Infection and immunity
Infections are the most common cause of acute illness in children.
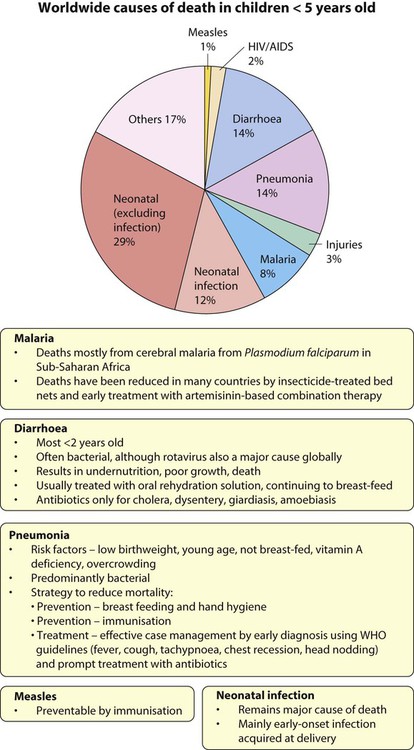
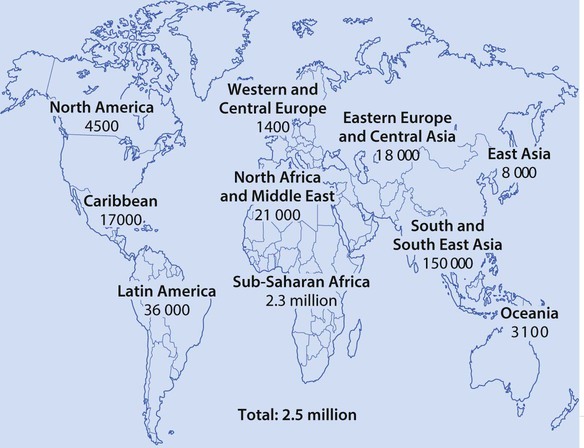
Worldwide, acute respiratory infections, diarrhoea, neonatal infection, malaria, measles and HIV infection, often accompanied by undernutrition, are responsible for the deaths of more than 4.5 million children <5 years old annually (Fig. 14.1).
The febrile child
Clinical features
When assessing a febrile child, consider the following.
(i) How is fever identified in children?
Parents usually know if their child has been febrile.
In hospital, it is measured at:
• <4 weeks old by an electronic thermometer in the axilla
• 4 weeks to 5 years by an electronic or chemical dot thermometer in the axilla or infrared tympanic thermometer.
In general, axillary temperatures underestimate body temperature by 0.5°C.
(ii) How old is the child?
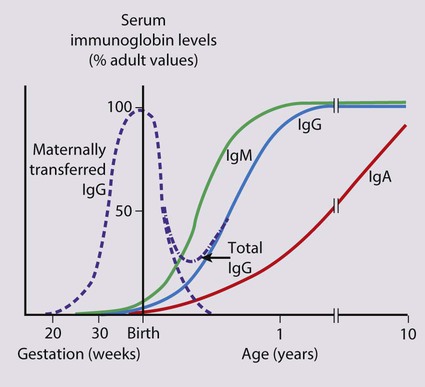
Febrile infants <3 months old present with non-specific clinical features (see Box 10.2) and often have a bacterial infection, which cannot be identified reliably on clinical examination alone. It is uncommon for them to have the common viral infections of older infants and children because of passive immunity from their mothers (Fig. 14.2). Unless a clear cause for the fever is identified, they require urgent investigation with a septic screen (Box 14.1) and intravenous antibiotic therapy given immediately to avoid the illness becoming more severe and to prevent rapid spread to other sites of the body. This is considered in more detail in the section on neonatal infection (Chapter 10 Neonatal medicine).
(iii) Are there risk factors for infection?
• Illness of other family members
• If a specific illness is prevalent in the community
• Recent travel abroad, e.g. malaria, typhoid
• Contact with animals, e.g. brucellosis.
• Increased susceptibility from immunodeficiency. This is usually secondary, e.g. post-autosplenectomy in sickle cell disease or splenectomy or nephrotic syndrome, resulting in increased susceptibility to encapsulated organisms (Streptococcus pneumoniae, Haemophilus influenzae and salmonella), or rarely, primary immune deficiency.
(vi) Is there a focus for infection?
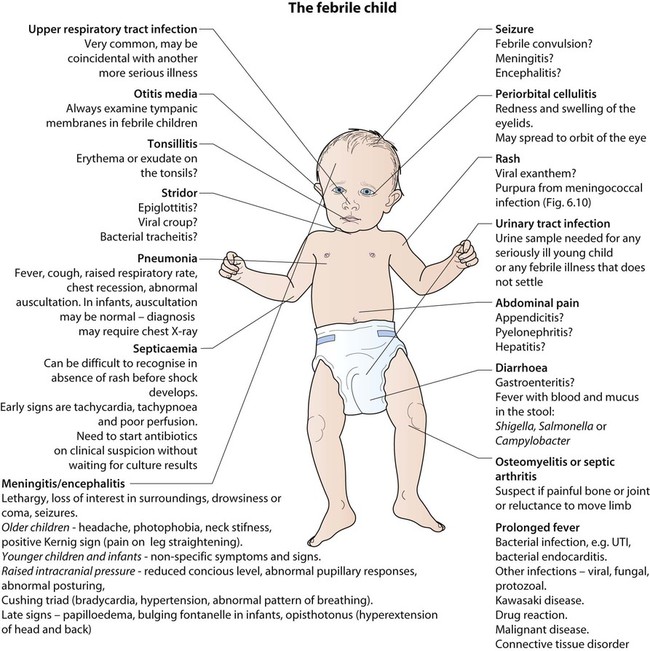
Examination may identify a focus of infection (Fig. 14.3). If identified, investigations and management will be directed towards its treatment. However, if no focus is identified, this is often because it is the prodromal phase of a viral illness, but may indicate serious bacterial infection, especially urinary tract infection or septicaemia.
Management
Children who are not seriously ill can be managed at home with regular review by the parents, as long as they are given clear instructions (e.g. what clinical features should prompt reassessment by a doctor). Children who are significantly unwell, particularly if there is no focus of infection, will require investigations and observation or treatment in a paediatric assessment unit or A&E department or children’s ward. A septic screen will be required (Box 14.1).
Serious life-threatening infections
Septicaemia
This is considered in Chapter 6 on Paediatric Emergencies.
Meningitis
Bacterial meningitis
Organisms
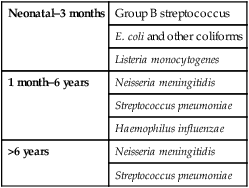
The organisms which commonly cause bacterial meningitis vary according to the child’s age (Table 14.1).
Table 14.1
Organisms causing bacterial meningitis according to age
| Neonatal–3 months | Group B streptococcus |
| E. coli and other coliforms | |
| Listeria monocytogenes | |
| 1 month–6 years | Neisseria meningitidis |
| Streptococcus pneumoniae | |
| Haemophilus influenzae | |
| >6 years | Neisseria meningitidis |
| Streptococcus pneumoniae |
Presentation
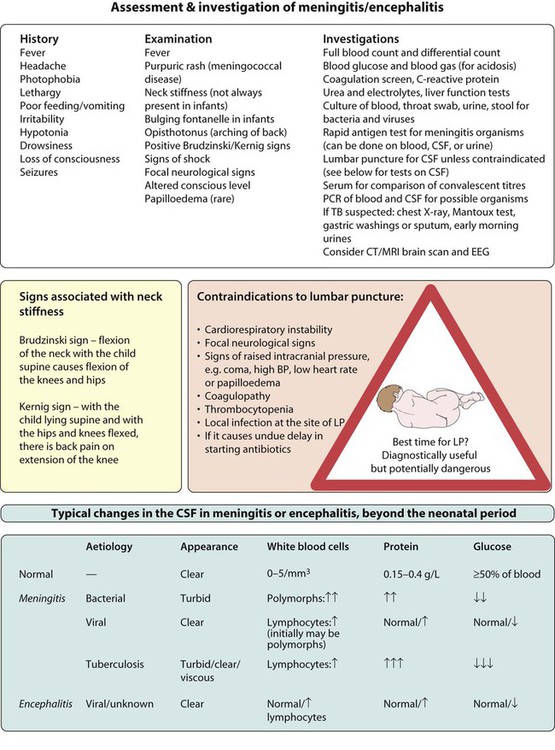
The clinical features are listed in Figure 14.4. The early signs and symptoms of meningitis are non-specific, especially in infants and young children. Only children old enough to talk are likely to describe the classical meningitis symptoms of headache, neck stiffness and photophobia. But neck stiffness may also be seen in some children with tonsillitis and cervical lymphadenopathy. As children with meningitis may also be septicaemic, signs of shock, such as tachycardia, tachypnoea, prolonged capillary refill time, and hypotension, should be sought. Purpura in a febrile child of any age should be assumed to be due to meningococcal sepsis, even if the child does not appear unduly ill at the time; meningitis may or may not be present.
Investigations
The essential investigations are listed in Figure 14.4. A lumbar puncture is performed to obtain CSF to confirm the diagnosis, identify the organism responsible, and its antibiotic sensitivity. If any of the contraindications listed in Figure 14.4 are present, a lumbar puncture should not be performed, as under these circumstances, the procedure carries a risk of coning of the cerebellum through the foramen magnum. In these circumstances, a lumbar puncture can be postponed until the child’s condition has stabilised. Even without a lumbar puncture, bacteriological diagnosis can be achieved in at least 50% of cases from the blood by culture or polymerase chain reaction (PCR), and rapid antigen screens can be performed on blood and urine samples. Throat swabs should also be obtained for bacterial and viral cultures. A serological diagnosis can be made on convalescent serum 4–6 weeks after the presenting illness if necessary.
Cerebral complications
• Hearing loss. Inflammatory damage to the cochlear hair cells may lead to deafness. All children who have had meningitis should have an audiological assessment promptly, as children who become deaf may benefit from hearing amplification or a cochlear implant.
• Local vasculitis. This may lead to cranial nerve palsies or other focal lesions.
• Local cerebral infarction. This may result in focal or multifocal seizures, which may subsequently lead to epilepsy.
• Subdural effusion. Particularly associated with Haemophilus influenzae and pneumococcal meningitis. This is confirmed by CT scan. Most resolve spontaneously but may require prolonged antibiotic treatment.
• Hydrocephalus. May result from impaired resorption of CSF (communicating hydrocephalus) or blockage of the ventricular outlets by fibrin (non-communicating hydrocephalus). A ventricular shunt may be required.
• Cerebral abscess. The child’s clinical condition deteriorates with the emergence of signs of a space-occupying lesion. The temperature will continue to fluctuate. It is confirmed on CT scan. Drainage of the abscess is required.
Encephalitis/encephalopathy
• Direct invasion of the cerebrum by a neurotoxic virus (such as herpes simplex virus, HSV)
• Delayed brain swelling following a disordered neuroimmunological response to an antigen, usually a virus (post-infectious encephalopathy), e.g. following chickenpox
• A slow virus infection, such as HIV infection or subacute sclerosing panencephalitis (SSPE) following measles.
The clinical features and investigation of encephalitis are described in Figure 14.4. Most children present with fever, altered consciousness and often seizures. Initially, it may not be possible to clinically differentiate encephalitis from meningitis, and treatment for both should be started. The underlying causative organism is only detected in 50% of cases. In the UK, the most frequent causes of encephalitis are enteroviruses, respiratory viruses and herpesviruses (e.g. herpes simplex virus, varicella and HHV6). Worldwide, microorganisms causing encephalitis include Mycoplasma, Borrelia burgdorferi (Lyme disease), Bartonella henselae (cat scratch disease), rickettsial infections (e.g. Rocky Mountain spotted fever) and the arboviruses.
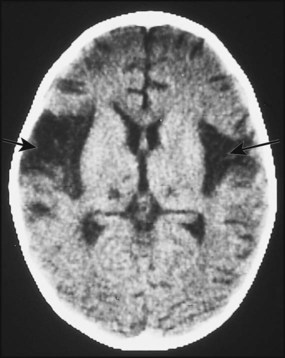
Herpes simplex virus (HSV) is a rare cause of childhood encephalitis but it may have devastating long-term consequences. All children with encephalitis should therefore be treated initially with high-dose intravenous aciclovir, since this is a very safe treatment. Most affected children do not have outward signs of herpes infection, such as cold sores, gingivostomatitis or skin lesions. The PCR of the CSF may be positive for HSV. As HSV encephalitis is a destructive infection, the EEG and CT/MRI scan may show focal changes, particularly within the temporal lobes (Fig. 14.5). These tests may be normal initially and need to be repeated after a few days if the child is not improving. Later confirmation of the diagnosis may be made from HSV antibody production in the CSF. Proven cases of HSV encephalitis or cases where there is a high index of suspicion should be treated with intravenous aciclovir for 3 weeks, as relapses may occur after shorter courses. Untreated, the mortality rate from HSV encephalitis is over 70% and survivors usually have severe neurological sequelae.
Specific bacterial infections
Meningococcal infection
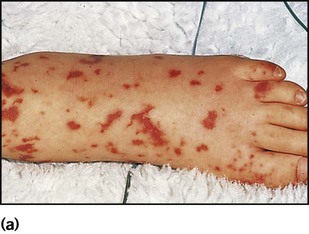
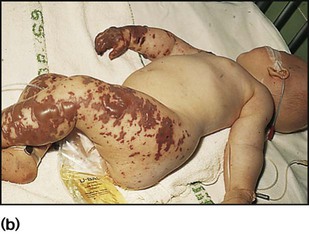
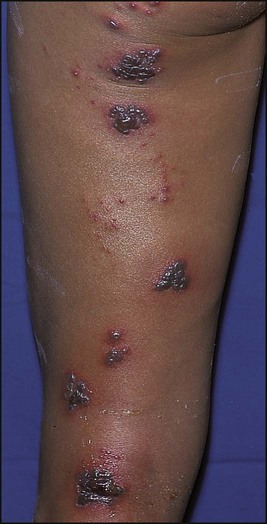
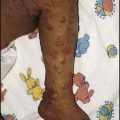
Meningococcal infection is a disease that strikes fear into both parents and doctors, as it can kill previously healthy children within hours (Case History 14.1). However, of the three main causes of bacterial meningitis, meningococcal has the lowest risk of long-term neurological sequelae, with most survivors recovering fully. The septicaemia is usually accompanied by a purpuric rash which may start anywhere on the body and then spread. The rash may or may not be present with meningococcal meningitis. Characteristic lesions are non-blanching on palpation, irregular in size and outline and have a necrotic centre (Fig. 14.8a,b). Any febrile child who develops a purpuric rash should be treated immediately, at home or in the general practitioner’s surgery, with systemic antibiotics such as penicillin before urgent admission to hospital. Although there are now polysaccharide conjugate vaccines against groups A and C meningococcus, there is still no effective vaccine for group B meningococcus, which accounts for the majority of isolates in the UK.
Staphylococcal and group A streptococcal infections
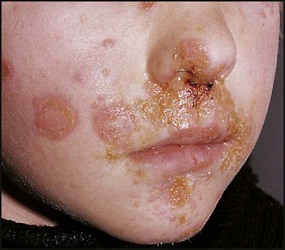
Impetigo
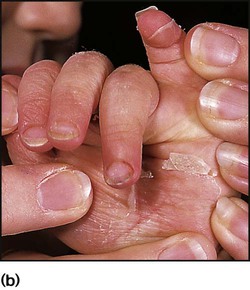
This is a localised, highly contagious, staphylococcal and/or streptococcal skin infection, most common in infants and young children. It is more common where there is pre-existing skin disease, e.g. atopic eczema. Lesions are usually on the face, neck and hands and begin as erythematous macules which may become vesicular/pustular or even bullous (Fig. 14.9). Rupture of the vesicles with exudation of fluid leads to the characteristic confluent honey-coloured crusted lesions. Infection is readily spread to adjacent areas and other parts of the body by autoinoculation of the infected exudate. Topical antibiotics (e.g. mupirocin) are sometimes effective for mild cases. Narrow-spectrum systemic antibiotics (e.g. flucloxacillin) are needed for more severe infections, although more broad-spectrum antibiotics such as co-amoxiclav or cefaclor have simpler oral administration regimens, taste better and therefore have better adherence. Affected children should not go to nursery or school until the lesions are dry. Nasal carriage is an important source of infection which can be eradicated with a nasal cream containing mupirocin or chlorhexidine and neomycin.
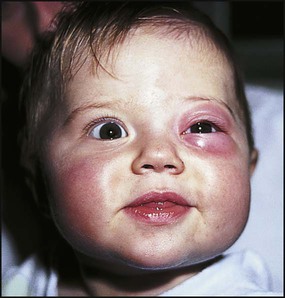
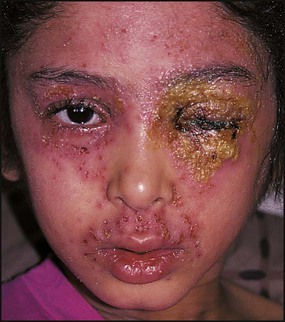
Periorbital cellulitis
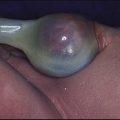
In periorbital cellulitis there is fever with erythema, tenderness and oedema of the eyelid (Fig. 14.10). It is almost always unilateral. In young, unimmunised children it may also be caused by Haemophilus influenzae type b which may also be accompanied by infection at other sites, e.g. meningitis. It may follow local trauma to the skin. In older children, it may spread from a paranasal sinus infection or dental abscess. Periorbital cellulitis should be treated promptly with intravenous antibiotics to prevent posterior spread of the infection to become an orbital cellulitis. In orbital cellulitis, there is proptosis, painful or limited ocular movement and reduced visual acuity. It may be complicated by abscess formation, meningitis or cavernous sinus thrombosis. Where orbital cellulitis is suspected, a CT scan should be performed to assess the posterior spread of infection and a lumbar puncture may be required to exclude meningitis.
Scalded skin syndrome
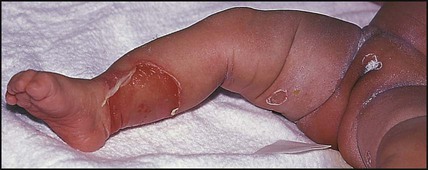
This is caused by an exfoliative staphylococcal toxin which causes separation of the epidermal skin through the granular cell layers. It affects infants and young children, who develop fever and malaise and may have a purulent, crusting, localised infection around the eyes, nose and mouth with subsequent widespread erythema and tenderness of the skin. Areas of epidermis separate on gentle pressure (Nikolsky sign), leaving denuded areas of skin (Fig. 14.11), which subsequently dry and heal without scarring. Management is with an intravenous anti-staphylococcal antibiotic, analgesia and monitoring of fluid balance.
Common viral infections
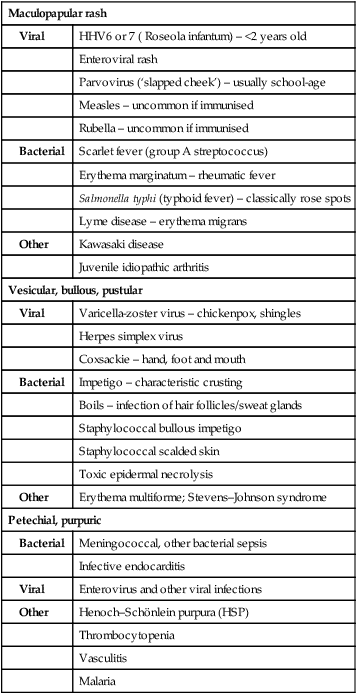
Many of the common childhood infections present with fever and a rash (Table 14.2). Incubation periods vary from 24 h for viral gastroenteritis, to about 2 weeks for chickenpox, but for some diseases, such as HIV, the length of time between exposure and the development of symptomatic illness may extend to many years. This is a reflection of host–pathogen interactions; an effective initial host response may result in a prolonged period of clinical latency, whereas an ineffective response permits rapid evolution of disease.
Table 14.2
| Maculopapular rash | |
| Viral | HHV6 or 7 ( Roseola infantum) – <2 years old |
| Enteroviral rash | |
| Parvovirus (‘slapped cheek’) – usually school-age | |
| Measles – uncommon if immunised | |
| Rubella – uncommon if immunised | |
| Bacterial | Scarlet fever (group A streptococcus) |
| Erythema marginatum – rheumatic fever | |
| Salmonella typhi (typhoid fever) – classically rose spots | |
| Lyme disease – erythema migrans | |
| Other | Kawasaki disease |
| Juvenile idiopathic arthritis | |
| Vesicular, bullous, pustular | |
| Viral | Varicella-zoster virus – chickenpox, shingles |
| Herpes simplex virus | |
| Coxsackie – hand, foot and mouth | |
| Bacterial | Impetigo – characteristic crusting |
| Boils – infection of hair follicles/sweat glands | |
| Staphylococcal bullous impetigo | |
| Staphylococcal scalded skin | |
| Toxic epidermal necrolysis | |
| Other | Erythema multiforme; Stevens–Johnson syndrome |
| Petechial, purpuric | |
| Bacterial | Meningococcal, other bacterial sepsis |
| Infective endocarditis | |
| Viral | Enterovirus and other viral infections |
| Other | Henoch–Schönlein purpura (HSP) |
| Thrombocytopenia | |
| Vasculitis | |
| Malaria | |
The infectious period characteristically begins a day or two before the rash appears and, for purposes of nursery/school exclusion, is generally considered to last until the rash has resolved or the lesions have dried up. For details about incubation and exclusion periods, see the Health Protection Agency website (http://www.hpa.org.uk).
The human herpesviruses
Herpes simplex infections
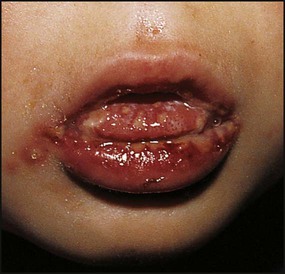
Gingivostomatitis
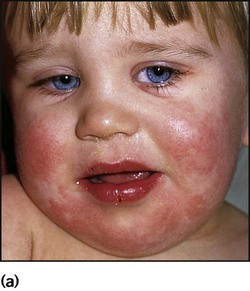
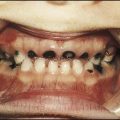
This is the most common form of primary HSV illness in children. It usually occurs from 10 months to 3 years of age. There are vesicular lesions on the lips, gums and anterior surfaces of the tongue and hard palate, which often progress to extensive, painful ulceration with bleeding (Fig. 14.12). There is a high fever and the child is very miserable. The illness may persist for up to 2 weeks. Eating and drinking are painful, which may lead to dehydration. Management is symptomatic, but severe disease may necessitate intravenous fluids and aciclovir.
Chickenpox (primary varicella zoster infection)
Clinical features
These are shown in Figure 14.14.
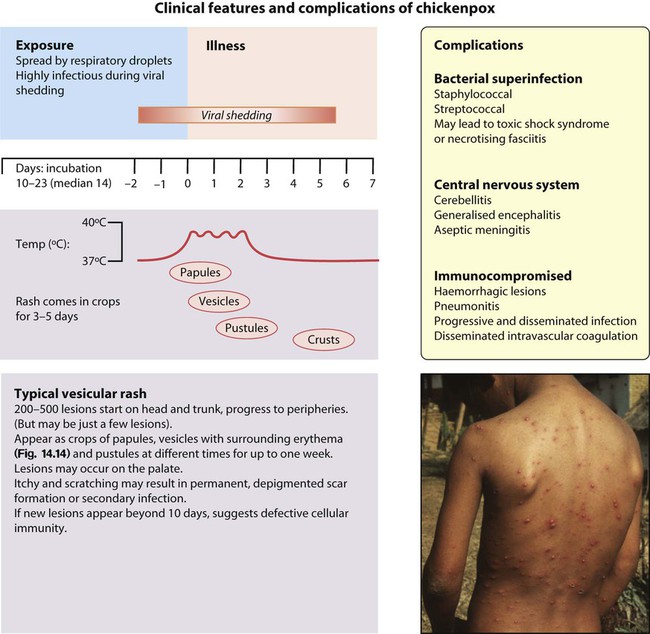
There are a number of rare but serious complications that can occur in previously healthy children:
• Secondary bacterial infection with staphylococci, group A streptococcal, or other organisms. May lead to further complications such as toxic shock syndrome or necrotising fasciitis. Secondary bacterial infection should be considered where there is onset of a new fever or persistent high fever after the first few days.
• Encephalitis. This may be generalised, usually occurring early during the illness. In contrast to the encephalitis caused by HSV, the prognosis is good. Most characteristic is a VZV-associated cerebellitis. This usually occurs about a week after the onset of rash. The child is ataxic with cerebellar signs. It usually resolves within a month.
• Purpura fulminans. This is the consequence of vasculitis in the skin and subcutaneous tissues. It is best known in relation to meningococcal disease and can lead to loss of large areas of skin by necrosis. It may rarely occur after VZV infection due to production of antiviral antibodies which cross-react and inactivate the coagulation factor protein S. There is subsequent dysregulation of fibrinolysis and an increased risk of clotting, most often manifest in the skin.
In the immunocompromised, primary varicella infection may result in severe progressive disseminated disease, which has a mortality of up to 20%. The vesicular eruptions persist and may become haemorrhagic. The disease in the neonatal period is described in Chapter 10.
Shingles (herpes zoster)
Shingles is uncommon in children. It is caused by reactivation of latent varicella-zoster virus (VZV), causing a vesicular eruption in the dermatomal distribution of sensory nerves (shingles). It occurs most commonly in the thoracic region, although any dermatome can be affected (Fig. 14.15). Children, unlike adults, rarely suffer neuralgic pain with shingles. Shingles in childhood is more common in those who had primary infection in the first year of life. Recurrent or multidermatomal shingles is strongly associated with underlying immune suppression, e.g. HIV infection. In the immunocompromised, reactivated infection can also disseminate to cause severe disease.
Epstein–Barr virus: infectious mononucleosis (glandular fever)
Older children, and occasionally young children, may develop a syndrome with:
• tonsillopharyngitis – often severe, limiting oral ingestion of fluids and food; rarely, breathing may be compromised
• lymphadenopathy – prominent cervical lymph nodes, often with diffuse adenopathy.
• atypical lymphocytes (numerous large T cells seen on blood film)
• a positive Monospot test (the presence of heterophile antibodies, i.e. antibodies that agglutinate sheep or horse erythrocytes but which are not absorbed by guinea pig kidney extracts – this test is often negative in young children with the disease)
• seroconversion with production of IgM and IgG to Epstein–Barr virus antigens.
Cytomegalovirus
As with EBV, CMV may cause a mononucleosis syndrome. Pharyngitis and lymphadenopathy are not usually as prominent as in EBV infections. Patients may have atypical lymphocytes on the blood film but are heterophile antibody-negative. Maternal CMV infection may result in congenital infection (see Ch. 9), which may be present at birth or develop when older. In the immunocompromised host, CMV can cause retinitis, pneumonitis, bone marrow failure, encephalitis, hepatitis, colitis and oesophagitis. It is a very important pathogen following organ transplantation. Organ recipients are closely monitored for evidence of CMV activation by sensitive tests such as blood polymerase chain reaction (PCR). Interventions used to reduce the risk of transmission of CMV disease are CMV-negative blood for transfusions and anti-CMV drug prophylaxis; also, if possible, CMV-positive organs are not transplanted into CMV-negative recipients.
CMV disease may be treated with ganciclovir or foscarnet, but both have serious side-effects.
Parvovirus B19
Parvovirus causes a range of clinical syndromes:
• Asymptomatic infection – common; about 5–10% of preschool children and 65% of adults have antibodies
• Erythema infectiosum – the most common illness, with a viraemic phase of fever, malaise, headache and myalgia followed by a characteristic rash a week later on the face (‘slapped-cheek’), progressing to a maculopapular, ‘lace’-like rash on the trunk and limbs; complications are rare in children, although arthralgia or arthritis is common in adults
• Aplastic crisis – the most serious consequence of parvovirus infection; it occurs in children with chronic haemolytic anaemias, where there is an increased rate of red cell turnover (e.g. sickle cell disease or thalassaemia); and in immunodeficient children (e.g. malignancy) who are unable to produce an antibody response to neutralise the infection
• Fetal disease – transmission of maternal parvovirus infection may lead to fetal hydrops and death due to severe anaemia, although the majority of infected fetuses will recover.
Uncommon viral infections
Measles
Health practitioners in the UK need to be aware of measles due to the rise in cases following public anxiety about the MMR vaccination (see the Immunisation section, below), as well as it continuing to be a major cause of morbidity and death worldwide. As with chickenpox and parvovirus, older children and adults tend to have more severe disease than the very young. For epidemiological tracking of infection, virological or serological confirmation of clinical cases of measles should be undertaken by testing either blood or saliva.
Clinical features
These are shown in Figure 14.16. There are a number of serious complications which can occur in previously healthy children:
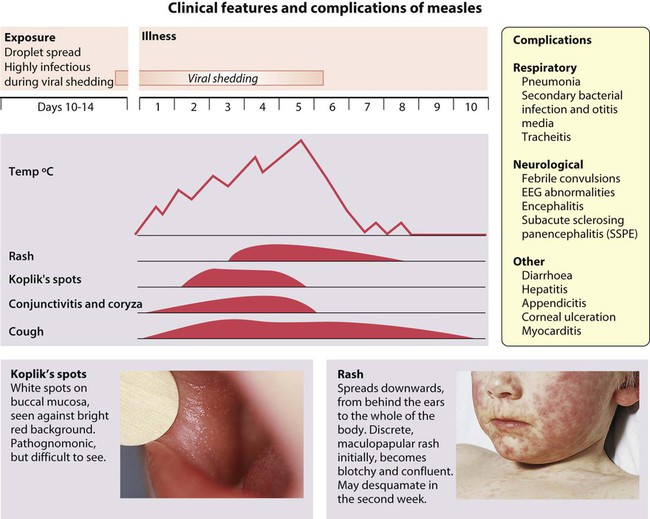
• Encephalitis occurs in about 1 in 5000, about 8 days after the onset of the illness. Initial symptoms are headache, lethargy and irritability, proceeding to convulsions and ultimately coma. Mortality is 15%. Serious long-term sequelae include seizures, deafness, hemiplegia and severe learning difficulties, affecting up to 40% of survivors.
• Subacute sclerosing panencephalitis (SSPE) is a rare but devastating illness manifesting, on average, 7 years after measles infection in about 1 in 100 000 cases. Most children who develop SSPE had primary measles infection before 2 years of age. SSPE is caused by a variant of the measles virus which persists in the central nervous system. The disorder presents with loss of neurological function, which progresses over several years to dementia and death. The diagnosis is essentially clinical, supported by finding high levels of measles antibody in both blood and cerebrospinal fluid and by characteristic EEG abnormalities. Since the introduction of measles immunisation, it has become extremely rare.
Rubella (German measles)
Rubella is generally a mild disease in childhood. It occurs in winter and spring. It is an important infection, as it can cause severe damage to the fetus (see Ch. 9). The incubation period is 15–20 days. It is spread by the respiratory route, frequently from a known contact. The prodrome is usually mild with a low-grade fever or none at all. The maculopapular rash is often the first sign of infection, appearing initially on the face and then spreading centrifugally to cover the whole body. It fades in 3–5 days. Unlike in adults, the rash is not itchy. Lymphadenopathy, particularly the suboccipital and postauricular nodes, is prominent. Complications are rare in childhood but include arthritis, encephalitis, thrombocytopenia and myocarditis. Clinical differentiation from other viral infections is unreliable. The diagnosis should be confirmed serologically if there is any risk of exposure of a non-immune pregnant woman. There is no effective antiviral treatment. Prevention therefore lies in immunisation.
Prolonged fever
Most childhood infections are acute and resolve in a few days. If not, the child needs to be reassessed for complications of the original illness, e.g. a secondary bacterial infection, or the source of infection may not have been identified, e.g. urinary tract infection. Often, the child has developed another unrelated febrile illness. Assessment of prolonged fever also needs to be made for prompt recognition of Kawasaki disease to avoid complications. Causes of prolonged fever are listed in Box 14.2.
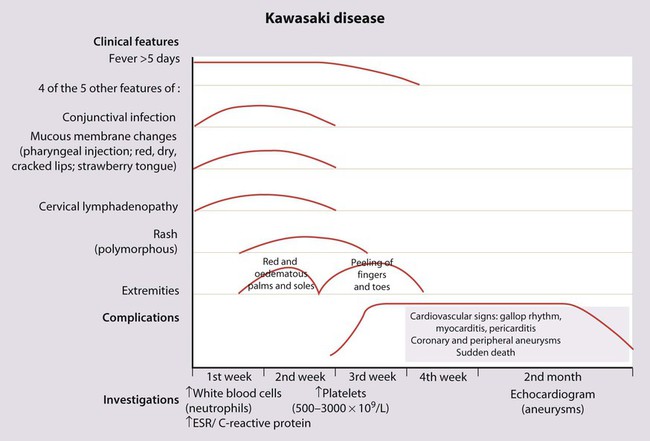
Kawasaki disease
There is no diagnostic test; instead, the diagnosis is made on clinical findings (Fig. 14.17). In addition to the classic features, affected children are strikingly irritable, have a high fever that is difficult to control, and may also have inflammation of their BCG vaccination site. They have high inflammatory markers (C-reactive protein, ESR, white cell count), with a platelet count that rises typically in the second week of the illness. The coronary arteries are affected in about one-third of affected children within the first 6 weeks of the illness. This can lead to aneurysms which are best visualised on echocardiography (see Case History 14.2). Subsequent narrowing of the vessels from scar formation can result in myocardial ischaemia and sudden death. Mortality is 1–2%.
HIV infection
Globally, HIV infection affects over 2 million children, mostly in sub-Saharan Africa (Fig. 14.21), and there are still 380 000 children becoming infected each year (Fig. 14.22). The major route of HIV infection in children is mother-to-child transmission (MTCT), which occurs during pregnancy (intrauterine), at delivery (intrapartum) or through breast-feeding (postpartum). The virus may also be transmitted to children by infected blood products, contaminated needles or through child sexual abuse, but this is uncommon.
Clinical features
A proportion of HIV-infected infants progress rapidly to symptomatic disease and onset of AIDS in the first year of life; however, other infected children remain asymptomatic for months or years before progressing to clinical disease. Some asymptomatic children will only be identified in adolescence at routine screening following diagnosis in another family member. Clinical presentation varies with the degree of immunosuppression. Children with mild immunosuppression may have lymphadenopathy or parotitis; if moderate, they may have recurrent bacterial infections, candidiasis, chronic diarrhoea and lymphocytic interstitial pneumonitis (LIP) (Fig. 14.23). This lymphocytic infiltration of the lungs may be caused by a response to the HIV infection itself, or it may be related to EBV infection. Severe AIDS diagnoses include opportunistic infections, e.g. Pneumocystis jiroveci (carinii) pneumonia (PCP), severe failure to thrive, encephalopathy (Fig. 14.24), and malignancy, although this is rare in children. More than one clinical feature is often present. An unusual constellation of symptoms, especially if infectious, should alert one to HIV infection.
Treatment
Other aspects of management include:
• Immunisation, which is important because of the higher risk of infections, and should follow the routine vaccination schedule, with the exception of BCG which should not be given as it is a live vaccine that can cause disseminated disease. Additional vaccination against influenza, hepatitis A, B and varicella zoster should be considered.
• Multidisciplinary management of children, if possible in a family clinic, where they can be seen together with other members of their family who may be HIV-infected and where the team includes an adult specialist. The team will need to address issues such as adherence to medication, disclosure of HIV diagnosis and planning for the future.
• Regular follow-up, with particular attention paid to weight, neurodevelopment and clinical signs and symptoms of disease. Effective antiretroviral therapy has transformed HIV infection into a chronic disease of childhood. Paediatric HIV clinics increasingly manage adolescents when there may be issues relating to maintaining ART adherence and address maternal issues such as safe sex practices, fertility and pregnancy.
Reduction of vertical transmission
• Use of maternal antenatal, perinatal and postnatal antiretroviral drugs to achieve an undetectable maternal viral load at the time of delivery
• Active management of labour and delivery, to avoid prolonged rupture of the membranes or unnecessary instrumentation
• Pre-labour Caesarean section if the mother’s viral load is detectable close to the time of delivery.
Immunisation
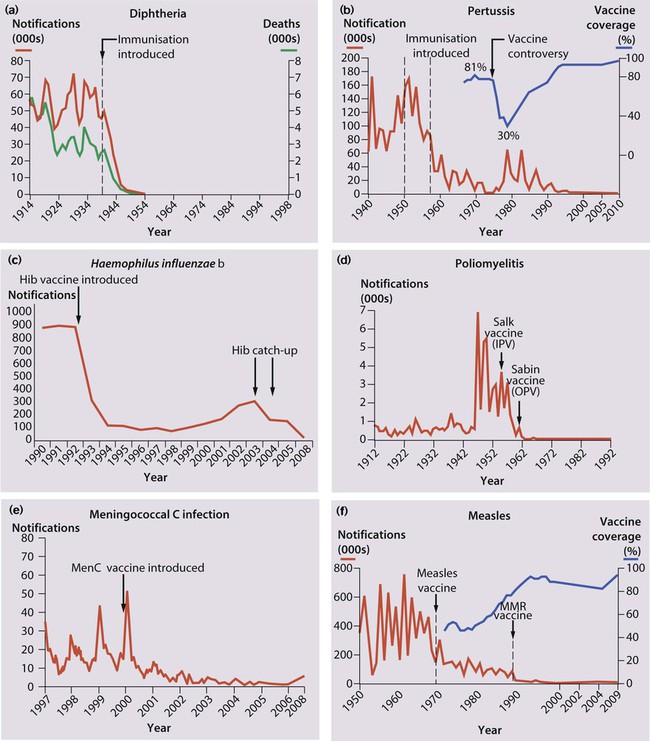
Differences exist in the composition and scheduling of immunisation programmes in different countries, and schedules change as new vaccines become available. The current UK schedule (Fig. 14.25) is available on the Department of Health website.
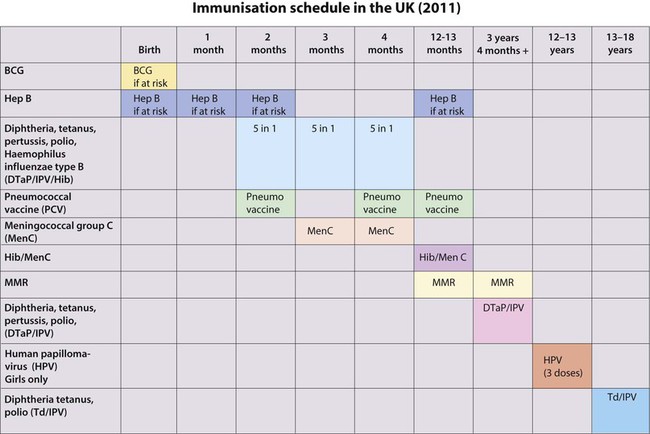
• In the newborn – BCG is given to infants at high risk of infection
• At 2, 3 and 4 months of age – the ‘5 in 1’ vaccine is given, against diphtheria, tetanus, pertussis, H. influenzae type b (Hib) and polio. The oral, live polio vaccine has been replaced by killed-vaccine given by injection, owing to the risk of vaccine-associated polio in unvaccinated family members or immune-deficient people following contact with gastrointestinal excretions of vaccine recipients
• At 2, 4 and 13 months, the pneumococcal conjugate vaccine (PCV13) is given
• At 2 months and 3 months, rotovirus vaccine given orally
• At 3 and 4 months, the conjugate vaccine against group C meningococcus (MenC) is given by separate injection
• At 12–13 months, a booster Hib vaccine is given, MenC and MMR (measles, mumps, rubella) is given
• At 12–13 years of age, the human papillomavirus (HPV) vaccine is given to girls. The rubella vaccine is no longer given to adolescent girls.
Rationale behind the immunisation programme
Diphtheria – infection causes local disease with membrane formation affecting the nose, pharynx or larynx or systemic disease with myocarditis and neurological manifestations. Immunisation has eradicated the disease in the UK (Fig. 14.26a).
Pertussis – clinical features described in Chapter 16. Huge decline in incidence with immunisation, but epidemics recur when immunisation rates fall (Fig. 14.26b).
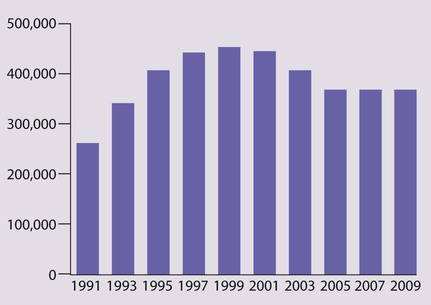
Haemophilus influenzae type b – causes invasive disease in young children The number of reports of infection dropped dramatically after the introduction of Hib vaccination (Fig. 14.26c), but a gradual rise from 1988 occurred because protection was not maintained throughout childhood. This was managed with a Hib catch-up programme, and to prevent a further resurgence, a Hib booster dose has been introduced at 12 months of age.
Poliovirus infection – Although most infected children are asymptomatic or have a mild illness, some develop aseptic meningitis and <1% develop paralytic polio. Almost eradicated worldwide (Fig. 14.26d).
Meningococcal C – The marked fall in the number of reports in all age groups is shown in Figure 14.26e.
Complications and contraindications
The controversy regarding a possible association between MMR vaccination and autism and inflammatory bowel disease has been discredited by a large number of well-conducted studies. However, public confidence in the immunisation programme was damaged, and uptake rates dropped (Fig. 14.26f). The MMR vaccine is only contraindicated in children with proven non-HIV-related immunodeficiency and those who are allergic to neomycin or kanamycin, which may be present in small quantities in the vaccine. Children with a history of anaphylaxis to egg (the virus is grown in fibroblast cultures generated from chick embryos) should be immunised with MMR under medical supervision. There is a 10% vaccine failure rate from primary vaccination with MMR at 12–13 months of age, but the proportion of susceptible school-age children in the UK has been reduced by the introduction of a preschool booster of MMR. Detailed information on MMR and other vaccines is available at: http://www.dh.gov.uk/en/Publichealth/Immunisation; further information about contraindications to vaccination can be found in the Department of Health Green Book, at: http://www.dh.gov.uk/en/Publichealth/Immunisation/Greenbook.
Immune deficiency
• Primary (uncommon) – an intrinsic defect in the immune system
• Secondary (more common) – caused by another disease or treatment, such as an intercurrent bacterial or viral infection, malignancy, malnutrition, HIV infection, immunosuppressive therapy, splenectomy or nephrotic syndrome.
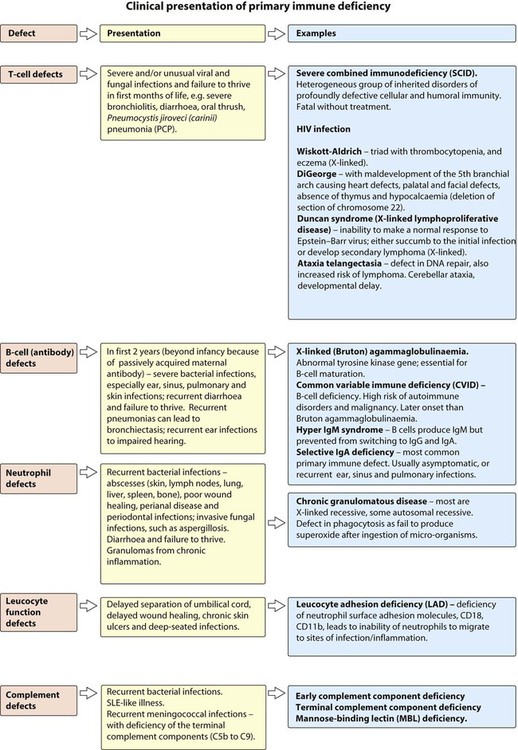
Primary immune deficiencies
Many of the primary immunodeficiencies are inherited as X-linked or autosomal recessive disorders. There may be a family history of parental consanguinity and unexplained death, particularly in boys. Immune deficiency should be considered in children who present with Severe, Prolonged, Unusual or Recurrent (SPUR) infections (Box 14.3). The clinical presentation of the different primary immune deficiencies is shown in Figure 14.27.
Full blood count
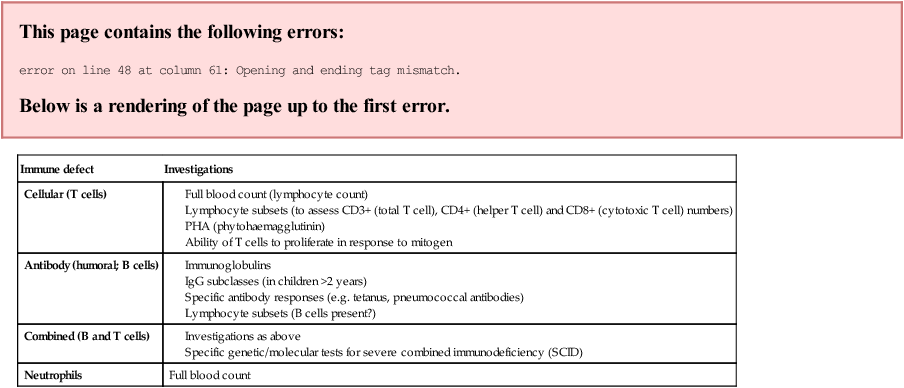
Management
– For T-cell and neutrophil defects – cotrimoxazole to prevent Pneumocystis jiroveci infection and itraconazole or fluconazole to prevent other fungal infections
– For B-cell defects – antibiotic prophylaxis (e.g. azithromycin) to prevent recurrent bacterial (e.g. chest, ear, sinus) infections
– Prompt treatment of infections
– Appropriate choice of antibiotics to cover likely organisms
– Generally longer courses, with lower threshold for intravenous therapy
• Screening for end-organ disease
• Immunoglobulin replacement therapy
– For children with antibody deficiency
– Can be given intravenously, which may require central venous (Portacath or Hickman) line insertion or subcutaneously