Neonatal medicine
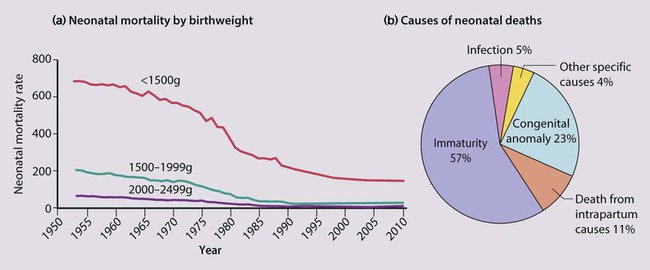
The dramatic reduction in neonatal mortality throughout the developed world has resulted from advances in the management of newborn infants together with improvements in maternal health and obstetric care. Neonatal intensive care became increasingly available in the UK from 1975, and it is since that time that the mortality of very low birthweight (VLBW) infants has fallen (Fig. 10.1).
In the UK, neonatal units are organised as networks, with units providing either:
Hypoxic-ischaemic encephalopathy
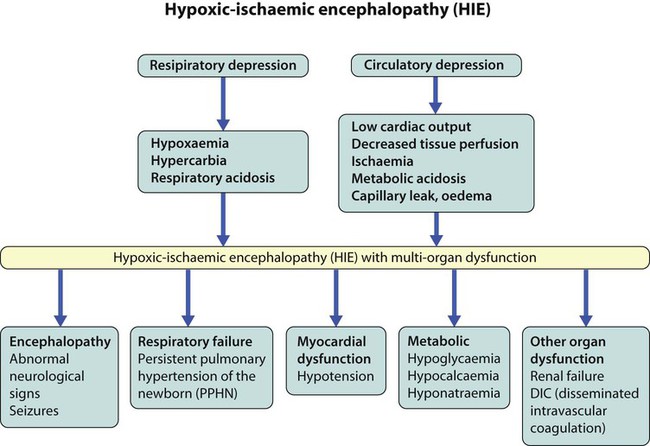
In perinatal asphyxia, gas exchange, either placental or pulmonary, is compromised or ceases altogether, resulting in cardiorespiratory depression. Hypoxia, hypercarbia and metabolic acidosis follow. Compromised cardiac output diminishes tissue perfusion, causing hypoxic-ischaemic injury to the brain and other organs. The neonatal condition is called hypoxic-ischaemic encephalopathy (HIE). It remains an important cause of brain damage, resulting in disability (Fig. 10.2) or death, and its prevention is one of the key aims of modern obstetric care. In developed countries, approximately 0.5–1/1000 liveborn term infants develop HIE and 0.3/1000 have significant neurologic disability. The incidence is higher in developing countries.
• Failure of gas exchange across the placenta – excessive or prolonged uterine contractions, placental abruption, ruptured uterus
• Interruption of umbilical blood flow – cord compression including shoulder dystocia, cord prolapse
• Inadequate maternal placental perfusion, maternal hypotension or hypertension – often with intrauterine growth restriction
• Compromised fetus – anemia, intrauterine growth restriction
• Failure of cardiorespiratory adaptation at birth – failure to breathe.
The clinical manifestations start immediately or up to 48 h after asphyxia, and can be graded:
• Mild – the infant is irritable, responds excessively to stimulation, may have staring of the eyes and hyperventilation and has impaired feeding
• Moderate – the infant shows marked abnormalities of tone and movement, cannot feed and may have seizures
• Severe – there are no normal spontaneous movements or response to pain; tone in the limbs may fluctuate between hypotonia and hypertonia; seizures are prolonged and often refractory to treatment; multi-organ failure is present.
Management
• recording of amplitude-integrated electroencephalogram (aEEG, cerebral function monitor) to detect abnormal background activity to confirm early encephalopathy or identify seizures
• treatment of clinical seizures with anticonvulsants
• fluid restriction because of transient renal impairment
• treatment of hypotension by volume and inotrope support
• monitoring and treatment of hypoglycaemia and electrolyte imbalance, especially hypocalcaemia.
Prognosis
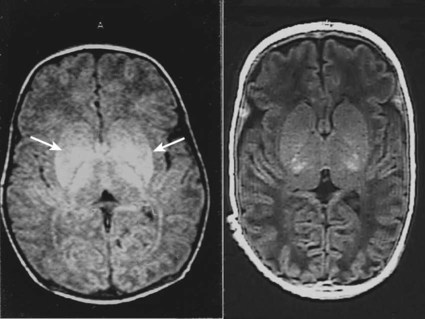
When HIE is mild, complete recovery can be expected. Infants with moderate HIE who have recovered fully on clinical neurological examination and are feeding normally by 2 weeks of age have an excellent long-term prognosis, but if clinical abnormalities persist beyond that time, full recovery is unlikely. Severe HIE has a mortality of 30–40%, and, of the survivors, over 80% have neurodevelopmental disabilities, particularly cerebral palsy. If magnetic resonance imaging (MRI) at 4–14 days in a term infant shows significant abnormalities (bilateral abnormalities in the basal ganglia and thalamus and lack of myelin in the posterior limb of the internal capsule), there is a very high risk of later cerebral palsy (Fig. 10.3).
Birth injuries
Soft tissue injuries
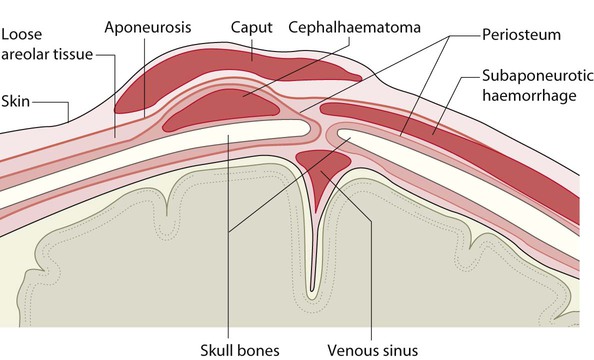
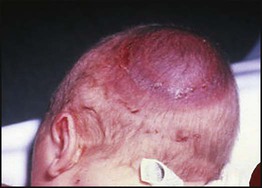
• Caput succedaneum (Fig. 10.4) – bruising and oedema of the presenting part extending beyond the margins of the skull bones; resolves in a few days
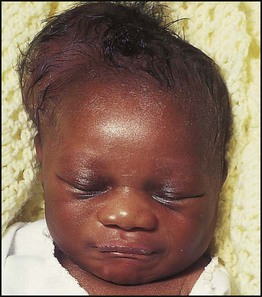
• Cephalhaematoma (Figs 10.4, 10.5) – haematoma from bleeding below the periosteum, confined within the margins of the skull sutures. It usually involves the parietal bone. The centre of the haematoma feels soft. It resolves over several weeks
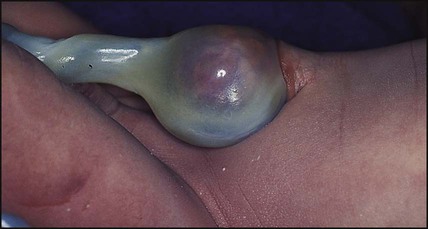
• Chignon (Fig. 10.6) – oedema and bruising from Ventouse delivery
• Bruising to the face after a face presentation and to the genitalia and buttocks after breech delivery. Preterm infants bruise readily from even mild trauma
• Abrasions to the skin from scalp electrodes applied during labour or from accidental scalpel incision at Caesarean section
• Forceps marks to face from pressure of blades – transient
• Subaponeurotic haemorrhage (Fig. 10.4) (very uncommon) – diffuse, boggy swelling of scalp on examination, blood loss may be severe and lead to hypovolaemic shock and coagulopathy.
Nerve palsies
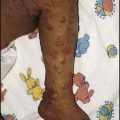
Brachial nerve palsy results from traction to the brachial plexus nerve roots. They may occur at breech deliveries or with shoulder dystocia. Upper nerve root (C5 and C6) injury results in an Erb palsy (Fig. 10.7). It may be accompanied by phrenic nerve palsy causing an elevated diaphragm. Most palsies resolve completely, but should be referred to an orthopaedic or plastic surgeon if not resolved by 2–3 months. Most recover by 2 years. A facial nerve palsy may result from compression of the facial nerve against the mother’s ischial spine. It is unilateral, and there is facial weakness on crying but the eye remains open. It is usually transient, but methylcellulose drops may be needed for the eye. Rarely, nerve palsies may be from damage to the cervical spine, when there is lack of movement below the level of the lesion.
The preterm infant
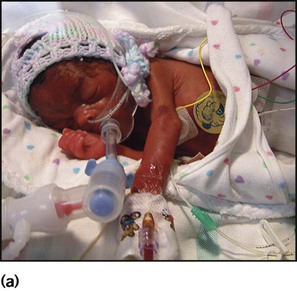
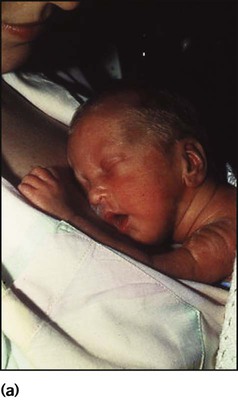
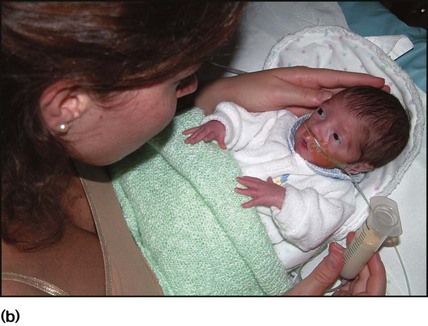
The appearance, the likely clinical course, chances of survival and long-term prognosis depend on the gestational age at birth. The appearance and maturational changes of very preterm infants are described in Table 10.1 and the importance of parental involvement shown in Figures 10.10a and b. The external appearance and neurological findings can be scored to provide an estimate of an infant’s gestational age (see Appendix).
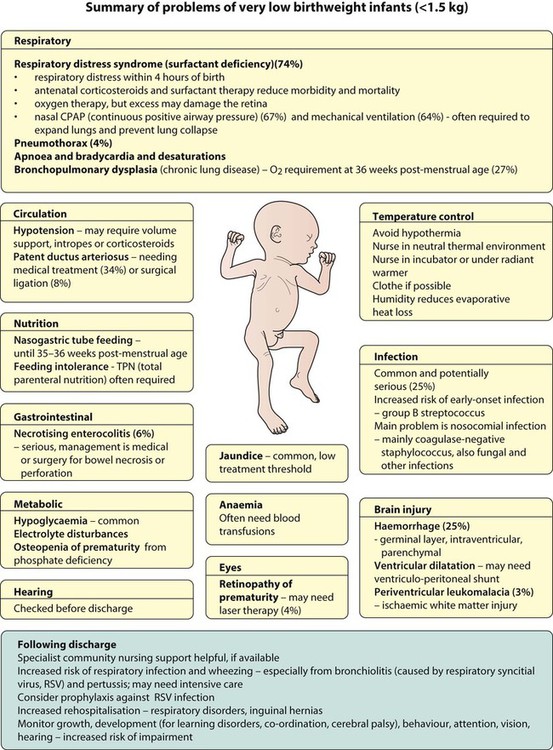
The rate and severity of problems associated with prematurity decline markedly with increasing gestation. Infants born at 23–26 weeks’ gestation encounter many problems (Box 10.1), require many weeks of intensive and special care in hospital and have a high overall mortality. With modern intensive care, the prognosis is excellent after 32 weeks’ gestational age. The severity of an infant’s respiratory disease and of any episodes of infection largely determine the neonatal course and outcome.
Respiratory distress syndrome
In respiratory distress syndrome (RDS), (also called hyaline membrane disease), there is a deficiency of surfactant, which lowers surface tension. Surfactant is a mixture of phospholipids and proteins excreted by the type II pneumocytes of the alveolar epithelium. Surfactant deficiency leads to widespread alveolar collapse and inadequate gas exchange. The more preterm the infant, the higher the incidence of RDS; it is common in infants born before 28 weeks’ gestation and tends to be more severe in boys than girls. Surfactant deficiency is rare at term but may occur in infants of diabetic mothers. The term hyaline membrane disease derives from a proteinaceous exudate seen in the airways on histology. Glucocorticoids, given antenatally to the mother, stimulate fetal surfactant production and are given if preterm delivery is anticipated (see Ch. 9.)
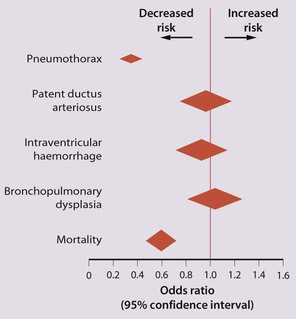
The development of surfactant therapy has been a major advance. The preparations are natural, derived from extracts of calf or pig lung. They are instilled directly into the lung via a tracheal tube. Multinational placebo-controlled trials show that surfactant treatment reduces mortality from RDS by about 40%, without increasing the morbidity rate (Fig. 10.11).
At delivery or within 4 h of birth, babies with RDS develop clinical signs of:
• laboured breathing with chest wall recession (particularly sternal and subcostal indrawing) and nasal flaring
• expiratory grunting in order to try to create positive airway pressure during expiration and maintain functional residual capacity
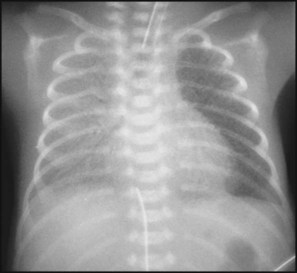
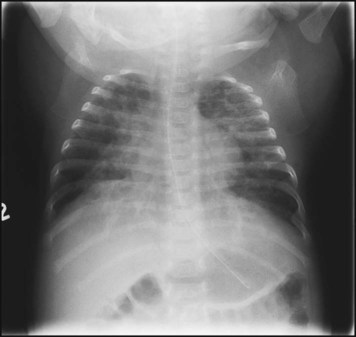
The characteristic chest X-ray appearance is shown in Figure 10.12. Treatment with raised ambient oxygen is required, which may need to be supplemented with continuous positive airway pressure (delivered via nasal cannulae) or artificial ventilation via a tracheal tube. The ventilatory requirements need to be adjusted according to the infant’s oxygenation (which is measured continuously), chest wall movements and blood gas analyses. Mechanical ventilation (with intermittent positive pressure ventilation or high-frequency oscillation) may be required. High-flow humidified oxygen therapy, via nasal cannulae, may be used to wean babies from added oxygen therapy.
Pneumothorax
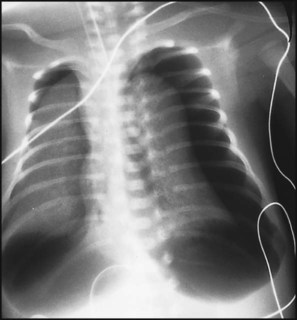
In respiratory distress syndrome, air from the overdistended alveoli may track into the interstitium, resulting in pulmonary interstitial emphysema (PIE). In up to 10% of infants ventilated for RDS, air leaks into the pleural cavity and causes a pneumothorax (Fig. 10.13). When this occurs, the infant’s oxygen requirement usually increases, and the breath sounds and chest movement on the affected side are reduced, although this can be difficult to detect clinically. A pneumothorax may be demonstrated by transillumination with a bright fibreoptic light source applied to the chest wall. A tension pneumothorax is treated by inserting a chest drain. In order to try and prevent pneumothoraces, infants are ventilated with the lowest pressures that provide adequate chest movement and satisfactory blood gases.
Temperature control
• they have a large surface area relative to their mass, so there is greater heat loss (related to surface area) than heat generation (related to mass)
• their skin is thin and heat permeable, so transepidermal water loss is important in the first week of life
• they have little subcutaneous fat for insulation
• they are often nursed naked and cannot conserve heat by curling up or generate heat by shivering.
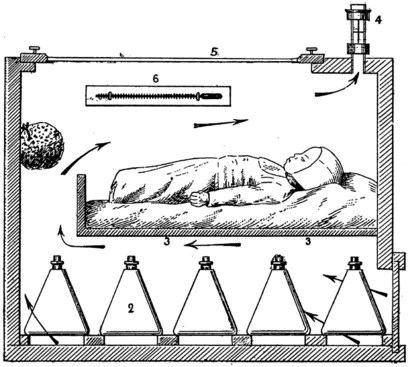
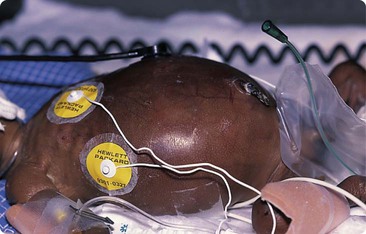
There is a neutral temperature range in which an infant’s energy consumption is at a minimum level. In the very immature baby, this neutral temperature is highest during the first few days of life and subsequently declines. The temperature of these small babies is maintained using incubators (Fig. 10.14) or initially with overhead radiant heaters. Incubators also allow ambient humidity to be provided, which reduces transepidermal heat loss.
Preterm brain injury
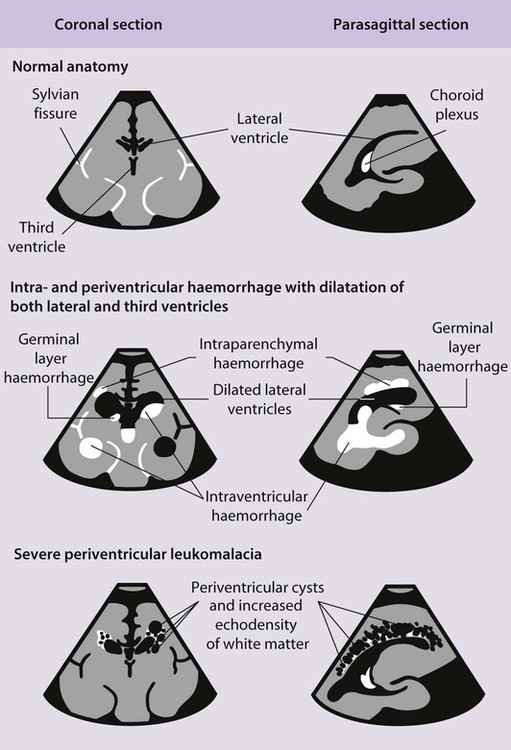
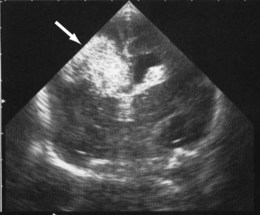
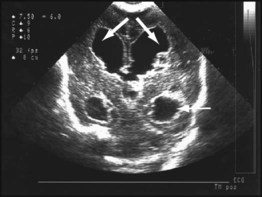
Haemorrhages in the brain occur in 25% of very low birthweight infants and are easily recognised on cranial ultrasound scans (Fig. 10.15a). Typically, they occur in the germinal matrix above the caudate nucleus, which contains a fragile network of blood vessels. Most haemorrhages occur within the first 72 h of life. They are more common following perinatal asphyxia and in infants with severe respiratory distress syndrome. Pneumothorax is a significant risk factor. Small haemorrhages confined to the germinal matrix do not increase the risk of cerebral palsy. Haemorrhage may occur in the ventricles. The most severe haemorrhage is unilateral haemorrhagic infarction involving the parenchyma of the brain; this usually results in hemiplegia (Fig. 10.15b).
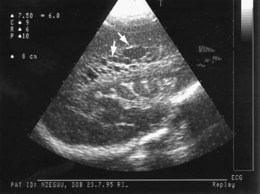
A large intraventricular haemorrhage may impair the drainage and reabsorption of cerebrospinal fluid (CSF), thus allowing CSF to build up under pressure. This dilatation (Fig. 10.15c) may resolve spontaneously or progress to hydrocephalus, which may cause the cranial sutures to separate, the head circumference to increase rapidly and the anterior fontanelle to become tense. A ventriculoperitoneal shunt may be required, but initially symptomatic relief may be provided by removal of CSF by lumbar puncture or ventricular tap. About half of infants with progressive post-haemorrhagic ventricular dilatation have cerebral palsy, a higher proportion if parenchymal infarction is also present.
Periventricular white matter brain injury may occur following ischaemia or inflammation and may occur in the absence of haemorrhage. It is more difficult to detect by cranial ultrasound. Initially there may be an echodense area or ‘flare’ within the brain parenchyma. This may resolve within a week (in which case the risk of cerebral palsy is not increased), but if cystic lesions become visible on ultrasound 2–4 weeks later, there is definite loss of white matter. Bilateral multiple cysts, called periventricular leukomalacia (PVL), have an 80–90% risk of spastic diplegia, often with cognitive impairment, if posteriorly sited (Fig. 10.15d).
Necrotising enterocolitis
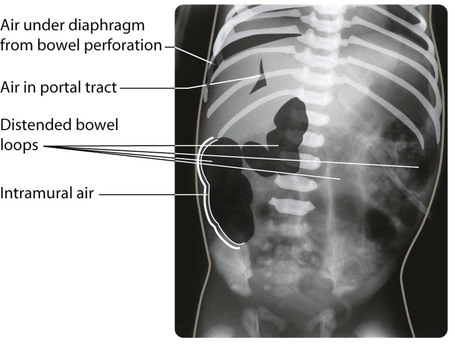
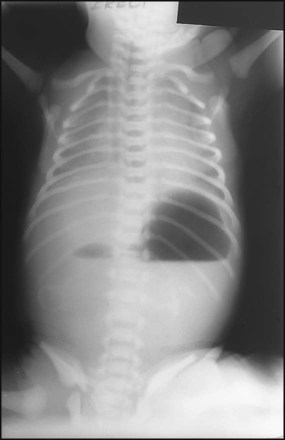
Necrotising enterocolitis is a serious illness mainly affecting preterm infants in the first few weeks of life. It is associated with bacterial invasion of ischaemic bowel wall. Preterm infants fed cow’s milk formula are more likely to develop this condition than if they are fed only breast milk. The infant stops tolerating feeds, milk is aspirated from the stomach and there may be vomiting, which may be bile-stained. The abdomen becomes distended (Fig. 10.16a) and the stool sometimes contains fresh blood. The infant may rapidly become shocked and require artificial ventilation because of abdominal distension and pain. The characteristic X-ray features are distended loops of bowel and thickening of the bowel wall with intramural gas, and there may be gas in the portal tract (Fig. 10.16b). The disease may progress to bowel perforation, which can be detected by X-ray or by transillumination of the abdomen.
Bronchopulmonary dysplasia
Infants who still have an oxygen requirement at a post-menstrual age of 36 weeks are described as having bronchopulmonary dysplasia (BPD) or chronic lung disease. The lung damage comes from pressure and volume trauma from artificial ventilation, oxygen toxicity and infection. The chest X-ray characteristically shows widespread areas of opacification, sometimes with cystic changes (Fig. 10.17). Some infants need prolonged artificial ventilation, but most are weaned onto continuous positive airways pressure (CPAP) followed by additional ambient oxygen, sometimes over several months. Corticosteroid therapy may facilitate earlier weaning from the ventilator and often reduces the infant’s oxygen requirements in the short term, but concern about increased risk of abnormal neurodevelopment including cerebral palsy limits use to those at highest risk and only short courses are given. Some babies go home while still receiving additional oxygen. A few infants with severe disease may die of intercurrent infection or pulmonary hypertension. Subsequent pertussis and RSV (respiratory syncytial virus) infection may cause respiratory failure necessitating intensive care.
Problems following discharge
Medical problems include increased risk of:
• poor growth – at discharge, over 90% of VLBW infants are below the 10th centile for weight, length and head circumference. Many show some catch-up growth in the first 2–3 years
• bronchiolitis from RSV (respiratory syncytial virus) infection (hospitalisation reduced by giving RSV monoclonal antibody, palivizumab)
• bronchopulmonary dysplasia – may require additional oxygen therapy for many months, when it may be provided at home
• gastro-oesophageal reflux – especially with bronchopulmonary dysplasia
• complex nutritional and gastrointestinal disorders – following necrotising enterocolitis or gastrointestinal surgery
About 5–10% of very low birthweight infants develop cerebral palsy, but the most common impairments are learning difficulties. The prevalence of cognitive impairment and of other associated difficulties increases with decreasing gestational age at birth, and is greatest if born at very early gestational age (<26 weeks’ gestation) (Fig. 10.18a,b). It becomes increasingly evident when the individual child is compared to their peers at nursery or school. In addition, children may have difficulties with:
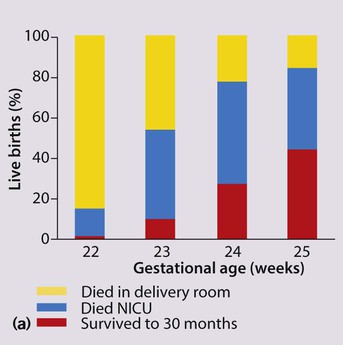
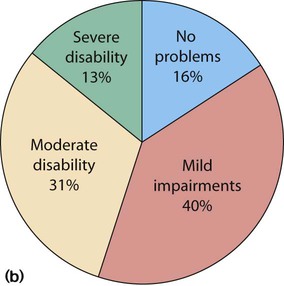
(b) Proportion of survivors with disability at 11 years of age. (Adapted from Johnson S et al. 2009. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics 124:e249–e257). Preterm babies born in 2006 show a 30% increase in numbers born at <26 weeks and increased survival. Long-term follow-up is in progress.
Jaundice
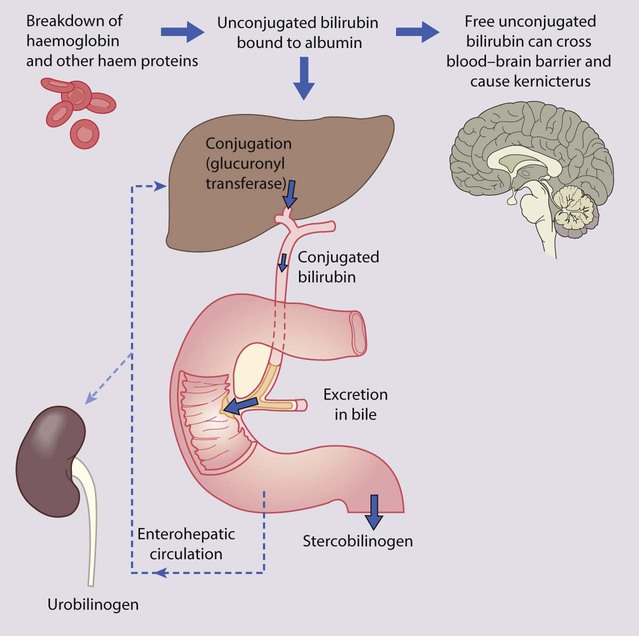
Over 50% of all newborn infants become visibly jaundiced. This is because:
• there is marked physiological release of haemoglobin from the breakdown of red cells because of the high Hb concentration at birth (Fig. 10.19)
• the red cell life span of newborn infants (70 days) is markedly shorter than that of adults (120 days)
• hepatic bilirubin metabolism is less efficient in the first few days of life.
Neonatal jaundice is important as:
• it may be a sign of another disorder, e.g. haemolytic anaemia, infection, metabolic disease, liver disease.
• unconjugated bilirubin can be deposited in the brain, particularly in the basal ganglia, causing kernicterus.
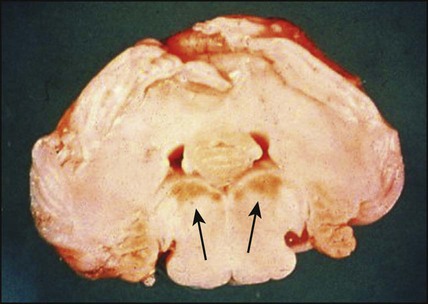
Kernicterus
This is the encephalopathy resulting from the deposition of unconjugated bilirubin in the basal ganglia and brainstem nuclei (Fig. 10.20). It may occur when the level of unconjugated bilirubin exceeds the albumin-binding capacity of bilirubin of the blood. As this free bilirubin is fat-soluble, it can cross the blood–brain barrier. The neurotoxic effects vary in severity from transient disturbance to severe damage and death. Acute manifestations are lethargy and poor feeding. In severe cases, there is irritability, increased muscle tone causing the baby to lie with an arched back (opisthotonos), seizures and coma. Infants who survive may develop choreoathetoid cerebral palsy (due to damage to the basal ganglia), learning difficulties and sensorineural deafness. Kernicterus used to be an important cause of brain damage in infants with severe rhesus haemolytic disease, but has become rare since the introduction of prophylactic anti-D immunoglobulin for rhesus-negative mothers. However, a few cases continue to occur, especially in slightly preterm infants (35–37 weeks), which has led NICE to issue guidelines on the management of neonatal jaundice.
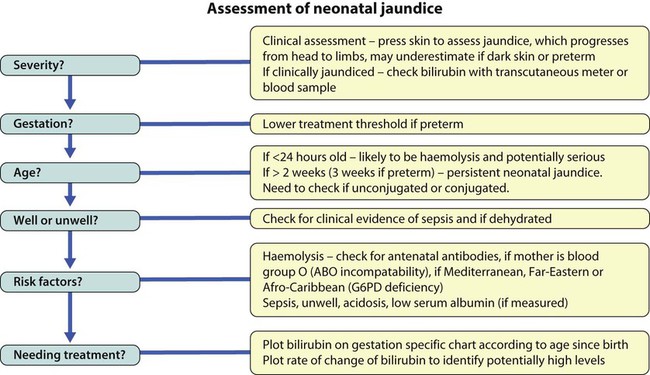
Clinical evaluation
Age at onset
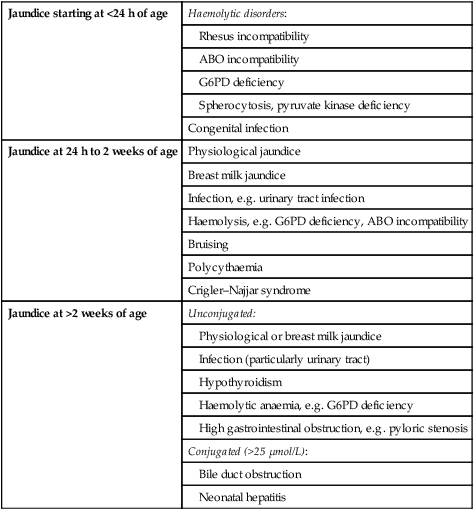
The age of onset is a useful guide to the likely cause of the jaundice (Table 10.2).
Table 10.2
| Jaundice starting at <24 h of age | Haemolytic disorders: |
| Rhesus incompatibility | |
| ABO incompatibility | |
| G6PD deficiency | |
| Spherocytosis, pyruvate kinase deficiency | |
| Congenital infection | |
| Jaundice at 24 h to 2 weeks of age | Physiological jaundice |
| Breast milk jaundice | |
| Infection, e.g. urinary tract infection | |
| Haemolysis, e.g. G6PD deficiency, ABO incompatibility | |
| Bruising | |
| Polycythaemia | |
| Crigler–Najjar syndrome | |
| Jaundice at >2 weeks of age | Unconjugated: |
| Physiological or breast milk jaundice | |
| Infection (particularly urinary tract) | |
| Hypothyroidism | |
| Haemolytic anaemia, e.g. G6PD deficiency | |
| High gastrointestinal obstruction, e.g. pyloric stenosis | |
| Conjugated (>25 µmol/L): | |
| Bile duct obstruction | |
| Neonatal hepatitis |
Jaundice <24 h of age
Haemolytic disorders
Rhesus haemolytic disease – Affected infants are usually identified antenatally and monitored and treated if necessary (see Ch. 9). The birth of a severely affected infant, with anaemia, hydrops and hepatosplenomegaly with rapidly developing severe jaundice, has become rare. Antibodies may develop to rhesus antigens other than D and to the Kell and Duffy blood groups, but haemolysis is usually less severe.
G6PD deficiency (see Ch. 22) – Mainly in people originating in the Mediterranean, Middle-East and Far East or in African-Americans. Mainly affects male infants, but some females develop significant jaundice. Parents of affected infants should be given a list of drugs to be avoided, as they may precipitate haemolysis.
Spherocytosis – This is considerably less common than G6PD deficiency (see Ch. 22). There is often, but not always, a family history. The disorder can be identified by recognising spherocytes on the blood film.
Management
Jaundice at >2 weeks of age
Jaundice in babies more than 2 weeks old (3 weeks if preterm), is called persistent or prolonged neonatal jaundice. The key feature is that it may be caused by biliary atresia, and it is important to diagnose biliary atresia promptly, as delay in surgical treatment adversely affects outcome (see Ch. 20 for further details).
In prolonged unconjugated hyperbilirubinaemia:
• ‘Breast milk jaundice’ is the most common cause, affecting up to 15% of healthy breast-fed infants; the jaundice gradually fades and disappears by 4–5 weeks of age.
• Infection, particularly of the urinary tract, needs to be considered.
• Congenital hypothyroidism may cause prolonged jaundice before the clinical features of coarse facies, dry skin, hypotonia and constipation become evident. Affected infants should be identified on routine neonatal biochemical screening (Guthrie test).
Conjugated hyperbilirubinaemia (>25 µmol/L) is suggested by the baby passing dark urine and unpigmented pale stools. Hepatomegaly and poor weight gain are other clinical signs that may be present. Its causes include neonatal hepatitis syndrome and biliary atresia, with improved prognosis of biliary atresia with early diagnosis (see Ch. 20 for further details).
Respiratory distress in term infants
Newborn infants with respiratory problems develop the following signs of respiratory distress:
• tachypnoea (>60 breaths/min)
• laboured breathing, with chest wall recession (particularly sternal and subcostal indrawing) and nasal flaring
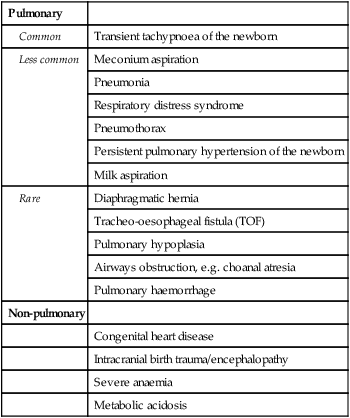
The causes in term infants are listed in Table 10.3.
Table 10.3
Causes of respiratory distress in term infants
| Pulmonary | |
| Common | Transient tachypnoea of the newborn |
| Less common | Meconium aspiration |
| Pneumonia | |
| Respiratory distress syndrome | |
| Pneumothorax | |
| Persistent pulmonary hypertension of the newborn | |
| Milk aspiration | |
| Rare | Diaphragmatic hernia |
| Tracheo-oesophageal fistula (TOF) | |
| Pulmonary hypoplasia | |
| Airways obstruction, e.g. choanal atresia | |
| Pulmonary haemorrhage | |
| Non-pulmonary | |
| Congenital heart disease | |
| Intracranial birth trauma/encephalopathy | |
| Severe anaemia | |
| Metabolic acidosis |
Diaphragmatic hernia
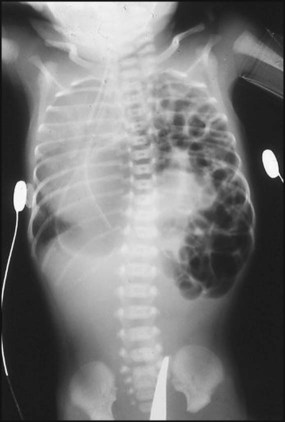
This occurs in about 1 in 4000 births. Many are now diagnosed on antenatal ultrasound screening. In the newborn period, it usually presents with failure to respond to resuscitation or as respiratory distress. In most cases, there is a left-sided herniation of abdominal contents through the posterolateral foramen of the diaphragm. The apex beat and heart sounds will then be displaced to the right side of the chest, with poor air entry in the left chest. Vigorous resuscitation may cause a pneumothorax in the normal lung, thereby aggravating the situation. The diagnosis is confirmed by X-ray of the chest and abdomen (Fig. 10.21). Once the diagnosis is suspected, a large nasogastric tube is passed and suction is applied to prevent distension of the intrathoracic bowel. After stabilisation, the diaphragmatic hernia is repaired surgically, but in most infants with this condition the main problem is pulmonary hypoplasia – where compression by the herniated viscera throughout pregnancy has prevented development of the lung in the fetus. If the lungs are hypoplastic, mortality is high.
Other causes
Other causes of respiratory distress are listed in Table 10.3. When due to heart failure, abnormal heart sounds and/or heart murmurs may be present on auscultation. An enlarged liver from venous congestion is a helpful sign. The femoral arteries must be palpated in all infants with respiratory distress, as coarctation of the aorta and interrupted aortic arch are important causes of heart failure in newborn infants.
Infection
Early-onset infection
The risk of early-onset infection is increased if there has been prolonged or premature rupture of the amniotic membranes, and when chorioamnionitis is clinically evident such as when the mother has fever during labour. Presentation is with respiratory distress, apnoea and temperature instability (Box 10.2). A chest X-ray is performed, together with a septic screen. A full blood count is performed to detect neutropenia, as well as blood cultures. An acute-phase reactant (C-reactive protein) is helpful but takes 12–24 h to rise, so one normal result does not exclude infection, but two consecutive normal values are strong evidence against infection. Antibiotics are started immediately without waiting for culture results. Intravenous antibiotics are given to cover group B streptococci, Listeria monocytogenes and other Gram-positive organisms (usually benzylpenicillin or amoxicillin), combined with cover for Gram-negative organisms (usually an aminoglycoside such as gentamicin). If cultures and CRP are negative and the infant has recovered clinically, antibiotics can be stopped after 48 h. If the blood culture is positive or if there are any neurological signs, CSF must be examined and cultured.
Late-onset infection
In late-onset infection (>48 h after birth), the source of infection is often the infant’s environment. The presentation is usually non-specific (Box 10.2). Nosocomially acquired infections are an inherent risk in a neonatal unit, and all staff must adhere strictly to effective hand hygiene measures to prevent cross-infection. In neonatal intensive care, the main sources of infection are indwelling central venous catheters for parenteral nutrition, invasive procedures which break the protective barrier of the skin, and tracheal tubes. Coagulase-negative staphylococcus (Staphylococcus epidermidis) is the most common pathogen, but the range of organisms is broad, and includes Gram-positive bacteria (Staphylococcus aureus and Enterococcus faecalis) and Gram-negative bacteria (Escherichia coli and Pseudomonas, Klebsiella and Serratia species). Initial therapy (e.g. with flucloxacillin and gentamicin) is aimed to cover most staphylococci and Gram-negative bacilli. If the organism is resistant to these antibiotics or the infant’s condition does not improve, specific antibiotics (e.g. vancomycin for coagulase-negative staphylococci or enterococci) or broad-spectrum antibiotics (e.g. meropenem) may be indicated. Use of prolonged or broad-spectrum antibiotics predisposes to invasive fungal infections (e.g. Candida albicans) in premature babies. Serial measurements of an acute-phase reactant (CRP) are useful to monitor response to therapy.
Neonatal meningitis, although uncommon, has a mortality of 20–50%, with one-third of survivors having serious sequelae. Presentation is non-specific (Box 10.2); a bulging fontanelle and hyperextension of neck and back (opisthotonos) are late signs and rarely seen in newborn infants. If meningitis is thought likely, ampicillin or penicillin and a third-generation cephalosporin (e.g. cefotaxime, which has CSF penetration) are given. Complications include cerebral abscess, ventriculitis, hydrocephalus, hearing loss and neurodevelopmental impairment.
Some specific infections
Group B streptococcal infection
Conjunctivitis
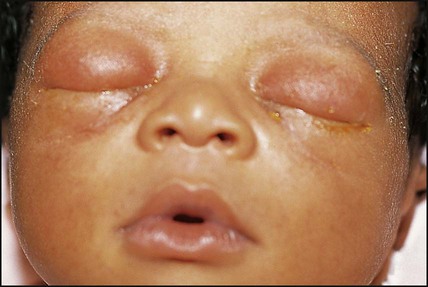
Chlamydia trachomatis eye infection usually presents with a purulent discharge, together with swelling of the eyelids (Fig. 10.22), at 1–2 weeks of age, but may also present shortly after birth. The organism can be identified with immunofluorescent staining. Treatment is with oral erythromycin for 2 weeks. The mother and partner also need to be checked and treated.
Neonatal seizures
Many babies startle or have tremors when stimulated or make strange jerks during active sleep. Seizures, on the other hand, are unstimulated. Typically, there are repetitive, rhythmic (clonic) movements of the limbs which persist despite restraint and are often accompanied by eye movements and changes in respiration. Many neonatal units now use continuous single channel EEG (amplified integrated EEG, also called a cerebral function monitor) to be able to confirm changes in electrical discharges in the brain. The causes of seizures are listed in Box 10.3.
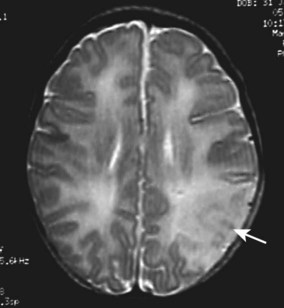
Cerebral infarction (neonatal stroke)
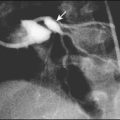
Infarction in the territory of the middle cerebral artery may present with seizures at 12–48 h in a term infant. The seizures may be focal or generalised. In contrast to infants with hypoxic-ischaemic encephalopathy, there are no other abnormal clinical features. The diagnosis is confirmed by MRI (Fig. 10.23). The mechanism is thought to be thrombotic, either thromboembolism from placental vessels or sometimes secondary to inherited thrombophilia. In spite of pronounced abnormalities on the MRI scans, the prognosis is relatively good, with only 20% having hemiparesis or epilepsy presenting later in infancy or early childhood.
Craniofacial disorders
Cleft lip and palate
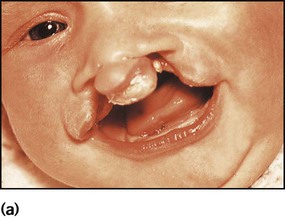
A cleft lip (Fig. 10.24a) may be unilateral or bilateral. It results from failure of fusion of the frontonasal and maxillary processes. In bilateral cases the premaxilla is anteverted. Cleft palate results from failure of fusion of the palatine processes and the nasal septum. Cleft lip and palate affect about 0.8 per 1000 babies. Most are inherited polygenically, but they may be part of a syndrome of multiple abnormalities, e.g. chromosomal disorders. Some are associated with maternal anticonvulsant therapy. They may be detected on antenatal ultrasound scanning.
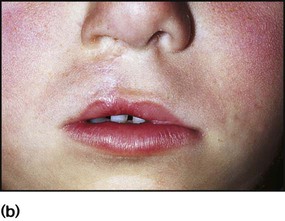
Surgical repair of the lip (Fig. 10.24b) may be performed within the first week of life for cosmetic reasons, although some surgeons feel that better results are obtained if surgery is delayed. The palate is usually repaired at several months of age. A cleft palate may make feeding more difficult, but some affected infants can still be breast-fed successfully. In bottle-fed babies, if milk is observed to enter the nose and cause coughing and choking, special teats and feeding devices may be helpful. Orthodontic advice and a dental prosthesis may help with feeding. Secretory otitis media is relatively common and should be sought on follow-up. Infants are also prone to acute otitis media. Adenoidectomy is best avoided, as the resultant gap between the abnormal palate and nasopharynx will exacerbate feeding problems and the nasal quality of speech. A multidisciplinary team approach is required, involving plastic and ENT surgeons, paediatrician, orthodontist, audiologist and speech therapist. Parent support groups can provide valuable support and advice for families (Cleft Lip and Palate Association, CLAPA).
Pierre Robin sequence
The Pierre Robin sequence is an association of micrognathia (Fig. 10.25), posterior displacement of the tongue (glossoptosis) and midline cleft of the soft palate. There may be difficulty feeding and, as the tongue falls back, there is obstruction to the upper airways which may result in cyanotic episodes. The infant is at risk of failure to thrive during the first few months. If there is upper airways obstruction, the infant may need to lie prone, allowing the tongue and small mandible to fall forward. Persistent obstruction can be treated using a nasopharyngeal airway. Eventually the mandible grows and these problems resolve. The cleft palate can then be repaired.
Gastrointestinal disorders
Oesophageal atresia
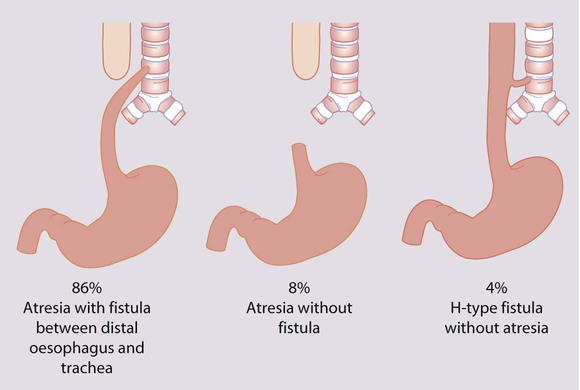
Oesophageal atresia is usually associated with a tracheo-oesophageal fistula (Fig. 10.26). It occurs in 1 in 3500 live births and is associated with polyhydramnios during pregnancy. If suspected, a wide-calibre feeding tube is passed and checked by X-ray to see if it reaches the stomach. If not diagnosed at birth, clinical presentation is with persistent salivation and drooling from the mouth after birth. If the diagnosis is not made at this stage, the infant will cough and choke when fed, and have cyanotic episodes. There may be aspiration into the lungs of saliva (or milk) from the upper airways and acid secretions from the stomach. Almost half of the babies have other congenital malformations, e.g. as part of the VACTERL association (Vertebral, Anorectal, Cardiac, Tracheo-oEsophageal, Renal and Radial Limb anomalies). Continuous suction is applied to a tube passed into the oesophageal pouch to reduce aspiration of saliva and secretions pending transfer to a neonatal surgical unit.
Small bowel obstruction
Small bowel obstruction may be caused by:
• Atresia or stenosis of the duodenum (Fig. 10.27) – one-third have Down syndrome and it is also associated with other congenital malformations
• Atresia or stenosis of the jejunum or ileum – there may be multiple atretic segments of bowel
• Malrotation with volvulus – a dangerous condition as it may lead to infarction of the entire midgut
• Meconium ileus – thick inspissated meconium, of putty-like consistency, becomes impacted in the lower ileum; almost all affected neonates have cystic fibrosis
• Meconium plug – a plug of inspissated meconium causes lower intestinal obstruction.
Large bowel obstruction
• Hirschsprung disease. Absence of the myenteric nerve plexus in the rectum which may extend along the colon. The baby often does not pass meconium within 48 h of birth and subsequently the abdomen distends. About 15% present as an acute enterocolitis (see Ch. 13).
• Rectal atresia. Absence of the anus at the normal site. Lesions are high or low, depending whether the bowel ends above or below the levator ani muscle. In high lesions, there is a fistula to the bladder or urethra in boys, or adjacent to the vagina or to the bladder in girls. Treatment is surgical.
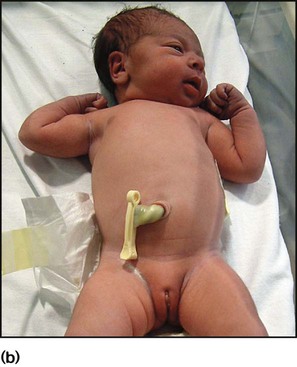
Exomphalos/gastroschisis
These lesions are often diagnosed antenatally (see Ch. 9). In exomphalos (also called omphalocele), the abdominal contents protrude through the umbilical ring, covered with a transparent sac formed by the amniotic membrane and peritoneum (Fig. 10.28). It is often associated with other major congenital abnormalities. In gastroschisis, the bowel protrudes through a defect in the anterior abdominal wall, adjacent to the umbilicus, and there is no covering sac (see Fig. 9.2). It is not associated with other congenital abnormalities.