access surgery in nasal and sinus tumours: lessons from initial experience. Clin Otolaryngol. 2008;33:42-46.
3 Robin PE, Powell DJ, Stansbie JM. Carcinoma of the nasal cavity and paranasal sinuses: incidence and presentation of different histological types. Clin Otolaryngol Allied Sci. 1979;4:431-456.
4 Alvarez I, Suarez C, Rodrigo JP, et al. Prognostic factors in paranasal sinus cancer. Am J Otolaryngol. 1995;16:109-114.
5 Euteneuer S, Sudhoff H, Bernal-Sprekelsen M, et al. [Malignomas of the nasal cavity and the paranasal sinuses: clinical characteristics, therapy and prognosis of different tumor types.]. Laryngorhinootologie. 2004;83:33-39.
6 Olsen JH. Epidemiology of sinonasal cancer in Denmark, 1943-1982. Acta Pathol Microbiol Immunol Scand [A]. 1987;95:171-175.
7 Zheng W, McLaughlin JK, Chow WH, et al. Risk factors for cancers of the nasal cavity and paranasal sinuses among white men in the United States. Am J Epidemiol. 1993;138:965-972.
8 ‘t Mannetje A, Kogevinas M, Luce D, et al. Sinonasal cancer, occupation, and tobacco smoking in European women and men. Am J Ind Med. 1999;36:101-107.
9 Doll R, Morgan LG, Speizer FE. Cancers of the lung and nasal sinuses in nickel workers. Br J Cancer. 1970;24:623-632.
10 Egedahl RD, Coppock E, Homik R. Mortality experience at a hydrometallurgical nickel refinery in Fort Saskatchewan, Alberta between 1954 and 1984. J Soc Occup Med. 1991;41:29-33.
11 Egedahl R, Carpenter M, Lundell D. Mortality experience among employees at a hydrometallurgical nickel refinery and fertiliser complex in Fort Saskatchewan, Alberta (1954-95). Occup Environ Med. 2001;58:711-715.
12 Pesch B, Pierl CB, Gebel M, et al. Occupational risks for adenocarcinoma of the nasal cavity and paranasal sinuses in the German wood industry. Occup Environ Med. 2008;65:191-196.
13 Bimbi G, Saraceno MS, Riccio S, et al. Adenocarcinoma of ethmoid sinus: an occupational disease. Acta Otorhinolaryngol Ital. 2004;24:199-203.
14 Acheson ED, Cowdell RH, Hadfield E, et al. Nasal cancer in woodworkers in the furniture industry. Br Med J. 1968;2:587-596.
15 Demers PA, Kogevinas M, Boffetta P, et al. Wood dust and sino-nasal cancer: pooled reanalysis of twelve case-control studies. Am J Ind Med. 1995;28:151-166.
16 Gordon I, Boffetta P, Demers PA. A case study comparing a meta-analysis and a pooled analysis of studies of sinonasal cancer among wood workers. Epidemiology. 1998;9:518-524.
17 Holmila R, Cyr D, Luce D, et al. COX-2 and p53 in human sinonasal cancer: COX-2 expression is associated with adenocarcinoma histology and wood-dust exposure. Int J Cancer. 2008;122:2154-2159.
18 Roush GC. Epidemiology of cancer of the nose and paranasal sinuses: current concepts. Head Neck Surg. 1979;2:3-11.
19 Luce D, Gerin M, Morcet JF, et al. Sinonasal cancer and occupational exposure to textile dust. Am J Ind Med. 1997;32:205-210.
20 Bonneterre V, Deschamps E, Persoons R, et al. Sino-nasal cancer and exposure to leather dust. Occup Med (Lond). 2007;57:438-443.
21 Battista G, Comba P, Orsi D, et al. Nasal cancer in leather workers: an occupational disease. J Cancer Res Clin Oncol. 1995;121:1-6.
22 Harrison DF. The management of malignant tumors of the nasal sinuses. Otolaryngol Clin North Am. 1971;4:159-177.
23 Fukuda K, Kojiro M, Hirano M, et al. Predominance of squamous cell carcinoma and rarity of adenocarcinoma of maxillary sinus among Japanese. Kurume Med J. 1989;36:1-6.
24 Fukuda K, Shibata A, Harada K. Squamous cell cancer of the maxillary sinus in Hokkaido, Japan: a case-control study. Br J Ind Med. 1987;44:263-266.
25 Mitarnun W, Suwiwat S, Pradutkanchana J. Epstein-Barr virus–associated extranodal non-Hodgkin’s lymphoma of the sinonasal tract and nasopharynx in Thailand. Asian Pac J Cancer Prev. 2006;7:91-94.
26 Lopategui JR, Gaffey MJ, Frierson HFJr, et al. Detection of Epstein-Barr viral RNA in sinonasal undifferentiated carcinoma from Western and Asian patients. Am J Surg Pathol. 1994;18:391-398.
27 Cerilli LA, Holst VA, Brandwein MS, et al. Sinonasal undifferentiated carcinoma: immunohistochemical profile and lack of EBV association. Am J Surg Pathol. 2001;25:156-163.
28 Batsakis J. Pathology of tumors of the nasal cavity and paranasal sinuses, 2nd ed. Thawley SE, Panje WR, Batsakis JG, et al, editors. Comprehensive Management of Head and Neck Tumors, Vol 1. Philadelphia: WB Saunders. 1999:522-539.
29 Myers LL, Oxford LE. Differential diagnosis and treatment options in paranasal sinus cancers. Surg Oncol Clin N Am. 2004;13:167-186.
30 Batsakis JG, Suarez P. Schneiderian papillomas and carcinomas: a review. Adv Anat Pathol. 2001;8:53-64.
31 Ferlito A, Devaney KO, Rinaldo A. Association between Schneiderian papilloma of the temporal bone and carcinoma: a critical review of the literature. Acta Otolaryngol. 2004;124:121-123.
32 Gaffey MJ, Frierson HF, Weiss LM, et al. Human papillomavirus and Epstein-Barr virus in sinonasal Schneiderian papillomas. An in situ hybridization and polymerase chain reaction study. Am J Clin Pathol. 1996;106:475-482.
33 Kim JY, Yoon JK, Citardi MJ, et al. The prevalence of human papilloma virus infection in sinonasal inverted papilloma specimens classified by histological grade. Am J Rhinol. 2007;21:664-669.
34 Choi HRSE, Sturgis EM, Rashid A, et al. Sinonasal adenocarcinoma: Evidence for histogenetic divergence of the enteric and non-enteric phenotypes. Hum Pathol. 2003;34:1101-1107.
35 Hyams VJ, Batsakis JG, Michaels L. Tumors of the Upper Respiratory Tract and Ear, 2nd ed. Washington, DC: Armed Forces Institute of Pathology; 1988.
36 Naficy S, Disher MJ, Esclamado RM. Adenoid cystic carcinoma of the paranasal sinuses. Am J Rhinol. 1999;13:311-314.
37 Ginsberg LE, De Monte F, Gillenwater AM. Greater superficial petrosal nerve: anatomy and MR findings in perineural tumor spread. AJNR Am J Neuroradiol. 1996;17:389-393.
38 Ginsberg LE. Imaging of perineural tumor spread in head and neck cancer. Semin Ultrasound CT MR. 1999;20:175-186.
39 Lupinetti AD, Roberts DB, Williams MD, et al. Sinonasal adenoid cystic carcinoma: the M. D. Anderson Cancer experience. Cancer. 2007;110:2726-2731.
40 Silva EG, Butler JJ, Mackay B, et al. Neuroblastomas and neuroendocrine carcinomas of the nasal cavity: a proposed new classification. Cancer. 1982;50:2388-2405.
41 Cohen ZR, Marmor E, Fuller GN, et al. Misdiagnosis of olfactory neuroblastoma. Neurosurg Focus. 2002;12(5):E3.
42 Austin JR, Cebrun H, Kershisnik MM, et al. Olfactory neuroblastoma and neuroendocrine carcinoma of the anterior skull base: treatment results at the M. D. Anderson Cancer. Skull Base Surg. 1996;6:1-8.
43 Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102:843-849.
44 Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37:1571-1576.
45 Morita A, Ebersold MJ, Olsen KD, et al. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32:706-714.
46 Dias FL, Sa GM, Lima RA, et al. Patterns of failure and outcome in esthesioneuroblastoma. Arch Otolaryngol Head Neck Surg. 2003;129:1186-1192.
47 Ejaz A, Wenig BM. Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv Anat Pathol. 2005;12:134-143.
48 Houston GD, Gillies E. Sinonasal undifferentiated carcinoma: a distinctive clinicopathologic entity. Adv Anat Pathol. 1999;6:317-323.
49 Kerrebijn JD, Tietze L, Mock D, et al. Sinonasal undifferentiated carcinoma. J Otolaryngol. 1998;27:40-42.
50 Mendenhall WM, Mendenhall CM, Riggs CEJr, et al. Sinonasal undifferentiated carcinoma. Am J Clin Oncol. 2006;29:27-31.
51 Rosenthal DI, Barker JL, El-Naggar AK, et al. Sinonasal malignancies with neuroendocrine differentiation. Patterns of failure according to histologic phenotype. Cancer. 2004;101:2567-2573.
52 Mishra A, El-Naggar AK, DeMonte F, et al. Endodermal sinus tumor of the paranasal sinuses. Head Neck. 2008;30:539-543.
53 Swain REJr, Kingdom TT, DelGaudio JM, et al. Meningiomas of the paranasal sinuses. Am J Rhinol. 2001;15:27-30.
54 Pfeiffer J, Arapakis I, Boedeker CC, et al. Malignant peripheral nerve sheath tumour of the paranasal sinuses and the anterior skull base. J Craniomaxillofac Surg. 2008;36:293-299.
55 Batsakis JG, Rice DH, Solomon AR. The pathology of head and neck tumors: squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Head Neck Surg. 1980;2:497-508.
56 Morshed K, Golqbek E, Drop A, et al. [Extension of nasal and paranasal sinus malignancies on the basis of computed tomography.]. Otolaryngol Pol. 2007;61:576-580.
57 Chaudhry AP, Gorlin RJ, Mosser DG. Carcinoma of the antrum: a clinical and histopathologic study. Oral Surg Oral Med Oral Pathol. 1960;13:269-281.
58 Weber AL, Stanton AC. Malignant tumors of the paranasal sinuses: radiologic, clinical, and histopathologic evaluation of 200 cases. Head Neck Surg. 1984;6:761-776.
59 Larsson LG, Martensson G. Maxillary antral cancers. JAMA. 1972;219:342-345.
60 Rice DH, Stanley RBJr. Surgical therapy of tumors of the nasal cavity, ethmoid sinus, and maxillary sinus, 2nd ed. Thawley SE, Panje WR, Batsakis JG, et al, editors. Comprehensive Management of Head and Neck Tumors, Vol 1. Philadelphia: WB Saunders. 1999:558-565.
61 DeMonte F, Ginsberg LE, Clayman GL. Primary malignant tumors of the sphenoidal sinus. Neurosurgery. 2000;46:1084-1091.
62 Cantu G, Bimbi G, Miceli R, et al. Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008;134:170-177.
63 Choussy O, Ferron C, Vedrine PO, et al. Adenocarcinoma of ethmoid: a GETTEC retrospective multicenter study of 418 cases. Laryngoscope. 2008;118:437-443.
64 Sisson GASr, Toriumi DM, Atiyah RA. Paranasal sinus malignancy: a comprehensive update. Laryngoscope. 1989;99:143-150.
65 Branstetter BF4th, Weissman JL. Role of MR and CT in the paranasal sinuses. Otolaryngol Clin North Am. 2005;38:1279-1299. x
66 Maroldi R, Ravanelli M, Borghesi A, et al. Paranasal sinus imaging. Eur J Radiol. 2008;66:372-386.
67 Lund VJ, Howard DJ, Lloyd GA, et al. Magnetic resonance imaging of paranasal sinus tumors for craniofacial resection. Head Neck. 1989;11:279-283.
68 Batra PS, Citardi MJ. Endoscopic management of sinonasal malignancy. Otolaryngol Clin North Am. 2006;39:619-637. x-xi
69 American Joint Committee on Cancer (AJCC) Staging Manual. 5th ed. Philadelphia: Lippincott-Raven. 1997.
70 Parsons JT, Mendenhall WM, Mancuso AA, et al. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys. 1988;14:11-22.
71 Cantu G, Solero CL, Mariani L, et al. Anterior craniofacial resection for malignant ethmoid tumors—a series of 91 patients. Head Neck. 1999;21:185-191.
72 Harbo G, Bundgaard T, Overgaard J, et al. Comparison of two T-classification systems for sino-nasal carcinoma. Clin Otolaryngol Allied Sci. 2002;27:254-259.
73 Dulguerov P, Allal AS. Nasal and paranasal sinus carcinoma: how can we continue to make progress? Curr Opin Otolaryngol Head Neck Surg. 2006;14:67-72.
74 Smith RR, Klopp CT, Williams JM. Surgical treatment of cancer of the frontal sinus and adjacent areas. Cancer. 1954;7:991-994.
75 Ketcham AS, Wilkins RH, Vanburen JM, et al. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106:698-703.
76 Ketcham AS, Chretien PB, Van Buren JM, et al. The ethmoid sinuses: a re-evaluation of surgical resection. Am J Surg. 1973;126:469-476.
77 Terz JJ, Alksne JF, Lawrence WJr. Craniofacial resection for tumors invading the pterygoid fossa. Am J Surg. 1969;118:732-740.
78 Blacklock JB, Weber RS, Lee YY, et al. Transcranial resection of tumors of the paranasal sinuses and nasal cavity. J Neurosurg. 1989;71:10-15.
79 McCutcheon IE, Blacklock JB, Weber RS, et al. Anterior transcranial (craniofacial) resection of tumors of the paranasal sinuses: surgical technique and results. Neurosurgery. 1996;38:471-479.
80 DeMonte F, Hanna EY. Transmaxillary exploration of the intracranial portion of the maxillary nerve in malignant perineural disease. J Neurosurg. 2007;107:672-677.
81 Suarez C, Ferlito A, Lund VJ, et al. Management of the orbit in malignant sinonasal tumors. Head Neck. 2008;30:242-250.
82 Perry C, Levine PA, Williamson BR, et al. Preservation of the eye in paranasal sinus cancer surgery. Arch Otolaryngol Head Neck Surg. 1988;114:632-634.
83 McCary WS, Levine PA, Cantrell RW. Preservation of the eye in the treatment of sinonasal malignant neoplasms with orbital involvement. A confirmation of the original treatise. Arch Otolaryngol Head Neck Surg. 1996;122:657-659.
84 Ketcham AS, Van Buren JM. Tumors of the paranasal sinuses: a therapeutic challenge. Am J Surg. 1985;150:406-413.
85 Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: report of an International Collaborative Study. Head Neck. 2005;27:575-584.
86 Namon AJ, Panje WR. Controversy in the management of tumors of the nasal cavity and paranasal sinuses, 2nd ed. Thawley SE, Panje WR, Batsakis JG, et al, editors. Comprehensive Management of Head and Neck Tumors, Vol 1. Philadelphia: WB Saunders. 1999:625-629.
87 Stern SJ, Goepfert H, Clayman G, et al. Orbital preservation in maxillectomy. Otolaryngol Head Neck Surg. 1993;109:111-115.
88 DeMonte F, Tabrizi P, Culpepper SA, et al. Ophthalmological outcome after orbital entry during anterior and anterolateral skull base surgery. J Neurosurg. 2002;97:851-856.
89 Chang DW, Langstein HN, Gupta A, et al. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107:1346-1355.
90 Terz JJ, Young HF, Lawrence WJr. Combined craniofacial resection for locally advanced carcinoma of the head and neck II. Carcinoma of the paranasal sinuses. Am J Surg. 1980;140:618-624.
91 Sundaresan N, Shah JP. Craniofacial resection for anterior skull base tumors. Head Neck Surg. 1988;10:219-224.
92 Kraus DH, Shah JP, Arbit E, et al. Complications of craniofacial resection for tumors involving the anterior skull base. Head Neck. 1994;16:307-312.
93 Shah JP, Kraus DH, Bilsky MH, et al. Craniofacial resection for malignant tumors involving the anterior skull base. Arch Otolaryngol Head Neck Surg. 1997;123:1312-1317.
94 Lund VJ, Howard DJ, Wei WI, et al. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses—a 17-year experience. Head Neck. 1998;20:97-105.
95 Richtsmeier WJ, Briggs RJ, Koch WM, et al. Complications and early outcome of anterior craniofacial resection. Arch Otolaryngol Head Neck Surg. 1992;118:913-917.
96 Danks RA, Kaye AH, Millar H, et al. Craniofacial resection in the management of paranasal sinus cancer. J Clin Neurosci. 1994;1:111-117.
97 Fukuda S, Sakai N, Kamata SE, et al. Surgical results of skull base surgery for the treatment of head and neck malignancies involving skull base: multi-institutional studies on 143 cases in Japan. Auris Nasus Larynx. 2001;28(suppl):S71-S75.
98 Gendeh BS, Salina H, Selladurai B, et al. Endoscopic-assisted craniofacial resection: a case series and post-operative outcome. Med J Malaysia. 2007;62:234-237.
99 Hemmerdinger SA, Jacobs JB, Lebowitz RA. Accuracy and cost analysis of image-guided sinus surgery. Otolaryngol Clin North Am. 2005;38:453-460.
100 Roh HJ, Batra PS, Citardi MJ, et al. Endoscopic resection of sinonasal malignancies: a preliminary report. Am J Rhinol. 2004;18:239-246.
101 Lund V, Howard DJ, Wei WI. Endoscopic resection of malignant tumors of the nose and sinuses. Am J Rhinol. 2007;21:89-94.
102 Bogaerts S, VanderPoorten V, Nuyts S, et al. Results of endoscopic resection followed by radiotherapy for primarily diagnosed adenocarcinomas of the paranasal sinuses. Head Neck. 2008;30:728-736.
103 Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(1):E3.
104 Hanna EY, Holsinger C, DeMonte F, et al. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133:1209-1214.
105 Gabriele P, Besozzi MC, Pisani P, et al. [Carcinoma of the paranasal sinuses. Results with radiotherapy alone or with a radio-surgical combination.]. Radiol Med (Torino). 1986;72:210-214.
106 Anniko M, Franzen L, Lofroth PO. Long-term survival of patients with paranasal sinus carcinoma. ORL J Otorhinolaryngol Relat Spec. 1990;52:187-193.
107 Osguthorpe JD. Sinus neoplasia. Arch Otolaryngol Head Neck Surg. 1994;120:19-25.
108 Cheesman AD, Lund VJ, Howard DJ. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses. Head Neck Surg. 1986;8:429-435.
109 Moss WT. Radiation therapy for tumors of the nasal cavity and paranasal sinus, 2nd ed. Thawley SE, Panje WR, Batsakis JG, et al, editors. Comprehensive Management of Head and Neck Tumors, Vol 1. Philadelphia: WB Saunders. 1999:601-607.
110 Parsons JT, Kimsey FC, Mendenhall WM, et al. Radiation therapy for sinus malignancies. Otolaryngol Clin North Am. 1995;28:1259-1268.
111 Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821-829.
112 Jansen EP, Keus RB, Hilgers FJ, et al. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys. 2000;48:27-35.
113 Robson A. Evidence-based management of sinonasal and nasopharyngeal tumours. Clin Otolaryngol Allied Sci. 2003;28:559-564.
114 Dirix P, Nuyts S, Geussens Y, et al. Malignancies of the nasal cavity and paranasal sinuses: long-term outcome with conventional or three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1042-1050.
115 Dirix P, Nuyts S, Vanstraelen B, et al. Post-operative intensity-modulated radiotherapy for malignancies of the nasal cavity and paranasal sinuses. Radiother Oncol. 2007;85:385-391.
116 Jiang GL, Ang KK, Peters LJ, et al. Maxillary sinus carcinomas: natural history and results of postoperative radiotherapy. Radiother Oncol. 1991;21:193-200.
117 Nagata Y, Okajima K, Murata R, et al. Three-dimensional treatment planning for maxillary cancer using a CT simulator. Int J Radiat Oncol Biol Phys. 1994;30:979-983.
118 Roa WH, Hazuka MB, Sandler HM, et al. Results of primary and adjuvant CT-based 3-dimensional radiotherapy for malignant tumors of the paranasal sinuses. Int J Radiat Oncol Biol Phys. 1994;28:857-865.
119 Stammberger H, Anderhuber W, Walch C, et al. Possibilities and limitations of endoscopic management of nasal and paranasal sinus malignancies. Acta Otorhinolaryngol Belg. 1999;53:199-205.
120 Habermann W, Zanarotti U, Groell R, et al. Combination of surgery and gamma knife radiosurgery—a therapeutic option for patients with tumors of nasal cavity or paranasal sinuses infiltrating the skull base. Acta Otorhinolaryngol Ital. 2002;22:74-79.
121 Duthoy W, Boterberg T, Claus F, et al. Postoperative intensity-modulated radiotherapy in sinonasal carcinoma: clinical results in 39 patients. Cancer. 2005;104:71-82.
122 LoRusso P, Tapazoglou E, Kish JA, et al. Chemotherapy for paranasal sinus carcinoma. A 10-year experience at Wayne State University. Cancer. 1988;62:1-5.
123 Kish JA, Weaver A, Jacobs J, et al. Cisplatin and 5-fluorouracil infusion in patients with recurrent and disseminated epidermoid cancer of the head and neck. Cancer. 1984;53:1819-1824.
124 Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705-1715.
125 Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695-1704.
126 Cruz JJ, Ocana A, Navarro M, et al. New options in the treatment of locally advanced head and neck cancer: role for induction chemotherapy. Cancer Treat Rev. 2008;34:268-274.
127 Lee YY, Dimery IW, Van Tassel P, et al. Superselective intra-arterial chemotherapy of advanced paranasal sinus tumors. Arch Otolaryngol Head Neck Surg. 1989;115:503-511.
128 Inuyama Y, Kawaura M, Toji M, et al. [Intra-arterial chemotherapy of maxillary sinus carcinoma.]. Gan To Kagaku Ryoho. 1989;16:2688-2691.
129 Arbit E, Sundaresan N, Galicich JH, et al. Craniofacial resection following chemotherapy. Surg Neurol. 1980;13:395-399.
130 Madison Michael L2nd, Sorenson JM, Samant S, et al. The treatment of advanced sinonasal malignancies with pre-operative intra-arterial cisplatin and concurrent radiation. J Neurooncol. 2005;72:67-75.
131 Choi KN, Rotman M, Aziz H, et al. Locally advanced paranasal sinus and nasopharynx tumors treated with hyperfractionated radiation and concomitant infusion cisplatin. Cancer. 1991;67:2748-2752.
132 Demonte F, McElroy EA, Buckner JC, et al. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998;42:1028.
133 Lee MM, Vokes EE, Rosen A, et al. Multimodality therapy in advanced paranasal sinus carcinoma: superior long-term results. Cancer J Sci Am. 1999;5:219-223.
134 Papadimitrakopoulou VA, Hong WK. Combined-modality therapy in paranasal sinus cancer. Cancer J Sci Am. 1999;5:208-210.
135 Kies MS. Induction chemotherapy for head and neck squamous cell carcinomas (SCCHN). Curr Treat Options Oncol. 2007;8:252-260.
136 Howard DJ, Lund VJ, Wei WI. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses: a 25-year experience. Head Neck. 2006;28:867-873.
137 Bridger GP, Kwok B, Baldwin M, et al. Craniofacial resection for paranasal sinus cancers. Head Neck. 2000;22:772-780.
138 Blanch JL, Ruiz AM, Alos L, et al. Treatment of 125 sinonasal tumors: prognostic factors, outcome, and follow-up. Otolaryngol Head Neck Surg. 2004;131:973-976.
139 Diaz EM, Johnigan RH, Pero E, et al. Olfactory neuroblastoma. The 22-year experience at one comprehensive cancer center. Head Neck. 2005;27(2):138-149.
140 Clayman GL, DeMonte F, Jaffe DM, et al. Outcome and complications of extended cranial-base resection requiring microvascular free-tissue transfer. Arch Otolaryngol Head Neck Surg. 1995;121(2):1253-1257.
141 Feiz-Erfan I, Suki D, Hanna E, et al. Prognostic significance of transdural invasion of cranial base malignancies in patients undergoing craniofacial resection. Neurosurgery. 2007;61:1178-1185.
142 Blanco AI, Chao KS, Ozyigit G, et al. Carcinoma of paranasal sinuses: long-term outcomes with radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:51-58.
143 Hentschel SJ, Nader R, Suki D, et al. Craniofacial resections in the elderly: an outcome study. J Neurosurg. 2004;101:935-943.
144 Konno A, Togawa K, Inoue S. Analysis of the results of our combined therapy for maxillary cancer. Acta Otolaryngol Suppl. 1980;372:1-16.
145 Murphy MA, Kaye AH, Hayes IP. Intracranial metastasis from carcinoma of the paranasal sinus. Neurosurgery. 1991;28:890-893.

 ,
,