CHAPTER 118 Navigation for Brain Tumors
Fundamentals
Historical Aspects
The development of surgical navigation was built on the foundation of 50 years of experience with frame stereotaxy and the additional decade of use of image-guided frame stereotaxy.1–15 The basic principles and methods of biopsy, as well as image-guided craniotomy, were established from this experience, as were standards for morbidity and mortality.
How It Works
Imaging and Fiducials
One or more volumes of image data (e.g., MRI, CT, functional magnetic resonance imaging [fMRI], positron emission tomography [PET]) are acquired containing most of the patient’s head (and, of course, the brain tumor). These data must be spatially accurate, and at least one of the volumes must contain some surface reference marks or features that can be accessed at surgery. Typically, these are adhesive markers (called fiducials) that are applied to the scalp in a wide distribution, but other markers can include anatomic features, skull-implanted fiducials, and feature contours. Scalp fiducials should be multiple and widely spaced, should not appear along a straight line, and should be located over relatively immobile scalp. With skull-implanted fiducials, the overall accuracy of navigation may be submillimetric and may match or exceed that obtained with stereotactic frames.16,17
Registration
The most common means of correlating (or “registering”) image data with the physical space of the patient’s head is called paired points.18,19 At surgery, the reference points are identified on the images and are touched with a pointing device. When surfaces are used, the physical surface is matched, or registered, to that of the radiographic surface, either by touching multiple random points on the surface (“cloud of points”) or by scanning the surface with laser beams.20
Pointing Devices
A variety of three-dimensional digitizers have been used to allow the navigation computer to locate the surgical pointing device in space. Historically, these have included mechanical arms with multiple articulations (both analog and digital), and ultrasonic, machine vision, and various magnetic devices.21–30 Today, most systems use active or passive (i.e., reflective) infrared markers on the pointing device, with the position determined by stereoscopic solid-state cameras that locate the markers trigonometrically.31–33 When the geometry of the markers and the pointing device are known to the computer, it can locate the tip and axis of the pointing device in the operating room. One disadvantage of this method is that it requires that line of sight be maintained between the probe markers and cameras. This can, at times, be logistically difficult, particularly when an operating microscope is to be used. Although a microscope can be adapted or designed to serve as such a pointing device, other technologies such as electromagnetic digitizers may be better suited for such applications.23,28,34
Display
When the registration process is completed, the computer can exhibit the location and orientation of the wand on the image data and can even be used to guide the surgeon to a preselected target along a prescribed trajectory. Common displays include one that shows a set of coronal, axial, and sagittal planes that converge at the point of interest and one that shows planes that are steered by the pointing device, including along the axis of the pointer or perpendicular to the axis.21,22 Three-dimensional presentations may also be made when using the system for guidance along a prescribed trajectory.
These images are typically displayed a few feet from the surgeon on a large flat panel display, or the older cathode ray tube technology may be used. Devices to improve the presentation of navigation data include various head-mounted displays, including light-emitting diode and laser technologies, but these have not been adopted widely.35
Brain Movement
Perhaps the greatest limitation to use of surgical navigation is movement of the brain during surgery compared with the preoperative state when the images were obtained. Gross movements of the brain occur after the dura is violated owing to loss of cerebrospinal fluid and are most prominent over the convexity and poles.18,36 Significant brain “shifting” is a problem that may occur during biopsy as well as during craniotomy for tumor. Fortunately, both these lobar displacements, as well as local distortions due to surgery, can usually be managed with some surgical foresight and are discussed later.37 In certain cases, however, intraoperative imaging may be required to compensate fully for these movements.
Procedures
Craniotomy
Minimal and Optimal Access Craniotomies
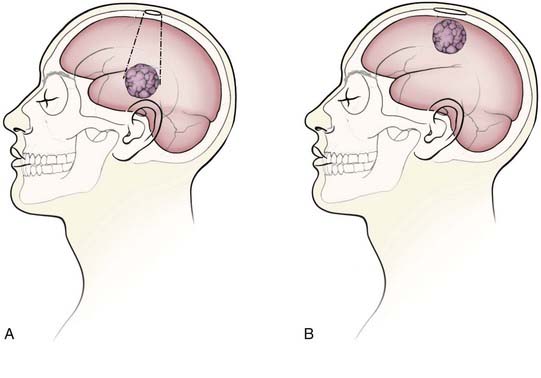
Minimal access craniotomies may have several advantages, including reduced length of surgery, lower incidence of wound infections, and shorter length of hospital stay.10,38 The minimum size of a craniotomy is, in part, dependant on the size and depth of the lesion as well as on surgical instrumentation. For intraparenchymal lesions at the cortical surface, the craniotomy generally should be large enough to encompass the extent of presentation of the tumor on the surface. For deeper lesions, the craniotomy may not need to be as large as if the lesion presented at the surface because the skull opening can be considered the apex of a working cone extending down to the tumor (Fig. 118-1). Of course, the opening must be large enough for the surgical instruments to fit, as well as for proper illumination and visualization of the region of work. Endoscopic procedures may be performed through very small openings (e.g., bur holes), whereas most microsurgical procedures require a minimum of 2- to 3-cm craniotomies. Extra-axial lesions such as meningiomas may require large craniotomies, but these can be optimized to account for dural tails, surface and draining veins, and intended extent of resection.39,40
Relationships to Critical Brain
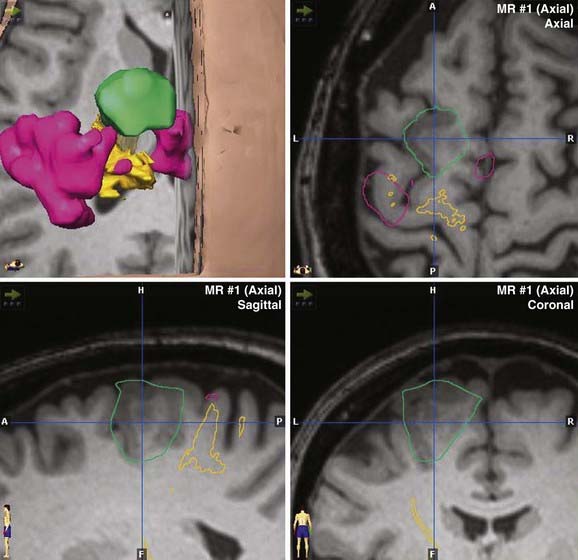
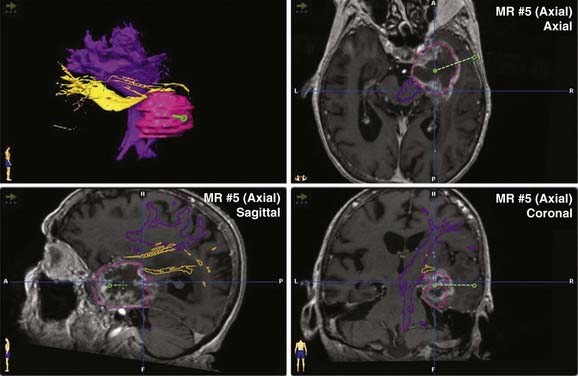
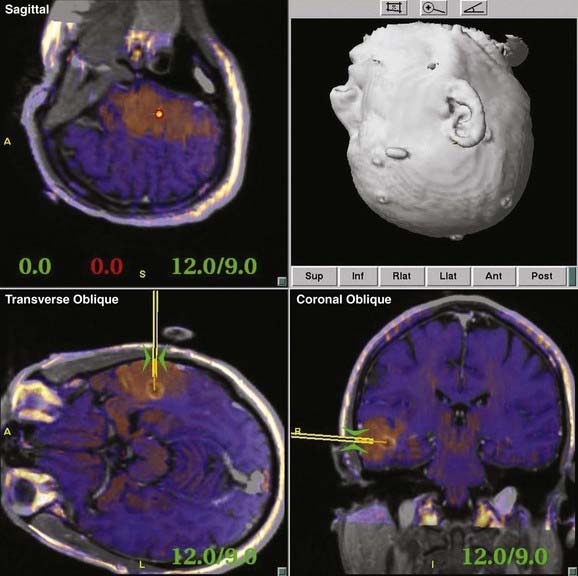
Most contemporary navigation systems allow for fusion of one or more image data sets (see the discussion of multimodality integration, later) for planning and navigation. Functional image data such as PET, fMRI, and magnetoencephalography may augment anatomic data when superimposed on high-resolution MRI (Fig. 118-2). Perhaps the greatest advance in navigation in recent years is the ability to incorporate diffusion tensor imaging (DTI) fiber tracking (Fig. 118-3) into the image data set.41 Although navigable anatomy was largely limited to cortical, periventricular, and lesion features, DTI fiber tracking has led to the era of subcortical navigation, particularly when used with subcortical stimulation of fiber tracks.
Guidance to Subcortical Lesions
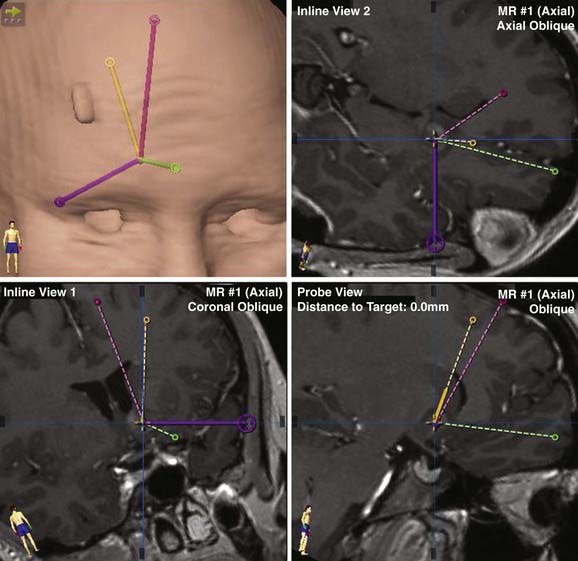
Because there are few, if any, landmarks within the substance of the brain, unambiguous guidance to subcortical tumors is another important function of surgical navigation. Contemporary systems manage this guidance function in one of several ways—usually by projection of the axis of the pointing device onto traditional axial, coronal, and sagittal displays or orthogonal views along and perpendicular to the axis of the pointing device.42 My experience has been that the latter is most useful because it shows all structures that will be encountered en route to the tumor, and thus the surgeon can “step down,” millimeter by millimeter, to the tumor with a view perpendicular to the pointing device or intended trajectory (Fig. 118-4).
Assistance with Resection Control
Assisting with resection control is perhaps the most misunderstood and underappreciated function of surgical navigation for intra-axial tumors. Some respected neurosurgeons have asserted that navigation is of no use in aiding determination of how much of the tumor has been removed, compared with the preoperative image data set, because of local tissue deformations caused by the procedure. Although local and lobar brain movements do occur after surgery, adjustment of the technique of tumor resection can allow navigation to be an important aid in resection control.38,43,44
On opening the dura, cerebrospinal fluid will begin to drain from the wound, resulting in a gradual drift of the brain as it deflates from loss of ventricular pressure and volume. With standard head positioning, this brain shifting can become pronounced during the course of a tumor resection and could lead to erroneous navigation using preoperatively acquired images. Fortunately, this problem can usually be managed, but not eliminated, by taking advantage of the fact that the shift is generally straight down toward the center of the Earth.45 By orienting the patient’s head so that the surgical trajectory is vertical, the surgeon need only compensate for brain shift in one direction (i.e., the brain and tumor are lower than expected) rather than for a complex three-dimensional slide that may occur when operating from a different direction. Also, minimizing the use of diuretics and compensating for volume loss by limiting or reversing hyperventilation may be useful strategies. When only part of the resection involves critical brain, the surgeon should work on that area first, while shift is minimal.
When the surgical plan suggests that these strategies will fail (e.g., a large cyst is overlying surgical access to the tumor), the surgeon may use navigational guidance to place several markers around the borders of the tumor.46,47 This “fence-posting” or “picket-fencing” may be time-consuming, however, and does not guarantee confinement or resection to the desired area.
Therefore, a surgeon with a willingness to modify his or her technique of tumor resection can make use of surgical navigation as an aid to resection control. A summary of these methods is provided in Table 118-1.
TABLE 118-1 Methods to Minimize “Brain Shift” and Local Tissue Deformation for Tumor Resection
Biopsy and Related Techniques
Surgical navigation systems provide accurate, safe intracranial access for the purpose of biopsy by a variety of techniques.48,49 Guidance may be provided by use of various devices, including some that are rigidly secured to the patient’s skull.50 A commercial semirigid multiarticulated instrument holder has been adapted for this purpose.48 In some cases, the orientation of these guides is set using the navigation system, and the biopsy instrument is then blindly passed to the prescribed depth. In other cases, tracking devices are attached to the biopsy instrument, and the navigation system reports the location of the tip on its way to the target.
For supratentorial lesions, diagnostic tissue is usually obtained in 95% or more of cases, with serious complications occurring in 5% or less of cases. The morbidity of infratentorial biopsies appears to be higher, however, and they are associated with a lower rate of diagnostic specimens.51 The use of skull-mounted fiducials may improve these results; I perform nearly all posterior fossa biopsies using these implantable devices.
The advent of target and trajectory guidance strategies has made target-directed procedures, such as biopsy, more user-friendly than more traditional image-guided frame stereotactic brain biopsy. Use of scalp-applied fiducials is also more comfortable for the patient than the application of a stereotactic frame and provides adequate accuracy for almost all supratentorial biopsies.48
Target Selection and Procedure
I prefer to select the center of the most radiographically abnormal region of the tumor as the target for biopsy.52 This approach applies to both enhancing and nonenhancing lesions. The surgeon may choose to augment targeting with PET, magnetic resonance spectroscopy (MRS), MRI cerebral blood volume, or other means so that the most abnormal area of the brain is targeted for biopsy and the risk for underestimating tumor grade because of sampling error is minimized.53
Bleeding during brain biopsy can often be managed without resorting to emergency craniotomy.54 When using a side-cutting biopsy instrument, the outer portion should be left in place to allow blood a route of exit from the site of bleeding. Irrigation and mechanical obturation of the cannula are essential to keep the device patent. Head elevation and blood pressure control may also be of benefit; the former is particularly important for venous bleeding. I have found that arterial bleeding may often be controlled by injecting a small net volume (about 0.5 mL) of thrombin (delivered by loading the inner biopsy apparatus with thrombin and replacing it in the outer sheath) and leaving the device occluded for 30 seconds before reopening. It is, again, essential to ensure that the bleeding has stopped as assessed by a patent cannula.
When adequate tissue is confirmed, the instrument is withdrawn, a few sutures are placed, the scalp is cleansed, and a dressing is applied. The patient is awakened and transferred for observation. A CT scan of the brain is obtained about 2 hours later to assess for occult intracranial blood. If the scan shows no more than 1 cm of blood (and is otherwise unchanged from baseline), undue bleeding did not occur at surgery, and the patient is neurologically unchanged, the patient may be safely discharged home to proper supervision.55
Related Procedures
Tumor cyst drainage, either episodic at surgery or by placement of an Ommaya reservoir, may be useful in the management of these disorders.56,57 Navigational endoscopes may allow for fenestration of tumor cysts or biopsy of intraventricular lesions.26,58 Conversely, radioactive liquids (e.g., P32, colloidal gold) may be instilled into cysts with the aim of reducing fluid production. With appropriate dosimetry software, navigation systems may be used to place temporary or permanent radioactive seeds for brachytherapy.59–61
Intraoperative Image Updates
Conventional surgical navigation relies on images acquired before surgery. As discussed earlier, gross or local brain movements after dural opening may render those images inaccurate representations of the brain during the procedure. Although controversial, many surgeons believe that gross or near-total resection translates to an improved prognosis for most common brain tumors.62,63 Considerable effort, therefore, has been directed toward devising methods of updating those images with data obtained at surgery. Investigators have used intraoperative ultrasound,64–66 CT,65 MRI,67–78 and even mathematical modeling45 to correct for brain movement during surgery. Although navigational ultrasound is cost-effective, it often has poor signal to noise, thereby limiting it usefulness in the operating room. Intraoperative CT usually provides better brain images than ultrasound but is cumbersome and slow and exposes operating room personnel to ionizing radiation. Mathematical modeling remains a research tool.
Intraoperative MRI promises the best visualization of brain structures but has been prohibitively expensive and too logistically demanding for widespread use. Recently, a new generation of intraoperative MRI has emerged, allowing surgery to be performed in a more normal operating room environment than was possible with previous generation imagers.52,79–81 It remains to be established whether this technology leads to improved patient outcome, however.82
Multimodality Imaging
The power and flexibility of these systems have been recently enhanced by incorporation of multimodality integration, whereby image data from different devices or times are fused. Fusions may be within modalities (e.g., different types of MRI) or across platforms (e.g., CT and PET). Instead of repeating the MRI study, fusion may help lower cost by allowing previously acquired nonstereotactic images such as MRI to be fused to a low-cost stereotactic scan such as CT without contrast. Fusion of MRI with CT may also help detect spatial inaccuracies in the former.83,84 The visualization of certain low-grade tumors may be enhanced by fusing color-encoded fluid-attenuated inversion recovery (FLAIR) images with high-resolution volume MRI (Fig. 118-5). PET, cerebral blood volume, or MRS maps may be fused with a stereotactic study to identify the ideal target for brain biopsy.
Economics of Navigation
Although sparse, most reports indicate that these devices are cost-effective, may reduce surgical morbidity, and enhance outcome.85,86 These studies, however, are potentially troubled by patient selection or they compare patients from different time periods. Randomized trials to evaluate this better are unlikely in the foreseeable future.
Future Applications
During the next decade, it is likely that there will be an expanded application of intraoperative MRI with integrated navigation into the neuro-oncology operating room. Strategic implantation of catheters using navigation for convection-enhanced or local delivery of new therapeutic agents, such as immunotoxins, gene therapies, and new receptor-targeted drugs, will likely be an important part of future brain tumor treatment. Although robotic navigation is not new,87,88 it may become more widely accepted as the need for these procedures increases.
Barnett GH, Kormos DW, Steiner CP, Weisenberger J. Use of a frameless, armless wand for brain tumor localization with two-dimensional and three-dimensional neuroimaging. Neurosurgery. 1993;33:674-678.
Barnett GH, Miller DW, Weisenberger J. Brain biopsy using frameless stereotaxy with scalp applied fiducials: Experience in 218 cases. J Neurosurg. 1999;91:569-576.
Barnett GH, Steiner CP, Kormos DW, Weisenberger J. Intracranial meningioma resection using interactive frameless stereotaxy-assistance. J Image Guid Surg. 1995;1:46-52.
Barnett GH, Steiner CP, Weisenberger J. Target and trajectory guidance for interactive surgical navigation systems. Stereotact Funct Neurosurg. 1996;66:91-95.
Berger MS, Deliganis AV, Dobbins J, Keles EG. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784-1791.
Bernstein M, Gutin PH. Interstitial irradiation of brain tumors: a review. Neurosurgery. 1981;9:741-750.
Black PM, Moriarty T, Alexander E3rd, et al. The development and implementation of intraoperative MRI and its neurosurgical applications. Neurosurgery. 1997;41:831-842.
Chimowitz MI, Barnett GH, Palmer J. Treatment of intractable arterial hemorrhage during stereotactic brain biopsy with thrombin: report of three patients. J Neurosurg. 1991;74:301-303.
Gutin PH, Phillips PL, Wara WM, et al. Brachytherapy of recurrent malignant brain tumors with removable high-activity iodine-125 sources. J Neurosurg. 1984;60:61-68.
Hadani M, Spiegelman R, Feldman Z, et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery. 2001;48:799-807.
Hassenbusch SJ, Anderson JS, Pillay PK. Brain tumor resection aided with markers placed using stereotaxis guided by magnetic resonance imaging and computed tomography. Neurosurgery. 1991;28:801-806.
Kaakaji W, Barnett GH, Bernhard D, et al. Clinical and economic consequences of early discharge after supratentorial stereotactic brain biopsy. J Neurosurg. 2001;94:892-898.
Kanner AA, Vogelbaum MA, Mayberg MR, et al. Intracranial navigation by using low-field intraoperative magnetic resonance imaging: preliminary experience. J Neurosurg. 2002;97:1115-1124.
Kelly PJ. Volumetric stereotactic surgical resection of intra-axial brain mass lesions. Mayo Clin Proc. 1988;63:1186-1198.
Kelly PJ, Earnest F4th, Kall BA, et al. Surgical options for patients with deep-seated brain tumors: computer-assisted stereotactic biopsy. Mayo Clin Proc. 1985;60:223-229.
Maciunas RJ, Galloway RLJr, Latimer J, et al. An independent application accuracy evaluation of stereotactic frame systems. Stereotact Funct Neurosurg. 1992;58:103-107.
Miga MI, Roberts DW, Kennedy FE, et al. Modeling of retraction and resection for intraoperative updating of images. Neurosurgery. 2001;49:75-84.
Nabavi A, Black PM, Gering DT, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48:787-797.
Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 2006;58(Suppl 2):292-303.
Pelizzari CA, Chen GTY, Spelbring DR, et al. Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr. 1989;13:20-26.
Roberts DW, Strohbehn JW, Friets EM, et al. The stereotactic operating microscope: accuracy refinement and clinical experience. Acta Neurochir Suppl (Wien). 1989;46:112-114.
Watanabe E, Watanabe T, Manaka S, et al. Three-dimensional digitizer (neuro-navigator): new equipment of CT-guided stereotaxic surgery. Surg Neurol. 1987;27:543-547.
Yoshikawa K, Kajiwara K, Morioka J, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006;78:91-97.
1 Apuzzo MLJ, Chandrasoma PT, Cohen D, et al. Computed imaging stereotaxy: experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930-937.
2 Barnett GH, McKenzie RL, Ramos L, Palmer J. Nonvolumetric stereotaxy-assisted craniotomy: results in 50 consecutive cases. Stereotact Funct Neurosurg. 1993;61:80-85.
3 Brown RA. A computerized tomography-computer graphics approach to stereotaxic localization. J Neurosurg. 1979;50:715-720.
4 Di Lorenzo N, Esposito V, Lunardi P, et al. A comparison of computerized tomography-guided stereotactic and ultrasound-guided techniques for brain biopsy [see comments]. J Neurosurg. 1991;75:763-765.
5 Goldstein S, Gumerlock MK, Neuwelt EA. Comparison of CT-guided and stereotactic cranial diagnostic needle biopsies. J Neurosurg. 1987;67:341-348.
6 Grunert P, Ungersbock K, Bohl J, et al. Results of 200 intracranial stereotactic biopsies. Neurosurg Rev. 1994;17:59-66.
7 Hahn JF, Levy WJ, Weinstein MJ. Needle biopsy of intracranial lesions guided by computerized tomography. Neurosurgery. 1979;5:11-15.
8 Kelly PJ, Earnest F4th, Kall BA, et al. Surgical options for patients with deep-seated brain tumors: computer-assisted stereotactic biopsy. Mayo Clin Proc. 1985;60:223-229.
9 Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated glial neoplasms. J Neurosurg. 1987;66:865.
10 Kelly PJ, Kall BA, Goerss SJ. Results of computed tomography-based computer-assisted stereotactic resection of metastatic intracranial tumors. Neurosurgery. 1988;22:7-17.
11 Kelly PJ. Computer-assisted stereotaxis: new approaches for the management of intracranial intra-axial tumors. Neurology. 1986;36:535-541.
12 Laitinen LV, Liliequist B, Fagerlund M, Eriksson AT. An adapter for computed tomography-guided stereotaxis. Surg Neurol. 1985;23:559-566.
13 Lee T, Kenny BG, Hitchcock ER, et al. Supratentorial masses: stereotactic or freehand biopsy? Br J Neurosurg. 1991;5:331-338.
14 Soo TM, Bernstein M, Provias J, et al. Failed stereotactic biopsy in a series of 518 cases. Stereotact Funct Neurosurg. 1995;64:183-196.
15 Whittle IR, Denholm SW, Elshunnar K. CT-guided stereotactic neurosurgery using the Brown-Roberts-Wells system: experience with 125 procedures. Aust N Z J Surg. 1991;61:919-928.
16 Maciunas RJ, Galloway RLJr, Latimer J, et al. An independent application accuracy evaluation of stereotactic frame systems. Stereotact Funct Neurosurg. 1992;58:103-107.
17 Wang MY, Maurer CRJr, Fitzpatrick JM, Maciunas RJ. An automatic technique for finding and localizing externally attached markers in CT and MR volume images of the head. IEEE Trans Biomed Eng. 1996;43:627-637.
18 Hill DL, Maurer CRJr, Maciunas RJ, et al. Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery. 1998;43:514-526.
19 Vrionis FD, Foley KT, Robertson JH, Shea JJ3rd. Use of cranial surface anatomic fiducials for interactive image-guided navigation in the temporal bone: a cadaveric study. Neurosurgery. 1997;40:755-763.
20 Pelizzari CA, Chen GTY, Spelbring DR, et al. Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr. 1989;13:20-26.
21 Barnett GH, Kormos DW, Steiner CP, Weisenberger J. Use of a frameless, armless wand for brain tumor localization with two-dimensional and three-dimensional neuroimaging. Neurosurgery. 1993;33:674-678.
22 Barnett GH, Kormos DW, Steiner CP, Weisenberger J. Intraoperative localization using an armless, frameless stereotactic wand. J Neurosurg. 1993;78:510-514.
23 Friets EM, Strohbehn JW, Hatch JF, Roberts DW. A frameless stereotaxic operating microscope for neurosurgery. IEEE Trans Biomed Eng. 1989;36:608-617.
24 Golfinos JG, Fitzpatrick BC, Smith LR, Spetzler RF. Clinical use of a frameless stereotactic arm: results in 325 cases. J Neurosurg. 1995;83:197-205.
25 Heilbrun MP, McDonald P, Wiker C, et al. Stereotactic localization and guidance using a machine vision technique. Stereotact Funct Neurosurg. 1992;58:94-98.
26 Manwaring KH. Magnetic field guided endoscopic dissection through a burr hole may avoid more invasive craniotomies—a preliminary report. ACTA Neurochir Suppl. 1993;61:34-39. 1994
27 Roberts DW, Strohbehn JW, Friets EM, et al. The stereotactic operating microscope: accuracy refinement and clinical experience. Acta Neurochir Suppl (Wien). 1989;46:112-114.
28 Roberts DW, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545-549.
29 Sipos EP, Tebo SA, Zinreich SJ, et al. In vivo accuracy testing and clinical experience with the ISG Viewing Wand. Neurosurgery. 1996;39:194-202.
30 Watanabe E, Watanabe T, Manaka S, et al. Three-dimensional digitizer (neuro-navigator): new equipment of CT-guided stereotaxic surgery. Surg Neurol. 1987;27:543-547.
31 Germano IM, Villalobos H, Silvers A, Post KD. Clinical use of the optical digitizer for intracranial neuronavigation. Neurosurgery. 1999;45:261-269.
32 Maciunas RJ, Galloway RLJr, Fitzpatrick JM, et al. A universal system for interactive image-directed neurosurgery. Stereotact Funct Neurosurg. 1992;58:108-113.
33 Smith KR, Frank KJ, Bucholz RD. The NeuroStation: a highly accurate, minimally invasive solution to frameless stereotactic neurosurgery. Comput Med Imaging Graph. 1994;18:247-256.
34 Roberts DW, Nakajima T, Brodwater B, et al. Further development and clinical application of the stereotactic operating microscope. Stereotact Funct Neurosurg. 1992;58:114-117.
35 Barnett GH, Steiner CP, Weisenberger J. Adaptation of personal projection television to a helmet-mounted display for intraoperative viewing of neuroimaging. Technical note. J Image Guid Surg. 1995;1:109-112.
36 Nabavi A, Black PM, Gering DT, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48:787-797.
37 Murphy MA, Barnett GH, Kormos DW, Weisenberger J. Astrocytoma resection using an interactive frameless stereotactic wand. An early experience. J Clin Neurosci. 1994;1:33-37.
38 Kelly PJ. Volumetric stereotactic surgical resection of intra-axial brain mass lesions. Mayo Clin Proc. 1988;63:1186-1198.
39 Barnett GH, Steiner CP, Kormos DW, Weisenberger J. Intracranial meningioma resection using interactive frameless stereotaxy-assistance. J Image Guid Surg. 1995;1:46-52.
40 Barnett GH. Use of surgical navigation systems for resection of intracranial meningiomas. In: Barnett GH, Roberts DW, Maciunas RJ, editors. Computer Assisted Neurosurgery. New York: Taylor & Francis Group, 2006.
41 Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurgery. 2006;58(Suppl 2):292-303.
42 Barnett GH, Steiner CP, Weisenberger J. Target and trajectory guidance for interactive surgical navigation systems. Stereotact Funct Neurosurg. 1996;66:91-95.
43 Barnett GH. The role of image-guided technology in the surgical planning and resection of gliomas. J Neurooncol. 1999;42:247-258.
44 Kelly PJ. Stereotactic resection: general principles. In: Tumor Stereotaxis. Philadelphia: Saunders; 1991:268-295.
45 Miga MI, Roberts DW, Kennedy FE, et al. Modeling of retraction and resection for intraoperative updating of images. Neurosurgery. 2001;49:75-84.
46 Hassenbusch SJ, Anderson JS, Pillay PK. Brain tumor resection aided with markers placed using stereotaxis guided by magnetic resonance imaging and computed tomography. Neurosurgery. 1991;28:801-806.
47 Yoshikawa K, Kajiwara K, Morioka J, et al. Improvement of functional outcome after radical surgery in glioblastoma patients: the efficacy of a navigation-guided fence-post procedure and neurophysiological monitoring. J Neurooncol. 2006;78:91-97.
48 Barnett GH, Miller DW, Weisenberger J. Brain biopsy using frameless stereotaxy with scalp applied fiducials: experience in 218 cases. J Neurosurg. 1999;91:569-576.
49 Barnett GH. Brain biopsy and related procedures using surgical navigation systems. In: Barnett GH, Roberts DW, Maciunas RJ, editors. Computer Assisted Neurosurgery. New York: Taylor & Francis Group, 2006.
50 Hall WA, Liu H, Truwit CL. Navigus trajectory guide. Neurosurgery. 2000;46:502-504.
51 Bernstein M, Parent A. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165-168.
52 Barnett GH. Compact MRI for intraoperative neurosurgical imaging and navigation. Acta Neurochir (Wien). 2000;142:1169-1210.
53 Chandrasoma PT, Smith MM, Apuzzo MLJ. Stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimen. Neurosurgery. 1989;24:160-165.
54 Chimowitz MI, Barnett GH, Palmer J. Treatment of intractable arterial hemorrhage during stereotactic brain biopsy with thrombin. Report of three patients. J Neurosurg. 1991;74:301-303.
55 Kaakaji W, Barnett GH, Bernhard D, et al. Clinical and economic consequences of early discharge after supratentorial stereotactic brain biopsy. J Neurosurg. 2001;94:892-898.
56 Bosch DA, Rahn D, Backlund EO. Treatment of colloid cysts of the third ventricle by stereotactic aspiration. Surg Neurol. 1978;9:15-18.
57 Rogers LR, Barnett GH. Percutaneous aspiration of brain tumor cysts via the Ommaya reservoir system. Neurology. 1991;41:279-282.
58 Rhoten RP, Luciano M, Barnett GH. Computer-assisted neuro-endoscopy (CANE). Neurosurgery. 1996;40:632-638.
59 Bernstein M, Gutin PH. Interstitial irradiation of brain tumors: a review. Neurosurgery. 1981;9:741-750.
60 Gutin PH, Phillips PL, Wara WM, et al. Brachytherapy of recurrent malignant brain tumors with removable high-activity iodine-125 sources. J Neurosurg. 1984;60:61-68.
61 Mundinger F, Ostertag CB, Birg W, Weigel K. Stereotactic treatment of brain lesions. Biopsy, interstitial radiotherapy (iridium-192 and iodine-125) and drainage procedures. Appl Neurophysiol. 1980;43:198-204.
62 Berger MS, Deliganis AV, Dobbins J, Keles EG. The effect of extent of resection on recurrence in patients with low-grade cerebral hemisphere gliomas. Cancer. 1994;74:1784-1791.
63 Curran WJJr, Scott CB, Horton J, et al. Does extent of surgery influence outcome for astrocytoma with atypical or anaplastic foci (AAF). A report from three Radiation Therapy Oncology Group (RTOG) trials. J Neurooncol. 1992;12:219-227.
64 Koivukangas J, Louhishalmi Y, Alakuijala J, Okarinene J. Ultrasound-controlled Nero navigator-guided brain surgery. J Neurosurg. 1993;79:36-42.
65 Lunsford LD, Kondziolka D, Bissonette DJ. Intraoperative imaging of the brain. Stereotact Funct Neurosurg. 1996;66:58-64.
66 Pallatroni H, Hartov A, McInerney J, et al. Coregistered ultrasound as a neurosurgical guide. Stereotact Funct Neurosurg. 1999;73:143-147.
67 Alexander E3rd, Moriarty TM, Kikinis R, et al. The present and future role of intraoperative MRI in neurosurgical procedures. Stereotact Funct Neurosurg. 1997;68:10-17.
68 Bernstein M, Al-Anazi AR, Kucharczyk W, et al. Brain tumor surgery with the Toronto open magnetic resonance imaging system: preliminary results for 36 patients and analysis of advantages, disadvantages, and future prospects. Neurosurgery. 2000;46:900-907.
69 Black PM, Moriarty T, Alexander E3rd, et al. The development and implementation of intraoperative MRI and its neurosurgical applications. Neurosurgery. 1997;41:831-842.
70 Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48:731-742.
71 Fahlbusch R, Ganslandt O, Buchfelder M, et al. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381-390.
72 Fahlbusch R, Ganslandt O, Nimsky C. Intraoperative imaging with open magnetic resonance imaging and neuronavigation. Childs Nerv Syst. 2000;16:829-831.
73 Hall WA, Liu H, Martin AJ, et al. Safety, efficacy, and functionality of high-field strength interventional magnetic resonance imaging for neurosurgery. Neurosurgery. 2000;46:632-641.
74 Knauth M, Wirtz CR, Tronnier VM, et al. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol. 1999;20:1642-1646.
75 Rubino GJ, Farahani K, McGill D, et al. Magnetic resonance imaging-guided neurosurgery in the magnetic fringe fields: the next step in neuronavigation. Neurosurgery. 2000;46:643-653.
76 Steinmeier R, Fahlbusch R, Ganslandt O, et al. Intraoperative magnetic resonance imaging with the MAGNETOM open scanner: concepts, neurosurgical indications, and procedures: a preliminary report. Neurosurgery. 1998;43:739-747.
77 Wirtz CR, Bonsanto MM, Knauth M, et al. Intraoperative magnetic resonance imaging to update interactive navigation in neurosurgery: method and preliminary experience. Comput Aided Surg. 1997;2:172-179.
78 Wirtz CR, Knauth M, Staubert A, et al. Clinical evaluation and follow-up results for intraoperative magnetic resonance imaging in neurosurgery. Neurosurgery. 2000;46:1112-1120.
79 Hadani M, Spiegelman R, Feldman Z, et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery. 2001;48:799-807.
80 Kanner AA, Vogelbaum MA, Mayberg MR, et al. Intracranial navigation by using low-field intraoperative magnetic resonance imaging: preliminary experience. J Neurosurg. 2002;97:1115-1124.
81 Schulder M, Liang D, Carmel PW. Cranial surgery navigation aided by a compact intraoperative magnetic resonance imager. J Neurosurg. 2001;94:936-945.
82 Berger MS. Intraoperative MR imaging: making an impact on outcomes for patients with brain tumors. AJNR Am J Neuroradiol. 2001;22:2.
83 Alexander E3rd, Kooy HM, van Herk M, et al. Magnetic resonance image-directed stereotactic neurosurgery: use of image fusion with computerized tomography to enhance spatial accuracy. J Neurosurg. 1995;83:271-276.
84 Hardy PA, Barnett GH. Spatial distortion in magnetic resonance imaging: impact on stereotactic localization. In: Gildenberg PL, Tasker RR, editors. Textbook of Stereotactic and Functional Neurosurgery. New York: McGraw Hill; 1998:271-280.
85 Bingaman WE, Barnett GH. Social and economic impact of surgical navigation systems. In: Barnett GH, Roberts DW, Maciunas RJ, editors. Image Guided Neurosurgery. Clinical Applications of Surgical Navigation. St. Louis: Quality Medical Publishing, 1998.
86 Krivoshapkin AL, Kanygin VV, Semin PA, Melidi EG. Results of radical removal of malignant cerebral gliomas, by using computer-assisted navigation, followed by adjuvant therapy. Zh Vopr Neirokhir Im N N Burdenko. 2006;4:10-13.
87 Glauser D, Fankhauser H, Epitaux M, et al. Neurosurgical robot Minerva: first results and current developments. J Image Guid Surg. 1995;1:266-272.
88 Koyama H, Uchida T, Funakubo H, et al. Development of a new microsurgical robot for stereotactic neurosurgery. Stereotact Funct Neurosurg. 1990;54-55:462-467.