Chapter 32 Nasopharyngeal Carcinoma
Etiology and Epidemiology
Nasopharyngeal carcinoma is an uncommon tumor in the United States, where the incidence is approximately 0.2 to 0.5 cases per 100,000 people.2 In comparison, the incidence is considerably greater in southern China and Hong Kong (25 to 50 cases per 100,000 people) and among the Inuit residing in Alaska and Greenland (15 to 20 cases per 100,000 people). The incidence of nasopharyngeal cancer is also higher in other parts of Southeast Asia (Taiwan, Vietnam, and Thailand), the Philippines, and Malaysia and in some Mediterranean and North African populations (8 to 12 per 100,000 people). These areas of increased incidence of nasopharyngeal cancer are considered endemic areas, and the incidence outside endemic areas is much lower and more often associated with tobacco use.
Several geographic-specific etiologic factors have been implicated in the incidence variations throughout the world. The high consumption of salted fish in southern China and Hong Kong has been implicated as a possible etiologic factor for nasopharyngeal carcinoma in these areas.3,4 It has been suggested that various macromolecular lignins associated with these foods in southern China (and perhaps other areas such as Alaska) may activate EBV,5 which has also been identified as a probable etiologic factor in nasopharyngeal carcinoma.6,7 Studies by Bouvier and colleagues5 involved the fractionation of harissa, a homemade spice mixture used in Tunisia on various foods, including salted fish. Harissa was separated into various macromolecular fractions by column chromatography. The lignin-containing complexes extracted from harissa induced the EBV promoter in Raji cells.5 The possibility that these environmental factors are important in the etiology of nasopharyngeal carcinomas is further supported by the finding that incidence rates decrease for successive generations of people who originally emigrated from southern China to California.8
Other potential environmental etiologic factors that have been associated with nasopharyngeal carcinoma include alcohol consumption and exposure to dust, fumes, formaldehyde, and cigarette smoke.9,10 Cigarette smoke and alcohol have long been associated with many other head and neck carcinomas, but their association with nasopharyngeal carcinoma has been controversial. Some studies have suggested that alcohol consumption and cigarette usage were not associated with nasopharyngeal carcinoma.9–11 Nam and associates10 conducted a case-control study using a National Mortality Follow-Back Survey based on death certificates and found that cigarette smoking and alcohol consumption are independent statistically significant risk factors for nasopharyngeal carcinoma. The increased risk of nasopharyngeal carcinoma with heavy smoking (adjusted for alcohol consumption) was threefold, and an excess risk of 80% was demonstrated for heavy alcohol consumption (adjusted for cigarette usage).
Prevention and Early Detection
The nasopharynx cannot be visualized externally, and tumors of this area often present after they have eroded into a vital structure and produced a presenting symptom. The association of EBV and nasopharyngeal cancer has led some investigators to hypothesize that serologic screening (EBV titer) may be useful in certain high-risk populations to identify groups of patients who might benefit from frequent nasopharyngeal examinations.12 In a study from China of 338,868 patients who underwent serologic screening for EBV titer, 9367 persons had immunoglobulin (Ig) A antibodies to EBV. Of these 9367 patients, 306 were positive for IgA to EBV early antigen. Nasopharyngeal cancer was detected in 113 of the 9367 patients (1.2%) and in 63 of the 306 (20.5%) who were positive for IgA to EBV early antigen. Most of the tumors (>85%) were early-stage lesions. Serologic EBV IgA screening is currently being used in endemic areas.
In addition to EBV serologic screening as a means of early detection and possible prevention of advanced-stage disease, several environmental and genetic predisposing factors are being explored as possible markers that could identify groups of patients at high risk for nasopharyngeal cancer.13 Real-time polymerase chain reaction (PCR) techniques show promise as screening tools for nasopharyngeal cancer.14 EBV DNA detection, in particular detection of EBV nuclear antigen, eliminates many false-positive results and improves the sensitivity and specificity of EBV IgA serologic screening.15 A retrospective analysis of blood samples from patients with disease relapse from previous clinical trials indicates that EBV nuclear antigen screening by PCR would have led to earlier detection of distant failure but, because of the quality of current imaging, would not have significantly affected detection of local recurrences.16 Real-time PCR screening for EBV latent membrane protein 1 (LMP1) in nasopharyngeal swabs has also been shown to be a promising screening tool for high-risk populations, with a sensitivity of 87% and specificity of 98%.17 These techniques are likely to replace serum IgA screening in endemic areas and have a promising role in screening for relapse in EBV-positive patients.18 The further study of other environmental and genetic markers may lead to the identification of patient populations that could benefit from screening or chemoprevention.13
Chemoprevention has been tested extensively in patients with a prior diagnosis of head or neck malignant tumors because second primary tumors in the respiratory and digestive tracts are common in that population.19–22 Hong and others19,20 conducted a prevention trial for patients with head and neck carcinoma after curative surgery or radiotherapy, or both. Patients were randomly assigned to receive 1 year of 13-cis-retinoic acid (isotretinoin) as a chemoprevention agent (see Khuri and associates13 for a discussion of the biologic factors) or placebo. The treated group had significantly fewer second primary tumors (4%) than the placebo group (24%) (p = .005); however, there was no difference in rates of survival or incidence of relapse between the two groups. In addition, the chemopreventive effect appeared to abate after 3 years; the incidence of second primary tumors was similar in the two groups after 3 years. Acute toxicity was significant.19
The results of the trial by Hong and colleagues19 were not confirmed by a controlled trial of 316 patients by Bolla and associates,21 in which patients were randomly assigned to 2 years of etretinate (a retinoic acid similar to isotretinoin) at a maintenance dosage of 25 mg/day. Subsequently, a large intergroup effort was mounted, and 1302 patients with previously treated head and neck malignant tumors were randomly assigned to receive 3 years of isotretinoin or placebo.22 Because of unacceptable toxicity seen with higher doses of isotretinoin (100 to 200 mg/m2 [≈150 to 400 mg total]) in prior studies, the dosage was decreased to 30 mg/day in this trial. No difference was seen in the occurrence of second primary tumors (4.6% in both arms), which was the primary endpoint of the study.23 A decrease in local recurrence was seen, however, and 13-cis-retinoic acid remains a topic of investigation.
The issue of whether retinoids or other chemopreventive agents should be used in the setting of premalignant lesions of the oral cavity has been considered, and many trials have suggested that retinoids can induce considerable responses in oral leukoplakia.24,25 Toxicity has been a limiting issue in these trials, however. It will be important to determine whether these chemoprevention strategies, developed for leukoplakia, can be extrapolated to prevention of nasopharyngeal cancer in subgroups of patients at high risk for such malignant tumors.
Blockade of cyclooxygenase-2 (COX-2) has also shown some promise as a chemopreventive strategy, and COX-2 inhibitors were recently the topic of investigation for various malignant tumors, including head and neck cancers.26 A phase II study at Fox Chase Cancer Center evaluated a 3-month course of celecoxib in patients with biopsy-proven dysplastic or hyperplastic leukoplakia, with results pending (NCT00101335). In another pilot randomized study of celecoxib in oral premalignant lesions conducted by the M.D. Anderson Cancer Center (MDACC), doses of 100 mg and 200 mg twice daily were shown to be ineffective in controlling these lesions.27 Most recent COX-2 inhibitor cancer prevention trials have been closed, except in very high risk settings such as familial polyposis, because of the increased rate of cardiovascular events seen in a few of the celecoxib and rofecoxib prevention trials.
Pathology
Malignant tumors of the nasopharyngeal area are generally carcinomas (90%), with lymphomas making up approximately 5% of lesions.28 Histologic classification of carcinomas continues to evolve as we find out more about the biologic characteristics of these tumors. The most current pathologic classification of nasopharyngeal carcinomas by the World Health Organization (WHO) includes three major categories: keratinizing squamous cell carcinoma (formerly, WHO type I), nonkeratinizing squamous cell carcinoma (including differentiated, or former WHO type II lesions, and undifferentiated, or former WHO type III lesions), as well as recently described basaloid squamous cell carcinoma.29
On histologic testing, keratinizing squamous cell carcinomas appear to be well differentiated with intercellular bridges. These lesions make up approximately 20% of carcinomas.29,30 Differentiated nonkeratinizing carcinomas, representing 30% to 40% of nasopharyngeal carcinomas, lack well-defined squamous cell characteristics but continue to show a “pavement stone pattern” characteristic of squamous cell histology.29 Undifferentiated nonkeratinizing carcinomas, comprising 40% to 50% of carcinomas, also formerly known as lymphoepitheliomas, are characterized by a lymphoplasmacytic infiltrate.29 On cytologic testing, the cells appear uniform, with round to oval nuclei and prominent nucleoli. The frequency of the different histologic types varies with geographic area. In the United States, the majority of nasopharyngeal carcinomas have keratinizing squamous cell histologic findings, whereas in Asia, the undifferentiated histologic type is by far the most common (Table 32-1).
Anatomy and Pathways of Spread
The nasopharynx is a musculofascial tube that connects the nasal cavity and oropharynx. The anatomic borders of the nasopharynx are (1) the anterior border, consisting of the posterior nasal apertures and nasal septum; (2) the posterior border, the pharyngeal mucosa; (3) the superior border, the pharyngeal mucosa and body of the sphenoid; and (4) the inferior border, the oropharynx. The lateral wall of the nasopharynx contains the pharyngeal opening of the auditory tube. The medial cartilaginous extension of the auditory tube forms a protrusion from the lateral wall of the nasopharynx at the superior and posterior aspects of the opening of the auditory tube. This protrusion, with its overlying mucosa, creates the torus tubarius. Just posterior to the torus tubarius lies the pharyngeal fossa, or the fossa of Rosenmüller, which is formed by the junction of the lateral and posterior walls of the nasopharynx (Fig. 32-1A to D).
The sensory innervation is shown in Figure 32-1D. The maxillary division of the trigeminal nerve supplies the upper nasopharynx, the posterior part of the nasal cavity, and most of the palate and upper gums of the oral cavity. The general sensory branches of the lingual and pharyngeal branches of the glossopharyngeal nerve supply the sensory innervation of the lower part of the nasopharynx, the posterior third of the tongue, and part of the soft palate and oropharynx.
Understanding the anatomic relationships of the nasopharynx and adjacent structures is important to correctly diagnose patients with nasopharyngeal carcinoma. The importance of superior extension into the sphenoid bone of the base of the skull is exemplified by the fact that cranial nerves can be involved at presentation. The frequency of involvement of cranial nerves has been described in two large series.31,32 Cranial nerves V and VI are the most commonly involved. These nerves traverse the sphenoid bone and can be involved when tumors erode superiorly through bone. Tumors of the nasopharynx can also gain access to the cranial nerves of the base of the skull by eroding superiorly through the foramen lacerum, which is bordered superiorly by the internal carotid artery (Fig. 32-1B).
Lateral extension of nasopharyngeal carcinomas can lead to erosion of the medial opening of the auditory tube and the medial pterygoid plate and can involve cranial nerves IX, X, XI, and XII (Fig. 32-1B and C). Lateral extension can lead to involvement of the carotid artery and internal jugular vein.
Lymph node involvement is common; 65% to 80% of patients present with clinically involved cervical neck nodes. The level VA and level II lymph nodes are commonly involved. The recently published frequency of involvement of these areas in two large series31,32 is presented later in the Clinical Manifestations section.
Biologic Characteristics and Molecular Biology
The potential etiologic link between EBV and nasopharyngeal carcinoma was first described more than 30 years ago6; however, recent advances in molecular biology have shed further light on this association. Earlier work focused on the serologic response to EBV and showed elevated levels of IgA and IgG antibodies to the viral capsid antigen and a replication protein called early antigen in patients with nasopharyngeal carcinoma. Other studies have demonstrated the presence of EBV DNA and encoded proteins directly in the nasopharyngeal carcinoma tissue samples.33 Latent infection of cells with EBV, initially studied in B lymphocytes, is associated with regulated expression of several viral genes, including latent membrane proteins (LMPs) 1, 2A, and 2B, six EBV nuclear antigens (EBNAs) 1, 2, 3A to C, and LP, and two small noncoding nuclear RNAs (EBERs).34 Some of the molecular mechanisms by which expression of these genes can lead to transformation have been elucidated. For example, LMP1 induces expression of epidermal growth factor receptor (EGFR) and may therefore influence cellular growth. A restricted pattern of these EBV antigens is present in nasopharyngeal cancer cells, which may in part explain why immunotherapy directed against these antigens has been more successful in other EBV-associated diseases.35 Allogeneic cytotoxic T cells to various EBV antigens have been developed and show some promise for treating locally recurrent nasopharyngeal carcinoma.36
Several studies have suggested that serologic responses to EBV may be more specific for the nonkeratinizing carcinoma (former WHO types II and III).37 This, however, was not confirmed by another group.38 Using the Southern blot technique to detect the viral DNA, Raab-Traub and others39 showed the presence of EBV in all former histologic variants of nasopharyngeal carcinoma, but the EBV copy number was lowest in keratinizing squamous cell carcinomas. The presence of EBV in keratinizing squamous cell carcinoma has been consistently detected by other groups.40–43 The differences in the rate of EBV detection by different investigators may stem from variability in the sensitivity of different assays. Alternatively, it may reflect differences in the sampled populations. The detection rate of EBV infection in keratinizing squamous cell nasopharyngeal carcinoma is highest in endemic areas.
Understanding the molecular basis of EBV-induced carcinogenesis and the role of the immune system response in this process will likely have a direct bearing on clinical practice. An association between decreasing antibody titers and response to therapy suggested that antibody titers could be used to assess a patient’s prognosis.44 Quantification of EBV DNA levels in pretreatment and postradiotherapy plasma samples from patients in Hong Kong has been shown to correlate with outcome,45 and, therefore, could prove to be a useful tool in risk stratification and planning of therapeutic interventions in high-risk areas.
In addition to environmental factors, the potential association of various genetic factors and nasopharyngeal cancer is a topic of current investigation. Earlier work implicated a disease susceptibility gene linked to the human leukocyte antigen (HLA) region, which has been associated with the increased incidence of nasopharyngeal carcinoma in southern China.46 Simons and associates47 originally described an association in Chinese patients with nasopharyngeal carcinoma and the HLA-A2 antigen with a deficit of the second antigen at the second locus (B locus). Later, Simons and co-workers48 reported on 110 Chinese patients in Singapore with nasopharyngeal carcinoma and hypothesized that the new B-locus antigen (Sin 2) may be associated with the tumor. It remains unclear whether specific HLA alleles may influence development of nasopharyngeal carcinoma directly, possibly by affecting the immune reaction to EBV, or whether, and perhaps more likely, the genetic susceptibility to nasopharyngeal carcinoma is encoded by a locus in close linkage disequilibrium. Various other genetic and epigenetic alterations have been reported in nasopharyngeal carcinoma. Deletions of the short arm of chromosome 3 (3p25, 3p14)29 and of chromosome 9 (9p21-22)49 are the most common cytogenetic changes. Candidate genes in these and other locations of interest are being identified. These findings may lead to a greater understanding of the genetic basis of nasopharyngeal carcinomas and the interplay of environmental factors with these genetic factors.
Clinical Manifestations, Patient Evaluation, and Staging
As mentioned, nasopharyngeal carcinoma is rare in the United States and, therefore, is not often suspected as a possible cause of a patient’s early symptoms. In addition, the list of possible early symptoms of nasopharyngeal carcinomas includes many symptoms that could have more common causes. In a series of 378 patients from the MDACC,32 the presenting symptoms included a neck mass in 41%; hearing loss, ear drainage, or otalgia in 27%; nasal bleeding or obstruction in 21%; cranial nerve deficits in 8%; and other nonspecific symptoms in 8%. Investigators from Washington University noted that the typical presentation involved multiple symptoms.31 In this series of 143 patients, presenting symptoms included otitis in 43%, throat pain in 39%, nasal obstruction in 37%, a neck mass in 35%, nasal bleeding in 29%, cranial nerve involvement in 24%, and trismus or other symptoms in 5%.
Similar to these data, most series have shown that neck masses, obstructive ear symptoms, and cranial nerve deficits are common presenting symptoms for nasopharyngeal carcinoma. In the Washington University series,31 66% of patients were found to have ipsilateral neck masses and 28% had contralateral neck masses on examination, although only 35% of patients had reported a neck mass as the reason for seeking medical advice. Of the patients with clinically involved neck disease, 60% had enlarged ipsilateral level II lymph nodes and 32% had enlarged ipsilateral level V lymph nodes. The most frequently involved cranial nerves in the series were cranial nerve VI in 15% of patients, V in 7.7%, and VIII, X, and XII in 5.6% of patients each.
In the series from the MDACC,32 the level VA lymph nodes were most commonly enlarged (54% of patients), followed by the level II nodes (49% of patients). The level III and lower level VA/upper level VB groups were involved in 24% and 22% of patients, respectively. The level IV lymph nodes, level VB lymph nodes, and supraclavicular lymph nodes were involved in 10%, 13%, and 10% of patients, respectively. Similar to the series from Washington University, cranial nerve VI was the most frequently involved nerve (6% of cases).
Patient Evaluation
The initial evaluation of patients with nasopharyngeal carcinoma should include a history and physical examination, with special attention to the level of nodal involvement (if any) (Fig. 32-2). It is helpful to diagram or digitally photograph the clinically involved lymph nodes in the neck because it can be helpful when the radiotherapy boost is considered.
CT and MRI of the head and neck are useful in the evaluation of both erosion of tumor into the bony structures of the base of the skull and retropharyngeal and cervical lymphadenopathy. Although the same information is often provided by CT and MRI, some investigations have suggested that MRI may be more useful in delineating soft tissue invasion outside the nasopharynx and the extent of retropharyngeal lymph node involvement,50 whereas CT may be most useful in delineating skull base erosion.51,52,53 Given the level of anatomic information required for the current T staging system, MRI is becoming the imaging study of choice in the evaluation of nasopharyngeal tumors.
Positron emission tomography (PET) can be useful for staging54,55 as well as for post-therapy management where other imaging is unclear. PET/CT, which provides additional anatomic information, is more commonly used now and may be helpful for radiotherapy planning.
The completion of the diagnostic evaluation involves acquiring routine complete blood counts, a chemistry panel, and a chest radiograph (see Fig. 32-2). Further evaluation of possible metastases should be done on the basis of the clinical presentation of the patient.
Staging
After a pathologic assessment has been made, the workup or evaluation leads to a clinical stage (Table 32-2). Clinical staging has been a matter of controversy, and the prognostic significance of the various TNM staging criteria remains under investigation. Several staging systems have been in use throughout the world. The most commonly used systems in North America and Europe are those of the American Joint Committee on Cancer (AJCC)56 and the International Union Against Cancer (Union Internationale Contre le Cancer [UICC]),57 which have become essentially analogous. In Asia, where nasopharyngeal carcinoma is endemic, the Ho58 classification system was initially developed in Hong Kong. In addition, in 1992, Chinese physicians adopted an independent system similar to the Ho system.59 All four systems, shown in Table 32-2, have limitations and continue to evolve, with researchers often drawing on each others’ experience. Updated versions of the Chinese system and the AJCC system were released in 200860,61 and 2010,62 respectively. The AJCC/UICC remains the most commonly used system in the English literature.
A well-recognized advantage of the Ho classification system lies in its approach to lymph node metastasis, namely, use of lymph node location in N-stage assignment and a more even distribution of nodal disease in stage grouping58,63 (see Table 32-2). Nodal involvement appears in stage II, and supraclavicular nodal metastasis, which is known to carry a worse prognosis, is represented in a separate stage IV category (see Table 32-2). The 1992 AJCC system used lymph node size and laterality in N-stage assignment, both of which carry some prognostic significance. However, only some patients with N1 disease were included in stage III, and most patients with nodal involvement were grouped into stage IV, together with patients with metastatic disease. Modifications to the AJCC classification implemented in 199764 and maintained in 200265 and 201062 have incorporated lymph node location into N-category assignment, designating involvement of the supraclavicular fossa, as originally defined by the Ho system, as N3 disease, and have placed all N3M0 lesions in a separate stage IVA grouping. In addition, stage groupings were changed to provide a more even stage distribution. These changes improved risk stratification of the 1997 AJCC classification in comparison to both the prior version of the AJCC system and the 1978 Ho system.66,67
Important changes to the most recent AJCC system targeted T-category assignment. Invasion into the soft tissues of the nasopharynx was used in the 1997 and 2002 AJCC classification to segregate T1 and T2 lesions, but studies have shown that this carries no prognostic significance.68–70 The parapharyngeal involvement used to segregate T2a and T2b lesions does,71,72,73 however, and therefore the new 2010 AJCC system segregates lesions with parapharyngeal involvement into the T2 subgroup, whereas all tumors either confined to the nasopharynx or extending into surrounding subsites but without parapharyngeal involvement are now in the T1 subgroup. Furthermore, cranial nerve involvement carries a significantly worse prognosis than base-of-skull involvement,31,69,74–76 but both were aspects of the T4 subgroup in the 1997 and 2002 AJCC systems and of the T3 subgroup in the Ho system. In the 2003 and 2010 versions, akin to the 2008 Chinese classification system, base-of-skull involvement has been downstaged to T3. Furthermore, data are emerging that extensive cranial nerve involvement, orbit involvement, and intracranial extension are associated with worse outcomes within the T4 subgroup.67,76 Therefore, some of the proposed modifications to the AJCC system include a separation of the T4 subgroup into two categories. T4a would include tumors with involvement of the masticator space (infratemporal fossae), asymptomatic radiographic cranial nerve involvement, and involvement of the hypopharynx. T4b would include tumors with intracranial extension, orbital involvement, and symptomatic cranial nerve palsies. However, more data need to be collected to validate this proposed modification.
With respect to N-category classification, the seventh edition of the AJCC staging manual for the first time includes disease that has spread to the retropharyngeal lymph nodes in staging, placing it in the N1 category. On MRI evaluation, these nodes are involved in 83% of patients with nasopharyngeal carcinoma compared with 74% involvement of the level II to IV nodes, and are considered to be the first echelon of nodal spread.77 Therefore, their inclusion into the staging system is expected to improve the prognostic accuracy.78,79
Primary Therapy
Traditionally, the nasopharyngeal area has not been easy to examine without fiberoptic technology and has been difficult to approach surgically. Surgical exposure of the area and resection of tumors with adequate tumor margins have long been challenging.80 For these reasons, primary surgical intervention fell out of favor in the 1950s; primary treatment has generally consisted of radiotherapy alone and, more recently, radiotherapy plus concurrent chemotherapy.
Single-Modality Therapy
Although histologic presentations of nasopharyngeal tumors vary throughout the world, with more undifferentiated tumors found in southern China and Hong Kong, the 10-year survival rates for patients treated with radiation therapy alone in the United States (MDACC),32 Denmark,81 and Hong Kong82 are similar, at 34%, 37%, and 43%, respectively (see Table 32-1). The rate from the Hong Kong study may be somewhat inflated because many patients had early-stage disease (node-negative disease in 39%) and few patients with the keratinizing histology (0.3%) were present in the series.82 However, elevation of EBV titers has been correlated with a poor prognosis,34 and higher titers would be expected in Hong Kong, an endemic area. The North American and European populations have a greater percentage of patients with keratinizing (WHO type I) histologic findings than with nonkeratinizing histologic findings (WHO types II and III), however, and the prognosis for the former has been poorer than for the latter.68 Therefore comparison of groups from various parts of the world is difficult, and our understanding of the role of EBV and histologic classifications is incomplete. Still, some general statements can be made.
The MDACC series elucidated the long-term outcome in a large population of patients treated with radiotherapy alone.32 Using the 1992 AJCC staging system (see Table 32-2), researchers found that advanced T category, squamous histologic typing, and cranial nerve deficits were predictive of poor prognosis for local control in univariate and multivariate analyses. Five-year local control rates for T1, T2, T3, and T4 categories were 93%, 79%, 68%, and 53%, respectively. Similar results were observed in other, smaller series.31,83–86
Combined-Modality Therapy
Irradiation Plus Chemotherapy
The randomized trial of Sanchiz and associates87 served as an introduction for using chemotherapy in nasopharyngeal cancer. However, only a small number of patients with nasopharyngeal cancer were included.
Brizel and co-workers88 reported a small randomized trial comparing twice-daily irradiation (1.25 to 75 Gy) to twice-daily irradiation (1.25 to 70 Gy) with concurrent cisplatin and 5-fluorouracil (5-FU) treatment. This trial included 121 patients with locally advanced (T3 and T4) squamous cell carcinomas of all head and neck sites (including some nasopharyngeal carcinomas). With a median follow-up of 41 months, the locoregional control rate at 3 years was 70% for the combined-modality arm versus 44% for the irradiation alone arm (p = .01). The overall survival (OS) at 3 years was 55% for the combined-modality arm versus 34% for the irradiation alone arm (p = .07). Mucositis appeared to be more prevalent in the combined-modality arm; 45% of patients required feeding tubes compared with 28% in the irradiation alone arm. However, the severe late complications of soft tissue necrosis or osteoradionecrosis were uncommon, with similar numbers in both arms (three patients in the irradiation group and five in the combined-modality group).
Several groups have conducted phase III trials specific to nasopharyngeal primary tumors (instead of a wide array of head and neck cancers). The trial design compared chemotherapy plus irradiation versus irradiation alone* (Table 32-3).
The intergroup study 0099 showed a dramatic improvement in survival rates with combined-modality treatment.1 The trial was closed at a planned interim analysis (October, 1995) after review of the preliminary results by the Data Safety and Monitoring Committee for the Southwest Oncology Group. The results are shown in Table 32-3. Patients treated with standard irradiation received 70 Gy to gross disease in daily fractions of 1.8 to 2 Gy. Patients enrolled in the combined-modality arm received 100 mg/m2 of cisplatin on days 1, 22, and 43 of irradiation, and cisplatin (80 mg/m2) and 5-FU (1000 mg/m2) on days 1 to 4 every 3 weeks for three courses after irradiation. A marked statistically significant improvement in both disease-free survival (DFS) and OS in the combined-modality arm was documented at both initial analysis and in the final update of the trial published in 2001.93 The 5-year OS for patients with the keratinizing squamous cell carcinoma histologic type (WHO type I) was 37%; the differentiated nonkeratinizing histologic type (WHO type II), 55%; and the undifferentiated nonkeratinizing histologic type (WHO type III), 60%.
Although the first reported results from a chemoradiation trial conducted in an endemic area were disappointing,91 the recently published final update94 as well as four additional independent trials conducted in Taiwan,89 Hong Kong,92,98 and Singapore96 all confirmed an advantage to cisplatin-based chemoradiation (see Table 32-3). A randomized trial from Hong Kong of concurrent weekly cisplatin chemotherapy (40 mg/m2), without adjuvant or neoadjuvant therapy, versus irradiation demonstrated a significant improvement in progression-free survival (PFS) at a median follow-up of 2.71 years91 and in OS at a median follow-up of 5.5 years (hazard ratio [HR], 0.51; p = .013)94 for a subset of patients with T3 to T4 disease. A trial conducted by Lin and colleagues89 in Taiwan randomized 284 patients to irradiation alone versus irradiation with two cycles of concurrent cisplatin (20 mg/m2/day) and 5-FU (400 mg/m2/day) by continuous infusion given over a course of 4 days. In this trial, combined-modality treatment was associated with a significant survival advantage. The differences in the degree of benefit derived from the combined-modality treatment in these two studies may stem from the different staging systems used to define entry criteria (AJCC system in the Lin study vs. Ho system in the Chan et al study91), different histologic makeup of the tumors (73.2% differentiated nonkeratinizing carcinoma in the Lin study vs. 93.7% undifferentiated carcinoma in the Chan study), or different chemotherapy and/or radiotherapy schedules. The role for concurrent chemotherapy in the management of nasopharyngeal carcinoma has been further supported by two other trials comparing concurrent and adjuvant chemotherapy with irradiation alone92,96 (see Table 32-3).
Many additional trials have been performed exploring combination chemotherapy and radiotherapy for nasopharyngeal carcinoma.59,90,95,99 So far, no benefit was shown to neoadjuvant or adjuvant chemotherapy in any of the randomized trials. Likewise, a recent meta-analysis using combined individual data from eight randomized trials enrolling 1753 patients showed no OS benefit with either neoadjuvant or adjuvant chemotherapy, although a meaningful treatment effect was seen for concurrent chemoradiation.100 However, the question remains whether addition of induction and/or adjuvant chemotherapy to concurrent chemotherapy may provide an additional advantage. Two ongoing trials are aimed at answering that question. A trial by the Nasopharyngeal Cancer Study Group of Hong Kong is designed to compare induction versus adjuvant chemotherapy with cisplatin and 5-FU in combination with concurrent chemoradiation (NCT00379262). A multicenter phase III trial opened by the Taiwan National Health Research Institutes is designed to compare a cisplatin-based multiagent induction regimen (mitomycin, epirubicin, cisplatin, 5-FU, and leucovorin) followed by concurrent chemoradiation with chemoradiation alone (NCT00201396). Both studies are expected to be completed by 2013.
In addition to evaluating the best sequencing for chemotherapy and irradiation, investigators are also examining the role for other chemotherapeutic agents in treatment of nasopharyngeal carcinoma. A recent study explored the efficacy of weekly carboplatin in the setting of concurrent chemoradiation for patients with locally advanced disease.101 At a median time of 26 months, OS and DFS were similar in the cisplatin and carboplatin groups, with less toxicity noted in the latter group. Therefore treatment with carboplatin is reasonable when use of cisplatin is precluded by patient comorbidities.
Setting the precedent, several landmark randomized controlled phase III studies have demonstrated that taxanes in the neoadjuvant setting greatly improve outcomes in other head and neck carcinomas.102,103 Phase II studies of patients with locally advanced nasopharyngeal cancer have already shown favorable results with paclitaxel, both in the neoadjuvant setting followed by concurrent therapy with platins or as a part of a concurrent regimen.104–106 To further address this question, a phase III trial was opened in 2009 by the international Groupe Oncologie Radiotherapie Tete et Cou (GORTEC), randomizing patients with nasopharyngeal carcinoma to receive induction chemotherapy with docetaxel, cisplatin, and 5-FU followed by concurrent chemoradiation with cisplatin or cisplatin-based concurrent chemoradiation alone (NCT00828386).
Molecular therapies are also under investigation. Although one trial showed a benefit to the use of cetuximab in other head and neck cancers,107 it remains to be seen whether these data will be confirmed for squamous cell carcinomas of the head and neck in general108 or for nasopharyngeal carcinoma in particular.
Altered versus Standard Fractionation with or without Chemotherapy
A large randomized trial by Sanchiz and colleagues87 addressing the role of twice-daily versus once-daily irradiation for head and neck cancer included tumors of the nasopharynx. In this study, 892 patients (852 evaluable patients) with advanced head and neck cancers (T3-4N0-3M0 by UICC staging), including 92 patients with nasopharyngeal cancer, were randomly assigned to once-daily irradiation (group A), twice-daily irradiation (group B), and once-daily irradiation with 5-FU (group C). No differences were seen in median duration of response or OS between groups B and C; however, when either group B or C was compared individually with group A, there were significant improvements in both measures.
An accelerated fractionation regimen using six 2-Gy fractions per week to a total dose of 66 to 68 Gy was compared with a conventional fractionation regimen to the same total dose in a large randomized Danish study of 1476 patients with head and neck cancer (435 patients with pharyngeal tumors, including nasopharyngeal tumors).109 In this trial, accelerated fractionation resulted in significantly improved locoregional control and disease-specific survival (DSS) but not OS.
The same fractionation scheme, either with or without concurrent chemotherapy, was evaluated in a trial from the Hong Kong Nasopharyngeal Study Group of 189 patients with advanced nasopharyngeal cancer.97 Patients were randomly assigned to one of four arms: (1) conventional fractionation alone, (2) accelerated fractionation alone, (3) conventional fractionation with concurrent cisplatin and 5-FU, and (4) accelerated fractionation with concurrent cisplatin and 5-FU. At 2.9 years of follow-up, accelerated fractionation with concurrent chemotherapy (arm 4) achieved the most substantial tumor control, with PFS of 97% versus 70% (arm 1), 63% (arm 2), and 74% (arm 3), without an improvement in OS. A significant increase in acute and late toxicity in the combined chemotherapy–accelerated fractionation arm was also noted.
Based on these and other experiences with altered fractionation schemes in nasopharyngeal carcinoma, as well as other head and neck cancers, it became apparent that both the total dose and overall treatment time were important determinants of outcome.110 With the advent of IMRT, dose escalation (delivery of adequate doses to the gross and subclinical disease) has become possible without concomitant changes in the toxicity profile, significantly improving the therapeutic ratio of concurrent chemoradiation and rendering the above fractionation schemes obsolete.
Intensity-Modulated Radiotherapy with or without Chemotherapy
The proximity of numerous critical structures within the nasopharynx often makes it challenging to provide an adequate dose to an irregularly shaped tumor while still keeping the dose to surrounding tissues within acceptable limits. Therefore the capacity of IMRT to provide highly conformal and precise coverage with sharp dose gradients has been widely embraced in the head and neck cancer community. In addition, the ability to assign different doses to a desired spatial distribution when using IMRT has allowed for simultaneous delivery of different fractionation schemes to different portions of the target. Multiple institutions both in the United States and the endemic regions have reported improved clinical outcomes since adoption of IMRT techniques for treatment of nasopharyngeal carcinoma (Table 32-4).
University of California San Francisco (UCSF) researchers were the first to report their experience with IMRT in 67 patients with nasopharyngeal cancer, including 45 patients with stage III to IV disease, treated between 1995 and 2000.111 Patients were prescribed 59.4 Gy in 1.8-Gy fractions to the planning target volume (PTV) encompassing microscopic and gross disease and typically received 65 to 69.96 Gy to the gross disease and involved lymph nodes in 2.12- to 2.25-Gy fractions. Twenty-six patients also had an intracavitary brachytherapy boost to a total dose of 5 to 7 Gy at the discretion of the treating physician. Patients with advanced disease received concurrent and adjuvant chemotherapy. The reported 4-year estimates of local PFS, locoregional PFS, and OS were 97%, 98%, and 88%, respectively.111 Long-term xerostomia was minimal at 2 years, with 66% of the patients having no xerostomia and no grade 2 or higher late xerostomia.
Locoregional control rates above 90% have since been noted in other single-institution prospective112–114 and retrospective115 series, as well as in a recently published multi-institutional Radiation Therapy Oncology Group (RTOG) 0225 study.116 Similar to the UCSF regimen, the RTOG 0225 trial used a simultaneous integration of the boost, with 2.12 Gy per fraction delivered to the gross disease with a minimal 5-mm margin (PTV70), while 1.8 Gy was delivered to the subclinical PTV (PTV59.4). Therefore, in 33 fractions, the PTV70 received a total dose of 69.96 Gy and the PTV59.4 received 59.4 Gy. A total dose of 50.4 Gy in 28 fractions was delivered to the supraclavicular nodes. After a median follow-up of 2.6 years, the estimated 2-year local PFS, locoregional PFS, and OS rates were 92.6%, 89.3%, and 80.2%, respectively. Importantly, while providing similar or better locoregional tumor control in comparison with conventional irradiation techniques, IMRT has resulted in significantly lower rates of late complications. Only nine patients (13.5%) had grade 2 xerostomia at 1 year from the start of IMRT, two patients had grade 3 xerostomia, and none had grade 4 xerostomia. The improved toxicity profile of IMRT compared with conformal radiotherapy in the treatment of nasopharyngeal carcinoma has been since confirmed in two prospective randomized trials.117,118
Locally Recurrent or Persistent Disease
Surgical Management
Surgical resection is the preferred treatment for persistent or recurrent lymph node involvement in the neck. However, surgical management of recurrent nasopharyngeal cancer at the primary site is hampered by the same factors that make surgery at this site difficult as a primary treatment, such as difficulties in obtaining adequate exposure and obtaining adequate surgical margins.80,119,120,121 Salvage nasopharyngectomy has been achieved by several different approaches, the most common of which are the transpalatal, transmandibular, and transmaxillary approaches. Recently, minimally invasive techniques, such as a transnasal approach and an endoscopic approach, have also been employed successfully in a select group of patients. The choice of surgical approach is dictated by the tumor size and its location. Generally, surgical treatment of recurrent nasopharyngeal carcinoma remains controversial, but it may offer a reasonable disease-free period in certain well-selected cases. Several recent surgical series reported locoregional control and OS of 40% to 72% and 30% to 54%, respectively.122–124,125
Reirradiation
In general, earlier stage at recurrence and longer time to recurrence are associated with better outcomes.126,127,128 Higher doses (≥60 Gy) are more effective than lower doses128 and can be accomplished with EBRT alone or in combination with radiosurgery, or a brachytherapy boost (intracavitary or interstitial treatment).126,128–130,131 IMRT is particularly well suited to this task, and reirradiation results with this method are promising.
At the Memorial Sloan-Kettering Cancer Center (MSKCC), 29 patients with recurrent nasopharyngeal carcinoma were treated with reirradiation between 1996 and 2008, in the era of modern techniques.132 Sixteen patients received EBRT alone to an average dose of 59.4 Gy and 13 received EBRT to 45 Gy followed by intracavitary brachytherapy to 20 Gy. Twenty-four patients, 14 in the EBRT alone group and 10 in the combined-modality group, underwent IMRT. Choice of radiotherapy technique was at the discretion of the treating physician but was often dictated by the size and location of the recurrence. Thus the combined-radiotherapy group was primarily composed of patients with rT1 or T2 disease (85%), while the EBRT group included patients with more advanced disease (31%, rT1 or T2; 69%, rT3 or T4); 93% of the patients received chemotherapy. Five-year local control and OS were 52% and 60%, respectively. Although no significant differences were seen in local control or survival rates between the combined-radiotherapy and EBRT groups, the combined-radiotherapy approach was associated with fewer late complications (8% rate of grade 3 or greater late effects compared with 73% rate in patients receiving EBRT alone).
Stereotactic radiosurgery (SRS), or stereotactic body radiotherapy (SBRT), treatment of recurrent head and neck lesions is another promising approach because the technique allows for rapid fall-off of the radiation dose outside of the tumor volume and nearby critical structures that surround this anatomic area. SRS has been used to treat recurrent nasopharyngeal lesions after prior irradiation, either alone or in combination with EBRT.133–135 When used as the only local treatment modality, it is generally reserved for smaller lesions, and doses of 11 to 35 Gy are used.133,135–137 Recent reports indicate that fractionated SBRT in the reirradiation setting can provide satisfactory control with an improved late toxicity profile.138
Concurrent Chemoradiation
Concurrent chemoradiation is being used increasingly for treatment of recurrent head and neck cancer after primary radiotherapy.139–142 In general, the late morbidity of these regimens is less severe than would be expected, given that reirradiation doses are comparable to the original radiotherapy doses.141,142 These reirradiation trials have usually included few nasopharyngeal cases. Historically, reirradiation without chemotherapy, possibly including brachytherapy, has given better results for nasopharyngeal cases than for other head and neck sites.139 However, given the superiority of chemoradiation over irradiation alone in the treatment of primary nasopharyngeal cancer, it is reasonable to extrapolate these results to recurrent disease. In fact, most of the recent salvage irradiation series have included cisplatin-based chemotherapy for advanced-stage disease (Table 32-5).
Irradiation Techniques and Tolerance
Conventional Irradiation
Treatment Fields
The radiation fields are arranged to cover the possible pathways of spread that account for the previously noted clinical manifestations (Fig. 32-3A to D). Field setup is designed to provide appropriate radiation doses to gross disease and areas at risk for microscopic extension, and this remains the case whether treatment is based on classically defined beam arrangements or on modern techniques such as IMRT. The Intergroup study 00991 (see Table 32-3) provides a structure for general irradiation guidelines with opposed lateral fields. The borders of this field arrangement are described below but can be modified on the basis of individual tumor characteristics as discussed in the subsequent section:
Irradiation Dose
With the intergroup study 00991 as a general guideline for doses delivered when conventional techniques are used, the primary head and neck fields should be treated to a dose of 40 Gy (1.8 to 2 Gy per day) before placement of the spinal cord block (see Fig. 32-3). The treatment then proceeds to 50 Gy, with the newly blocked posterior neck portion of the field treated with electron fields. Electron energies should be determined by CT simulation and treatment planning to allow adequate treatment of the posterior neck with minimal dose to the spinal cord. After 50 Gy, the fields are reduced to include a margin of 1.5 to 2 cm around the primary tumor and any enlarged lymph nodes (>1 cm on CT scan of the neck). This reduced field arrangement is treated to a dose of 66 Gy; consideration can be given for a second reduction around gross disease to a final dose of 70 Gy.
Field Modifications and Boost Techniques
In addition to the opposed lateral field arrangement described above, several other techniques have been used to optimize target dose delivery. For example, patients may initially be treated with opposed lateral fields to the primary site and upper neck, and then continue with a boost to the primary site with either oblique fields, a single central field, or a three-field technique. The use of oblique and smaller opposed lateral fields is generally accepted and has been shown to have acceptable tolerance32 (see Fig. 32-3). Interestingly, the use of three-dimensional conformal techniques to optimize the final or boost portion of treatment has not been shown to lead to a decrease in complications.143,144
Another modification is to shield the pituitary and the anterior part of the hypothalamus in tumors with no evidence of erosion of the base of the skull or sphenoid and no extension of disease to the nasal fossa and ethmoid sinuses.145 This approach allows for a decreased number of endocrine and temporal lobe complications without a compromise in tumor control in a well-selected patient population.
Brachytherapy
Brachytherapy can be used to deliver a boost to T1 to T2 lesions after EBRT or alone in the setting of reirradiation. Intracavitary* and interstitial153–155 techniques have been used. Multiple applicators have been developed for intracavitary insertions. The Rotterdam Nasopharynx Applicator, developed in the Netherlands, is most commonly used today in conjunction with a remote-controlled high-dose-rate afterloading device156 (Fig. 32-4A and B). The device, made out of pliable silicone and shaped to conform to the nasopharynx, is guided into position through the oral cavity. After it is immobilized and dummy sources are inserted, orthogonal radiographs are obtained to define the anatomy for treatment planning (Fig. 32-4C and D). Dose is prescribed to a fixed anatomic point. In cases where brachytherapy is used as a boost for EBRT, 5 to 6 Gy in two fractions given 5 to 6 hours apart is generally given. Given the excellent results with IMRT in treatment of nasopharyngeal carcinoma, however, many institutions employ brachytherapy almost exclusively for reirradiation.
Permanent interstitial implantation of either iodine-125 (125I) seeds or gold-198 (198Au) grains should be considered for localized lesions and can be accomplished through several approaches, depending on the location of disease.153,154,157 For lesions in the low to mid posterior wall, transoral or transnasal approaches have been used, whereas a transpalatal approach is favored for lesions in the high posterior wall.153,154 Alternatively, a split-palate approach can be used.157
Intensity-Modulated Radiation Therapy
In the 1970s and 1980s it was becoming clear that advancing technology would make it possible to optimize radiotherapy by deriving the set of optimal incident stationary and moving beams from a desired dose distribution.158,159 These techniques deliver a set of beamlets of varying weights, with the weights chosen to optimally conform the resulting distribution of dose to a target.160,161 More generally, the optimization can be performed to optimize the biologic or physical objective functions, based on minimizing complications and maximizing tumor control probabilities.162 The technique of optimizing beam delivery with inverse planning has become known as intensity-modulated radiation therapy (IMRT) for historical reasons, although typically the beam intensity is not modulated.
Several institutions have shown a potential dosimetric improvement for IMRT over conventional techniques and three-dimensional conformal techniques in the setting of nasopharyngeal cancer.144,163,164,165,166 As discussed previously, several single-institution series as well as a phase II RTOG trial have shown the efficacy of IMRT in the treatment of nasopharyngeal cancer.116
Simulation and Treatment Planning
CT simulation is necessary for IMRT treatment planning. The patient’s head, neck, and shoulders are immobilized with a thermoplastic mask. The head should be hyperextended to allow for maximal separation between the primary nasopharyngeal tumor and lymphatics.111 A limited CT scan or orthogonal radiographs are obtained to allow for isocenter localization, after which planning CT images are acquired, with contiguous slices of 3 mm or less in the region of the tumor and of 5 mm elsewhere. Some treatment planning systems require equal spacing of slices throughout the entire scan. Alternatively, the CT images can be obtained first and then a treatment isocenter placed within the treatment volume.
IMRT treatment planning requires precise spatial information on the full extent of gross disease and thorough knowledge of the potential sanctuaries of microscopic disease along the lymphatic routes of tumor spread, because the therapeutic dose will only be delivered to the designated areas. Therefore, MRI, CT, and/or PET imaging is instrumental in supplementing the information from clinical examination when delineating the tumor volume. It is recommended that fusion of diagnostic MRI and/or PET scans to the planning CT is performed to accurately define the gross tumor volume (GTV) and surrounding critical structures. Several clinical target volumes (CTVs) are delineated, CTVgross disease and CTVsubclinical disease, which will receive different doses per fraction and total doses (Table 32-6). This practice is known as simultaneous integrated boost or dose painting. In addition, in the absence of low neck lymphadenopathy, a third dose schedule may be administered to the low neck, either with separate anterior-posterior fields matched to the IMRT plan (split-field technique) or with extension of the IMRT plan (extended whole-field technique). If the extended whole-field technique is used, then a third CTVlow-risk subclinical disease may be outlined. However, in the presence of any pathologic low neck lymph node, the uninvolved low neck lymph nodes are incorporated into the CTVsubclinical disease.
TABLE 32-6 IMRT Target Delineation for Nasopharyngeal Carcinoma
| Primary Tumor and Upper Neck | |
| CTV70 or gross disease Dose: 2.12 Gy/Fr to 69.96 Gy |
|
Dose: 1.8 Gy/Fr to 59.4 Gy
Can consider 54 Gy in N0 disease
Based on MSKCC guidelines. Expansion is 3 to 5 mm for planning tumor volume, except near brainstem or spinal cord, where 1 mm is acceptable.
CTV, clinical target volume; Fr, fraction(s); GTV, gross tumor volume; Gy, Gray.
The primary tumor in the nasopharynx and any cervical or retropharyngeal lymph nodes larger than 1 cm or with evidence of necrosis on imaging are considered GTVgross disease. CTVgross disease is composed of GTVgross disease with a 5- to 10-mm margin to account for possible microscopic disease (see Table 32-6). A notable exception is near the brain stem or spinal cord, where normal tissue tolerances necessitate that the margin be reduced, sometimes to as little as 1 mm. The CTVsubclinical disease includes all areas at risk for microscopic spread, including the entire nasopharynx, clivus, base of skull, pterygoid fossae, parapharyngeal space, sphenoid sinus, posterior one-third to one-half of the nasal cavity, posterior ethmoid sinuses, posterior one-third of the maxillary sinuses, and the following lymph node regions: bilateral upper deep jugular (junctional, parapharyngeal), level Ib, level II, level III, level V, and retropharyngeal nodes. In a patient with no clinically or radiographically detectable cervical lymph node involvement, no coverage of the submandibular region is required. The delineation of cervical lymph node stations on the planning CT scans is based on the published image-based consensus guidelines.167,168,169,170 The PTV is generally a 3- to 5-mm expansion of all the CTVs to account for potential setup errors and patient motion. Once again, limiting the margin to 1 mm around the CTV is allowed near the brainstem and spinal cord.
Treatment Planning and Delivery
Several commercial treatment planning systems are available.171–174 Inverse planning is generally used to generate a treatment plan. However, in the absence of sufficient experience with inverse planning, forward planning can be used with comparable clinical outcomes.175 At the MSKCC, IMRT is delivered with the dynamic multileaf collimator system using a sliding-window approach and megavoltage 6-MV or greater x rays are used. Megavoltage beams with energy above 6 MV should not be used for cervical lymph node irradiation.
Figure 32-5B to D provides an example of an IMRT plan for a patient with nasopharyngeal cancer treated in the RTOG 0225 trial. Seven coplanar nonopposed fields were used with a 0.5-cm multileaf collimator using the sliding-window technique, optimized with a gradient inverse planning engine. For comparison, a conventional plan for the same patient is also illustrated (Fig. 32-5A to C). The conventional ports used for the comparison are shown in Figure 32-6. Considerable sparing of normal tissues, in particular the parotid glands, is achieved with IMRT, and there is better coverage of the base of the skull than can be achieved with lateral fields. Figure 32-7 shows the dose-volume histograms for this case, again comparing conventional technique with IMRT.
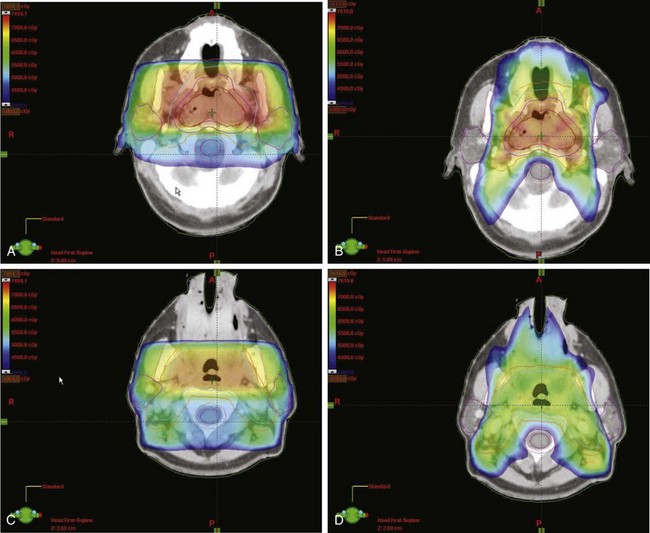
Figure 32-5 Dose colorwash comparison of coverage on a representative patient with stage T2bN0M0 nasopharyngeal cancer treated in the RTOG 0225 intensity-modulated radiotherapy (IMRT) trial. (For comparison, Figure 32-6 illustrates the conventional lateral ports used.) Computed tomography cuts are shown at the level of the pterygoid plates for conventional technique (A) and IMRT (B). The level of the uvula is also shown for conventional radiotherapy (C) and IMRT (D). The conventional plan is a composite of comprehensive head and neck fields with a boost to the primary lesion. Sparing of the parotid glands is the primary benefit of IMRT in this case. For the IMRT plan, seven coplanar nonopposed fields were used with a 0.5-cm multileaf collimator using the sliding-window technique, optimized with a gradient inverse planning algorithm.
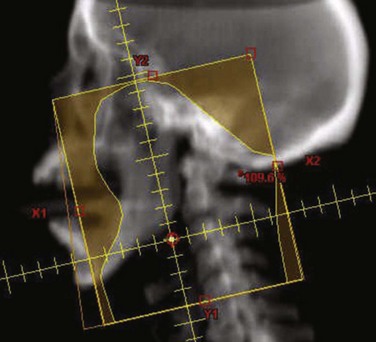
Figure 32-6 Digitally reconstructed radiograph of the conventional ports used in the comparison of intensity-modulated radiotherapy (IMRT) with conventional therapy illustrated (see Figs. 32-5 and 32-7). The posterior neck was blocked and treated with electrons at 40 Gy, and the boost treated the primary tumor with margins using 15-MV photons.
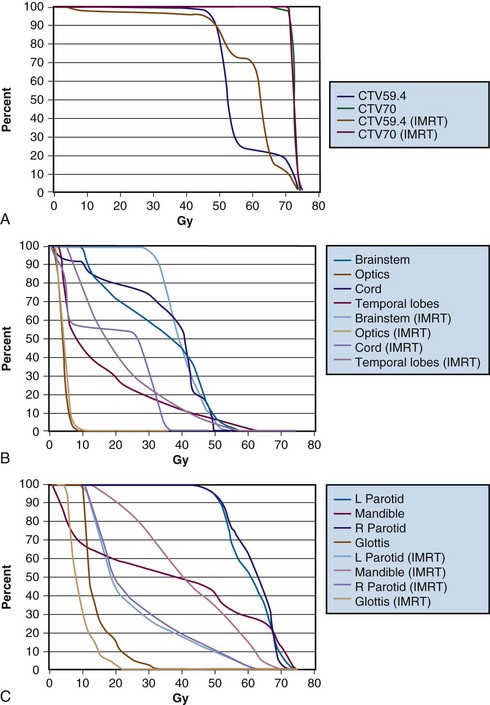
Figure 32-7 Dose-volume histograms (DVHs) for the patient in Figures 32-5 and 32-6, comparing intensity-modulated radiotherapy (IMRT) with conventional technique. Substantial gains can be made with IMRT in terms of target coverage and normal tissue sparing. A, Target coverage. Differences in the CTV59.4 (clinical target volume to be covered by 59.4 Gy) target coverage reflect the relatively high dose given to subclinical risk areas per RTOG 0225, with much of this target volume being intentionally prescribed a dose of 50.4 Gy in the conventional plan. Also, a conventional anterior low-neck field was used in the IMRT plan, with a midline block over the spinal cord and larynx in that region, which blocked a portion of the CTV59.4, a portion that was not included in the optimization but was included in the DVH. The DVHs for central nervous system (CNS) structures (B) and other normal structures (C) are also shown. Greater relative sparing of CNS structures by IMRT would be seen for higher-stage patients.
Figure 32-8 illustrates the dosimetric coverage that can be obtained with modern IMRT techniques in a sample reirradiation case. This is a situation in which the critical need to spare normal structures makes IMRT essential.
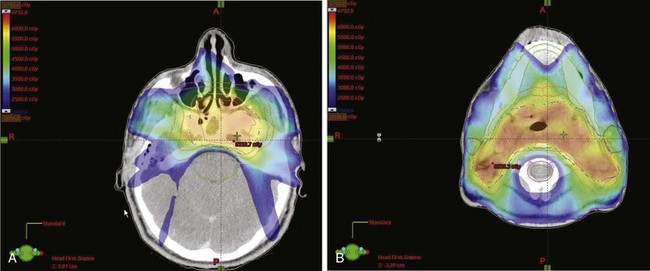
Figure 32-8 Dose colorwash illustrating radiotherapy coverage near the base of the skull for a case of recurrent nasopharyngeal cancer, in which intensity-modulated radiotherapy (IMRT) was used to minimize reirradiation risk to normal structures. Colorwash ranges between 20 Gy and maximum dose. The tolerance of the brainstem and temporal lobes places constraints on the dosimetry of the base of the skull (A). The mandible and spinal cord tolerance constrains the neck coverage (B). The IMRT technique used was similar to that in another case (see Figs. 32-5 and 32-6) but with nine coplanar nonopposed fields.
Radiotherapy Dose
Based on the early experience with IMRT (see Table 32-4) and the consensus opinion used in and subsequently validated by the RTOG 0225, the prescribed dose to the PTVgross disease is 69.96 Gy in 2.12-Gy fractions, the dose to the PTVsubclinical disease is 59.4 Gy in 1.8-Gy fractions, and the dose to the PTVlow-risk subclinical disease (if the extended whole-field technique is used) is 54.12 Gy in 1.64-Gy fractions. If the split-field technique is used, the anterior-posterior fields receive 50.4 Gy in 1.8-Gy fractions or 50 Gy in 25 fractions.
The following normal tissue dose constraints are currently used at the MSKCC:
| Critical | |
| Brainstem | Dmax = 54 Gy or 60 Gy to 1% of the volume |
| Optic nerves | Dmax = 54 Gy or 60 Gy to 1% of the volume |
| Optic chiasm | Dmax = 54 Gy or 60 Gy to 1% of the volume |
| Spinal cord | Dmax = 45 Gy or 50 Gy to 1% of the volume |
| Temporal lobes | Dmax = 60 Gy or 65 Gy to 1% of the volume |
| Mandible and TM joint | Dmax = 70 Gy or 75 Gy to 1 mL of the volume |
| Noncritical | |
| Parotid glands | Dmean = 26 Gy (in at least one gland) |
| Submandibular glands | As low as possible |
| Oral cavity | Dmean = 40 Gy |
| Larynx | Dmean = 40 to 45 Gy or Dmax = 70 Gy or = 105% of prescription |
| Cochlea | Dmax = 50 Gy |
| Retinas (globes) | Dmax = 45 Gy |
| Lenses | Dmax = 5 Gy |
| Brachial plexus | Dmax = 65 Gy and D05 Dmax = 70 Gy |
Normal Tissue Tolerance and Complications
Because of the anatomic location and requirement for large treatment fields and high doses, conventional treatment of nasopharyngeal carcinoma was associated with complication rates of 31% to 66%32,84,176 (see Table 32-1). Severe late complications included xerostomia, neck fibrosis, trismus, thyroid and hypothalamic-pituitary dysfunction, soft tissue and bone necrosis, hearing loss, cranial nerve dysfunction, and transverse radiation myelitis.
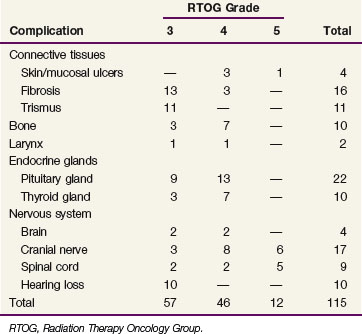
The incidence and types of complications of irradiation for nasopharyngeal cancer at the MDACC between 1954 and 1992 are listed in Table 32-7. The actuarial frequency of grade 3 to 5 complications was 16%, 19%, and 29% at 5, 10, and 20 years, respectively.32 However, the actuarial frequency of grade 3 to 5 complications at 10 years decreased steadily over three consecutive time periods included in the study: 1954 to 1971, 14%; 1972 to 1982, 10%; and 1983 to 1992, 5%, respectively. Of nine severe spinal cord injuries, eight occurred in the early time period (1954 to 1971). On univariate analysis, the frequency of moderate-to-severe complications was dependent on treatment technique. The actuarial incidence of moderate-to-severe complications at 10 years was 58% for the single central field technique compared with 12% and 25% for the oblique fields and opposed lateral fields techniques, respectively (p <.001). It should be noted that patients treated with oblique and opposed lateral fields were treated in more recent periods, and the decreased frequency of complications may also be related to the use of custom blocking and CT treatment planning.
TABLE 32-7 Serious Complications of Radiotherapy Alone for Nasopharyngeal Carcinoma in 378 Patients31,32
The use of conformal techniques for the final boost portion of radiotherapy could potentially decrease the risk of complications, but this has not been shown to be the case. Conformal techniques are associated with a 25% incidence of grade 3 to 4 morbidity, including hearing loss, trismus, dysphagia, chronic sinusitis, and cranial neuropathy.143 This result has led to an interest in delivering the full course of therapy with intensity modulation.111,143,144,177
Xerostomia is the most common toxicity associated with conventional therapy and may lead to dental caries. Strict dental hygiene and prophylactic fluoride treatment should be instituted to guard against dental decay. The use of IMRT allows for improved preservation and faster recovery of parotid gland function.111,114,117,118
Functional hearing loss was reported in up to 8% of patients treated with conventional technique, and at least 30% of those evaluated with audiograms showed a high-frequency sensorineural hearing loss.176,178,179 This radiation sequel may also be negatively affected by concurrent treatment with ototoxic chemotherapy. Dose-dependent cochlear damage is the likely mechanism of radiation-induced ototoxicity and can be minimized by keeping the mean dose to the cochlea below 48 to 50 Gy.178,179 Lowering the dose to the external auditory canal also reduces the incidence and severity of acute external otitis. Excluding the mastoid air cells reduces the incidence and severity of chronic serous otitis media. Unfortunately, given the location of the primary tumor in the nasopharynx, chronic eustachian tube dysfunction is unavoidable.
When treating lesions with base-of-skull involvement, it is important to consider the potential for optic neuropathy. A University of Florida review of 106 patients receiving treatment encompassing the optic nerves reported that patients who received more than 60 Gy and daily fractions of more than 1.9 Gy had a 47% incidence of optic nerve injury, compared with 11% for patients treated with daily fractions of less than 1.9 Gy.180
Clinically significant endocrine dysfunction has been described in 5% to 10% of patients.32,176 Given the availability of pharmacologic treatment, one should have a low threshold for evaluating long-term survivors for the presence of hypothalamic-pituitary or thyroid endocrinopathies. Routine evaluation of survivors with serum sodium, cortisol, thyroid-stimulating hormone (TSH), follicle-stimulating hormone, luteinizing hormone, and testosterone levels may be considered.
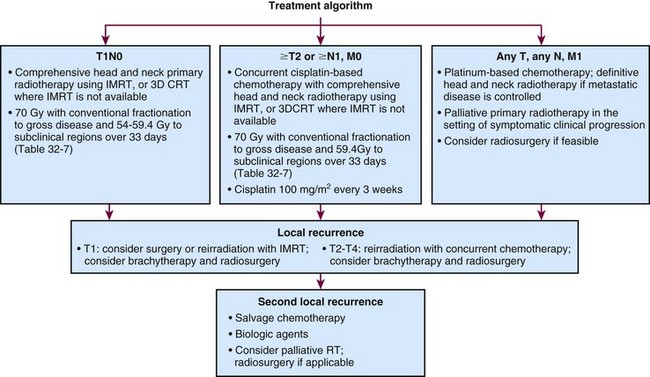
Treatment Algorithm, Conclusions, and Future Possibilities
A preferred treatment algorithm for patients with nasopharyngeal carcinoma is illustrated in Figure 32-9. With early-stage disease, irradiation alone with once-daily fractionation generally yields excellent local control results. For locally advanced disease (nonmetastatic stage ≥T2 or ≥N1 in the current AJCC staging system), concomitant chemotherapy and radiotherapy results are encouraging, and entry of patients into further trials testing combined-modality treatment is indicated. The advancement in radiotherapy optimization and the integration of functional imaging with radiotherapy planning promise improvement in both tumor control and decreased morbidity of treatment.
1 Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer. Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310-1317.
2 Yu MC. Nasopharyngeal carcinoma. Epidemiology and dietary factors. IARC Sci Publ. 1991;105:39-47.
28 Batsakis JG, Solomon AR, Rice DH. The pathology of head and neck tumors. Carcinoma of the nasopharynx. Part 11. Head Neck Surg. 1981;3:511-524.
32 Sanguineti G, Geara FB, Garden AS, et al. Carcinoma of the nasopharynx treated by radiotherapy alone. Determinants of local and regional control. Int J Radiat Oncol Biol Phys. 1997;37:985-996.
34 Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431-441.
45 Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461-2470.
46 Lu SJ, Day NE, Degos L, et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature. 1990;346:470-471.
50 Poon PY, Tsang VH, Munk PL. Tumour extent and T stage of nasopharyngeal carcinoma. A comparison of magnetic resonance imaging and computed tomographic findings. Can Assoc Radiol J. 2000;51:287-295. quiz 286
51 Chua DT, Sham JS, Kwong DL, et al. Retropharyngeal lymphadenopathy in patients with nasopharyngeal carcinoma. A computed tomography-based study. Cancer. 1997;79:869-877.
59 Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:1350-1357.
62 Pharynx. In Edge SB, Byrd DR, Compton CC, et al, editors: AJCC Cancer Staging Manual, ed 7, New York: Springer, 2010.
67 Au JS, Law CK, Foo W, et al. In-depth evaluation of the AJCC/UICC 1997 staging system of nasopharyngeal carcinoma. Prognostic homogeneity and proposed refinements. Int J Radiat Oncol Biol Phys. 2003;56:413-426.
72 Mao YP, Xie F, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73:1326-1334.
80 Wilson CP. The approach to the nasopharynx. Proc R Soc Med. 1951;44:353-358.
81 Johansen LV, Mestre M, Overgaard J. Carcinoma of the nasopharynx. Analysis of treatment results in 167 consecutively admitted patients. Head Neck. 1992;14:200-207.
82 Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985. Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261-270.
88 Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798-1804.
89 Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma. Positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631-637.
90 Chua DT, Sham JS, Choy D, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer. 1998;83:2270-2283.
91 Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. Progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038-2044.
92 Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma. NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966-6975.
93 Al-Sarraf M, LeBlanc M, Giri P, et al. Superiority of 5-year survival with chemoradiotherapy vs. radiotherapy in patients with locally advanced nasopharyngeal cancer. Intergroup 0099 Phase III Study. Final report. Proc Am Soc Clin Oncol. 2001;20:2279.
94 Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536-539.
95 Chan AT, Teo PM, Leung TW, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1995;33:569-577.
109 Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck. DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933-940.
110 Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas. First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7-16.
111 Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma. An update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12-22.
112 Kwong DL, Pow EH, Sham JS, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma. A prospective study on disease control and preservation of salivary function. Cancer. 2004;101:1584-1593.
113 Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy. The Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440-1450.
114 Wolden SL, Chen WC, Pfister DG, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer. Update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64:57-62.
115 Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy. The National Cancer Centre Singapore experience. Int J Radiat Oncol Biol Phys. 2009;75:1481-1486.
116 Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma. Radiation Therapy Oncology Group phase II trial 0225. J Clin Oncol. 2009;27:3684-3690.
117 Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma. Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981-991.
121 Fee WEJr, Roberson JBJr, Goffinet DR. Long-term survival after surgical resection for recurrent nasopharyngeal cancer after radiotherapy failure. Arch Otolaryngol Head Neck Surg. 1991;117:1233-1236.
125 Wei WI. Cancer of the nasopharynx. Functional surgical salvage. World J Surg. 2003;27:844-888.
126 Lee AW, Foo W, Law SC, et al. Reirradiation for recurrent nasopharyngeal carcinoma. Factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys. 1997;38:43-52.
131 Hwang JM, Fu KK, Phillips TL. Results and prognostic factors in the retreatment of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41:1099-1111.
132 Koutcher L, Lee N, Zelefsky M, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:130-137.
144 Hunt MA, Zelefsky MJ, Wolden S, et al. Treatment planning and delivery of intensity-modulated radiation therapy for primary nasopharynx cancer. Int J Radiat Oncol Biol Phys. 2001;49:623-632.
148 Levendag PC, Lagerwaard FJ, Noever I, et al. Role of endocavitary brachytherapy with or without chemotherapy in cancer of the nasopharynx. Int J Radiat Oncol Biol Phys. 2002;52:755-768.
165 Sultanem K, Shu HK, Xia P, et al. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma. The University of California-San Francisco experience. Int J Radiat Oncol Biol Phys. 2000;48:711-722.
169 Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck. DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227-236.
176 Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976-1985. Late complications following megavoltage irradiation. Br J Radiol. 1992;65:918-928.
1 Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer. Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310-1317.
2 Yu MC. Nasopharyngeal carcinoma. Epidemiology and dietary factors. IARC Sci Publ. 1991;105:39-47.
3 Yu MC, Ho JH, Lai SH, et al. Cantonese-style salted fish as a cause of nasopharyngeal carcinoma. Report of a case-control study in Hong Kong. Cancer Res. 1986;46:956-961.
4 Ho JH, Huang DP, Fong YY. Salted fish and nasopharyngeal carcinoma in southern Chinese. Lancet. 1978;2:626.
5 Bouvier G, Hergenhahn M, Polack A, et al. Characterization of macromolecular lignins as Epstein-Barr virus inducer in foodstuff associated with nasopharyngeal carcinoma risk. Carcinogenesis. 1995;16:1879-1885.
6 Henle G, Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976;17:1-7.
7 Neel HB3rd, Pearson GR, Taylor WF. Antibodies to Epstein-Barr virus in patients with nasopharyngeal carcinoma and in comparison groups. Ann Otol Rhinol Laryngol. 1984;93:477-482.
8 Buell P. The effect of migration on the risk of nasopharyngeal cancer among Chinese. Cancer Res. 1974;34:1189-1191.
9 Henderson BE, Louie E, Soo Hoo Jing J, et al. Risk factors associated with nasopharyngeal carcinoma. N Engl J Med. 1976;295:1101-1106.
10 Nam JM, McLaughlin JK, Blot WJ. Cigarette smoking, alcohol, and nasopharyngeal carcinoma. A case-control study among U.S. whites. J Natl Cancer Inst. 1992;84:619-622.
11 Lin TM, Chen KP, Lin CC, et al. Retrospective study on nasopharyngeal carcinoma. J Natl Cancer Inst. 1973;51:1403-1408.
12 Deng H, Zeng Y, Lei Y, et al. Serological survey of nasopharyngeal carcinoma in 21 cities of south China. Chin Med J (Engl). 1995;108:300-303.
13 Khuri FR, Lippman SM, Spitz MR, et al. Molecular epidemiology and retinoid chemoprevention of head and neck cancer. J Natl Cancer Inst. 1997;89:199-211.
14 Lo YM, Chan LY, Lo KW, et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59:1188-1191.
15 Leung SF, Tam JS, Chan AT, et al. Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating Epstein-Barr virus DNA and anti-Epstein-Barr viral capsid antigen IgA antibody. Clin Chem. 2004;50:339-345.
16 Hong RL, Lin CY, Ting LL, et al. Comparison of clinical and molecular surveillance in patients with advanced nasopharyngeal carcinoma after primary therapy. The potential role of quantitative analysis of circulating Epstein-Barr virus DNA. Cancer. 2004;100:1429-1437.
17 Hao SP, Tsang NM, Chang KP. Screening nasopharyngeal carcinoma by detection of the latent membrane protein 1 (LMP-1) gene with nasopharyngeal swabs. Cancer. 2003;97:1909-1913.
18 Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100:1162-1170.
19 Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795-801.
20 Benner SE, Pajak TF, Lippman SM, et al. Prevention of second primary tumors with isotretinoin in patients with squamous cell carcinoma of the head and neck. Long-term follow-up. J Natl Cancer Inst. 1994;86:140-141.
21 Bolla M, Lefur R, Ton Van J, et al. Prevention of second primary tumours with etretinate in squamous cell carcinoma of the oral cavity and oropharynx. Results of a multicentric double-blind randomised study. Eur J Cancer. 1994;30A:767-772.
22 Benner SE, Pajak TF, Stetz J, et al. Toxicity of isotretinoin in a chemoprevention trial to prevent second primary tumors following head and neck cancer. J Natl Cancer Inst. 1994;86:1799-1801.
23 Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441-450.
24 Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501-1505.
25 Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15-20.
26 Lin DT, Subbaramaiah K, Shah JP, et al. Cyclooxygenase-2. A novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24:792-799.
27 Papadimitrakopoulou VA, William WNJr, Dannenberg AJ, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14:2095-2101.
28 Batsakis JG, Solomon AR, Rice DH. The pathology of head and neck tumors. Carcinoma of the nasopharynx, Part 11. Head Neck Surg. 1981;3:511-524.
29 Vasef MA, Ferlito A, Weiss LM. Nasopharyngeal carcinoma, with emphasis on its relationship to Epstein-Barr virus. Ann Otol Rhinol Laryngol. 1997;106:348-356.
30 Shanmugaratnam K, Chan SH, de-The G, et al. Histopathology of nasopharyngeal carcinoma. Correlations with epidemiology, survival rates and other biological characteristics. Cancer. 1979;44:1029-1044.
31 Perez CA, Devineni VR, Marcial-Vega V, et al. Carcinoma of the nasopharynx. Factors affecting prognosis. Int J Radiat Oncol Biol Phys. 1992;23:271-280.
32 Sanguineti G, Geara FB, Garden AS, et al. Carcinoma of the nasopharynx treated by radiotherapy alone. Determinants of local and regional control. Int J Radiat Oncol Biol Phys. 1997;37:985-996.
33 Klein G, Giovanella BC, Lindahl T, et al. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974;71:4737-4741.
34 Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431-441.
35 Straathof KC, Bollard CM, Rooney CM, et al. Immunotherapy for Epstein-Barr virus-associated cancers in children. Oncologist. 2003;8:83-98.
36 Comoli P, De Palma R, Siena S, et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol. 2004;15:113-117.
37 Pearson GR, Weiland LH, Neel HB3rd, et al. Application of Epstein-Barr virus (EBV) serology to the diagnosis of North American nasopharyngeal carcinoma. Cancer. 1983;51:260-268.
38 Sam CK, Prasad U, Pathmanathan R. Serological markers in the diagnosis of histopathological types of nasopharyngeal carcinoma. Eur J Surg Oncol. 1989;15:357-360.
39 Raab-Traub N, Flynn K, Pearson G, et al. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987;39:25-29.
40 Chang YS, Tyan YS, Liu ST, et al. Detection of Epstein-Barr virus DNA sequences in nasopharyngeal carcinoma cells by enzymatic DNA amplification. J Clin Microbiol. 1990;28:2398-2402.
41 Niedobitek G, Agathanggelou A, Barber P, et al. P53 overexpression and Epstein-Barr virus infection in undifferentiated and squamous cell nasopharyngeal carcinomas. J Pathol. 1993;170:457-461.
42 Dictor M, Siven M, Tennvall J, et al. Determination of nonendemic nasopharyngeal carcinoma by in situ hybridization for Epstein-Barr virus EBER1 RNA. Sensitivity and specificity in cervical node metastases. Laryngoscope. 1995;105:407-412.
43 Murono S, Yoshizaki T, Tanaka S, et al. Detection of Epstein-Barr virus in nasopharyngeal carcinoma by in situ hybridization and polymerase chain reaction. Laryngoscope. 1997;107:523-526.
44 Henle W, Ho JH, Henle G, et al. Nasopharyngeal carcinoma. Significance of changes in Epstein-Barr virus-related antibody patterns following therapy. Int J Cancer. 1977;20:663-672.
45 Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350:2461-2470.
46 Lu SJ, Day NE, Degos L, et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature. 1990;346:470-471.
47 Simons MJ, Wee GB, Day NE, et al. Immunogenetic aspects of nasopharyngeal carcinoma. I. Differences in HLA antigen profiles between patients and control groups. Int J Cancer. 1974;13:122-134.
48 Simons MJ, Wee GB, Goh EH, et al. Immunogenetic aspects of nasopharyngeal carcinoma. IV. Increased risk in Chinese of nasopharyngeal carcinoma associated with a Chinese-related HLA profile (A2, Singapore 2). J Natl Cancer Inst. 1976;57:977-980.
49 Golovleva I, Birgander R, Sjalander A, et al. Interferon-alpha and p53 alleles involved in nasopharyngeal carcinoma. Carcinogenesis. 1997;18:645-647.
50 Poon PY, Tsang VH, Munk PL. Tumour extent and T stage of nasopharyngeal carcinoma. A comparison of magnetic resonance imaging and computed tomographic findings. Can Assoc Radiol J. 2000;51:287-295. quiz 286
51 Chua DT, Sham JS, Kwong DL, et al. Retropharyngeal lymphadenopathy in patients with nasopharyngeal carcinoma. A computed tomography-based study. Cancer. 1997;79:869-877.
52 Mancuso AA, Bohman L, Hanafee W, et al. Computed tomography of the nasopharynx. Normal and variants of normal. Radiology. 1980;137:113-121.
53 Olmi P, Fallai C, Colagrande S, et al. Staging and follow-up of nasopharyngeal carcinoma. Magnetic resonance imaging versus computerized tomography. Int J Radiat Oncol Biol Phys. 1995;32:795-800.
54 Liu FY, Lin CY, Chang JT, et al. 18F-FDG PET can replace conventional work-up in primary M staging of nonkeratinizing nasopharyngeal carcinoma. J Nucl Med. 2007;48:1614-1619.
55 Gordin A, Golz A, Daitzchman M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography imaging in patients with carcinoma of the nasopharynx. Diagnostic accuracy and impact on clinical management. Int J Radiat Oncol Biol Phys. 2007;68:370-376.
56 Pharynx. In Beahrs OH, Hulter RVP, Kennedy BJ, et al, editors: Manual of Staging of Cancer, ed 4, Philadelphia: JB Lippincott, 1992.
57 . Pharynx. 2nd rev. Union Internationale Contre le Cancer: TNM Atlas, ed 3. Springer-Verlag, Berlin, 1992..
58 Teo PM, Leung SF, Yu P, et al. A comparison of Ho’s, International Union Against Cancer, and American Joint Committee stage classifications for nasopharyngeal carcinoma. Cancer. 1991;67:434-439.
59 Ma J, Mai HQ, Hong MH, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19:1350-1357.
60 Committee of Chinese Clinical Staging of Nasopharyngeal Carcinoma. Report on the revision of nasopharyngeal carcinoma ’92 staging. Chin J Radiat Oncol. 2009;18:2-6.
61 Mao YP, Li WF, Chen L, et al. A clinical verification of the Chinese 2008 staging system for nasopharyngeal carcinoma. Chin J Cancer. 2009;28:1022-1028.
62 Pharynx. In Edge SB, Byrd DR, Compton CC, et al, editors: AJCC Cancer Staging Manual, ed 7, New York: Springer, 2010.
63 Ho JH. Stage classification of nasopharyngeal carcinoma. A review. IARC Sci Publ. 1978;20:99-113.
64 Pharynx. In Fleming ID, Cooper JS, Henson DE, editors: AJCC Cancer Staging Manual, ed 5, Philadelphia: Lippincott-Raven, 1997.
65 Pharynx. In Greene FL, Page DL, Fleming ID, et al, editors: AJCC Cancer Staging Manual, ed 6, New York: Springer-Verlag, 2002.
66 Ozyar E, Yildiz F, Akyol FH, et al. Comparison of AJCC 1988 and 1997 classifications for nasopharyngeal carcinoma. American Joint Committee on Cancer. Int J Radiat Oncol Biol Phys. 1999;44:1079-1087.
67 Au JS, Law CK, Foo W, et al. In-depth evaluation of the AJCC/UICC 1997 staging system of nasopharyngeal carcinoma. Prognostic homogeneity and proposed refinements. Int J Radiat Oncol Biol Phys. 2003;56:413-426.
68 Neel HB3rd, Taylor WF. New staging system for nasopharyngeal carcinoma. Long-term outcome. Arch Otolaryngol Head Neck Surg. 1989;115:1293-1303.
69 Sham JS, Cheung YK, Choy D, et al. Cranial nerve involvement and base of the skull erosion in nasopharyngeal carcinoma. Cancer. 1991;68:422-426.
70 Liu MZ, Tang LL, Zong JF, et al. Evaluation of sixth edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement. Int J Radiat Oncol Biol Phys. 2008;70:1115-1123.
71 Xiao GL, Gao L, Xu GZ. Prognostic influence of parapharyngeal space involvement in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:957-963.
72 Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73:1326-1334.
73 Ho HC, Lee MS, Hsiao SH, et al. Prognostic influence of parapharyngeal extension in nasopharyngeal carcinoma. Acta Otolaryngol. 2008;128:790-798.
74 Teo P, Shiu W, Leung SF, et al. Prognostic factors in nasopharyngeal carcinoma investigated by computer tomography—an analysis of 659 patients. Radiother Oncol. 1992;23:79-93.
75 Huang SC. Nasopharyngeal cancer. A review of 1605 patients treated radically with cobalt 60. Int J Radiat Oncol Biol Phys. 1980;6:401-407.
76 Roh JL, Sung MW, Kim KH, et al. Nasopharyngeal carcinoma with skull base invasion. A necessity of staging subdivision. Am J Otolaryngol. 2004;25:26-32.
77 King AD, Ahuja AT, Leung SF, et al. Neck node metastases from nasopharyngeal carcinoma. MR imaging of patterns of disease. Head Neck. 2000;22:275-281.
78 Mao YP, Liang SB, Liu LZ, et al. The N staging system in nasopharyngeal carcinoma with Radiation Therapy Oncology Group guidelines for lymph node levels based on magnetic resonance imaging. Clin Cancer Res. 2008;14:7497-7503.
79 Tang L, Li L, Mao Y, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma detected by magnetic resonance imaging. Prognostic value and staging categories. Cancer. 2008;113:347-354.
80 Wilson CP. The approach to the nasopharynx. Proc R Soc Med. 1951;44:353-358.
81 Johansen LV, Mestre M, Overgaard J. Carcinoma of the nasopharynx. Analysis of treatment results in 167 consecutively admitted patients. Head Neck. 1992;14:200-207.
82 Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985. Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261-270.
83 Chu AM, Flynn MB, Achino E, et al. Irradiation of nasopharyngeal carcinoma. Correlations with treatment factors and stage. Int J Radiat Oncol Biol Phys. 1984;10:2241-2249.
84 Hoppe RT, Goffinet DR, Bagshaw MA. Carcinoma of the nasopharynx. Eighteen years’ experience with megavoltage radiation therapy. Cancer. 1976;37:2605-2612.
85 Mesic JB, Fletcher GH, Goepfert H. Megavoltage irradiation of epithelial tumors of the nasopharynx. Int J Radiat Oncol Biol Phys. 1981;7:447-453.
86 Bailet JW, Mark RJ, Abemayor E, et al. Nasopharyngeal carcinoma. Treatment results with primary radiation therapy. Laryngoscope. 1992;102:965-972.
87 Sanchiz F, Milla A, Torner J, et al. Single fraction per day versus two fractions per day versus radiochemotherapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 1990;19:1347-1350.
88 Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798-1804.
89 Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma. Positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631-637.
90 Chua DT, Sham JS, Choy D, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer. 1998;83:2270-2283.
91 Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. Progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20:2038-2044.
92 Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma. NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966-6975.
93 Al-Sarraf M, LeBlanc M, Giri P, et al. Superiority of 5-year survival with chemoradiotherapy vs. radiotherapy in patients with locally advanced nasopharyngeal cancer. Intergroup 0099 Phase III Study. Final report. Proc Am Soc Clin Oncol. 2001;20:2279.
94 Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536-539.
95 Chan AT, Teo PM, Leung TW, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1995;33:569-577.
96 Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730-6738.
97 Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:142-151.
98 Kwong DL, Sham JS, Au GK, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma. A factorial study. J Clin Oncol. 2004;22:2643-2653.
99 Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(≥ N2, M0) undifferentiated nasopharyngeal carcinoma. A positive effect on progression-free survival. International Nasopharynx Cancer Study Group. VUMCA I trial. Int J Radiat Oncol Biol Phys. 1996;35:463-469.
100 Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma. An individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47-56.
101 Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer. Randomised, non-inferiority, open trial. Eur J Cancer. 2007;43:1399-1406.
102 Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695-1704.
103 Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705-1715.
104 Chan AT, Ma BB, Lo YM, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma. Therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22:3053-3060.
105 Wong AS, Soo RA, Lu JJ, et al. Paclitaxel, 5-fluorouracil and hydroxyurea concurrent with radiation in locally advanced nasopharyngeal carcinoma. Ann Oncol. 2006;17:1152-1157.
106 Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242-249.
107 Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567-578.
108 Koutcher L, Fury S, Wolden S, et al. Comparison of cisplatin (CDDP) and radiation (RT) to cetuximab (C) and RT for locally advanced head and neck cancer (LAHNC). A preliminary analysis. Abstract #6042. In 2009 ASCO Annual Meeting Proceedings, 2009. Orlando, Florida. J Clin Oncol. 2009;27(Suppl 15):6010.
109 Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck. DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933-940.
110 Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas. First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7-16.
111 Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma. An update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12-22.
112 Kwong DL, Pow EH, Sham JS, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma. A prospective study on disease control and preservation of salivary function. Cancer. 2004;101:1584-1593.
113 Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy. The Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440-1450.
114 Wolden SL, Chen WC, Pfister DG, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer. Update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64:57-62.
115 Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy. The National Cancer Centre Singapore experience. Int J Radiat Oncol Biol Phys. 2009;75:1481-1486.
116 Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma. Radiation Therapy Oncology Group phase II trial 0225. J Clin Oncol. 2009;27:3684-3690.
117 Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma. Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981-991.
118 Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873-4879.
119 Hsu MM, Ko JY, Sheen TS, et al. Salvage surgery for recurrent nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:305-309.
120 Fee WEJr, Gilmer PA, Goffinet DR. Surgical management of recurrent nasopharyngeal carcinoma after radiation failure at the primary site. Laryngoscope. 1988;98:1220-1226.
121 Fee WEJr, Roberson JBJr, Goffinet DR. Long-term survival after surgical resection for recurrent nasopharyngeal cancer after radiotherapy failure. Arch Otolaryngol Head Neck Surg. 1991;117:1233-1236.
122 Chang KP, Hao SP, Tsang NM, et al. Salvage surgery for locally recurrent nasopharyngeal carcinoma. A 10-year experience. Otolaryngol Head Neck Surg. 2004;131:497-502.
123 Hao SP, Tsang NM, Chang KP, et al. Nasopharyngectomy for recurrent nasopharyngeal carcinoma. A review of 53 patients and prognostic factors. Acta Otolaryngol. 2008;128:473-481.
124 Danesi G, Zanoletti E, Mazzoni A. Salvage surgery for recurrent nasopharyngeal carcinoma. Skull Base. 2007;17:173-180.
125 Wei WI. Cancer of the nasopharynx. Functional surgical salvage. World J Surg. 2003;27:844-848.
126 Lee AW, Foo W, Law SC, et al. Reirradiation for recurrent nasopharyngeal carcinoma. Factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys. 1997;38:43-52.
127 Teo PM, Kwan WH, Chan AT, et al. How successful is high-dose (≥60 Gy) reirradiation using mainly external beams in salvaging local failures of nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys. 1998;40:897-913.
128 Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma—treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987;13:953-956.
129 Lee N, Chan K, Bekelman JE, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:731-740.
130 Chua DT, Sham JS, Leung LH, et al. Re-irradiation of nasopharyngeal carcinoma with intensity-modulated radiotherapy. Radiother Oncol. 2005;77:290-294.
131 Hwang JM, Fu KK, Phillips TL. Results and prognostic factors in the retreatment of locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1998;41:1099-1111.
132 Koutcher L, Lee N, Zelefsky M, et al. Reirradiation of locally recurrent nasopharynx cancer with external beam radiotherapy with or without brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:130-137.
133 Cmelak AJ, Cox RS, Adler JR, et al. Radiosurgery for skull base malignancies and nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:997-1003.
134 Xiao J, Xu G, Miao Y. Fractionated stereotactic radiosurgery for 50 patients with recurrent or residual nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:164-170.
135 Chua DT, Sham JS, Kwong PW, et al. Linear accelerator-based stereotactic radiosurgery for limited, locally persistent, and recurrent nasopharyngeal carcinoma. Efficacy and complications. Int J Radiat Oncol Biol Phys. 2003;56:177-183.
136 Chua DT, Sham JS, Hung KN, et al. Stereotactic radiosurgery as a salvage treatment for locally persistent and recurrent nasopharyngeal carcinoma. Head Neck. 1999;21:620-626.
137 Chua DT, Wei WI, Sham JS, et al. Stereotactic radiosurgery versus gold grain implantation in salvaging local failures of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:469-474.
138 Wu SX, Chua DT, Deng ML, et al. Outcome of fractionated stereotactic radiotherapy for 90 patients with locally persistent and recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:761-769.
139 Chmura SJ, Milano MT, Haraf DJ. Reirradiation of recurrent head and neck cancers with curative intent. Semin Oncol. 2004;31:816-821.
140 Spencer S, Wheeler R, Peters G, et al. Phase 1 trial of combined chemotherapy and reirradiation for recurrent unresectable head and neck cancer. Head Neck. 2003;25:118-122.
141 Spencer SA, Harris J, Wheeler RH, et al. RTOG 96-10. Reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys. 2001;51:1299-1304.
142 Kao J, Garofalo MC, Milano MT, et al. Reirradiation of recurrent and second primary head and neck malignancies. A comprehensive review. Cancer Treat Rev. 2003;29:21-30.
143 Wolden SL, Zelefsky MJ, Hunt MA, et al. Failure of a 3D conformal boost to improve radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2001;49:1229-1234.
144 Hunt MA, Zelefsky MJ, Wolden S, et al. Treatment planning and delivery of intensity-modulated radiation therapy for primary nasopharynx cancer. Int J Radiat Oncol Biol Phys. 2001;49:623-632.
145 Sham J, Choy D, Kwong PW, et al. Radiotherapy for nasopharyngeal carcinoma. Shielding the pituitary may improve therapeutic ratio. Int J Radiat Oncol Biol Phys. 1994;29:699-704.
146 Teo P, Leung SF, Choi P, et al. Afterloading radiotherapy for local persistence of nasopharyngeal carcinoma. Br J Radiol. 1994;67:181-185.
147 Levendag PC, Schmitz PI, Jansen PP, et al. Fractionated high-dose-rate brachytherapy in primary carcinoma of the nasopharynx. J Clin Oncol. 1998;16:2213-2220.
148 Levendag PC, Lagerwaard FJ, Noever I, et al. Role of endocavitary brachytherapy with or without chemotherapy in cancer of the nasopharynx. Int J Radiat Oncol Biol Phys. 2002;52:755-768.
149 Wang CC. Improved local control of nasopharyngeal carcinoma after intracavitary brachytherapy boost. Am J Clin Oncol. 1991;14:5-8.
150 Zhang YW, Liu TF, Fi CX. Intracavitary radiation treatment of nasopharyngeal carcinoma by the high dose rate afterloading technique. Int J Radiat Oncol Biol Phys. 1989;16:315-318.
151 Lee N, Hoffman R, Phillips TL, et al. Managing nasopharyngeal carcinoma with intracavitary brachytherapy. One institution’s 45-year experience. Brachytherapy. 2002;1:74-82.
152 Pryzant RM, Wendt CD, Delclos L, et al. Re-treatment of nasopharyngeal carcinoma in 53 patients. Int J Radiat Oncol Biol Phys. 1992;22:941-947.
153 Harrison LB, Sessions RB, Fass DE, et al. Nasopharyngeal brachytherapy with access via a transpalatal flap. Am J Surg. 1992;164:173-175.
154 Vikram B. Permanent iodine-125 (I-125) boost after teletherapy in primary cancers of the nasopharynx is safe and highly effective. Long-term results. Int J Radiat Oncol Biol Phys. 1997;38:1140.
155 Choy D, Sham JS, Wei WI, et al. Transpalatal insertion of radioactive gold grain for the treatment of persistent and recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1993;25:505-512.
156 Levendag PC, Peters R, Meeuwis CA, et al. A new applicator design for endocavitary brachytherapy of cancer in the nasopharynx. Radiother Oncol. 1997;45:95-98.
157 Kwong DL, Wei WI, Cheng AC, et al. Long term results of radioactive gold grain implantation for the treatment of persistent and recurrent nasopharyngeal carcinoma. Cancer. 2001;91:1105-1113.
158 Brahme A. Optimization of stationary and moving beam radiation therapy techniques. Radiother Oncol. 1988;12:129-140.
159 Cormack AM. A problem in rotation therapy with X rays. Int J Radiat Oncol Biol Phys. 1987;13:623-630.
160 Gustafsson A, Lind BK, Brahme A. A generalized pencil beam algorithm for optimization of radiation therapy. Med Phys. 1994;21:343-356.
161 Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy. A new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709-1719.
162 Soderstrom S, Gustafsson A, Brahme A. The clinical value of different treatment objectives and degrees of freedom in radiation therapy optimization. Radiother Oncol. 1993;29:148-163.
163 Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for head-and-neck cancer. The UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57:49-60.
164 Munter MW, Debus J, Hof H, et al. Inverse treatment planning and stereotactic intensity-modulated radiation therapy (IMRT) of the tumor and lymph node levels for nasopharyngeal carcinomas. Description of treatment technique, plan comparison, and case study. Strahlenther Onkol. 2002;178:517-523.
165 Sultanem K, Shu HK, Xia P, et al. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma. The University of California-San Francisco experience. Int J Radiat Oncol Biol Phys. 2000;48:711-722.
166 Verhey LJ. Comparison of three-dimensional conformal radiation therapy and intensity-modulated radiation therapy systems. Semin Radiat Oncol. 1999;9:78-98.
167 Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000;174:837-844.
168 Nowak PJ, Wijers OB, Lagerwaard FJ, et al. A three-dimensional CT-based target definition for elective irradiation of the neck. Int J Radiat Oncol Biol Phys. 1999;45:33-39.
169 Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck. DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol. 2003;69:227-236.
170 Gregoire V, Coche E, Cosnard G, et al. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol. 2000;56:135-150.
171 Lichter AS, Sandler HM, Robertson JM, et al. Clinical experience with three-dimensional treatment planning. Semin Radiat Oncol. 1992;2:257-266.
172 Carol M, Grant WH3rd, Pavord D, et al. Initial clinical experience with the Peacock intensity modulation of a 3-D conformal radiation therapy system. Stereotact Funct Neurosurg. 1996;66:30-34.
173 Boyer AL, Geis P, Grant W, et al. Modulated beam conformal therapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 1997;39:227-236.
174 Welsh JS, Patel RR, Ritter MA, et al. Helical tomotherapy. An innovative technology and approach to radiation therapy. Technol Cancer Res Treat. 2002;1:311-316.
175 Lee N, Akazawa C, Akazawa P, et al. A forward-planned treatment technique using multisegments in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:584-594.
176 Lee AW, Law SC, Ng SH, et al. Retrospective analysis of nasopharyngeal carcinoma treated during 1976-1985. Late complications following megavoltage irradiation. Br J Radiol. 1992;65:918-928.
177 Xia P, Fu KK, Wong GW, et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:329-337.
178 Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820-829.
179 Grau C, Moller K, Overgaard M, et al. Sensori-neural hearing loss in patients treated with irradiation for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1991;21:723-728.
180 Parsons JT, Bova FJ, Fitzgerald CR, et al. Radiation optic neuropathy after megavoltage external-beam irradiation. Analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755-763.
181 Chua DT, Sham JS, Kwong DL, et al. Locally recurrent nasopharyngeal carcinoma. Treatment results for patients with computed tomography assessment. Int J Radiat Oncol Biol Phys. 1998;41:379-386.
182 Leung TW, Tung SY, Sze WK, et al. Salvage radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:1331-1338.
183 Lu TX, Mai WY, Teh BS, et al. Initial experience using intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:682-687.

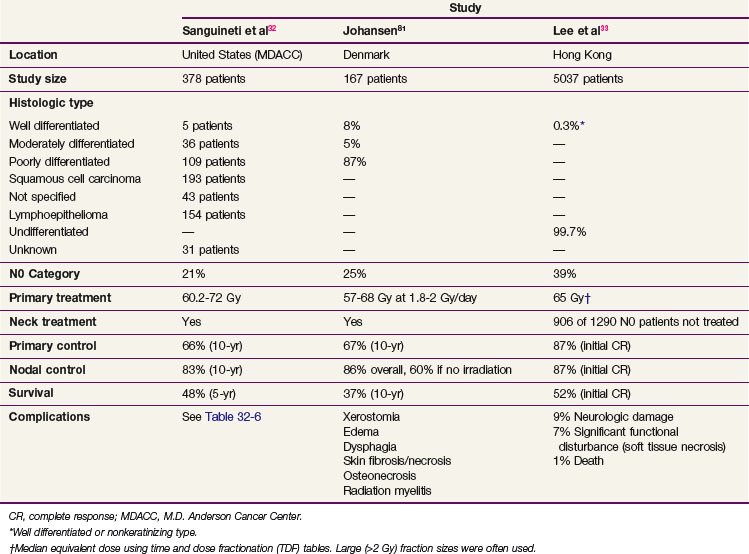
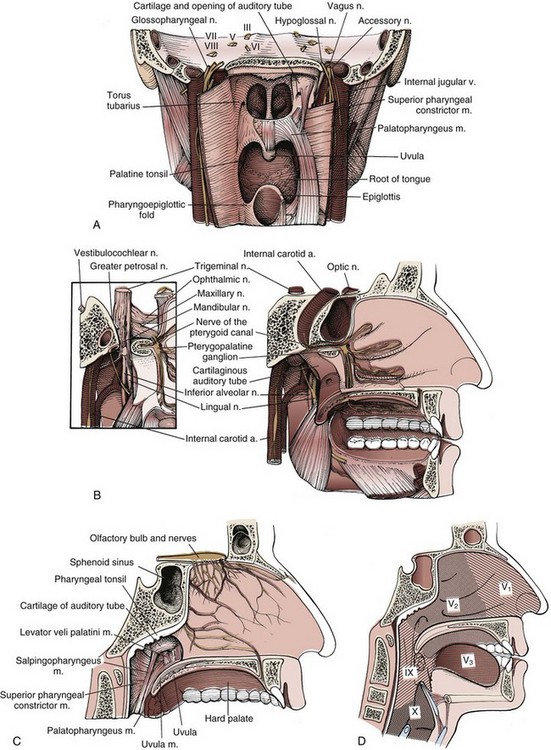
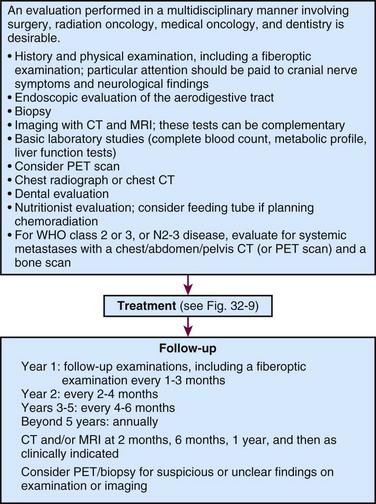
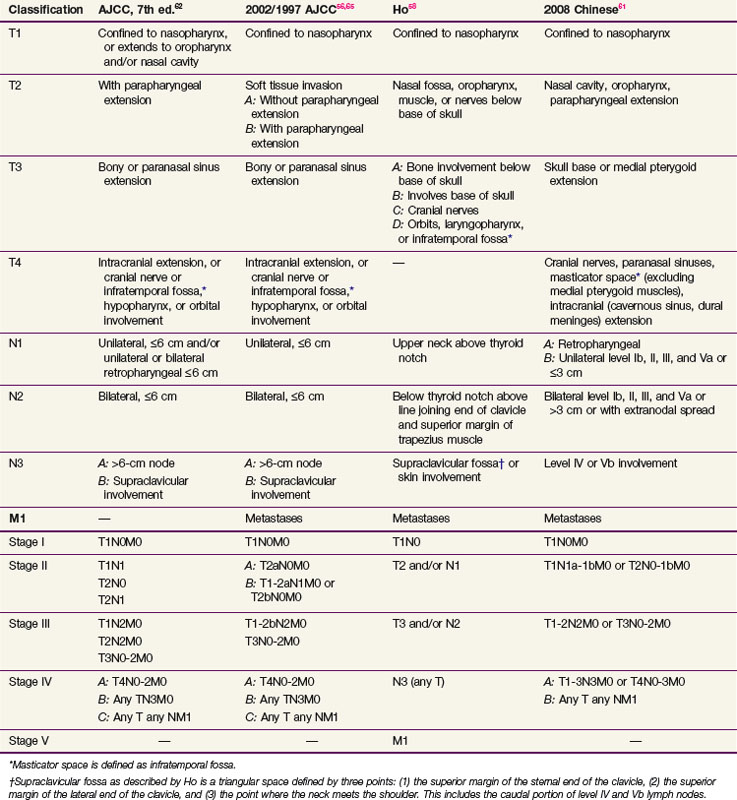
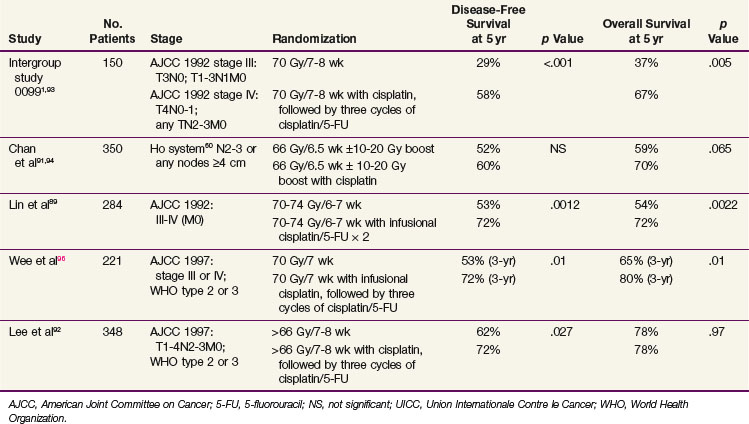
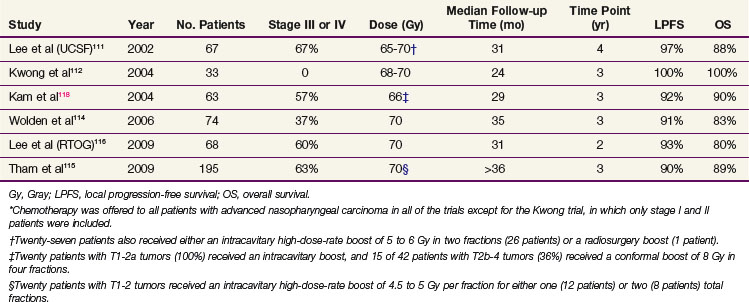
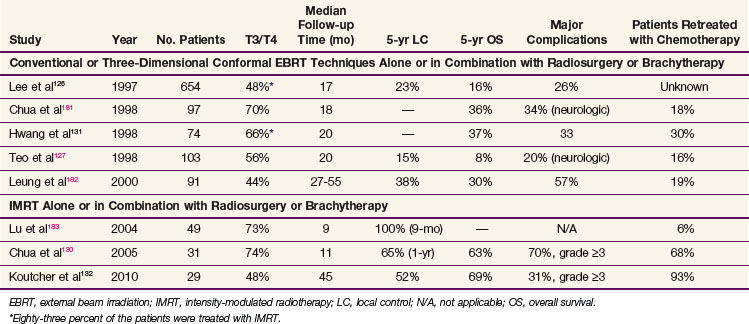
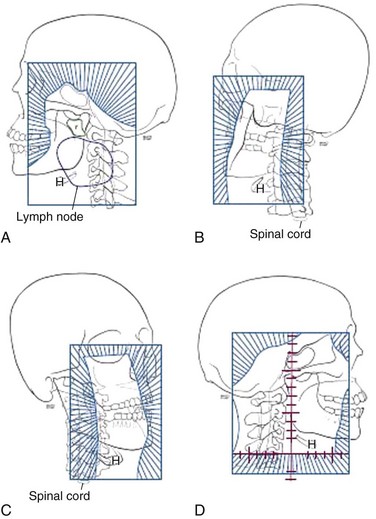
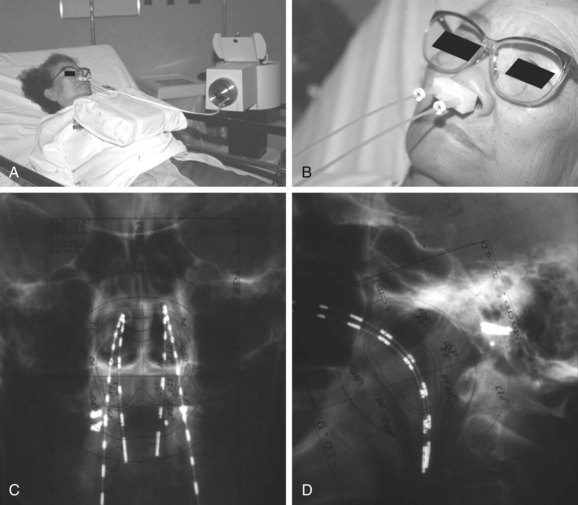
 to
to  of nasal cavity
of nasal cavity of maxillary sinuses
of maxillary sinuses