Neonatal Brain Injury
Adverse events during the neonatal period (the first month of life) account for a large proportion of child deaths and permanent neurologic disability. Preterm neonates are particularly vulnerable to brain injury during the first weeks of life. Imaging has been used widely not only to diagnose and understand brain injury in neonates, but also to predict the neurodevelopmental outcome.1 Because of its portability, cranial ultrasound is usually the first imaging modality to be performed and can be used serially to monitor the evolution of certain injuries. Ultrasound is usually sufficient for evaluation of germinal matrix hemorrhage (GMH) and intraventricular hemorrhage (IVH), hydrocephalus and serial assessment of ventricular size, cystic white matter injury of prematurity, and severe brain malformations. Ultrasound is less sensitive than computed tomography (CT) or magnetic resonance imaging (MRI) in the detection of small calcifications and is less sensitive than MRI in the detection of hypoxic-ischemic injury and subtle brain malformations. MRI also is excellent for the evaluation of punctate white matter lesions, which are seen in the setting of premature white matter. Because of the associated ionizing radiation, the use of CT is mainly restricted to instances in which there is a suspicion of skull fracture or to confirm the presence of intracranial calcifications, lesions that contain fat, and acute intracranial hemorrhage. MRI is the most sophisticated modality to evaluate the neonatal brain, and with advanced imaging techniques such as diffusion-weighted imaging (DWI), functional magnetic resonance imaging (fMRI), and magnetic resonance spectroscopy (MRS), it has the advantage of providing information regarding physiology, function, and metabolism. With the development of arterial spin labeling techniques, it is now possible to assess brain perfusion in the neonate without the use of intravenous contrast material.
Imaging Techniques
Dedicated neonatal MR head coils improve image contrast and resolution at the smaller field of view optimal for neonatal imaging.2,3 These dedicated coils improve gray-white matter differentiation and provide better visualization of the brainstem and posterior fossa.
Patient Preparation, Safety, and Hazards
Because of the MR environment and length of most MRI examinations, MR safety is a particular concern for neonates. Before the MR examination, any patient, including neonates, should be screened for possible cardiac devices, implants, non–MR-compatible leads, or surgically implanted wires. The compatibility of any device must always be verified with the manufacturer. Additionally, Shellock and Kanal3a provide useful information about the attraction/deflection forces of many items exposed to static magnetic fields. Continuous monitoring and support for respiratory and cardiovascular functions can be achieved with MR-compatible equipment. Thermoregulation, which can be a particular concern for preterm neonates, can be supported through use of MR-compatible incubators and monitored through use of an MR-compatible temperature probe.
Germinal Matrix and Intraventricular Hemorrhage
Hemorrhagic brain injury of prematurity has been classified into four groups. Grade I is hemorrhage confined to the GM; grade II is GMH extending into the ventricles without evidence of ventricular dilatation; grade III is IVH with evidence of ventriculomegaly; and grade IV is IVH with an associated parenchymal infarction, as a result of congestion of the venous outflow (Table 30-1). Grades I and II of IVH have a low morbidity and mortality, whereas grades III and IV have higher mortality rates and a substantial risk of poor neurodevelopmental outcome among survivors (Fig. 30-1). Posthemorrhagic ventricular dilation can be managed with a temporizing neurosurgical procedure, including a ventricular reservoir, a subgaleal shunt, or a ventriculoperitoneal shunt.
Table 30-1
Classification of Germinal Matrix Hemorrhage/Intraventricular Hemorrhage
| Grade | Definition |
| 1 | Hemorrhage confined to germinal matrix |
| 2 | Intraventricular without ventriculomegaly |
| 3 | Intraventricular with ventriculomegaly |
| 4 | Parenchymal hemorrhage related to venous infarction (not directly related to grades 1-3) |
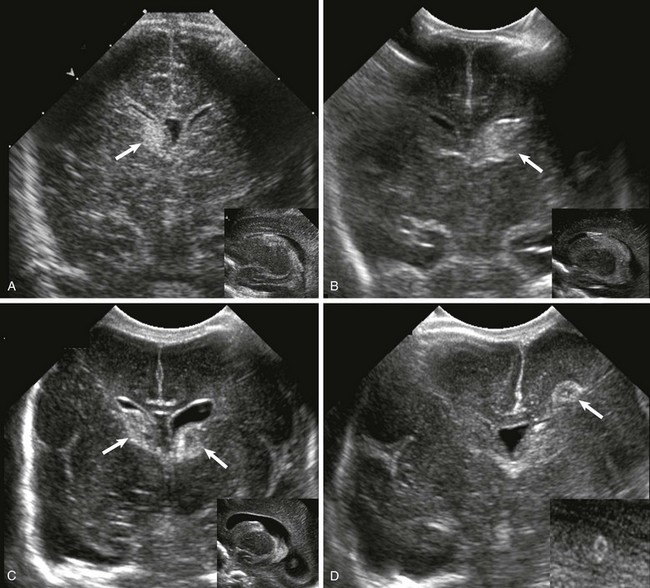
Figure 30-1 Ultrasound of germinal matrix hemorrhage.
A, Grade 1, hemorrhage confined to the germinal matrix (arrow). B, Grade 2, germinal matrix hemorrhage extending into the ventricles (arrow). C, Grade 3, germinal matrix and intraventricular hemorrhage with ventriculomegaly (arrows). D, Intraparenchymal hemorrhage (arrow).
Premature White Matter Injury
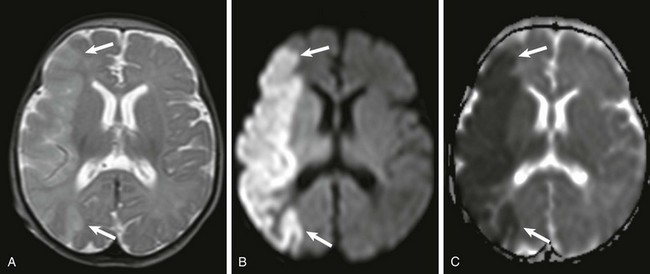
For more than a century, it has been recognized that the developing white matter is exceptionally vulnerable to injury during the second half of fetal gestation (i.e., the period during which preterm infants are born, and in most cases, survive beyond infancy). However, more recently it increasingly has been recognized that injury to the preterm neonate can involve many regions in the central nervous system (CNS), from gray matter (thalamus, cortex, and basal ganglia) to white matter to the brainstem and cerebellum. As a result, it has been argued that more comprehensive terms such as “encephalopathy of prematurity” should be used when referring to injury in the preterm neonate (see Suggested Readings). At the same time, the pattern of injury in preterm neonates has changed during the past several decades in parallel with advances in neonatal intensive care. Historically, cystic periventricular leukomalacia, characterized by multiple areas of cavitary necroses in the periventricular and deep white matter with surrounding astrogliosis, was a frequent observation, particularly among neonates who underwent autopsy. In the modern era, subtle changes in the white matter are more often observed on neuroimaging (e.g., “diffuse excessive high signal intensity”) and at autopsy (e.g., gliosis) with or without accompanying microscopic (1 to 2 mm or less) necroses (visualized on MRI as punctate lesions with high T1-signal intensity in the periventricular and deep white matter).4,5 A low concentration of lactate may be detected in preterm neonates and neonates who are small for gestational age using MRS and is often considered a “normal” finding unless it persists beyond term-equivalency or is associated with other findings (Fig. 30-2).
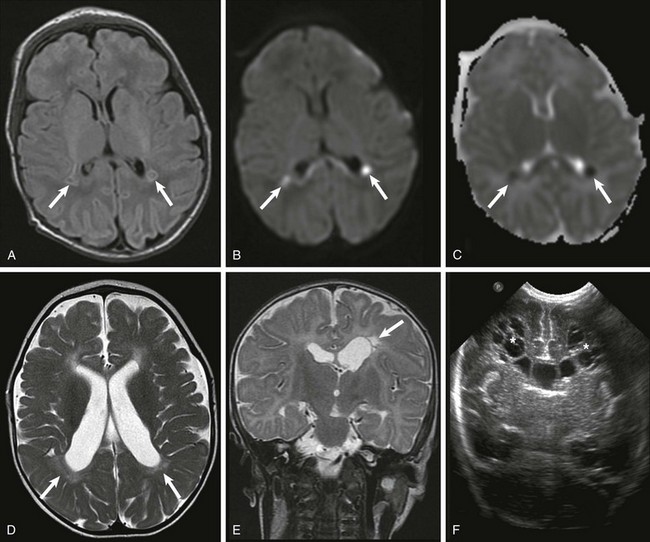
Figure 30-2 White matter injury of prematurity.
Acutely, focal areas of necrosis with increased signal are seen on T1-weighted images (A), with restricted diffusion (B) and low apparent diffusion coefficient (arrows) (C). D, A different patient at 2 years of age has increased T2 signal and volume loss involving the periventricular white matter bilaterally (arrows). E, A unilateral white matter injury with small cysts (arrow) seen on T2-weighted imaging. F, A bilateral cystic white matter injury (asterisks) on cranial ultrasound.
Hypoxic-Ischemic Encephalopathy
Central Pattern of Hypoxic-Ischemic Encephalopathy
The central pattern of hypoxic-ischemic encephalopathy (HIE) usually occurs with profound asphyxia, when there is an abrupt interruption of the blood supply, depriving the neonatal brain of oxygen and glucose. Highly metabolic structures such as the thalami, basal ganglia, and brainstem are more vulnerable to hypoxia and ischemia, more specifically the ventral lateral thalami, posterolateral lentiform nuclei, posterior midbrain, hippocampi, lateral geniculate nuclei, and perirolandic cerebral cortex (Fig. 30-3).5 Quadriparesis, choreoathetosis, seizures, mental retardation, and cerebral palsy have been associated with profound asphyxia.6 Ultrasound and CT have low sensitivity to detect early ischemic changes in the deep structures of the brain. The most common pattern on ultrasound is transient or persistent hyperechogenicity, which may progress to cavitation in the basal ganglia and thalami, particularly in the globus pallidus and ventral lateral nuclei of the thalamus. With more severe insults involving the cortex and subcortical white matter, ultrasound and CT may depict indirect evidence of edema, such as effacement of the sulci, loss of gray-white matter differentiation, and compressed lateral ventricles.
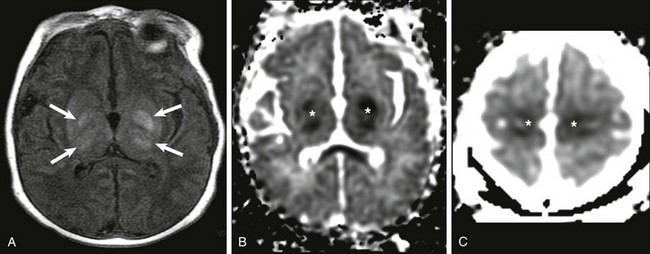
Figure 30-3 Central hypoxic-ischemic injury.
A, Increased T1 signal in the globi pallidi and ventrolateral thalami bilaterally (arrows). B and C, Reduced apparent diffusion coefficient in the globi pallidi and thalami and in the regions of the perirolandic cortex bilaterally (*).
MRI is the imaging modality of choice for neonatal encephalopathy. MRS performed 24 hours after the insult is considered sensitive for hypoxic-ischemic brain injury. Elevated lactate and diminished N-acetyl-aspartate (NAA) are the most common MRS findings in neonates with neurologic and developmental abnormalities (e-Fig. 30-4). Lactate rises after the hypoxic-ischemic event, peaking at 3 to 5 days, whereas NAA starts to decline around the third day. Although a minimally elevated lactate level (lactate/choline ratio <0.15) may be detected in the normal neonatal brain at term, an increased lactate level relative to the total creatine peak in the basal ganglia provides an early indication of brain injury before changes may be apparent on conventional T1- and T2-weighted imaging. It has been suggested that resuscitation may rapidly clear lactate and that a secondary increase in lactate may occur after 12 to 24 hours. The metabolites tend to normalize after about day 5, although in some cases abnormal metabolite ratios persist. Persistently elevated lactate levels in the basal ganglia provide prognostic information about the severity of the brain injury and the subsequent neurodevelopmental outcome. False-negative MRS findings also may occur, with normal spectral findings but abnormal outcomes.
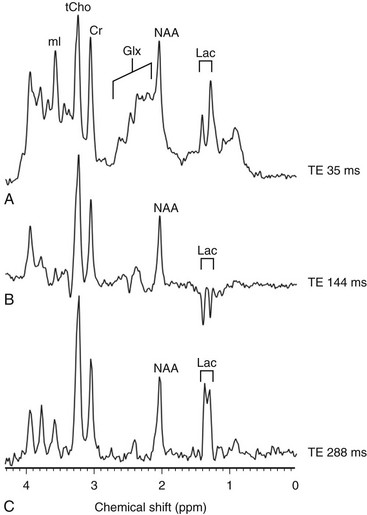
e-Figure 30-4 Magnetic resonance spectroscopy (MRS).
An increased level of lactate is seen on MRS performed with a time echo of 35 ms (A) and 288 ms (C) and inverted with 144 ms (B). Cr, Creatine; Glx, glutamic acid; Lac, lactate; mI, myo-inositol; NAA, N-acetylaspartate; ppm, parts per million; tCho, total choline; TE, echo time.
DWI is a sensitive technique for assessment of acute brain injury. DWI also shows deep gray matter and perirolandic gray matter lesions before they are seen with conventional MRI. If DWI is performed in the first few hours after the injury, it may underestimate the extent of the injury or even show normal results. Paralleling the clinical presentation of HIE, in which neonates may actually transiently improve before demonstrating a more permanent decline in neurologic functioning because of delayed cell death via apoptotic mechanisms, some patients demonstrate mild brain damage for the first few days and then proceed to demonstrate extensive brain involvement around 5 days after the injury. Apparent diffusion coefficient (ADC) values always evolve over time; they decrease initially after the injury, with the nadir around 3 to 5 days, and increase (via facilitated diffusion) later in the chronic phase. As ADC values increase, a point of transient “pseudonormalization” exists in which injured tissue can be misdiagnosed as “normal.” Conventional MRI findings are usually unremarkable during the first few days but then begin to demonstrate first an increased T1-weighted signal and a decreased T2-weighted signal in the subacute period followed by an increased T2-weighted signal in the more chronic period. Evidence suggests that hypothermia therapy delays the onset of MR changes (in metabolic, diffusion, and conventional imaging) associated with injury and, in particular, delays the onset of pseudonormalization in the ADC signal.7
Peripheral Pattern of Hypoxic-Ischemic Encephalopathy
The peripheral pattern of HIE usually results from a period of decreased blood supply to the brain (rather than a near total and abrupt interruption) and is thought to develop as a result of a compensatory shunting of blood to vital brain structures, such as the brainstem, thalami, basal ganglia, hippocampi, and cerebellum, at the expense of less metabolically active structures, namely the cerebral cortex and white matter (Fig. 30-5). Therefore the brainstem, cerebellum, and deep gray matter structures generally are spared from injury in mild to moderate hypoxic-ischemic insults. More prolonged insults result in injury to the intervascular border (watershed) zones, which are relatively hypoperfused as a result of this shunting. Neurologic examination varies depending on the severity of the insult, from asymptomatic in mild cases to proximal extremity weakness or spasticity and cerebral palsy in persons who sustain severe insults.6 Increasing severity of watershed-distribution injury is associated with impaired neurocognitive functioning, including language, visuoperceptual, and executive functioning impairments.
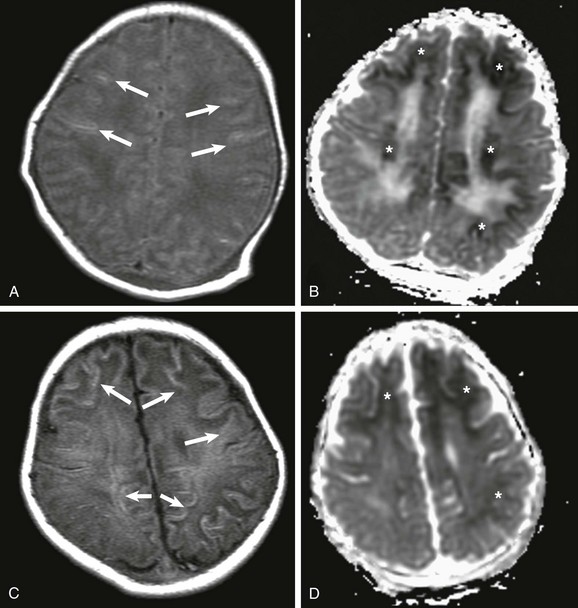
Figure 30-5 Peripheral pattern of hypoxic-ischemic injury.
A, At day 2 after the ischemic event, subtle findings are seen on T1-weighted imaging (arrows), but reduced apparent diffusion coefficient (ADC), mainly involving the subcortical white matter, is marked (*) (B). C, At 7 days after the insult, T1-weighted images demonstrate progression on the areas of increased T1 signal, mainly involving the subcortical white matter (arrows), and less conspicuously reduced ADC (*) (D).
Neonatal Arterial Infarction
Infarctions involving the vascular territory of a major artery, most commonly the middle cerebral artery (L>R), are more common in term than in preterm neonates. Symptoms are subtle and nonspecific, and many neonatal arterial infarcts may be unrecognized until motor or cognitive symptoms develop later in infancy or childhood. In the neonatal period, the most common presenting symptom is a focal motor seizure involving the contralateral limbs, which may secondarily generalize. Infarcts of the anterior and posterior cerebral arteries are underdetected because they may be asymptomatic and difficult to visualize on ultrasound. Several causes have been described, including sepsis, bacterial meningitis, inherited or acquired coagulopathies (heterozygosity for factor V Leiden and disseminated intravascular coagulation), and cardiac abnormalities, but often no specific cause is identified. Initial imaging shows a loss of gray-white matter differentiation, which in severe instances may be detected on ultrasound but is most readily visualized on MRI as increased signal on DWI with low ADC values (Fig. 30-6). Later (after 24 to 48 hours) the edema becomes apparent as increased echogenicity on ultrasound, hypoattenuation on CT, increased signal on T2-weighted MRI, and decreased signal on T1-weighted MRI. Although the high water content of the unmyelinated neonatal brain poses significant challenges for the detection of edema on CT or conventional MRI sequences, loss of gray-white matter differentiation frequently is present. This lack of contrast between the normal brain and the infarction is particularly relevant at 5 to 10 days after infarction when the ADC may be pseudonormalized. With time, ADC values increase (via facilitated diffusion); after a few weeks, volume loss and encephalomalacia become apparent. Hemorrhagic transformation of an ischemic stroke is rare in neonates, but increased signal on T1-weighted imaging along the cortex related to laminar necrosis is not uncommon. Interestingly, acutely after a perinatal ischemic infarct, hyperperfusion as demonstrated on arterial spin label sequences frequently is observed in the regions corresponding to areas of low ADC on diffusion-weighted MRI. Occasionally, focal areas of hyperperfusion also can be seen after seizures (epileptiform activity) in the neonate.
Vascular Malformations
Vein of Galen Malformation
Malformations involving the vein of Galen are true congenital arteriovenous connections between thalamic perforating, choroidal, and anterior cerebral arteries with the embryonic median prosencephalic vein. Associated cardiovascular anomalies may be present, such as aortic coarctation and secundum atrial septal defect.8 The most common clinical presentation is high-output cardiac failure. The prognosis is mainly related to the number of abnormal arteriovenous connections and the amount of associated parenchymal injury from arterial steal and hypoperfusion. The most common is the choroidal type with innumerous connections, which usually is fatal. The mural type with one to four connections has a better prognosis and is less likely to present in the neonatal period. This type usually presents during infancy as developmental delay, hydrocephalus, and seizures, or in older children as hemorrhage. Endovascular therapy is the treatment of choice. Prenatal and postnatal spontaneous thrombosis has been reported.
Birth Trauma
Extracranial Hematomas
The major types of extracranial hematomas are subgaleal hematoma, caput succedaneum, and cephalohematoma (also see Chapter 23). Subgaleal hematomas may enlarge rapidly and lead to potentially life-threatening hypovolemia. Anemia, coagulopathy, metabolic acidosis, renal impairment, and skull fracture have been reported as predictors of poor outcome. Early recognition and management are essential. An extracranial hematoma is caused by rupture of the emissary veins between the dural sinuses and the scalp veins; the hemorrhage accumulates between the epicranial aponeurosis of the scalp and the periosteum. Upon imaging, extracranial hematomas appear as a hemorrhagic collection overlying the calvarium and crossing the sutures. They may extend underneath the attachments of the occipitofrontalis muscle to the orbital margins anteriorly, the temporal fascia laterally, and the nuchal ridge posteriorly.
Congenital and Neonatal Infection
Bacterial Infections
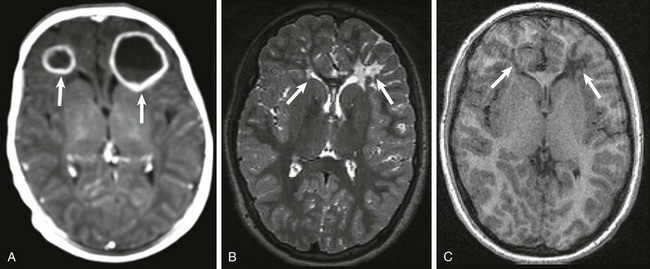
Ventricular enlargement resulting from impaired CSF absorption by fibrinous inflammatory exudate is the most common imaging finding in persons with meningitis and does not necessarily indicate ventriculitis. Ventriculitis usually presents with ependymal enhancement and sometimes with a pus-fluid level. Ventriculitis often is associated with hydrocephalus and sometimes with intraventricular abscess. Sterile subdural effusions are not uncommon in neonates with meningitis; however, in a very small percentage of cases (approximately 2%), empyemas develop. Subdural collection with a signal intensity different from that of CSF, peripheral enhancement sometimes with septa, and signal abnormality involving the brain parenchyma are suggestive of empyema. Cerebritis is not uncommon in autopsies of neonates with meningitis despite normal findings of in vivo imaging. When imaging findings are present, cerebritis appears as an area of vasogenic edema with or without patchy areas of enhancement and/or cortical enhancement that may resolve with treatment. If the infection progresses, the enhancing areas become more confluent, and later an abscess forms with restricted water diffusivity and an enhancing capsule. Neonatal Citrobacter koseri (diversus) meningitis is rare but often is complicated by formation of abscesses, with a predilection for the frontal lobes. Neonatal pneumocephalus also has been reported as a complication of C. koseri meningitis. Citrobacter meningitis usually has a poor neurologic outcome (e-Fig. 30-7).
Herpes Simplex Virus Type 2 Infections
Women who have their first outbreak of herpes simplex virus type 2 genital herpes during pregnancy are at high risk of miscarriage or delivering a baby with a low birth weight. The infection can be passed to the neonate in utero or, most commonly, during birth. The most serious risk to the infant is herpes simplex virus type 2 encephalitis. Signs and symptoms in the newborn are nonspecific, with irritability, a high-pitched cry, fever, and poor feeding. Diagnosis is made via a viral culture or polymerase chain reaction (PCR) of the CSF. If the infection occurs early in pregnancy, it may cause hydranencephaly, basal ganglia calcifications, and microphthalmia (the classic TORCH pattern). Late pregnancy or intrapartum transmission involves the bilateral gray and white matter diffusely, which rapidly develops into whole brain necrosis. Multiple cortical hemorrhagic foci and sometimes leptomeningeal enhancement often are present. With time, cystic white matter changes develop (Fig. 30-8).9
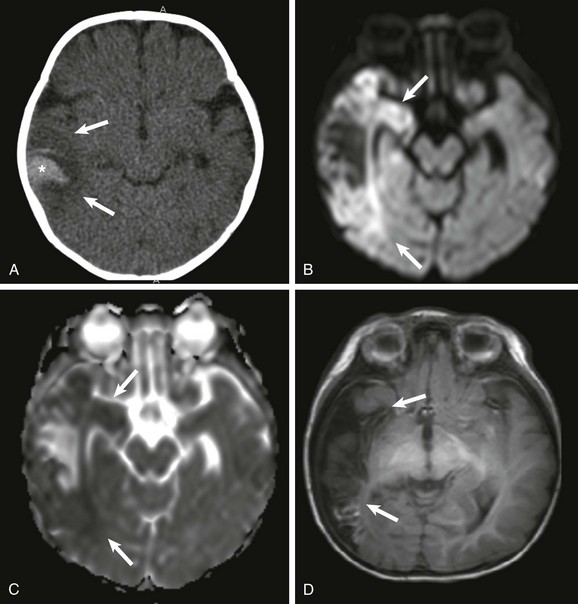
Figure 30-8 Herpes encephalitis.
A, A noncontrast computed tomography (CT) image showing a large area of parenchymal edema in the right temporal lobe (arrows). A peripheral area of increased attenuation (*) represents confluent petechial hemorrhages. A subsequent magnetic resonance image demonstrates restricted diffusion on diffusion-weighted imaging (B) and low apparent diffusion coefficient values (arrows) (C). Of note, the medial temporal lobe, mainly the hippocampus, is usually spared in middle cerebral artery infarcts but is commonly involved in persons with herpes encephalitis. D, Thirty days after the first CT scan, encephalomalacia of the involved area is seen (arrows).
Enterovirus and Parechovirus Infection
Enterovirus and parechovirus belong to the family Picornaviridae. Enteroviruses are well known for causing neonatal hepatitis, myocarditis, and meningoencephalitis, which can lead to death or severe long-term morbidity. Parechoviruses recently have been reported as causing severe neonatal infection, including involvement of the CNS. The diagnosis is made by PCR; however, PCR for enterovirus does not detect parechovirus. Meningoencephalitis in the neonatal period causes extensive white matter abnormalities, which appear as hyperechogenicity on ultrasound, hypoattenuation on CT, increased signal on T2-weighted MRI images, and decreased signal on T1-weighted MRI images, with or without restricted water diffusivity (e-Fig. 30-9). This pattern of injury easily may be misinterpreted as white matter injury of prematurity or as being caused by hypoxic-ischemic injury, particularly the partial prolonged type.10
Congenital Cytomegalovirus Infection
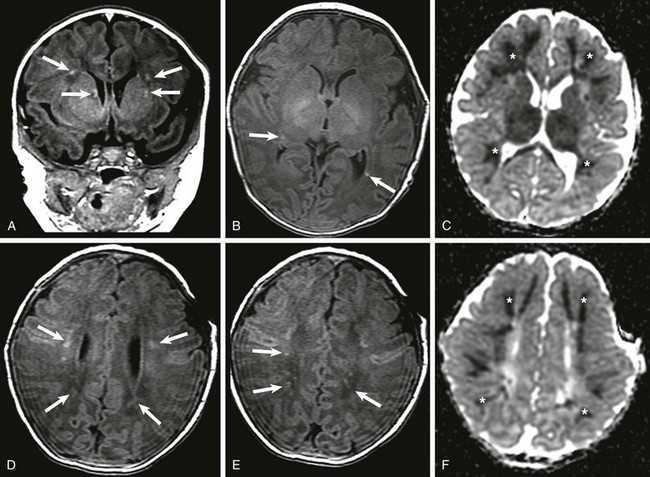
The brain is the most commonly affected organ. CMV interferes with normal fetal brain development and is associated long-term with mental retardation, blindness, deafness, or epilepsy. Depending on the timing of infection during fetal development, neonates with congenital CMV may have microcephaly, predominately periventricular calcifications, ventriculomegaly, lissencephaly, polymicrogyria, cerebellar hypoplasia, dysplastic white matter, and porencephaly.11 Other findings include intrauterine growth restriction, hepatosplenomegaly, cardiomyopathy, echogenic bowel, and hydrops. CT can detect and characterize diminutive periventricular and subependymal calcifications. CMV is also the leading infectious cause of sensorineural hearing loss; CT may demonstrate a Mondini malformation with an absent interscalar septum, a large vestibule, and an enlarged vestibular aqueduct.
Congenital Varicella Infection
The incidence of congenital varicella syndrome after maternal varicella infection during the first two trimesters is <1%. As with CMV and toxoplasmosis, intrahepatic and intracranial calcifications are very common. Intrauterine encephalitis, cortical atrophy, and porencephaly have been reported in cases of congenital varicella infection before 20 weeks of gestational age. Other possible fetal abnormalities are polyhydramnios, limb hypoplasia, and contractures, as well as paradoxical diaphragmatic motion as a result of unilateral paralysis. Neonates may demonstrate dysfunction of the autonomic nervous system (neurogenic bladder), hydroureter, esophageal dilation, aspiration pneumonia, and cutaneous lesions in a dermatomal distribution.12
Acquired Metabolic Disorders
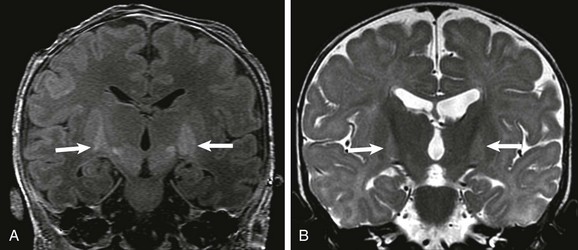
Kernicterus (Bilirubin Encephalopathy)
Pathologically, kernicterus causes damage to the globi pallidi, subthalamic nuclei, and hippocampi (CA2 and CA3 regions). Ultrasound and CT are not sensitive in detecting early abnormalities in persons with kernicterus. On MRI, an increased signal initially is seen on T1-weighted images; this signal is inconsistent. Increased signal on T2-weighted images of affected areas develops later (Fig. 30-10), when hyperechogenicity may appear on ultrasound and hypoattenuation may appear on CT.13 Restricted diffusion usually is not seen in persons with kernicterus unless it is associated with other conditions, such as hypoxia-ischemia. Conventional T1 spin echo images appear to be more reliable than 3D T1 spoiled gradient echo images, because the latter are associated with a high signal in normal subthalamic nuclei, which may cause false-positive results. A metabolite signature of acute kernicterus has been proposed on MRS using an echo time of 35 ms with elevated ratios of taurine, glutamate, glutamine, and myo-inositol and a decreased ratio of choline relative to creatine with no significant elevation of lactate.
Hypoglycemia
Neonatal encephalopathy resulting from hypoglycemia typically occurs when glucose concentrations are less than 30 mg/dL in the term infant and less than 20 mg/dL in the preterm infant. The clinical presentation of neonatal hypoglycemia may be subtle, including stupor, jitteriness, seizures, respiratory abnormalities, and hypotonia. MRI is the imaging modality of choice, showing abnormalities in the posterior parietal and occipital cortex and adjacent white matter. Acutely, there is restricted diffusion on DWI as well as edema on T1- and T2-weighted imaging. MRS with an echo time of 30 ms exhibits an increased lactate-lipid peak and a decreased NAA peak in the involved areas. In the chronic phase, the involved areas evolve into encephalomalacia and atrophy. If the hypoglycemia is severe and prolonged, progression to diffuse brain damage occurs with involvement of the hippocampus, corpus striatum, and cerebellum.14
Inborn Errors of Metabolism
Inborn errors of metabolism present with signs and symptoms related to the involvement of one or more of the organ systems, including the CNS. Inborn errors of metabolism are classified into organic acidemia, disorders of amino acid oxidation, disorders of fatty acid oxidation, primary lactic acidosis, mitochondria function, lysosomal storage disorders, and peroxisome disorders (also see Chapter 33). Whereas some inborn errors of metabolism manifest immediately at birth (e.g., primary lactic acidosis, type 2 glutaric aciduria, long-chain acyl coenzyme A dehydrogenase, hydroxymethylglutaryl-CoA lyase, ornithine transcarbamylase, and carbamyl phosphatase synthetase deficiencies), in others, it takes a few days for signs and symptoms to develop (e.g., isovaleric acidemia, methylmalonic acidemia, propionic acidemia, nonketotic hyperglycinemia, citrullinemia, argininosuccinic aciduria, and maple syrup urine disease).
The inborn errors of metabolism that affect the nervous system exclusively (namely l-2-hydroxyglutaric aciduria, glutaric aciduria type 1, 4-hydroxybutyric aciduria, alpha-ketoglutaric aciduria, mevalonic aciduria, and N-acetylaspartic aciduria [Canavan disease]) present later in life and are not encountered in neonates. MRS can provide valuable information regarding specific metabolites in certain neonatal metabolic disorders: increased branched-chain amino acids (e.g., l-leucine, l-isoleucine, and valine) in persons with maple syrup urine disease, increased glycine in persons with nonketotic hyperglycinemia, and absence of creatine in persons with guanidinoacetate methyltransferase deficiency (also see Chapter 25).
Amino Acid Disorders
Maple syrup urine disease results from an interruption of the metabolism of the essential amino acids leucine, isoleucine, and/or valine. The most severe form manifests in the first week of life with seizures, vomiting, dystonia, fluctuating ophthalmoplegia, and coma. The imaging findings are almost pathognomonic. Conventional MR shows edema in the deep cerebellar white matter, brainstem tegmentum, posterior limb of the internal capsule, perirolandic white matter, and precentral and postcentral gyrus. Restricted water diffusivity is seen acutely in the areas of vacuolating edema in the myelinated white matter. MRS shows a peak at 0.9 parts per million (ppm) related to the accumulation of abnormal branched-chain amino acids and branched-chain apha-ketoacids. The imaging findings by diffusion imaging and MRS may resolve with treatment.15
Barkovich, AJ. MR imaging of the neonatal brain. Neuroimaging Clin N Am. 2006;16(1):117–135. viii-ix
Barkovich, AJ. Pediatric neuroradiology, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
Huang, BY, Castillo, M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28(2):417–439. quiz 617
Rutherford, MA. MRI of the neonatal brain. (website) www.mrineonatalbrain.com. Accessed October 19, 2012
Volpe, JJ. The encephalopathy of prematurity—brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16(4):167–178.
References
1. Lemons, JA, Bauer, CR, Oh, W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107(1):E1.
2. Panigrahy, A, Borzage, M, Bluml, S. Basic principles and concepts underlying recent advances in magnetic resonance imaging of the developing brain. Semin Perinatol. 2010;34(1):3–19.
3. Blüml, S, Friedlich, P, Erberich, S, et al. MR imaging of newborns by using an MR-compatible incubator with integrated radiofrequency coils: initial experience. Radiology. 2004;231(2):594–601.
3a. Kanal, E, Shellock, FG. Safety reference manual on magnetic resonance imaging contrast agents. New York: Lippincott-Raven; 1996.
4. Dyet, LE, Kennea, N, Counsell, SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548.
5. Sie, LT, van der Knapp, MS, Oosting, J, et al. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics. 2000;31(3):128–136.
6. Martinez-Biarge, M, Diez-Sebastian, J, Kapellou, O, et al. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76(24):2055–2061.
7. Bednarek, N, Mathur, A, Inder, T, et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78(18):1420–1427.
8. McElhinney, DB, Halbach, W, Silverman, NH, et al. Congenital cardiac anomalies with vein of Galen malformations in infants. Arch Dis Child. 1998;78(6):548–551.
9. Whitley, R. Neonatal herpes simplex virus infection. Curr Opin Infect Dis. 2004;17(3):243–246.
10. Gupta, S, Fernandez, D, Siddiqui, A, et al. Extensive white matter abnormalities associated with neonatal Parechovirus (HPeV) infection. Eur J Paediatr Neurol. 2010;14(6):531–534.
11. Barkovich, AJ, Lindan, CE. Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. AJNR Am J Neuroradiol. 1994;15(4):703–715.
12. Smith, CK, Arvin, AM. Varicella in the fetus and newborn. Semin Fetal Neonatal Med. 2009;14(4):209–217.
13. Gkoltsiou, K, Tzoufi, M, Counsell, S, et al. Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev. 2008;84(12):829–838.
14. Kim, SY, Goo, HW, Lim, KH, et al. Neonatal hypoglycaemic encephalopathy: diffusion-weighted imaging and proton MR spectroscopy. Pediatr Radiol. 2006;36(2):144–148.
15. Jan, W, Zimmerman, RA, Wang, ZJ, et al. MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology. 2003;45(6):393–399.