Neisseria and Moraxella catarrhalis
1. Identify the clinical specimens or sources for the isolation of pathogenic Neisseria spp.
2. List the Neisseria species considered normal flora and the sites where they colonize the human body.
3. Explain the routes of transmission for the organisms discussed in this chapter; include the clinical relevance of asymptomatic carriers.
4. Define and describe the diseases associated with Moraxella catarrhalis and the pathogenic Neisseria spp., Neisseria gonorrhoeae and Neisseria meningitidis (i.e., pelvic inflammatory disease, disseminated gonococcal infection, ophthalmia neonatorum, pharyngitis, meningitis, and septicemia); include the signs and symptoms, treatments, and prognosis.
5. Describe the method of transport that yields optimal recovery of N. gonorrhoeae, including transport media, growth temperatures, and atmospheric conditions.
6. Describe the benefits of amplified testing for N. gonorrhoeae over nonamplified testing as it relates to financial, diagnostic and clinical efficacy, and control measures.
7. Identify the optimal growth conditions for the Neisseria species.
8. Name the appropriate biochemical tests for differentiating the Neisseria species and explain the chemical principle for each test.
9. Biochemically differentiate the organisms in this chapter using carbohydrate utilization (cysteine trypticase agar [CTA]) and orthonitrophenyl galactoside (ONPG).
10. Describe the appropriate therapeutic agents for N. gonorrhoeae.
11. Compare and contrast the laboratory identification of M. catarrhalis and Neisseria spp.
12. Analyze laboratory data and disease signs and symptoms for correlation and identification of the etiologic agents discussed in this chapter.
General Characteristics
Species of the family Neisseriaceae, genus Neisseria, are discussed in this chapter, along with family Moraxellaceae, species Moraxella catarrhalis, because of their biochemical and morphologic similarities. The organisms are all oxidase-positive, gram-negative diplococci that do not elongate when exposed to subinhibitory concentrations of penicillin. The rodlike Neisseria spp. are described in Chapter 28.
Epidemiology
Except for Neisseria gonorrhoeae, the organisms considered in Table 40-1 are normal inhabitants of the upper respiratory tract of humans. Humans are the only natural host for N. gonorrhoeae, primarily a clinically significant pathogen found in the urogenital tract and never considered normal flora. Asymptomatic carriers of gonorrhea are the primary reservoir for dissemination in the human population. The number of reported and identified cases of infection with N. gonorrhoeae is probably significantly higher than statistical data indicate because of the number of unreported cases.
TABLE 40-1
| Organism | Habitat (Reservoir) | Mode of Transmission |
| Moraxella catarrhalis | Normal human flora of upper respiratory tract; occasionally colonizes female genital tract | Spread of patient’s endogenous strain to normally sterile sites. Person-to-person nosocomial spread by contaminated respiratory droplets also can occur |
| Neisseria gonorrhoeae | Not part of normal human flora. Only found on mucous membranes of genitalia, anorectal area, oropharynx, or conjunctiva at time of infection | Person-to-person spread by sexual contact, including rectal intercourse and orogenital sex. May also be spread from infected mother to newborn during birth. Asymptomatic carriers are a significant reservoir for increased disease transmission. |
| Neisseria meningitidis | Colonizes oropharyngeal and nasopharyngeal mucous membranes of humans. Humans commonly carry the organism without symptoms | Person-to-person spread by contaminated respiratory droplets, usually in settings of close contact |
| Other Neisseria spp. | Normal human flora of the upper respiratory tract | Spread of patient’s endogenous strain to normally sterile sites. Person-to-person spread may also be possible, but these species are not common causes of human infections |
| Neisseria animaloris | Not part of normal human flora. Animal oral and respiratory commensal organism | Animal contact, particularly bites or scratches from dogs and cats |
Pathogenesis and Spectrum of Disease
As noted in Table 40-2, infections caused by M. catarrhalis are usually localized to the respiratory tract and rarely disseminate.
TABLE 40-2
Pathogenesis and Spectrum of Disease
| Organism | Virulence Factors | Spectrum of Disease and Infections |
| Moraxella catarrhalis | Uncertain; factors associated with cell envelope probably facilitate attachment to respiratory epithelial cells | Most infections are localized to sites associated with the respiratory tract and include otitis media, sinusitis, and pneumonia. Lower respiratory tract infections often target elderly patients and those with chronic obstructive pulmonary disease. Rarely causes disseminated infections such as bacteremia or meningitis. |
| Neisseria gonorrhoeae | Several surface factors, such as pili (types T1-T2 virulent and T3-T5 avirulent), mediate the exchange of genetic material between strains and attachment to human mucosal cell surface, invasion of host cells, and survival through the inhibition of phagocytosis in the presence neutrophils. Genetic-phase variation of pilus structure between types T1 through T5 allows the organism to vary its antigenic structure, preventing recognition by host immune cells. Capsule, lipooligosaccharide (endotoxin), and outer cell membrane proteins I-III are important in antigenic variation and for eliciting an inflammatory response. Protein II (Opa) facilitates adherence to phagocytic and epithelial cells. Protein II (RMP) blocks the bactericidal effect of host IgG. Outer membrane porin (PorB) provides protection from the host’s immune response, including serum complement–mediated cell death. |
A leading cause of sexually transmitted diseases. Genital infections include acute purulent urethritis, prostatitis, and epididymitis in males and acute cervicitis in females. These infections also may be asymptomatic in females. Other localized infections include pharyngitis, anorectal infections, and conjunctivitis (e.g., ophthalmia neonatorum of newborns acquired during birth from an infected mother). Disseminated infections result when the organism spreads from a local infection to cause pelvic inflammatory disease or disseminated gonococcal infection that includes bacteremia, arthritis, and metastatic infection at other body sites. Pelvic inflammatory disease (PID) may cause sterility, ectopic pregnancy or perihepatitis also referred to as Fitz-Hugh–Curtis syndrome. |
| Neisseria meningitidis | Surface structures, perhaps pili, facilitate attachment to mucosal epithelial cells and invasion to the submucosa. Once in the blood, survival is mediated by production of a polysaccharide capsule. Endotoxin release mediates many of the systemic manifestations of infection, such as shock. Cellular proteins are similar to those described for N. gonorrhoeae, including Por and Opa. Two porin proteins are produced (PorA and PorB). IgA protease degrades membrane-associated IgA, increasing the host’s susceptibility to invasion. |
Life-threatening, acute, purulent meningitis. Meningitis may be accompanied by appearance of petechiae (i.e., rash) that is associated with meningococcal bacteremia (i.e., meningococcemia). Bacteremia leads to thrombocytopenia, disseminated intravascular coagulation, and shock. Disseminated disease is often fatal. Less common infections include conjunctivitis, pneumonia, and sinusitis. |
| Other Neisseria spp. | Unknown; probably of low virulence | Rarely involved in human infections. When infections occur, they can include bacteremia, endocarditis, and meningitis. |
| Neisseria animaloris | Unknown | Cellulitis or abscess formation secondary to infected bite wounds; systemic infection (rare). |
N. gonorrhoeae is a leading cause of sexually transmitted disease, and infections caused by this organism usually are localized to the mucosal surfaces where the host is initially exposed to the organism (e.g., cervix, conjunctiva, pharyngeal surface, anorectal area, or urethra of males). Localized infections may be asymptomatic or acute with a pronounced purulent response. Not all infections remain localized, and dissemination from the initial infection site can lead to severe disseminated disease (see Table 40-2). Isolates with nutritional requirements for arginine, hypoxanthine, and uracil (AHU strains) are often isolated from disseminated infections, most often from women although also from asymptomatic males.
N. meningitidis is a leading cause of fatal bacterial meningitis. However, the virulence factors responsible for the spread of this organism from a patient’s upper respiratory tract to the bloodstream and meninges, causing life-threatening infections, are not fully understood (see Table 40-2).
Laboratory Diagnosis
Specimen Collection and Transport
The pathogenic Neisseria spp. described in this chapter are very sensitive to drying and temperature extremes. In addition to general information on specimen collection and transport provided in Table 5-1, there are some special requirements for isolation of N. gonorrhoeae and N. meningitidis.
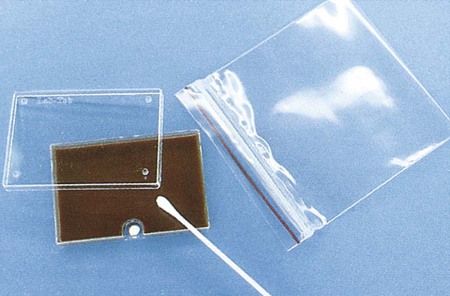
Swabs are acceptable for N. gonorrhoeae testing if the specimen will be plated within 6 hours; however, reduced recovery may result within 30 minutes of collection. If cotton swabs are used, the transport medium should contain charcoal (Ames medium) to inhibit toxic fatty acids present in the fibers. Calcium alginate has also been found to be inhibitory. Dacron or rayon fibers are recommended. N. gonorrhoeae should be inoculated to growth media immediately after specimen collection. The sample should then be placed in a container able to sustain an atmosphere of increased carbon dioxide (CO2) during transport. Specially packaged media consisting of selective agar in plastic trays that contain a CO2-generating system are commercially available (JEMBEC plates). The JEMBEC system (Figure 40-1) is transported to the laboratory at room temperature. Upon receipt in the laboratory, the agar surface is cross-streaked to obtain isolated colonies, and the plate is incubated at 35°C in 3% to 5% CO2. Additional commercial transport systems that may be useful, particularly when the collection site is separate from the diagnostic laboratory, are the Bio-Bag, Gono-Pak, and Transgrow.
Direct Detection Methods
Gram Stain
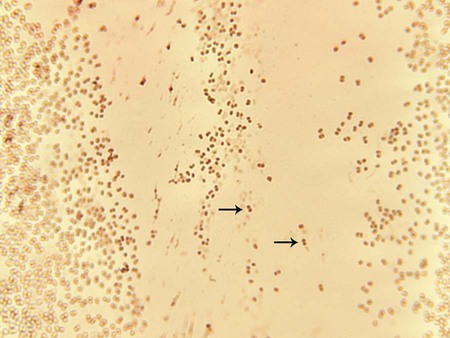
The members of the genus Neisseria discussed in this chapter and M. catarrhalis appear as gram-negative diplococci (Figure 40-2) with adjacent sides flattened. They are often referred to as “kidney bean”–shaped diplococci. Direct Gram staining of urethral discharge from symptomatic males with urethritis is an important test for gonococcal disease. The appearance of gram-negative diplococci inside polymorphonuclear leukocytes is diagnostic in this situation. However, because the normal vaginal and rectal flora are composed of gram-negative coccobacilli, which can resemble Neisseria spp., direct examination of endocervical secretions in symptomatic women is only presumptive evidence of gonorrhea, and the diagnosis must be confirmed by culture. In addition, avirulent strains (i.e., pili types 3 to 5) may be present as extracellular diplococci; these are not pathogenic. Pharyngeal specimens should not be Gram stained, because nonpathogenic, commensal Neisseria spp. may be present, and these are not diagnostic of infection.
Commercial Molecular Assays
Molecular assays have replaced old enzyme-linked immunosorbent assay systems for rapid diagnosis of N. gonorrhoeae. The U.S. Food and Drug Administration (FDA) has cleared a number of amplified and nonamplified tests. (For a discussion of molecular technology, see Chapter 8.) The nonamplified DNA probe assay, PACE 2 (Hologic, Inc., Bedford, MA), has a chemiluminescent detection system for direct detection of gonococcal ribosomal RNA (rRNA) in genital and conjunctival specimens. This test performs well in high-risk patients, is rapid (results are available in 2 hours), and is suitable for screening many patients simultaneously. The Gen-Probe Accuprobe test targets rRNA after lysis of bacteria; the rRNA is detected using a single-strand chemiluminescent DNA probe. The hybrids are then detected in a luminometer. In addition, the Digene CT/GC Dual ID HC2 (HC2; Qiagen) detects RNA-DNA hybrids using antibody-mediated recognition of the hybrids and visualization of a chemiluminescent substrate.
Cultivation
Media of Choice
New York City (NYC) medium, a transparent medium containing lysed horse blood, horse plasma, yeast dialysate, and the same antibiotics as MTM, also has been used. The advantage of NYC medium is that genital mycoplasmas (Mycoplasma hominis and Ureaplasma urealyticum; see Chapter 45) also grow on this agar. Some strains of N. gonorrhoeae are inhibited by the concentration of vancomycin in the selective media, so the addition of nonselective chocolate agar is recommended, especially in suspect cases that are culture negative or for sterile specimens (e.g., joint fluid).
Colonial Appearance
Table 40-3 describes the colonial appearance and other distinguishing characteristics (e.g., pigment) of M. catarrhalis and the Neisseria spp. on chocolate agar.
TABLE 40-3
Colonial Appearance and Other Characteristics on Chocolate Agar*
| Organism | Appearance |
| Moraxella catarrhalis | Large, nonpigmented or gray, opaque, smooth; friable “hockey puck” consistency; colony may be moved intact over surface of agar |
| Neisseria gonorrhoeae | Small, grayish white, convex, translucent, shiny colonies with either smooth or irregular margins; may be up to five different colony types on primary plates |
| N. meningitidis | Medium, smooth, round, moist, gray to white; encapsulated strains are mucoid; may be greenish cast in agar underneath colonies |
| N. animaloris | Some strains exhibit yellow to tan pigment; odor resembles popcorn |
| N. cinerea | Small, grayish white; translucent; slightly granular |
| N. flavescens | Medium, yellow, opaque, smooth |
| N. lactamica | Small, nonpigmented or yellowish, smooth, transparent |
| N. mucosa | Large, grayish white to light yellow, translucent; mucoid because of capsule |
| N. polysaccharea | Small, grayish white to light yellow, translucent, raised |
| N. sicca | Large, nonpigmented, wrinkled, coarse and dry, adherent |
| N. subflava | Medium, greenish yellow to yellow, smooth, entire edge |
Approach to Identification
Various commercial systems are available for the rapid identification of the coccoid Neisseria spp. and M. catarrhalis. Some of these systems are described briefly in Table 13-1. These systems employ biochemical or enzymatic substrates and work very well for the pathogenic species (N. gonorrhoeae, N. meningitidis, and M. catarrhalis). A heavy inoculum of the organism is required, but because these systems detect the activity of preformed enzymes, viability of the organisms in the inoculum is not essential. Manufacturers’ instructions should be followed exactly; several systems have been developed only for strains isolated on selective media and should not be used to test other gram-negative diplococci.
Biochemical Identification
Table 40-4 presents some conventional biochemical tests that traditionally have been used to identify these organisms definitively. The extent to which identification of isolates is carried out depends on the source of the specimen and the suspected species of the organism involved.
TABLE 40-4
Biochemical and Physiologic Characteristics of Moraxella catarrhalis and Coccoid Neisseria spp.
| GROWTH ON: | RAPID FERMENTATION SUGARS | ||||||||
| Organism | Modified Thayer-Martin* | Nutrient Agar at 35°C | Blood or Chocolate Agar at 25°C | Glucose | Maltose | Lactose | Nitrate Reduction | Gas from Nitrate Reduction | 0.1% Nitrite Reduction |
| Moraxella catarrhalis† | v | + | + | − | − | − | + | − | v |
| Neisseria cinerea‡ | v | + | − | −§ | − | − | − | − | + |
| N. flavescens | − | + | + | − | − | − | − | − | +|| |
| N. gonorrhoeae¶ | + | − | − | + | − | − | − | − | − |
| N. lactamica | + | v | v | + | + | + | − | − | + |
| N. meningitidis | + | − | − | + | + | − | − | − | v |
| N. mucosa | − | + | + | + or (+) | + | − | + | + | + |
| N. sicca# | − | + | + | + or (+) | + | − | − | − | + |
| N. subflava# | − | + | + | v | + | − | − | − | + |
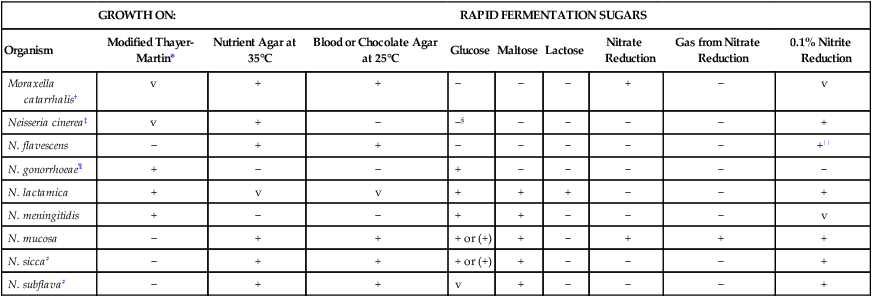
+, >90% of strains positive; (+), >90% of strains positive but reaction may be delayed (i.e., 2 to 7 days); −, >90% of strains negative; v, variable.
*Growth defined as >10 colonies.
‡Neisseria cinerea may be differentiated from N. flavescens by a positive reaction with the amylosucrase test.
§Some strains of N. cinerea may appear glucose-positive in some rapid systems and be mistaken for N. gonorrhoeae. However, N. cinerea grows on nutrient agar at 35°C and reduces nitrite, unlike the gonococcus.
||Only 2 of 10 strains were tested.
¶Kingella denitrificans may grow on modified Thayer-Martin agar and be mistaken for N. gonorrhoeae on microscopic examination. However, K. denitrificans can reduce nitrate and is catalase-negative, unlike the gonococcus.
#Neisseria subflava produces a yellow pigment on Loeffler’s agar; N. sicca does not.
Data compiled from Janda WM, Knapp JS: Neisseria and Moraxella catarrhalis. In Murray PR, Baron EJ, Jorgensen JH et al, editors: Manual of clinical microbiology, ed 8, Washington, DC, 2003, ASM Press; and Weyant RS, Moss CW, Weaver RE et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1996, Williams & Wilkins.
An isolate from a child or a person involved in a case of sexual abuse must be identified unequivocally because of the medicolegal ramifications of these results. It is recommended that these organisms be identified using at least two different types of tests; that is, biochemical, immunologic, enzymatic, or the nonamplified DNA probe previously discussed. Isolates from normally sterile body fluids should also be completely identified. However, isolates from genital sites in adults at risk of sexually transmitted disease (STD) can be identified presumptively; that is, oxidase-positive, gram-negative diplococci that grow on gonococcal selective agar. Likewise, an oxidase-positive, gram-negative diplococcus that hydrolyzes tributyrin from an eye or ear culture can be identified as M. catarrhalis (see Figure 13-8).
Comments About Specific Organisms
Determination of carbohydrate utilization patterns historically has been performed in cysteine trypticase soy agar (CTA) with 1% dextrose, maltose, lactose, and sucrose (see Procedure 40-1 on the Evolve site). This medium is no longer widely used, because it does not work well for oxidative Neisseria spp., specifically N. gonorrhoeae and N. meningitidis. Therefore, carbohydrate utilization patterns are currently determined by inoculating an extremely heavy suspension of the organism to be tested in a small volume of buffered, low-peptone substrate with the appropriate carbohydrate. These methods do not require subculture or growth, and results are available in approximately 4 hours. Commercially available methods include the Rim-Neisseria Test (Rapid Identification Method–Neisseria) (Remel Laboratories), the Neisseria Kwik Test (Micro-Biologics) and the Gonobio Test (I.A.F Production).
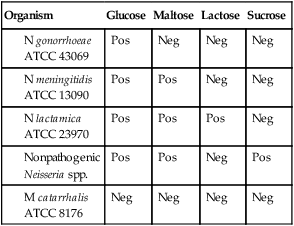
N. lactamica may grow on selective media and may be confused with N. meningitidis. The ONPG test (Procedure 13-33) is used to determine an organism’s ability to produce beta-galactosidase, which is an indicator of lactose utilization. N. lactamica is ONPG positive, and N. gonorrhoeae is ONPG negative.
The eugonic fermenter N. animaloris propagates well on routine laboratory media and ferments glucose; this distinguishes it from dysgonic fermenters that grow poorly on blood and chocolate agars (see Chapter 31). N. animaloris ferments no carbohydrates other than glucose and is indole negative and arginine dihydrolase positive.
Serotyping
Twelve different serogroups are distinguishable for N. meningitidis. Antisera are commercially available for identifying N. meningitidis serogroups A, B, C, H, I, K, L, X, Y, Z, W135, and 29E. Serologic identification is usually performed by slide agglutination. A, B, C, Y, and W135 are the serotypes that most frequently cause systemic disease in the United States. Serotyping has been replaced in many laboratories by DNA sequence typing methods related to the hypervariable outer membrane proteins. Information is available at http://neisseria.org.
Antimicrobial Susceptibility Testing and Therapy
Although beta-lactamase production is common among M. catarrhalis isolates, many beta-lactam antibiotics maintain activity. Because several other agents are also effective, susceptibility testing to guide therapy is not routinely required (Table 40-5).
TABLE 40-5
Antimicrobial Therapy and Susceptibility Testing
| Species | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods* | Comments |
| Moraxella catarrhalis | Several beta-lactams are effective, including beta-lactam/beta-lactamase–inhibitor combinations, cephalosporins, macrolides, quinolones, and trimethoprim- sulfamethoxazole | Commonly produce beta-lactamases that mediate resistance to ampicillin. Although not common, resistance to erythromycin and trimethoprim-sulfamethoxazole may occur | See CLSI document M45 methods | Testing to guide therapy is not routinely needed |
| Neisseria gonorrhoeae | Recommended therapy includes ceftriaxone and other broad-spectrum cephalosporins. Macrolides also may be used |
Penicillin resistance by beta- lactamase production is common | As documented in Chapter 12: disk diffusion, agar dilution, limited commercial methods | Testing by disk diffusion may not detect decrease in quinolone activity. No ceftriaxone resistance has been documented |
| Neisseria meningitidis | Supportive therapy for shock and antimicrobial therapy using penicillin, ceftriaxone, cefotaxime, or chloramphenicol | Subtle increases in beta-lactam resistance have been described, but clinical relevance is uncertain. Beta-lactamase production is extremely rare Reduced fluoroquinolone susceptibility possible |
As documented in Chapter 12: broth dilution, agar dilution and CLSI guidelines | Testing to guide therapy is not routinely needed |
| Other Neisseria spp. | Usually susceptible to penicillin and other beta-lactams | Uncertain; potential for beta-lactamase production | Not available | |
| Neisseria animaloris | Not well characterized; purported susceptibility to penicillin, ampicillin, ciprofloxacin, and ofloxacin | Unknown; first-generation cephems appear less active than penicillins | Not available |
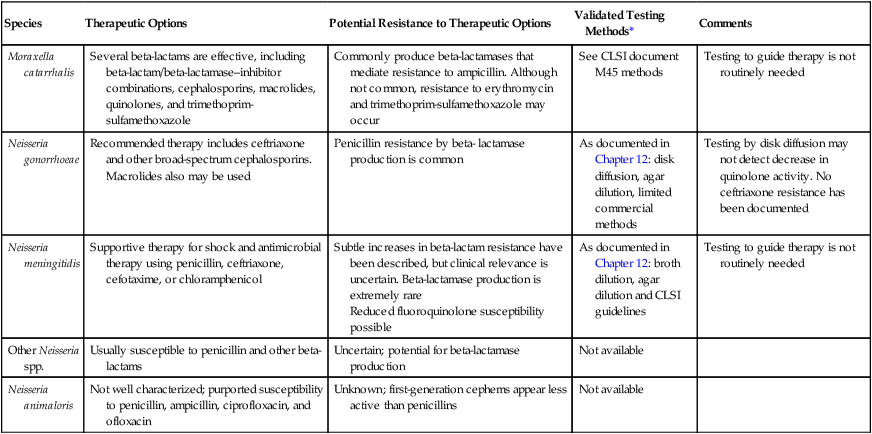
*Validated testing methods include standard methods recommended by the Clinical and Laboratory Standards Institute (CLSI) and commercial methods approved by the U.S. Food and Drug Administration (FDA).
Standard methods have been established for performing in vitro susceptibility testing with N. gonorrhoeae and N. meningitidis (see Chapter 12). The Clinical and Laboratory Standards Institute (CLSI) recommends the use of agar dilution for minimum inhibitory concentration (MIC) measurements and GC agar containing 1% growth supplement for N. gonorrhoeae disk diffusion methods.