Chapter 6 Natural product chemistry
Natural products in drug discovery
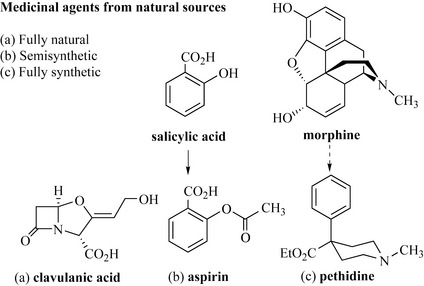
A survey of any pharmacopoeia will show that natural products have a key role as biologically active agents; in fact, it has been estimated that 20–25% of all medicines are derived from such sources. In this definition, the medicinal agent may be a natural product isolated straight from the producing organism (e.g. the β-lactamase inhibitor clavulanic acid isolated from the bacterium Streptomyces clavuligerus), a natural product that has undergone a minor chemical modification (semisynthetic) (e.g. aspirin, derived from salicylic acid, which occurs as esters and glycosides in Salix spp.), or a compound that was totally synthesized based on a particular natural product possessing biological activity (e.g. pethidine, which was based on morphine from the opium poppy, Papaver somniferum). It is sometimes difficult to see how the fully synthetic compound was modelled on the natural product (Fig. 6.1).
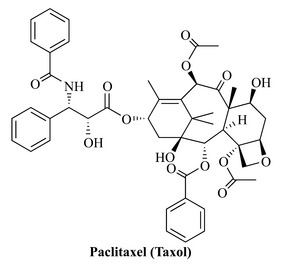
Natural products are historically the core of medicines and they are still a major source of drug leads, which is a term used to describe compounds that may be developed into medicines. A particular example of a natural product that is currently one of the best selling drugs is paclitaxel, marketed as Taxol (Fig. 6.2). This drug was developed by BristolMyers Squibb and marketed for the treatment of ovarian and mammary cancers, and became available for use in the USA in 1993. The compound was initially isolated from the bark of the Pacific yew tree, Taxus brevifolia, and demonstrates the best possible qualities of a natural product, being highly functional and chiral. Additionally, paclitaxel occurs in the bark with a wide range of structurally related compounds (taxanes diterpenes); this is a further important and valuable quality of natural products when they are considered as a source in the search for biologically active drug leads. Paclitaxel has many functional groups and chiral centres (11) and these qualities give rise to its distinct shape and fascinating biological activity. It is important not to be overwhelmed at such a complex molecule, but to look at the functional groups that make up the total structure of the compound. Even a natural product that is as structurally complex as paclitaxel can be broken down into the simple chemical features of functional groups and chiral centres.
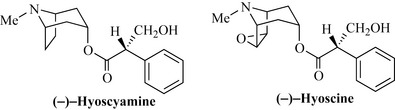
In the chemotaxonomic approach, knowledge that a particular group of plants contains a certain class of natural product may be used to predict that taxonomically related plants may contain structurally similar compounds. This approach is highly useful when the chemistry and biological activity of a compound is well described and compounds with similar chemical structure are needed for further biological testing. A good example of this is the plant family Solanaceae, which is a rich source of alkaloids of the tropane type. The knowledge that deadly nightshade (Atropa belladonna) produces hyoscyamine (a smooth muscle relaxant) would enable one to predict that the thorn apple (Datura stramonium) would contain structurally related compounds, and this is certainly the case, with hyoscine being the major constituent of this solanaceous plant (Fig. 6.3).
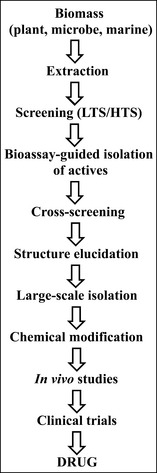
The discovery of drugs from nature is complex and is depicted schematically in Fig. 6.4. The biomass (plant, microbe, marine organism) is collected, dried and extracted into a suitable organic solvent to give an extract, which is then screened in a bioassay to assess its biological activity (bioactivity). Screening or assessment of biological activity is generally divided into two formats depending on the number of extracts to be assessed. In low-throughput screening (LTS), small numbers of extracts (a single extract up to hundreds of extracts) are dispensed into a format that is compatible with the bioassay (e.g. a microtitre plate, sample tubes). This approach is used widely in academic laboratories where only a relatively low number of extracts are assessed. In high-throughput screening (HTS), thousands of extracts are dispensed into a format (usually microtitre plates with many wells, e.g. 384 wells per plate) and screened in the bioassay. This approach is favoured by the pharmaceutical industry, which may have hundreds of thousands of samples (both natural and synthetic) for biological evaluation. This large-scale approach means that decisions can be made rapidly about the status of an extract, which has an impact on the cost of the discovery process.
The polyketides
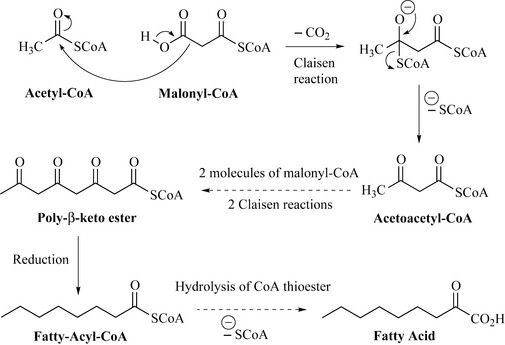
Polyketides are mainly acetate (C2) derived metabolites and occur throughout all organisms (as fatty acids and glycerides), but it is the microbes, predominantly the filamentous bacteria of the genus Streptomyces, that produce structurally diverse types of polyketides, especially as antibiotic substances. The biosynthesis of these compounds begins (Fig. 6.5) with the condensation of one molecule of malonyl-CoA (CoA is short for coenzyme A) with one molecule of acetyl-CoA to form the simple polyketide acetoacetyl-CoA. In this reaction (Claisen reaction), one molecule of CO2 and one molecule of HSCoA are generated. The reaction occurs because the carbon between both carbonyl groups of malonyl-CoA (the acidic carbon) is nucleophilic and can attack an electropositive (electron-deficient) centre (e.g. the carbon of a carbonyl group).
The curved arrows in Fig. 6.5 indicate the movement of a pair of electrons to form a bond. Further condensation reactions between another molecule of malonyl-CoA and the growing polyketide lead to chain elongation, in which every other carbon in the chain is a carbonyl group. These chains are known as poly-β-keto esters and are the reactive intermediates that form the polyketides. Using these esters, large chains such as fatty acids can be constructed and, in fact, reduction of the carbonyl groups and hydrolysis of the -SHCoA thioester leads to the fatty acid class of compounds. The expanding polyketide chain may be attached as a thioester to either CoA or to a protein called an acyl-carrier protein. Multiple Claisen reactions with additional molecules of malonyl-CoA can generate long-chain fatty acids such as stearic acid and myristic acid.
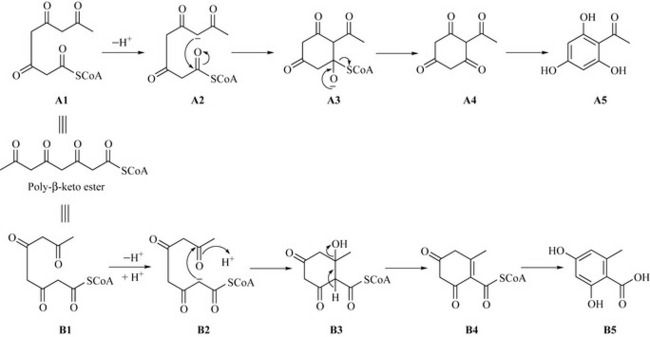
The poly-β-keto ester can also cyclize to give aromatic natural products, and the way in which the poly-β-keto ester folds determines the type of natural product generated (Fig. 6.6). If the poly-β-keto ester folds as A1, then loss of a proton, followed by an intramolecular Claisen reaction of intermediate A2 (by attack of the acidic carbon on the carbonyl), would result in the formation of a cyclic polyketide enolate A3 which will rearrange to the keto compound with expulsion of the SCoA anion, resulting in the ketone A4. This ketone would readily undergo keto-enol tautomerism to the more favoured aromatic triphenol A5 (phloroacetophenone).
Fatty acids and glycerides
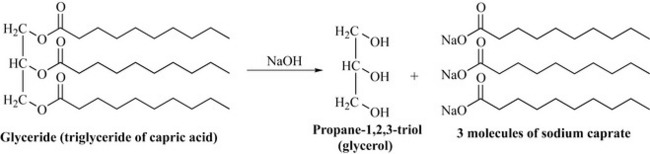
This group of polyketides is widely distributed and present as part of the general biochemistry of all organisms, particularly as components of cell membranes. They are usually insoluble in water and soluble in organic solvents such as hexane, diethyl ether and chloroform. These natural products are sometimes referred to as fixed oils (liquid) or fats (solid), although these terms are imprecise as both fixed oils and fats contain mixtures of glycerides and free fatty acids and the state of the compound (i.e. liquid or solid) will depend on the temperature as well as the composition. Glycerides are fatty acid esters of propane-1,2,3-triol (glycerol). They are sometimes referred to as saponifiable natural products, meaning that they can be converted into soaps by a strong base (NaOH). The term saponifiable comes from the Latin word sapo meaning ‘soap’. Saponification of fatty acids and glycerides with sodium hydroxide results in the formation of the sodium salts of the fatty acids (Fig. 6.7).
Glycerides can be very complicated mixtures as, unlike the example given in Fig. 6.7, the substituents on the glycerol alcohol may be different from each other, and it is not uncommon for lipophilic plant extracts to contain many types of glycerides.
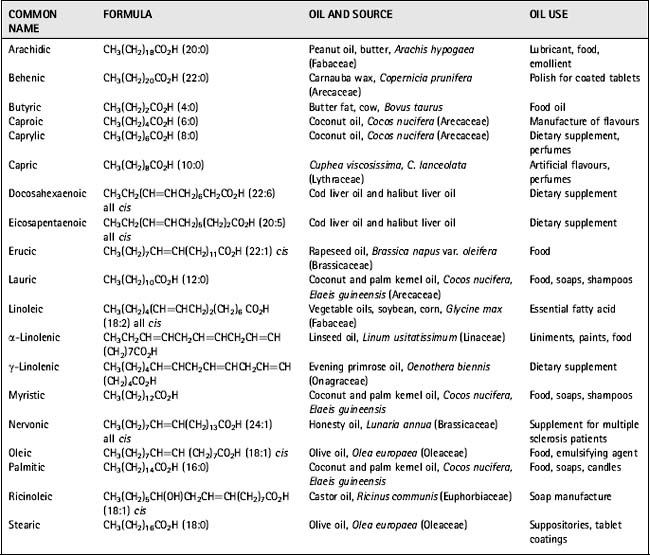
Fatty acids are very important as formulation agents and vehicles in pharmacy and as components of cosmetics and soaps. Table 6.1 lists the common names, chemical formulae, sources and uses of the more common fatty acids.
The tetracyclines
These polyketide-derived natural products are tetracyclic (i.e. have four linear six-membered rings, from which the group was named) and were discovered as part of a screening programme of extracts produced by filamentous bacteria (Actinomycetes), which are common components of soil. The most widely studied group of actinomycetes are species of the genus Streptomyces, which are very adept at producing many types of polyketide natural products of which the antibiotic tetracycline (Fig. 6.8) and the anthracyclic antitumour agents (see Chapter 8) are excellent examples.
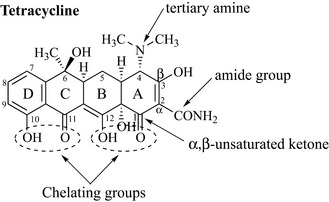
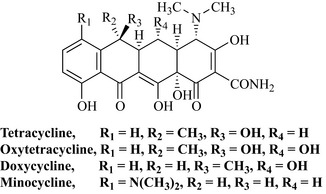
The key features of this class of compound are shown in Fig. 6.8. Although tetracycline has numerous functional groups, including a tertiary amine, hydroxyls, an amide, a phenolic hydroxy and keto groups, it is still possible to see that tetracycline is a member of the polyketide class of natural products by looking at the lower portion of the molecule. C10, C11, C12 and C1 are oxygenated, indicating that the precursor of this compound was a poly-β-keto ester. C10 and C11 and C12 and C1 form part of a chelating system that is essential for antibiotic activity and may readily chelate metal ions such as calcium, magnesium, iron or aluminium and become inactive. This is one of the reasons why oral formulations of the tetracycline antibiotics are never given with foodstuffs that are high in these ions (e.g. Ca2+ in milk) or with antacids, which are high in cations such as Mg2+. This group of antibiotics has been long known and they have a very broad spectrum of activity against Gram-positive and Gram-negative bacteria, spirochetes, mycoplasmae, rickettsiae and chlamydiae. Tetracycline comes from mutants of Streptomyces aureofaciens, and the related analogue oxytetracycline from S. rimosus (Fig. 6.9). These antibiotics are widely used as topical formulations for the treatment of acne, and as oral/injection preparations.
Griseofulvin
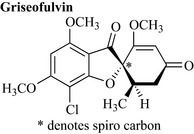
Another polyketide antibiotic is griseofulvin (Grisovin) from the fungus (mould) Penicillium griseofulvum (Fig. 6.10). This compound was originally isolated by researchers at the London School of Hygiene and Tropical Medicine.
Erythromycin A
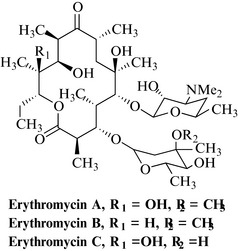
Erythromycin A is a complex polyketide from Saccharopolyspora erythrea (Actinomycetes), which is a filamentous bacterium, originally classified in the genus Streptomyces. This compound is a member of the natural product class of macrolide antibiotics; these can contain 12 or more carbons in the main ring system. The term macrolide is derived from the fact that erythromycin is a large ring structure (macro) and is also a cyclic ester referred to as an olide (a lactone). As can be seen from Fig. 6.11, erythromycin A has the best features of natural products, being highly chiral (possessing many stereochemical centres) and having many different functional groups, including a sugar, an amino sugar, lactone, ketone and hydroxyl groups.
The statins
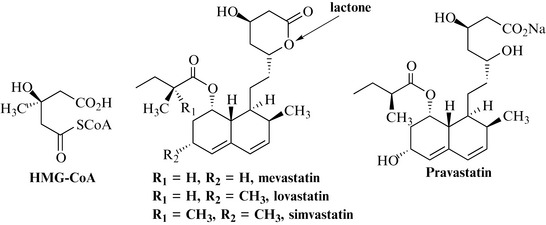
A further group of polyketide-derived natural products is the statins, so named for their ability to lower (bring into stasis) the production of cholesterol, high levels of which are a major contributing factor to the development of heart disease. The rationale behind the use of these compounds is as inhibitors of the enzyme hydroxymethylglutaryl-CoA (HMG-CoA) reductase, which catalyses the conversion of HMG-CoA (Fig. 6.12) to mevalonic acid, one of the key intermediates in the biosynthesis of cholesterol. HMG-CoA reductase became a target for the discovery of the natural product inhibitor mevastatin, which was initially isolated from cultures of the fungi Penicillium citrinum and Penicillium brevicompactum (Fig. 6.12).
Shikimic-acid-derived natural products
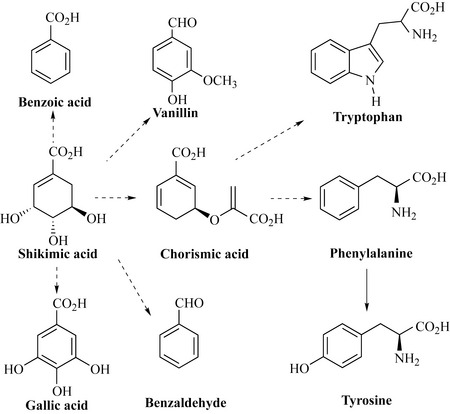
Shikimic acid, sometimes referred to as shikimate, is a simple acid precursor for many natural products and aromatic amino acids, including phenylalanine, tyrosine, tryptophan, the simple aromatic acids that are common in nature (e.g. benzoic and gallic acids) and aromatic aldehydes such as vanillin and benzaldehyde that contribute to the pungent smell of many plants (Fig. 6.13).
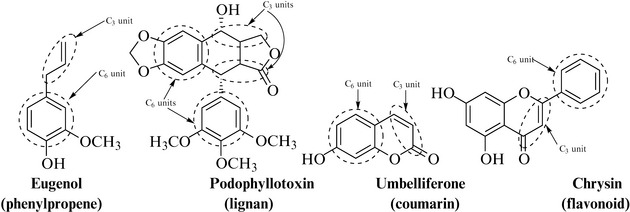
A number of natural product groups can be constructed from the amino acid phenylalanine, in particular the phenylpropenes, lignans, coumarins and flavonoids, all of which possess a common substructure based on an aromatic 6-carbon ring (C6 unit) with a 3-carbon chain (C3 unit) attached to the aromatic ring (Fig. 6.14). Many reactions can occur to this 9-carbon unit, including oxidation, reduction, methylation, cyclization, glycosylation (addition of a sugar) and dimerization, all of which contribute to the value of natural products as a resource of biologically active compounds and enhance the qualities of structural complexity with the presence of chirality and functionality.
Phenylpropenes
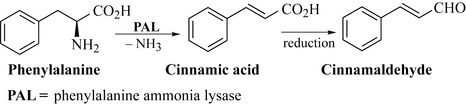
The phenylpropenes are the simplest of the shikimic-acid-derived natural products and consist purely of an aromatic ring with an unsaturated 3-carbon chain attached to the ring. They are biosynthesized by the oxidation of phenylalanine by the enzyme phenylalanine ammonia lyase, which through the loss of ammonia results in the formation of cinnamic acid. Cinnamic acid may then undergo a number of elaboration reactions to generate many of the phenylpropenes. For example, in Fig. 6.15, cinnamic acid is reduced to the corresponding aldehyde, cinnamaldehyde, which is the major component of cinnamon oil derived from the bark of Cinnamomum zeylanicum (Lauraceae) and used as a spice and flavouring. Cinnamon has a rich history, being used by the ancient Chinese as a treatment for fever and diarrhoea and by the Egyptians as a fragrant ingredient in embalming mixtures.
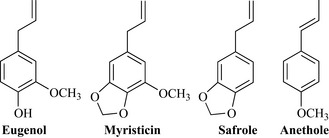
Cinnamon leaf also contains eugenol, the major constituent of oil of cloves derived from Syzygium aromaticum (Myrtaceae). Clove oil was used as a dental anaesthetic and antiseptic, both properties of which are due to eugenol, and the oil is still widely used as a short-term relief for dental pain. These phenylpropenes may have many different functional groups (e.g. OCH3, O-CH2-O, OH) and the double bond may be in a different position in the C3 chain (e.g. eugenol versus anethole) (Fig. 6.16). They are common components of spices, have highly aromatic pungent aromas and many are broadly antimicrobial, with activities against yeasts and bacteria. Some members of this class can also cause inflammation.
Lignans
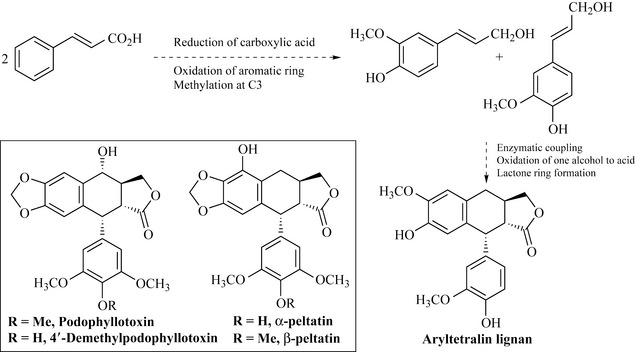
Lignans are low molecular weight polymers formed by the coupling of two phenylpropene units through their C3 side-chains (Fig. 6.17) and between the aromatic ring and the C3 chain. A common precursor of lignans is cinnamyl alcohol, which can readily form free radicals and enzymatically dimerize to form aryltetralin-type lignans of which the compounds podophyllotoxin, 4′-demethylpodophyllotoxin and α- and β-peltatin (from Podophyllum peltatum and Podophyllum hexandrum, Berberidaceae) are examples (Fig. 6.17).
Coumarins
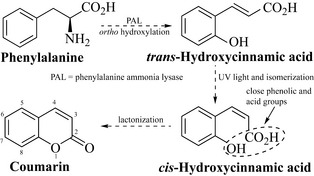
The coumarins are shikimate-derived metabolites formed when phenylalanine is deaminated and hydroxylated to trans-hydroxycinnamic acid (Fig. 6.18). The double bond of this acid is readily converted to the cis form by light-catalysed isomerization, resulting in the formation of a compound that has phenol and acidic groups in close proximity. These may then react intramolecularly to form a lactone and the basic coumarin nucleus, typified by the compound coumarin itself, which contributes to the smell of newly mown hay.
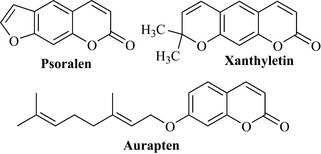
Coumarins have a limited distribution in the plant kingdom and have been used to classify plants according to their presence (chemotaxonomy). They are commonly found in the plant families Apiaceae, Rutaceae, Asteraceae and Fabaceae and, as with all of the natural products mentioned so far, undergo many elaboration reactions, including hydroxylation and methylation and, particularly, the addition of terpenoid-derived groups (C2, C5 and C10 units) (Fig. 6.19).
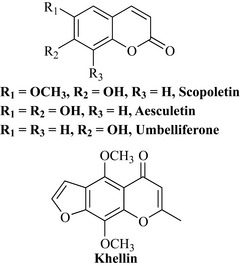
Some coumarins are phytoalexins and are synthesized de novo by the plant following infection by a bacterium or fungus. These phytoalexins are broadly antimicrobial; for example, scopoletin is synthesized by the potato (Solanum tuberosum) following fungal infection. Aesculetin occurs in the horse chestnut (Aesculus hippocastanum) and phytotherapeutic preparations of the bark of this species are used to treat capillary fragility. Hieracium pilosella (Asteraceae), also known as mouse ear, contains umbelliferone and was used to treat brucellosis in veterinary medicine and the antibacterial activity of this plant drug may in part be due to the presence of this simple phenol (Fig. 6.20). Khellin is an isocoumarin (chromone) natural product from Ammi visnaga (Apiaceae) and has activity as a spasmolytic and vasodilator.
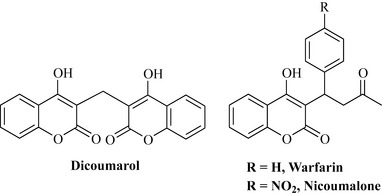
It has long been known that animals fed sweet clover (Melilotus officinalis, Fabaceae) die from haemorrhaging. The poisonous compound responsible for this adverse effect was identified as the bishydroxycoumarin (hydroxylated coumarin dimer) dicoumarol (Fig. 6.21).
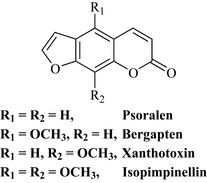
The psoralens are coumarins that possess a furan ring and are sometimes known as furocoumarins or furanocoumarins because of this ring. Examples are psoralen, bergapten, xanthotoxin and isopimpinellin (Fig. 6.22).
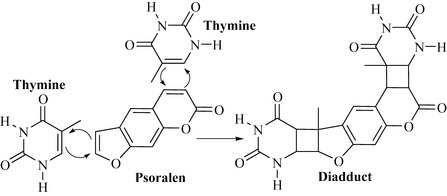
The mechanism of this phototoxicity has yet to be fully elucidated, but it is known that the psoralens are carcinogenic and mutagenic due to the formation of adducts with pyrimidine bases of DNA, such as thymine, via cycloaddition (Fig. 6.23). This reaction can occur with one (monoadduct) or two (diadduct) pyrimidine bases and may result in cross-linking of DNA.
Flavonoids
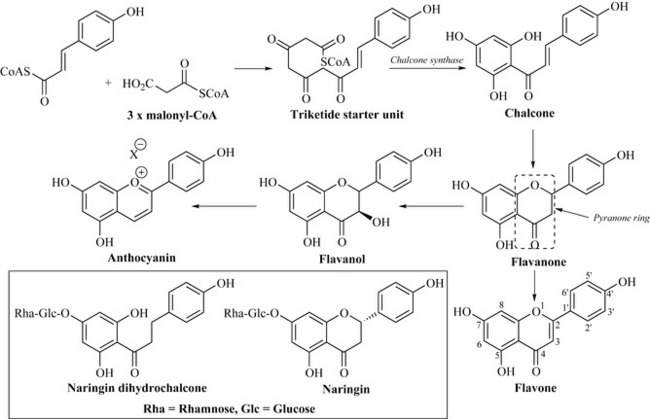
The flavonoids are derived from a C6-C3 (phenylpropane) unit which has as its source shikimic acid (via phenylalanine) and a further C6 unit that is derived from the polyketide pathway. This polyketide fragment is generated by three molecules of malonyl-CoA, which combine with the C6-C3 unit (as a CoA thioester) to form a triketide starter unit (Fig. 6.24). Flavonoids are, therefore, of mixed biosynthesis, consisting of units derived from both shikimic acid and polyketide pathways.
The triketide starter unit undergoes cyclization by the enzyme chalcone synthase to generate the chalcone group of flavonoids. Cyclization can then occur to give a pyranone ring-containing flavanone nucleus, which can either have the C2-C3 bond oxidized (unsaturated) to give the flavones or be hydroxylated at position C3 of the pyranone ring to give the flavanol group of flavonoids. The flavanols may then be further oxidized to yield the anthocyanins, which contribute to the brilliant blues of flowers and the dark colour of red wine. The flavonoids contribute to many of the other colours found in nature, particularly the yellow and orange of petals; even the colourless flavonoids absorb light in the UV spectrum (due to their extensive chromophores) and are visible to many insects. It is likely that these compounds have high ecological importance in nature as colour attractants to insects and birds as an aid to plant pollination. Certain flavonoids also markedly affect the taste of foods; for example, some are very bitter and astringent such as the flavanone glycoside naringin (Fig. 6.24), which occurs in the peel of grapefruit (Citrus paradisi). Interestingly, the closely related compound naringin dihydrochalcone (Fig. 6.24), which lacks the pyranone ring of naringin, is exceptionally sweet, being some 1000 times sweeter than table sugar (sucrose).
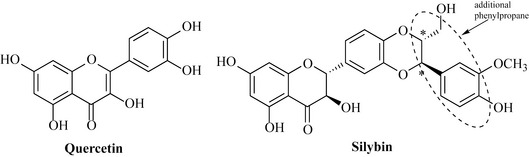
It is likely that the flavonoids have important dietary significance because, being phenolic compounds, they are strongly antioxidant. Many disease states are known to be exacerbated by the presence of free radicals such as superoxide and hydroxyl, and flavonoids have the ability to scavenge and effectively ‘mop up’ these damaging oxidizing species. Foods rich in this group have, therefore, been proposed to be important in ameliorating diseases such as cancer and heart disease (which can be worsened by oxidation of low-density lipoprotein); quercetin (Fig. 6.25), a flavonoid present in many foodstuffs, is a strong antioxidant. Components of milk thistle (Silybum marianum), in particular silybin (Fig. 6.25), are antihepatotoxins; extracts of milk thistle are generally known as silymarin and are used to reduce the effects of poisoning by fungi of the genus Amanita, which produces the deadly peptide toxins the amanitins. The mechanism of action of these antihepatotoxins is not entirely clear, but it has been proposed that they protect liver cells by reducing entry of the toxic peptides through the cell membrane and by acting as broad-spectrum antioxidants by scavenging the free radicals that can lead to hepatotoxicity. Silybin is a flavanol that has an additional phenylpropane unit joined to it as a di-ether and it exists in the extract as a mixture of enantiomers at one of the positions where this additional unit is joined (* in Fig. 6.25).
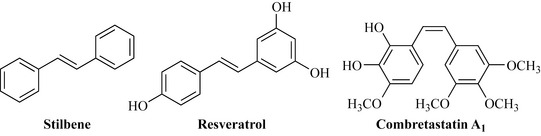
The stilbenes, sometimes referred to as bisbenzyls or stilbenoids, are related to the flavonoids and have the basic structure C6-C2-C6 (Fig. 6.26) arising from the loss of one carbon (as CO2) from the triketide starter unit. The simplest member of this class is stilbene. There is much interest in this class of compounds, especially in resveratrol, a component of red wine that has antioxidant, anti-cancer and anti-inflammatory activity. There is a low incidence of heart disease among the French population where large concentrations of fatty acids are sometimes present in the diet. It has been suggested that this low rate of heart disease is due to the consumption of red wine, which is rich in resveratrol and other flavonoids, and that the presence of these antioxidant compounds is cardioprotective. This phenomenon is known as ‘the French paradox’ and cardiologists advise patients who have a history of heart disease to consume a glass of red wine per day. Another group of stilbenoids of current interest are the combretastatins. For example, combretastatin A1, which is a cytotoxic drug lead, is a potent inhibitor of microtubule assembly and thought to have antitumour activity as a result of specifically targeting the vasculature of tumours. Combretastatin A1 is derived from Combretum caffrum (Combretaceae) and there has been much work on the Combretaceae to look for other biologically active members of this class.
Tannins
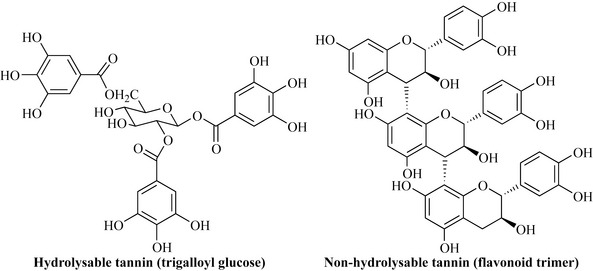
In addition to the flavonoids, another class of natural products that gives rise to the astringency and bitterness in plants and food are the tannins. This group comprises water-soluble polyphenolic compounds, which may have a high molecular weight. They are broadly divided into two groups: the hydrolysable tannins, which are formed by the esterification of sugars (e.g. glucose) with simple phenolic acids that are shikimate-derived (e.g. gallic acid), and the non-hydrolysable tannins, which are sometimes referred to as condensed tannins, that occur due to polymerization (condensation) reactions between flavonoids (Fig. 6.27).
The terpenes
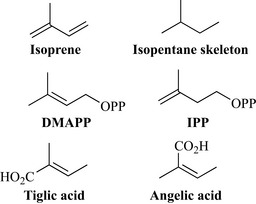
The terpenes are very widespread in nature and occur in most species, including man. They are sometimes referred to as isoprenes because a common recurring motif in their structure (the branched repeating C5 unit, the isopentane skeleton) is similar to isoprene (Fig. 6.28). Terpenes (hemiterpenes, monoterpenes and sesquiterpenes) contribute to many of the aromas associated with plants and range in complexity from simple C5 units (hemiterpenes) up to the polyisoprenes, which include latex, leaf waxes and rubber. Terpenes are derived from a number of extensive reactions between two C5 units [dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP)] (Fig. 6.28); the products of these reactions will, therefore, have multiples of five carbons. DMAPP and IPP are biosynthesized from two sources (mevalonic acid or deoxyxylulose phosphate).
The terpenes are a perfect example of a natural product class that is highly structurally diverse, has many members that are chiral and have extensive functional group chemistry. The simplest are the hemiterpenes (C5) produced by modification reactions to either DMAPP or IPP and include simple acids such as the structural isomers tiglic acid and angelic acid (Fig. 6.28), which form esters with many natural products. The monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes and steroids (C30-derived) and the tetraterpenes (carotenoids, C40) are all important medicinally and thus will be dealt with in more detail.
Monoterpenes (C10)
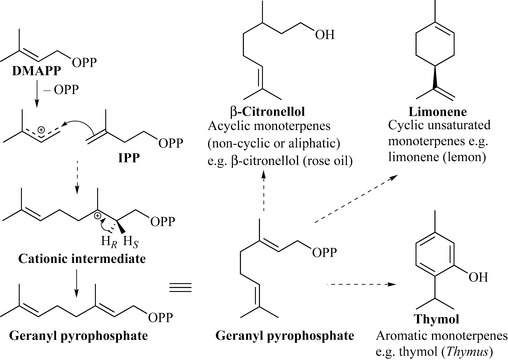
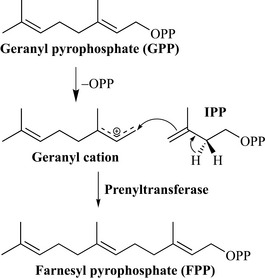
Biosynthetically, the monoterpenes are produced by the reaction between DMAPP and IPP in the presence of the enzyme prenyltransferase (Fig. 6.29). The first step of this reaction is thought to be the ionization of DMAPP to a cation (through the loss of pyrophosphate), which is then attacked by the double bond of IPP to generate a further cationic intermediate. Loss of a proton from the carbon neighbouring the cation (resulting in double bond formation) occurs in a stereospecific fashion (the R proton is lost) and this generates geranyl pyrophosphate (a C10 unit).
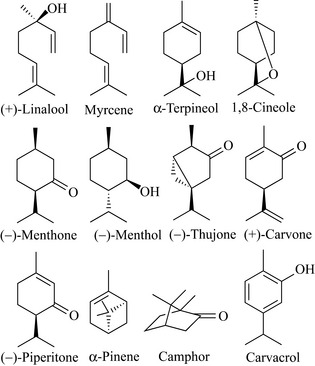
Geranyl pyrophosphate can then undergo many reactions to generate the variety of monoterpenes observed, such as simple modification to give the acyclic monoterpene β-citronellol, which is a component of rose oil. Geranyl pyrophosphate can be cyclized to give cyclic monoterpenes, which may be fully saturated, partially unsaturated or fully aromatic products of which menthol, piperitone and carvacrol are examples, respectively (Fig. 6.30).
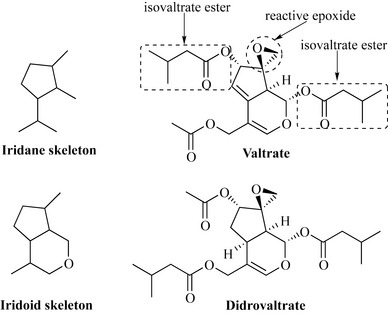
The iridoids are monoterpenes derived from the iridane skeleton, which is derived from geranyl pyrophosphate and, when oxidized, produces the iridoid skeleton (Fig. 6.31). These natural products are normally esterified and are common in the plant families Lamiaceae, Gentianaceae and Valerianaceae. The compounds are highly oxygenated and the esters are often derived from hemiterpenes; for example, valeric acid is esterified to form valtrate and didrovaltrate.
Sesquiterpenes (C15)
These natural products have properties similar to those of the monoterpenes, are constituents of many of the volatile oils and in some cases are broadly antimicrobial and anti-insecticidal, therefore contributing to the overall chemical defence of the producing organism. The starting unit for these compounds is farnesyl pyrophosphate (FPP), which is produced by the reaction of GPP (the monoterpene precursor) with a molecule of IPP (Fig. 6.32). The reaction is analogous to that for the formation of the monoterpenes in which a cationic intermediate is formed that reacts with IPP with elimination of a hydrogen ion.
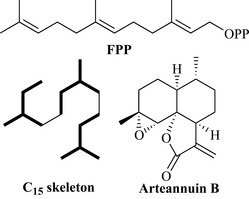
As with the monoterpenes, FPP can cyclize to form linear (acyclic) and cyclic sesquiterpenes. A key feature of these metabolites is their ability to undergo extensive elaboration chemistry, where they are highly functionalized, thus giving rise to the high structural diversity seen within this group of natural products. It is not always easy to see that these complex, functional, cyclic chiral compounds are derived from FPP due to these elaboration reactions. However, if the C15 skeleton of FPP is compared to arteannuin B, it can be seen how even complex structures are constructed (Fig. 6.33).
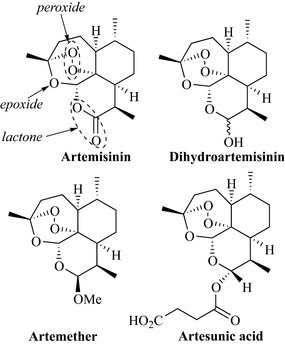
The most important sesquiterpene from the pharmaceutical perspective is the antimalarial product artemisinin (Fig. 6.34) from sweet wormwood (Artemisia annua, Asteraceae). This herb is widely distributed throughout Europe but also has a long history of use for the treatment of fevers and malaria in China where the drug is known as Qinghao. Artemisinin has a number of interesting features, including an ether, a lactone (cyclic ester) and an unusual peroxide functional group.
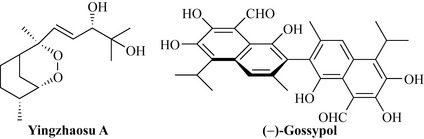
Interestingly, another Chinese medicinal plant used for treating malaria, Artabotrys uncinatus (Annonaceae), also contains a series of sesquiterpene peroxides (typically, yingzhaosu A; Fig. 6.35), which are responsible for the antimalarial activity.
Diterpenes
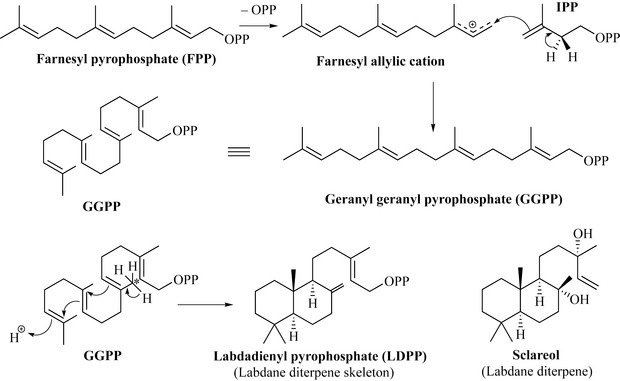
Members of the diterpene class are formed by the reaction of farnesyl pyrophosphate (FPP), a C15 unit, with isopentenyl pyrophosphate (IPP), the C5 unit that is the common building block for all of the terpenes. The first step of this reaction is the formation of a farnesyl allylic cation (analogous to the other examples of terpenes seen) which then reacts with IPP with stereospecific loss of a proton, resulting in the formation of geranyl geranyl pyrophosphate (GGPP). Depending on how GGPP folds and cyclizes, a very large number of products may result (Fig. 6.36).
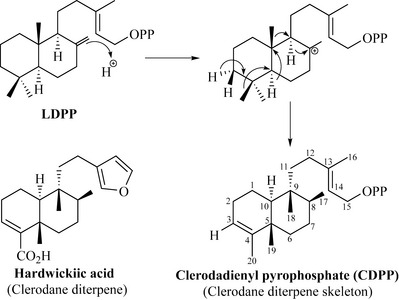
Loss of a proton from an allylic methyl (* in Fig. 6.36) and migration of bonds to form a bicyclic structure results in the formation of labdadienyl pyrophosphate (LDPP), which is a member of the labdane class of diterpenes of which sclareol from the clary sage (Salvia sclarea, Lamiaceae) is widely used in the perfumery industry. Sclareol is generated by hydrolysis of LDPP. If the exomethylene of LDPP reacts with a proton to form a cationic intermediate, this may undergo a series of Wagner–Meerwein hydride and methyl shifts (Fig. 6.37).
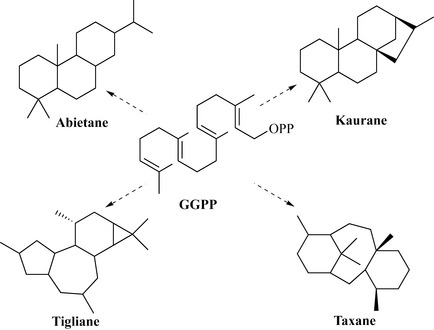
GGPP can cyclize to give an extraordinarily wide range of diterpene groups, some of which are shown in Fig. 6.38.
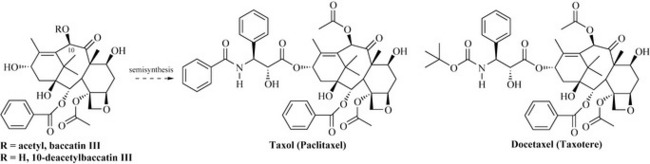
It is important to understand that, once a simple skeleton has been produced, a wide array of further elaboration reactions can occur, resulting in the highly complicated natural products of this class (e.g. paclitaxel; Fig. 6.39). This antitumour diterpene was discovered in 1971 by Monroe Wall and Mansukh Wani at the Research Triangle Institute as part of a programme funded by the National Cancer Institute. This compound is dealt with in further detail in Chapter 8. It was not until the 1980s that further work on the mode of action of this compound prompted its development and release onto the US market in 1993 under the trade name Taxol for the treatment of ovarian cancers.
Paclitaxel is present in the bark of the Pacific yew (Taxus brevifolia, Taxaceae), a slow growing tree from the forests of north-west Canada and the USA that takes 100 years before it can be exploited for processing. The wood of T. brevifolia is not suitable for timber production and was in danger of replacement by faster growing conifers, but this practice has been stopped. The yield of paclitaxel is also low (0.01–0.02%) as it takes three 100-year-old trees to produce 1 g of the drug. Thus, with a course of treatment being 2 g, it was quickly realized that the supply of paclitaxel had to come from another source. Taxus brevifolia produces a wide range of taxane diterpenes, and related compounds are also found in the common English yew, Taxus baccata. Paclitaxel belongs to a small class of taxanes that possess a four-membered ether (also called an oxirane) and a complex nitrogen-containing ester side-chain; both of these functional groups are essential for antitumour activity. The solution to the problem of low concentration of the drug came from the knowledge that related compounds, such as baccatin III and 10-deacetylbaccatin III (Fig. 6.39), were present in greater concentrations than paclitaxel and could be converted to paclitaxel by simple reactions.
Docetaxel (Taxotere) (Fig. 6.39), a related semi-synthetically produced taxane diterpene, is also used clinically for the treatment of ovarian cancers and has a modified side-chain to that of paclitaxel.
Triterpenes
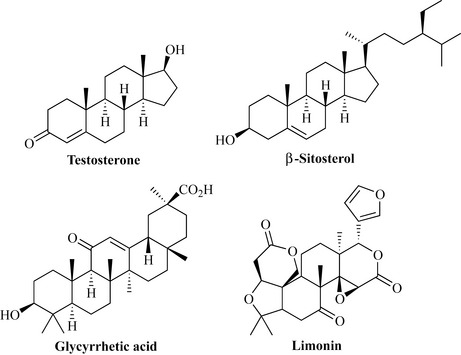
The triterpenes are C30-derived terpenoids with an exceptionally wide distribution, including man, plants, fungi, bacteria, soft corals and amphibia. The triterpenes include some very important molecules, such as the steroids (e.g. testosterone), which are degraded triterpenes with many important functions in mammals, notably as sex hormones. Other types include the sterols (e.g. β-sitosterol), which are common tetracyclic steroidal alcohols with ubiquitous distribution in plants, the pentacyclic triterpenes such as glycyrrhetic acid found in liquorice and the limonoids (e.g. limonin), which are highly oxidized bitter principles present in the Citrus plant family (Rutaceae) (Fig. 6.40).
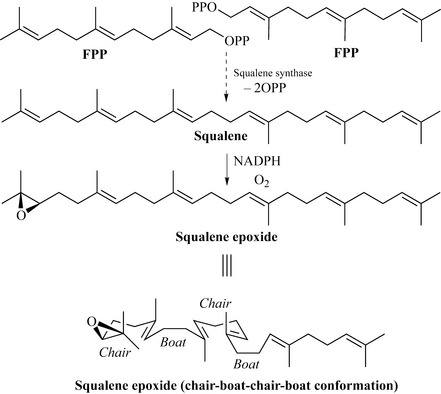
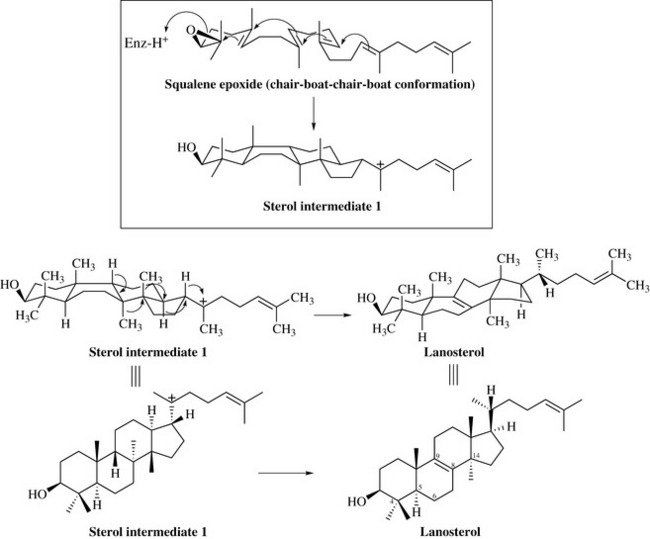
Their biosynthesis starts with the reaction between two molecules of farnesyl pyrophosphate (FPP) to form the true precursor of all triterpenes, squalene (Fig. 6.41). Squalene is then enzymatically epoxidized to squalene epoxide which, when folded in a particular conformation such as the ‘chair-boat-chair-boat’ conformation, can cyclize to give sterol intermediate 1 which is the precursor of the steroids and sterols (Fig. 6.42). This intermediate can undergo a series of Wagner–Meerwein shifts to give lanosterol, a common component of plants and of wool fat.
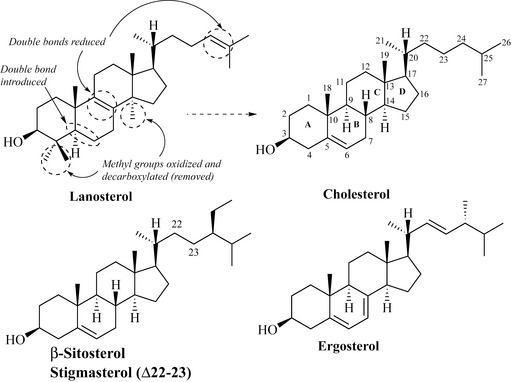
Oxidation and loss of methyls at positions C4 and C14, introduction of a C5-C6 double bond (oxidation) and loss of two double bonds (one at C8-C9 and one in the side-chain) would result in the formation of cholesterol. Cholesterol is the main animal sterol, a component of cell membranes and gallstones, and control of the levels of this sterol is important in the management of heart disease. The basic steroid nucleus and numbering of the ring system depicting the A, B, C and D rings is given for cholesterol (Fig. 6.43).
Other common sterols include the phytosterols (plant sterols) β-sitosterol and stigmasterol (which differs from β-sitosterol only by the presence of a double bond at position C22-C23), which are widespread in plants, and ergosterol, which is ubiquitous in fungi as a cell wall component (Fig. 6.43).
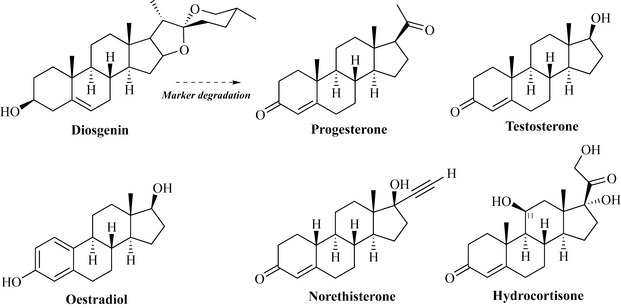
There is a great need for steroids in the pharmaceutical industry and this is met by using the plant sterol diosgenin from the wild yam (Dioscorea sp.). Diosgenin also occurs naturally as a glycoside (a sugar is attached at the hydroxyl position) and without the sugar the compound is referred to as a genin. Unlike the other plant sterols mentioned, the side-chain that is normally present at position C17 has been formed into two ring structures. Diosgenin can be converted into progesterone via a chemical process known as the Marker degradation, which gives access to many important steroids such as testosterone (a male sex hormone) and oestradiol (a female sex hormone) which has had the A ring aromatized, resulting in the loss of a methyl group from C10 (Fig. 6.44).
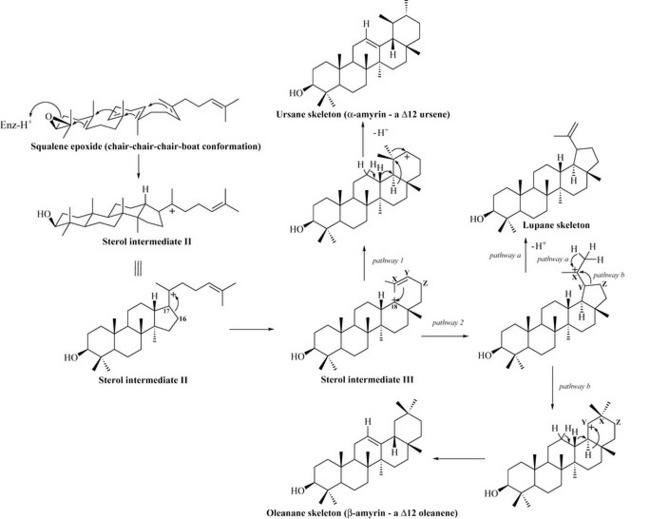
If squalene is folded in a different conformation (chair-chair-chair-boat), then cyclization mediated by a cyclase enzyme results in the formation of a different intermediate, sterol intermediate II, which is the precursor of the pentacyclic triterpenes (Fig. 6.45).
Migration of the C16-C17 bond to satisfy the positive charge results in the formation of sterol intermediate III. This may undergo several rearrangements to give different triterpene skeletons. Pathway 1 involves formation of a bond between C18 and CX, resulting in a positive charge on CY (through removal of one pair of electrons from the double bond to form the C18-CX bond). This may be satisfied by a series of Wagner–Meerwein methyl and hydride shifts with loss of a proton from C12 resulting in a C12 double bond. This pathway gives us the ursane-type triterpenes of which α-amyrin is an example, possessing a double bond in position C12 (referred to as a Δ12 ursene) (Fig. 6.45).
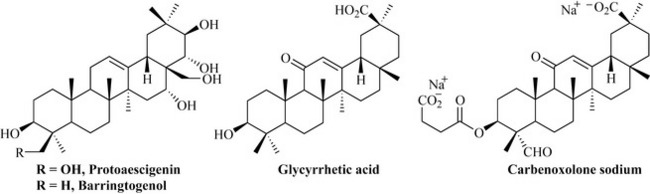
Pentacyclic triterpenes are common in plants and herbal remedies such as horse chestnut (Aesculus hippocastanum) and liquorice (Glycyrrhiza glabra). Examples such as protoaescigenin, baringtogenol (both from horse chestnut) and glycyrrhetic acid (liquorice) (Fig. 6.46) have a high degree of functionality and chirality, and usually occur in the plant material in the form of glycosides.
Tetraterpenes (C40)
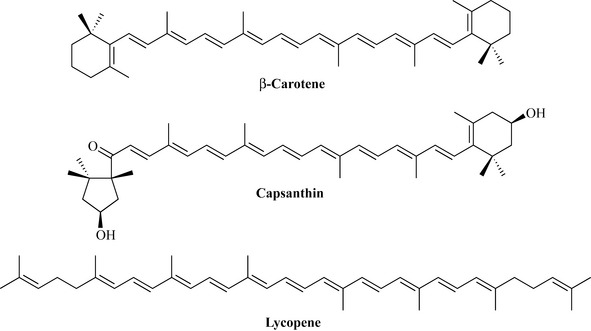
The final class of terpenoids that will be dealt with are the tetraterpenes, which are C40 natural products derived from the reaction of two molecules of geranyl geranyl pyrophosphate (C20). Members of this class are sometimes referred to as carotenes or carotenoids because of their occurrence in the carrot (Daucus carota). As with the flavonoids, the tetraterpenes are highly pigmented natural products and are responsible for the very bright colours of certain plants, in particular the orange of carrots due to β-carotene, and the brilliant red colour of tomatoes (Lycopersicon esculentum) and peppers (Capsicum anuum), which is due to lycopene and capsanthin, respectively (Fig. 6.47). These compounds are highly conjugated and strongly UV light absorbing, and are involved in photosynthesis as light accessory pigments. They are widely distributed in plants and may also act as a protection factor against UV light damage. Because of their high colouration they are employed as colouring agents in foods, pharmaceuticals and cosmetics.
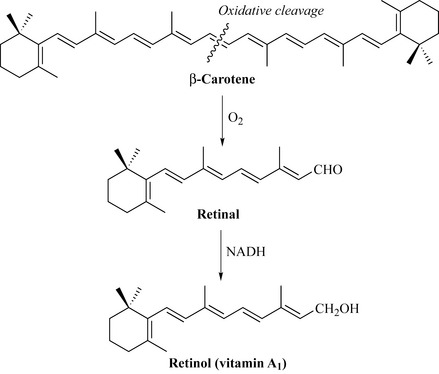
The tetraterpenes are precursors of vitamin A1 (retinol), a deficiency of which results in a reduction in sight efficiency through changes to the cornea and conjunctiva. Vitamin A1 occurs naturally in fish liver oils, carrots, green and yellow vegetables, and dairy products. It is biosynthesized by the oxidative cleavage of β-carotene to retinal, which is then reduced to retinol (vitamin A1) (Fig. 6.48).
The glycosides
The glycosides are discussed in a separate section here as they enhance the structural diversity of other natural product classes. The term glycoside is a generic term for a natural product that is chemically bound to a sugar. Thus the glycoside is composed of parts: the sugar and the aglycone. The aglycone may be a terpene, a flavonoid, a coumarin or practically any other natural product. If the aglycone is a triterpene, it is sometimes referred to as a genin (e.g. protoaescigenin; Fig. 6.46). Glycosides are very common in nature and provide extra chemical diversity and structural complexity in natural products.
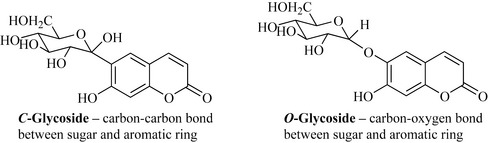
There are two basic classes of glycosides: the C-glycosides, in which the sugar is attached to the aglycone through a carbon–carbon bond, and the O-glycosides in which the sugar is connected to the aglycone through an oxygen–carbon bond (Fig. 6.49).
Cyanide glycosides
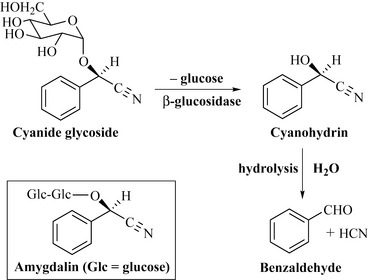
Some glycosides are undoubtedly used by plants as a chemical defence and this is certainly so with the cyanide glycosides. These compounds, in the presence of enzymes such as β-glucosidase, lose their sugar portion to form a cyanohydrin which, in the presence of water, can undergo hydrolysis to give benzaldehyde and the highly toxic hydrogen cyanide (HCN) (Fig. 6.50).
Cyanide glycosides such as amygdalin (Fig. 6.50) are present in many species of the genus Prunus, which includes commercially important fruit such as peaches, cherries, plums and apricots. Fortunately, the enzymes that convert these compounds to the cyanohydrins are localized in different parts of the plant or are absent. In the case of sweet almonds (Prunus amygdalus var. dulcis), the enzymes are present but there are no cyanide glycosides present.
Glucosinolates
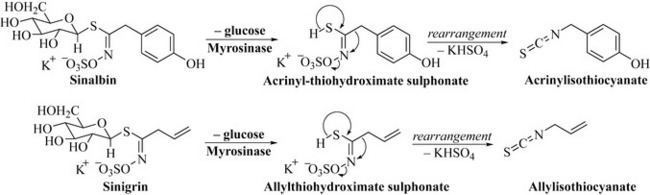
The plant family Brassicaceae includes cabbages, sprouts and the mustards and produces a group of glycosides known as glucosinolates. These are sulphur- and nitrogen-containing glycosides previously referred to as nitrogen mustards. A common example of this group is sinalbin from white mustard (Sinapis alba), which in the presence of the enzyme myrosinase is converted into a thiohydroximate, which rearranges with the loss of a hydrogen sulphate salt to the isothiocyanate, acrinylisothiocyanate (Fig. 6.51).
These isothiocyanates are exceptionally pungent and impart a strong aroma to mustards, which can be described as hot or even acrid to the taste. In black mustard (Brassica nigra), the simple glucosinolate sinigrin is converted in the same fashion to allylisothiocyanate (Fig. 6.51), which is an oil and far more volatile than acrinylisothiocyanate. The oils derived from mustards are rich in these isothiocyanates and are mildly irritant; they are used medicinally as externally applied treatments for muscular pain.
Cardiac glycosides
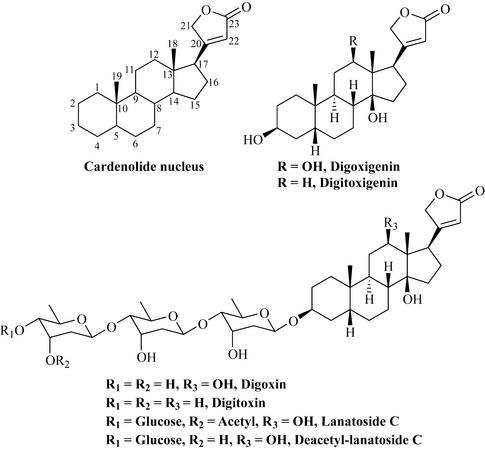
Many plants contain cardioactive or cardiac glycosides, which have a profound effect on heart rhythm. They are commonly found in the genera Convallaria, Nerium, Helleborus and Digitalis. The aglycone portion is steroidal in nature and is sometimes referred to as a cardenolide, being cardioactive and possessing an alkene and an olide (a cyclic ester) (Fig. 6.52).
Being ‘steroid-like’, the aglycone (genin) portion is derived from the triterpenes and these compounds may have a wide variety of sugars attached to the steroid portion. The most widely studied plant that contains these compounds is the foxglove (Digitalis purpurea) of the plant family Scrophulariaceae, which was used as long ago as the 18th century for the treatment of heart disease described as ‘dropsy’. The basis of this use was well founded as this plant contains the medicinal agents digoxin and digitoxin (Fig. 6.52). Digoxin is the most widely used cardiac glycoside in congestive heart failure and is now produced by isolation from the related species Digitalis lanata. Related cardiac glycosides, which because they are very fast-acting compounds are used in emergencies via the intravenous route, are lanatoside C and deacetyl-lanatoside C.
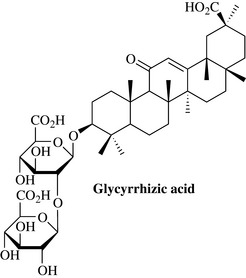
Triterpene glycosides have widespread distribution in plants and are sometimes referred to as saponins as they have soap-like properties and readily form foams. Medicinally important examples include glycyrrhizic acid from liquorice (Glycyrrhiza glabra) (Fig. 6.53), which is used as a treatment for stomach ulcers and the salts of which are intensely sweet. The sugars in Fig. 6.53 are of the glucuronic acid type and are shown as their Fisher projections.
Anthraquinone glycosides
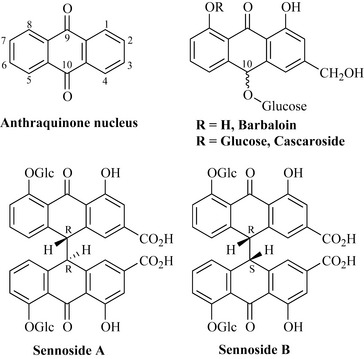
A number of plants that contain anthraquinone or anthrone glycosides (Fig. 6.54) have long been known for their laxative properties. They include cascara (Rhamnus purshiana), aloe (Aloe vera) and senna; the latter is divided into two species (Cassia angustifolia, known as Tinnevelly senna, and Cassia senna, known as Alexandrian senna). Aloe is used as a laxative as well as a treatment for minor burns. It contains a mixture of anthraquinone glycosides of which barbaloin is the major component and is a mixture of 10R and 10S isomers; the purified components are referred to as aloin A and B. The gel or mucilage from aloe is rich in polysaccharides and these anthraquinone glycosides, and is incorporated into creams and ointments to treat abrasions, burns and skin irritation.
There is little difference in the chemistry of the two senna species. The active constituents are sennosides A and B (Fig. 6.54). These natural products are dianthrones (dimers) of the anthrone skeleton. The fresh leaves of senna contain glycosides with additional sugar groups present and these are naturally hydrolysed to sennosides A and B. In vivo, the sennosides are then hydrolysed to the dianthrones (lacking the glucose sugars). Senna is widely prescribed for constipation; an example of a marketed product is Senokot.
The alkaloids
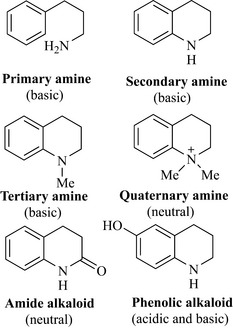
No other group of natural products has contributed more to medicines and pharmaceutical preparations than the alkaloids. As a group, they display an exceptionally wide array of biological activities and have an equally wide distribution, being present in plants, fungi, bacteria, amphibia, insects, marine animals and man. Plants and fungi rich in these natural products were used by early man to relieve pain, as recreational stimulants or, in religious ceremonies, to enter a psychological state to achieve ‘communication’ between his ancestors or God. The German pharmacist Karl Friedrich Wilhelm Meissner first coined the term ‘alkaloid’ in 1818, to describe substances that had alkaline (hence alkaloid) properties. Many alkaloids are, indeed, alkaline in nature (Fig. 6.55) as they possess either a primary, secondary or tertiary amine functional group and the alkaline (basic) properties of these groups may be exploited to aid their extraction and purification (see Chapter 7). However, some alkaloids exist as quaternary amine salts in which a lone pair of electrons from the nitrogen atom is used to form a bond with another group (e.g. methyl) and, therefore, a positive charge resides on the nitrogen making this group essentially neutral (neither basic nor acidic). Care must, therefore, be taken with the alkali or base definition of alkaloids as some are neutral, especially the amides (Fig. 6.55), and some alkaloids possess phenolic groups which actually contribute to the acidity of the molecule.
Alkaloids may also naturally exist as salts, which are the product of a reaction of a base (alkaloid) and an acid (e.g. sulphuric acid to give the sulphate, or hydrochloric acid to give the hydrochloride). A further definition of this group is that they are heterocyclic natural products containing nitrogen, but in our definition we will include compounds that contain nitrogen in an aliphatic chain (e.g. the phenyl-alkylamines; see below). Biosynthetically, the alkaloids are produced from several different amino acids thereby giving rise to a diverse group of fundamental structures (Fig. 6.56). A biosynthetic treatment of this class is outside the scope of this chapter; consequently this group of natural products will be dealt with by alkaloid class.
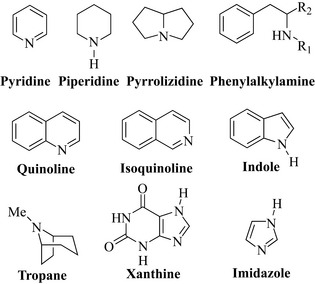
Pyridine, piperidine and pyrrolizidine alkaloids
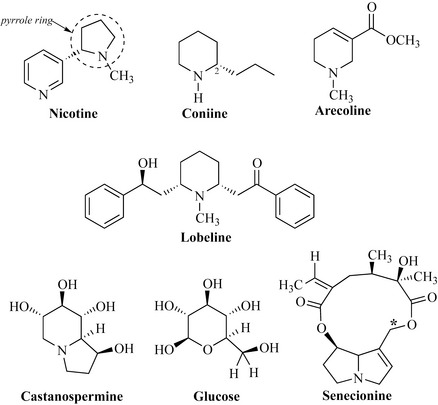
The most widely studied member of the pyridine class is nicotine, the stimulant alkaloidal component of tobacco (Nicotiana tabacum, Solanaceae) (Fig. 6.57), which is responsible for the addictive nature of cigarettes and other tobacco preparations. Nicotine is used as a model for addiction to other drugs such as heroin. The compound has a pyrrole ring attached to the pyridine ring. Pharmaceutically, nicotine is formulated into chewing gum as an aid to cessation of smoking in products such as Nicorette.
Lobeline is found in the leaves and tops of Lobelia inflata (Campanulaceae), which is also known as wild tobacco or pukeweed. It has similar effects to those of nicotine and arecoline and has been used as a smoking deterrent. Much work has been done to find alkaloids with activity against HIV of which castanospermine from Castanospermum australe (Fabaceae) is exceptional. This compound is an inhibitor of α-glucosidase, an enzyme involved in glycoprotein processing, which is important in the formation of viral coating, abnormalities of which stop infection of white blood cells. Castanospermine is a polyhydroxylated alkaloid (PHA) and is in fact a sugar analogue (compare with glucose in Fig. 6.57), which explains its activity against the glucosidase enzymes involved in the formation of glycoproteins. The compound is sometimes classified as an indolizidine alkaloid, but, as it also has a piperidine ring system, it is included in this section for convenience.
Senecionine is a member of the pyrrolizidine class of alkaloids, which have gained notoriety due to their hepatotoxic properties. These compounds possess a reactive carbon (* in Fig. 6.57), which is readily alkylated by reactive thiol groups present in many enzymes found in the liver. This accounts for the withdrawal of comfrey (Symphytum officinale, Boraginaceae), which has a long history of use as a medicinal plant but also contains these toxic alkaloids. Senecionine occurs in groundsel (Senecio vulgaris, Asteraceae), which is problematic in farms and paddocks where it can cause poisoning of livestock and horses.
Phenylalkylamine alkaloids
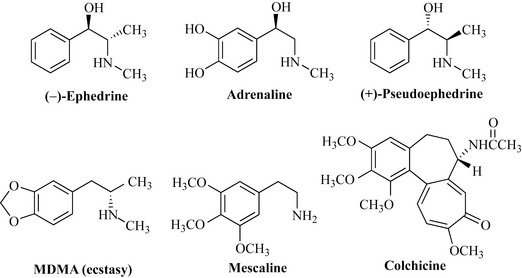
The natural products of this group do not have a cyclic nitrogen atom but have either a free amine or an alkyl-substituted amine. In Chinese medicine, Ma Huang (Ephedra sinica, Ephedraceae) has a long tradition of use as a treatment for colds, asthma and other bronchial conditions. The biologically active component of this species is ephedrine (Fig. 6.58), which possesses CNS stimulatory, vasoconstrictive and bronchodilatory properties. These effects are similar to those of the natural hormone adrenaline (epinephrine), which is structurally similar (Fig. 6.58). Ephedrine has two stereogenic (chiral) centres and, therefore, has four possible isomers. Injections of (–)-ephedrine are used for severe asthma and life-threatening anaphylactic shock. Another isomer of ephedrine, (+)-pseudoephedrine, is used in cough preparations such as Sudafed for its bronchodilatory properties. Herbal Ephedra has recently gained notoriety as ‘herbal ecstasy’, with a number of sources selling plant material over the Internet and in magazines. Claims of the stimulant’s ‘ecstasy-like’ properties are not unfounded due to the high similarity in structure of ephedrine and ecstasy (methylenedioxy-methylamphetamine, MDMA). These herbal preparations are dangerous and should therefore be avoided.
Quinoline alkaloids
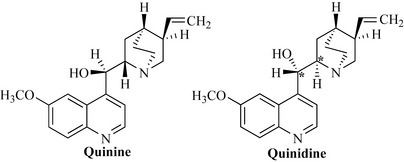
The Spanish conquistadors who invaded Peru in the latter part of the 16th century discovered that the indigenous Incas of this area used a preparation of the bark of a rain-forest tree to treat fevers, especially malaria. The Jesuit priests accompanying the invading force collected large amounts of this bark and used it to prevent and treat malaria. The bark was shipped back to Europe where it became known as Jesuit bark or Peruvian bark and gained great fame as a treatment for malaria. The trees responsible for this biological activity are of the genus Cinchona (Rubiaceae), which produce the quinoline alkaloid quinine, first isolated in 1820 by the French pharmacists Pelletier and Caventou (Fig. 6.59). The structure of this compound was not known, however, until 1908 and total synthesis was only achieved in the mid-1940s. The pure compound was used extensively as an antimalarial and was a template for synthetic antimalarials such as quinacrine, chloroquine and mefloquine. Resistance to these agents, particularly chloroquine, has become increasingly widespread, in particular through removal of the antimalarial from the cell by plasmodial membrane-bound efflux mechanisms, resulting in a low intracellular (ineffective) concentration of the drug. Interestingly, quinine is active in many cases against chloroquine-resistant malaria and there has been increased use of this drug. It is thought that quinine and other quinoline antimalarials exert their effects by binding to haem, a degradation product of haemoglobin. This haem–quinoline conjugate is toxic and leads to death of the parasite. In the absence of quinine, haem is converted into a polymeric form known as haemozoin or malaria pigment which is non-toxic. Plasmodia are highly adaptable organisms and at present there is a need for new antimalarials to counter multidrug resistance in Plasmodium falciparum.
Quinidine is an isomer of quinine and has different configuration at the positions marked * in Fig. 6.59. It was observed that patients suffering from malaria who also had atrial fibrillation were cured of arrhythmias by quinine and quinidine. Quinidine is used to treat type I cardiac arrhythmias.
Isoquinoline alkaloids
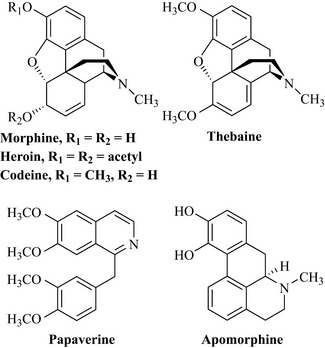
Opium is the gummy exudate of the unripe capsules of the opium poppy (Papaver somniferum, Papaveraceae) and contains more than 30 alkaloids, of which the major components are morphine, codeine, thebaine, papaverine and noscapine (Fig. 6.60).
The majority of opium, which is produced for illegal drug use, now originates in Afghanistan.
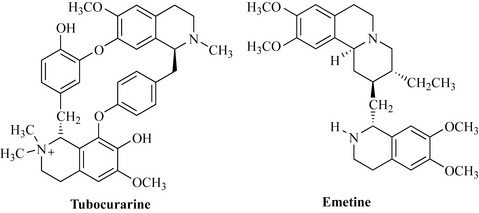
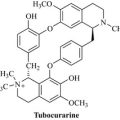
Indigenous peoples of South America use a variety of arrow poisons for hunting purposes, of which curare is a strong muscle relaxant. This poison is prepared from plants of the family Menispermaceae, notably Chondrodendron tomentosum, which kills by paralysis of the muscles required to breathe. The major active component of this species is the isoquinoline alkaloid tubocurarine, so named because the curare poison was carried in bamboo ‘tubes’ prior to use (Fig. 6.61).
Ipecac (Caephaelis ipecacuanha, Rubiaceae) is a shrub indigenous to Brazil and produces rhizomes (underground stems) that were used by the indigenous peoples to treat diarrhoea. The main alkaloidal components of this species are emetine, psychotrine and cephaeline. Ipecac was used to treat amoebic dysentery, but the side effects (vomiting, nausea and severe gastrointestinal disturbance) stopped its use. However, it is used as an emetic in the form of a syrup to induce vomiting after poisoning and drug overdose. In addition to its emetic and amoebicidal properties, emetine (Fig. 6.61) is an expectorant and is added to many cough medicines.
Indole alkaloids
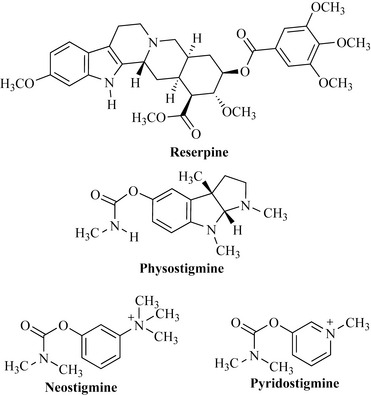
Like the isoquinolines, the indole alkaloids are a very important source of bioactive compounds. Snake root (Rauvolfia serpentina, Apocynaceae) is a shrub common to the Indian subcontinent; it has been used as a panacea in the Ayurvedic system of medicine, with uses described for the treatment of snakebite and madness. Reserpine, the major component of this species, was used as an antihypertensive agent, but due to side effects (neurotoxicity, cytotoxicity and depression) it is now not in use (Fig. 6.62).
British missionaries working in the Calabar coast area of West Africa (Nigeria and Cameroon) reported that criminal trials were conducted using the Calabar bean (Physostigma venenosum, Fabaceae). The beans of this plant are highly toxic and, when an individual was accused of a crime, they were forced to consume an extract of the bean. This practice was ‘trial by ordeal’ and accounts for the other name for the Calabar bean, the ‘ordeal bean’. Should the individual live then they were innocent of the crime, but death indicated guilt. The margin between innocence and guilt was probably a result of the completeness or incompleteness of extraction of the toxic chemicals from the plant! The toxic component of this species is physostigmine (Fig. 6.62), which is an inhibitor of acetylcholinesterase, resulting in an enhancement of the activity of acetylcholine (which is degraded by acetylcholinesterase). There is interest in this compound in the treatment of Alzheimer’s disease in which a low concentration of acetylcholine in the brain is observed. Synthetic compounds based on physostigmine include neostigmine and pyridostigmine, which are used to treat myasthenia gravis, a rare disease characterized by severe muscle weakness.
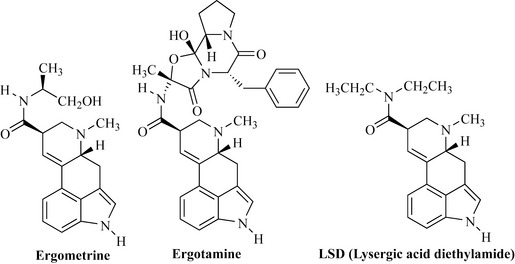
Poisoning through contamination of rye grain by fungi, in particular by Claviceps purpurea, has been described since the Middle Ages. This fungus produces dark-coloured structures (sclerotia) known as ergot on rye plants; these structures are rich in indole alkaloids. The poisoning from ingestion of bread made from contaminated grain is highly unpleasant, with victims complaining of burning, ‘fire-like’ sensations throughout their extremities and of vivid highly coloured hallucinations. These poisons can cause massive constriction of blood vessels, leading to ‘blackened’ limbs and gangrene. This condition became known as St Anthony’s fire after the saint who spent much of his life meditating in the fire-like heat of the Sinai desert. Because bread was the main staple diet in the Middle Ages, it is likely that this condition was widespread, especially as the damp surroundings in which grain was kept are conducive to the growth of the fungus. Ergot was used as an obstetric preparation in the 1500s to shorten labour during childbirth. It contains several groups of indole alkaloids such as the ergometrine type, which have simple amide side-chains, and the ergotamine group, which possess complex amino acid derived side-chains (Fig. 6.63).
Ergometrine is an oxytocic used to expel the placenta after childbirth or to increase contractions. This compound also acts on the pituitary as well as on the uterine muscles. Ergotamine was first used in the 1920s for the relief of migraine and is still used today. It reduces vasodilation, which can occur in throbbing migraine headache. The ergot alkaloids were used as a template for the semi-synthesis of bromocriptine, pergolide and cabergolide, which have use in neurological disorders such as Parkinson’s disease. Ergot can cause hallucinations, and the hallucinogenic drug of abuse LSD (lysergic acid diethylamide) is structurally related to these compounds (Fig. 6.63).
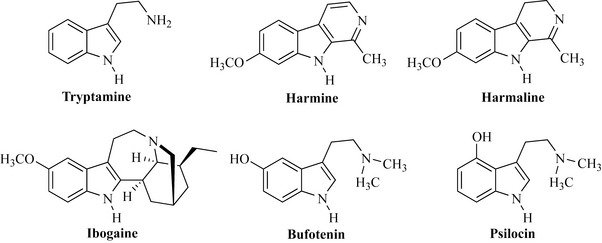
Many of the psychoactive compounds (including LSD) are structurally related to tryptamine, as are the harmine and harmaline alkaloids from Peganum harmala (Syrian Rue, Nitrariaceae) and the yahé or ayahuasca preparations (Bansiteriopsis caapi and B. inebrians, Malpighiaceae), which are prepared by Amazonian shamen. Ayahuasca is used as part of the community rituals of some Peruvian groups to preserve their traditional ways and to promote bonding and the establishment of social order. Ibogaine, from iboga (Tabernanthe iboga, Apocynaceae), is hallucinogenic and anticonvulsant, and has recently been studied as a treatment for heroin addiction. Psychoactive indole derivatives are even found in amphibia, notably in the skin of species of the genus Bufo, which produce bufotenin (Fig. 6.64).
Mushrooms of the genera Psilocybe, Panaeolus, Conocybe and Stropharia are known to produce psychoactive substances such as psilocybin, which is a phosphate salt in the fungi and is converted into psilocin in vivo (Fig. 6.64). The Aztecs of Mexico revered certain fungi (Psilocybe mexicana, Strophariaceae) as the ‘flesh of the Gods’ and gave it the name Teonanactl. The reverence for these mushrooms is presumably attributed to the profound hallucinogenic effects they exert, and, in Europe, many related species such as the liberty cap (Psilocybe semilanceata) are collected illegally for recreational abuse. These fungi are colloquially referred to as ‘magic mushrooms’, but as fungal taxonomy is highly complex there are risks of collecting poisonous species and the outcome may not be ‘magic’ at all.
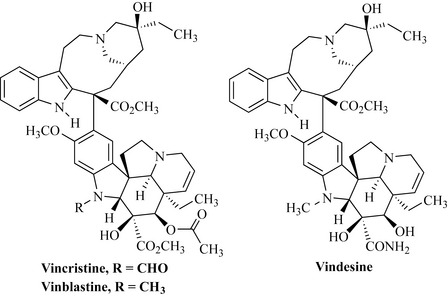
The most important alkaloids of the indole group are the anticancer agents vincristine and vinblastine from the Madagascar periwinkle (Catharanthus roseus, Apocynaceae). These are complex bisindole (dimeric indole) natural products present in small quantities in the plant material. Vindesine is a semi-synthetic derivative which is also used clinically. These compounds are used for the treatment of Hodgkin’s lymphoma, acute leukaemia and some solid tumours (Fig. 6.65) and are dealt with in further detail in Chapter 8.
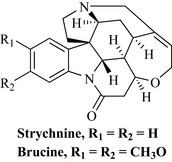
Strychnine and brucine (Fig. 6.66) are intensely bitter indoles from the seeds of nux-vomica or ‘vomiting nut’ (Strychnos nux-vomica, Loganiaceae), which is a tree indigenous to India. Preparations of nux-vomica were used as a stimulant tonic until the middle of the 20th century. However, these compounds are highly poisonous (strychnine is used as a rodenticide) and they are responsible for occasional poisoning incidents. They are of historical interest only in pharmacy and are now used as research tools.
Tropane alkaloids
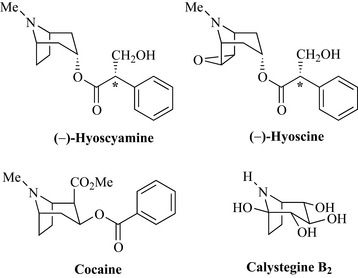
The European plant deadly nightshade (Atropa belladonna, Solanaceae) produces hyoscyamine (Fig. 6.67), which occurs in the plant as a racemic mixture [(+) and (–) isomers, sometimes denoted (±)] at the chiral centre denoted * in Fig. 6.67. This mixture is often referred to as atropine. The generic name of the plant refers to Atropos, the ancient Greek Fate who, in mythology, cut the thread of life, and belladonna, meaning beautiful lady in Italian and refers to the use of the juice of the berries of this plant by ladies in the 16th century to dilate the pupils of their eyes which was considered an attractive feature. Hyoscyamine is an anticholinergic and also has been used to treat acute arrhythmias and to dilate the pupil of the eye (a mydriatic) for ophthalmic examinations. Semi-synthetic derivatives are also used (such as tropicamide) that are less longer-acting. Hyoscyamine also occurs in other species of Solanaceae, notably henbane (Hyoscyamus niger) and thornapple (Datura stramonium), together with hyoscine, also known as scopolamine, which is the epoxide derivative of hyoscyamine. Hyoscine is widely used as a premedication prior to operations to dry up secretions produced by inhalant anaesthetics and reduce nausea caused by the opiates. It is also a component of many travel (motion) sickness preparations.
Xanthine alkaloids
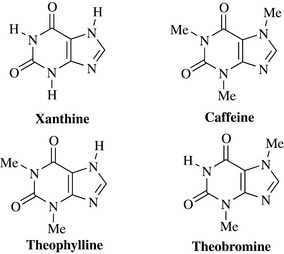
The xanthine alkaloids are probably the most widely known (and used) group of alkaloids, being constituents of popular daily beverages such as tea (Camellia sinensis, Theaceae) and coffee (Coffea arabica, Rubiaceae). Coffee contains the xanthine (or purine) alkaloid caffeine (1–2%) (Fig. 6.68); typically a cup of instant coffee contains approximately 50 mg of caffeine. The caffeine content is appreciably higher in Turkish or Arabic coffees, which are highly concentrated and may contain up to 300 mg of caffeine per cup. Caffeine is a CNS stimulant and is a component of Proplus, a highly popular product amongst students to counter fatigue and drowsiness. It is also a diuretic and is used in combination with analgesics.
Together with caffeine, theophylline and theobromine (Fig. 6.68) are minor components of tea; theobromine also occurs in cocoa (Theobroma cacao, Malvaceae). All three alkaloids differ only in the number and position of methyl substituents around the xanthine ring system. Theophylline is a diuretic and its derivatives (e.g. aminophylline) are used to relax the smooth muscle of the bronchi for relief of asthma.
Imidazole alkaloids
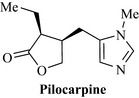
The only member of this class that is of pharmaceutical merit is pilocarpine from jaborandi (Pilocarpus jaborandi, Rutaceae), a tree common to South America (Fig. 6.69). Pilocarpine is a cholinergic agent and is used to stimulate muscarinic receptors of the eye in the treatment of glaucoma. In the eye, this compound and derivatives (salts such as the hydrochloride and nitrate) cause pupillary constriction (miosis) and relieve eye pressure by facilitating better ocular drainage. Currently, there is interest in this class of alkaloid as muscarinic agonists in the treatment of Alzheimer’s disease.
Appendino G., Fontana G., Pollastro F. Natural products drug discovery. In: Mander L., Lui H.W., editors. Comprehensive Natural Products II, vol. 3. Oxford: Elsevier; 2010:205-236.
Bruneton J. Pharmacognosy, phytochemistry, medicinal plants. Berlin: Springer-Verlag; 1995.
Dewick P.W. Medicinal natural products: a biosynthetic approach, third ed. Chichester: Wiley; 2009.
Evans W.C. Trease and Evans’ pharmacognosy, fifteenth ed. London: Baillière Tindall; 2002.
Luckner M. Secondary metabolism in microorganisms, plants and animals, third ed. Berlin: Springer-Verlag; 1990.
Mann J. Chemical aspects of biosynthesis. In Oxford chemistry primers. Oxford: Oxford University Press; 1994.
Robbers J.E., Speedie M.K., Tyler V.E. Pharmacognosy and pharmacobiotechnology. Baltimore: Williams & Wilkins; 1996.
Samuelsson G., Bohlin L. Drugs of natural origin: a treatise of pharmacognosy. London: Taylor & Francis; 2010.
Thadani M.B. Medicinal and pharmaceutical uses of natural products. Winnipeg: Cantext Publications; 1996.
Torsell K.B.G. Natural product chemistry: a mechanistic, biosynthetic and ecological approach, second ed. Stockholm: Swedish Pharmaceutical Press; 1997.