4 Natural History of the Degenerative Cascade
KEY POINTS
Natural History of the Degenerative Cascade
The natural history of degenerative disc disease has been studied for many years. Lees and Turner, in 1963, followed 51 patients with cervical radiculopathy for 19 years and found that 25% had worsening of the symptoms, 45% had no recurrence, and 30% had what they classified as mild symptoms.1 Nurick studied the nonsurgical treatment of 36 patients with cervical myelopathy over 20 years.2 Sixty-six percent of the patients who presented with early symptoms did not progress, and approximately 66% of patients with moderate to severe symptoms did not progress either. The patients who progressed tended to be the younger patients.
Anatomy and General Mechanisms of Pain
Cortical bone, bone marrow, and periosteum have been found to be innervated by nerves containing nociceptive neuropeptides such as calcitonin, gene-related peptides, and substance P. Periosteal elevation, such as in cases of infection, tumor, or hematoma, can be painful. Periosteal tears in cases such as fractures, inflammation, or subsidence (e.g., in osteoarthritic conditions) can cause pain. Vascular congestion from bone infarcts or sickle cell can cause the intramedullary nerve fibers to initiate a painful response. Nociceptive nerve fibers have been identified in varying concentrations within the fibrous tissue of spondylolytic pars defects as well.
Biochemical Changes
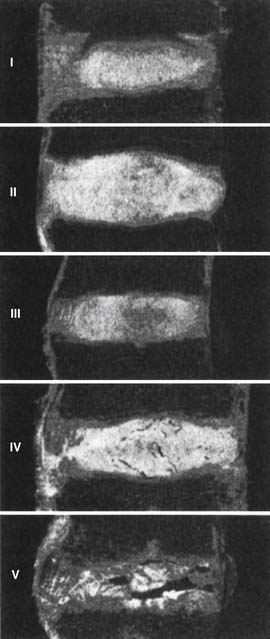
Numerous biochemical changes occur in the disc as a result of aging. The gelatinous nature of the disc degenerates into a more fibrotic state due to loss of water content. It is important to understand that a normal disc is composed of 80% water and 20% collagen and proteoglycans. The negatively charged glycosaminoglycans are what allows the nucleus to retain its water content and osmotic pressure. The actual cascade of nucleus degeneration occurs in the following order. First, there is loss of distinction between the nuclear and annular fibers and an increase in the collagen content of the disc, followed by the loss of the negative charges mentioned earlier and loss of water content, greatly reducing the proteoglycan aggregates. In fact, during the breakdown of the glycosaminoglycans, there is also a significant loss of chondroitin sulfate in comparison to keratin sulfate. The annulus degenerates by a decrease in cellularity and metabolic activity. The annulus is the only portion of the disc that in its healthy state has vascularity. This vascularity decreases with degeneration, which may hinder the healing process. Proteoglycan content decreases and large collagen fibrils appear. The large fibrils when present in a biomechanically vulnerable portion of the annulus may increase the likelihood of annular tears. Such tears generally occur due to a rotational force and occur in the posterolateral annulus. With annular disruption, changes take place within the disc itself. Vascularized granulation tissue forms along the margins of the annular ruptures and may pass as far as into the nucleus.3 Unlike discs from asymptomatic subjects, among discs taken from back pain patients, nerve endings extended deep into the annulus and in some cases into the nucleus. Such nerves produced substance P.4 These changes within the disc likely play a role in discogenic pain. Also, such changes may challenge disc regeneration as a pain-relieving intervention.
The cartilaginous endplate serves as a nutrition gradient for the healthy disc. Degeneration of the disc has been associated with a decrease in the diffusion capability across the endplate and sclerosis of the endplate, which in turn negatively affects the nutrition of the disc.5 This is thought to at least have a negative impact on the biochemical medium within the disc, if it is not the actual cause. These types of degenerative and nutritional changes within the disc will likely pose a significant challenge to disc regenerative therapies.
Kirkaldy-Willis et al. inspected 50 lumbar cadaveric specimens and also analyzed morphologic changes in 161 patients’ lumbar spines intraoperatively.6 It is such observations that have provided links between the different aspects of the degenerative cascade, leading to a better understanding of the transformation of a healthy level in the spine to a stenotic level with spondylolisthesis and instability.
Biomechanical Changes
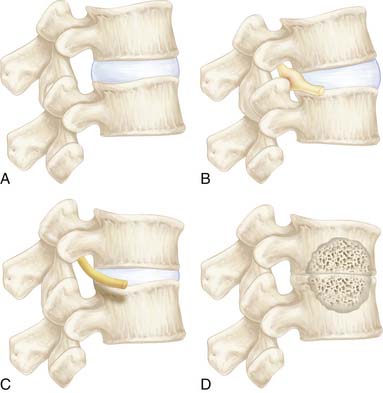
The theory of the three joint complex, and the interdependence of these elements, was recognized and described by Farfan and co-workers.7 This interdependence and sequence of degeneration is outlined in Figure 4-1. Furthermore, the increased risk of the lower two levels for degeneration, secondary to their increased lordotic shape of the disc as well as their increased vulnerability to rotational injuries due to the exaggerated obliquity of their facet joints, was recognized. The two mechanisms of propagation of degeneration that were described consisted of a minor rotational injury causing facet injuries and annular tears and a repetitive compressive injury causing minor damage to the cartilage plate, which would serve as an early stimulus for progressive disc degeneration over time. Additionally, it was postulated that the abnormal stresses of a degenerated segment will affect the adjacent levels. The biochemical changes are accompanied and potentiated by biomechanical factors. The healthy disc has hydrostatic properties that allow the nucleus to convert axial compressive forces to tensile strain on the annular fibers as well as evenly share the load over the endplates. The oblique arrangement of the crossing collagen fibrils in the annulus allow it to convert the axial loads to tensile strains. In fact, the annulus is largely made of type I collagen which provides the tensile strength seen in tendons, whereas the nucleus is largely made of type II collagen. In the degenerative cascade, loss of hydrostatic properties occurs in the annulus and nucleus, and the osmotic pressure of the disc decreases, allowing an increase in creep by a factor of two. The disc loses its ability to imbibe water and to evenly distribute the loads that it is under. This is due to changes in the molecular meshwork of the proteoglycan collagens. Annular fissures occur, and, as a result of repetitive trauma, coalesce together and become radial tears. Radial tears render the disc even more incompetent. Such factors, particularly when potentiated by biochemical changes, cause resorption of disc material, and facilitate adjacent endplate sclerosis. Rarely may resorption lead to spontaneous fusion of the disc. Herniations are generally more likely in the earlier stages of degeneration when the intradiscal pressures are higher than in the more advanced stages. Offending osteophytes, however, are more likely in the more advanced stages of degeneration.
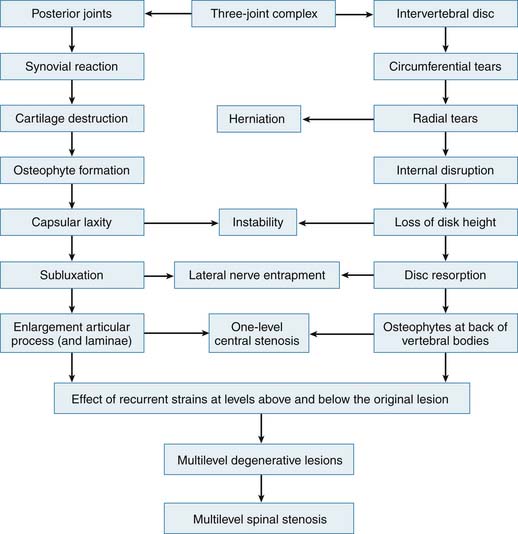
FIGURE 4-1 Overview of the interrelation of disc and posterior element degeneration.
(From Kirkaldy-Willis WH, et al: Pathology and pathogenesis of lumbar spondylosis and stenosis, Spine 3:320, 1978.)
The medial and anterior facet joint capsules are made of approximately 80% elastin and 20% collagen. Degeneration starts by a synovial inflammatory response and fibrillation of the articular cartilage of the joint. This progresses to gross irregularity of the articular cartilage and formation of osteophytes. Eventually, one of the articular processes may fracture and become a loose body as well as contribute to capsular laxity, which will allow excessive motion of the joint and instability. The facet and discchanges cause mechanical incompetence of a motion segment and may lead to abnormal sagittal translation, further compromising the neural elements (Figure 4-2). Compensatory posturing is observed in the elderly with spinal stenosis as a forward flexed posture in an attempt to put the spine into flexion and increase the space available for the neural elements. This posturing will offload the degenerated facets and potentially decrease facet pain as well.
The Three Stages of Instability
The theory of biomechanical degenerative instability was described by Kirkaldy-Willis and Farfan in 1982.8 They defined instability as a clinical entity when the patient changes from mild symptoms to severe symptoms acutely with minimal activity or provocation. This was explained as abnormal joint deformation with stress, which produces a symptomatic reaction in the affected area, hence causing pain. The factors that affect such instability are primarily the increased motion of the joint and, secondarily, the physical changes that occur within the joint with repetitive trauma. They divided the clinical symptoms into three phases. First, a stage of temporary dysfunction, second, an unstable phase, and lastly, a stabilization phase. In the temporary dysfunction stage, the increased abnormal motion may actually manifest itself as decreased overall motion secondary to acute inflammation, muscle spasm, or guarding. The spinous processes may be held in midline or to one side secondary to spasm and hence limit lateral bending and rotation. Vertebral tilting and rotation are coupled in the spine and produce lateral bending. Abnormal excursion of the facets may be seen on lateral flexion and extension radiographs. Generally, significant abnormal shear or translation does not occur if there is a healthy disc present. In the second stage, the changes become more constant and long-lasting, yet the spine still has increased motion present. As stage two progresses the changes become more irreversible. Stage three is accompanied by advanced degeneration and loss of disc height as well as the presence of stabilizing osteophytes. This stage is generally more stable and less prone to instability. Some of the key clinical findings of each stage are summarized in Table 4-1.
TABLE 4-1 Clinical observations seen in the Kirkaldy-Willis classification stages of spinal degeneration

In this context, injury is defined as any force that is too great for the joint to withstand. Such forces do not necessarily have to be from a significant traumatic episode or from lifting a heavy object, but simply from uncoordinated muscle activity supporting the patient’s body weight. Injury can cause trauma to the articular surface and capsule of the facets, as well as to the endplates and disc annulus. . However, much larger external trauma is required for injury to the other ligamentous tissues and muscles. Facet joint articular surface injuries will start with fibrillation and progress to erosion and eburnation. Finally, subchondral fractures can lead to complete fractures and loose bodies as alluded to earlier in this chapter. By the same token, the synovial membrane will thicken through this inflammatory process and develop an effusion, which can become exudative and create fibrosis. If capsular tears occur, they may cause initial instability. Recovery with minor trauma is usually complete, though it can lead to a more prolonged vulnerable (unstable) phase.
Clinical Instability and Diagnostic Imaging
Careful attention to x-rays can identify signs of instability, such as McNab traction spurs, which occur below the rims of the endplates, or the presence of gas in the disc space, sometimes referred to as Knutsson sign. Lateral flexion/extension x-rays can help identify instability by revealing a dynamic spondylolisthesis or retrolisthesis. Such malalignments can cause narrowing of the neural foramen, especially in the presence of decreased discspace height. If flexion/extension radiographs demonstrate an exaggerated increase in posterior heights of the disc along with decreased anterior height of the disc of one level in comparison to the other levels, this may also be a sign of instability. This finding is sometimes referred to as “rockering.” Less commonly evaluated radiographic modalities include anterior/posterior side bending films, which may demonstrate asymmetric tilting of the vertebral body, or decreased bending to one side (which stems from decreased tilt and rotation in a coupled fashion) with a paradoxical increase in disc height on the side to which the patient is bending. Exaggerated closure of the disc on the ipsilateral side as the bending can also occur. Lateral listhesis is due to abnormal rotation of the vertebral body during side bending, which is yet another sign of instability. Spinous process malalignment and pedicle asymmetry are important to be noted on the AP films as well. CT scanning a patient while rotated to the left and right side (with similar positioning to that of Judet views) can show gapping of the facet joint on the side opposite to the rotation of the vertebral body. This causes the superior articulating process to shift anteriorly and narrow the lateral recess on the ipsilateral side as the gapping. Such a finding can be consistent with dynamic nerve entrapment in the lateral recess.
Conclusion
In summation, we have to compile the degenerative changes of each of the different parts of the spine, and apply them to the theory of the interrelated three-joint (tripod) complex. Injury to one part of the spine can cause abnormal motion and load transfers, and hence affect the other parts of the spine over time. Loss of disc height causes the posterior facets to sublux and the superior articular process of the level below to migrate upward and anteriorly, hence narrowing the lateral recess and possibly impinging on the traversing root. This is especially true when there is concomitant hypertrophy of the superior articular process. Depending on the amount of loss of disc height, the neural foramen can be narrowed as well and cause exiting root impingement. If the initial injury was asymmetric with respect to one facet joint, then that facet can degenerate, hypertrophy, stretch the capsule, and become more lax than the other side. In such a case scenario, a rotational deformity begins to occur which can simultaneously cause eccentric bulging of the disc due to its rotational instability, and cause unilateral lateral recess stenosis. Experimental work supports the concept that abnormal motion at one level causes nonphysiologic strains at the adjacent levels which can lead to multi-level involvement. This can explain why degeneration is typically seen in multiple adjacent levels of the spine in different stages of the cascade (Figure 4-3). Posterior element laxity and increased motion can exert additional forces on an already partially degenerated disc, render the segment incompetent to physiologic loads, and cause a degenerative spondylolisthesis. Certainly the reverse order of events can occur as well, possibly more often. When formulating a surgical treatment plan for a patient, it is of paramount importance to diagnose which of the stages of instability best fits the patient’s spine at the time of treatment (Figure 4-4). Most stage I and early stage II will respond to conservative treatment. However, decompression alone for late stage II can lead to further instability and may be better accompanied by a fusion. Stage III, on the other hand, may best be treated with decompression alone without fusion.
1. Lees F., Turner J.W. Natural history and prognosis of cervical spondylosis. BMJ. 1963;2:1607-1610.
2. Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
3. Peng B., Hao J., Hou S., et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31:560-566.
4. Freemont A.J., Peacock T.E., Goupille P., et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
5. Urban J.P., Smith S., Fairbank J.C. Nutrition of the intervertebral disc. Spine. 2004;29:2700-2709.
6. Kirkaldy-Willis W.H., et al. Pathology and pathogenesis of spondylosis and stenosis. Spine. 1978;3:319-328.
7. Farfan H.F. Effects of torsion on the intervertebral disc lesions. Can J Surg. 1969;12:336.
8. Kirkaldy-Willis W.H., Farfan H.F. Instability of the lumbar spine. Clin. Orthop. Relat. Res. 165. 1982:110-123.