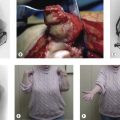
Chapter 50 Myositis Ossificans of the Elbow
Introduction
According to the World Health Organization classification of tumours myositis ossificans is:
A non-neoplastic condition, sometimes associated with trauma. The lesion may occur on the external surface of a bone or in soft tissue at a distance from the periosteal surface. The abnormal tissue is characterized by proliferation of fibrous tissue and by formation of large amounts of new bone. Cartilage may also be present.1
In its rare, autosomal dominant form, fibrodysplasia ossificans progressiva (FOP), it is characterized by skeletal malformation and progressive, disabling heterotopic osteogenesis. The condition was first described by Guy Patin in 16922 and in 1868 it was named by Theodore von Dusch3 (1824–1890). Because muscle is not always involved and an inflammatory reaction is not always present, the term ‘myositis’ is perhaps misleading and the alternative nomenclature of heterotopic ossification or ‘extraosseous localized, non-neoplastic bone and cartilage formation’4 is preferable. Others, however, do not agree as heterotopic ossification is defined by some as the formation of lamellar bone inside soft tissue structures where bone normally does not exist, whereas myositis ossificans refers to a condition in which ectopic bone is formed within muscles and other soft tissues. This is differentiated from ectopic calcification, which is the mineralization of soft tissue structures that usually follows chemical or physical trauma, as in tendinitis calcarea. Histologically, a calcium deposit rather than new bone would be formed. As yet, there is unfortunately no consensus on the definition and classification of heterotopic ossification.5
Martin et al regard myositis ossificans and heterotopic ossification as subsets of the process of ectopic bone formation: myositis ossificans being confined to muscle and heterotopic ossification as the ossification of capsular structures and joints.6 Although some authors have regarded them as separate processes,4 for the purpose of this chapter the term ectopic bone formation (EBF) will be used to encompass both. Also known as myositis ossificans circumscripta, myositis ossificans traumatica, extraosseous localized non-neoplastic bone, myo-osteosis, ossifying haematoma and traumatic ossifying myositis,3 it is a localized, self-limiting, reparative lesion that is composed of reactive hypercellular fibrous tissue and bone.1 A preceding traumatic incident is believed to be the initiating event in most cases. It is regarded by some as a rare, aberrant response to soft tissue trauma that passes through the same histological phases that are seen with fracture callus.7
Background/aetiology
The formation of bone in soft tissue requires inductive signalling pathways, inducible osteoprogenitor cells and a heterotopic environment conducive to osteogenesis.8 This triggers the transformation of mesenchymal cells into bone-forming cells.9 Differentiation of pluripotential mesenchymal cells into osteoblastic stem cells peaks at approximately 32 hours after surgery in mice.10 Thus, in this model, the crucial pathophysiological events occur in the immediate postoperative period, even though the EBF is not clinically or radiologically detectable for many weeks. Although it is known that this differentiation is induced by bone morphogenic protein,11 little is known about the molecular pathogenesis of this condition. Research into the genetic forms of this disease has revealed that there is overexpression of bone morphogenetic protein-4 and underexpression of multiple antagonists.12
Although the exact aetiology of EBF is unknown, there are a number of recognized associations or triggers. The first and most common association is the posttraumatic type with a single traumatic event or repetitive trauma being noted in 40–75% of the published cases.1,7 Thompson and Garcia have given a figure of 3% for the number of patients that develop EBF following simple elbow dislocations.13 Ilahi et al reported that the incidence rose to almost 50% in patients with fractures about the elbow (n = 41).14 After a direct blow to a muscle the incidence has been reported between 9% and 17%.15 A similar but atraumatic type has also been described. The proposed mechanisms for this type include non-documented trauma, repeated small mechanical injuries or ‘microtrauma’,3 and non-mechanical injuries as a result of ischaemia or inflammation.2,16 This type is usually confined to one muscle or muscle group. Factors that predispose to developing EBF after an injury to an elbow include a previous history of EBF, male gender, age greater than 60 years, Paget’s disease, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis (Forestier’s disease) and hypertrophic osteoarthrosis.12
Circumscribed heterotopic new bone formation without a history of trauma is sometimes regarded as a separate entity and is termed pseudomalignant EBF due to the diagnostic confusion between this benign lesion and other malignant conditions. The patient presents with a localized expanding soft tissue mass that exhibits peripheral calcification surrounding a radiolucent centre on plain radiographs and CT scans.17 The condition is benign and self-limiting and is indistinguishable from atraumatic EBF. A variant that is associated with long periods of immobility or a vegetative state has been described in paraplegics (the prevalence being reported as between 20% and 25%)18 and traumatic brain injury patients,19 and also as a rare complication in patients with tetanus, poliomyelitis and burns.6 Karapinar and Yagdi reported a case of a female with tetanus whose elbows became ankylosed in extension.20 Tompkins and Lachiewicz also described the development of disabling myositis ossificans of the posterior aspect of the elbow in a 66-year-old man as a sequela of tetanus.21
EBF following distal repair or reattachment of biceps brachialis is also well recognized. Of the two approaches to the repair the anterior repair alone has the advantage of a minimal risk of heterotopic bone formation, although it carries a greater chance of injury to the posterior interosseous nerve. Conversely, the two-incision technique markedly diminishes the risk of posterior interosseous nerve palsy, but is associated with a greater likelihood of EBF.22
Pathology
EBF is considered to be a pseudosarcomatous condition by virtue of its clinical and histological features,23 including reactive hypercellular fibrous tissue and bone. Although it is clearly a benign lesion, its clinical, radiological and histological appearance may sometimes mimic a malignant tumour.24 Histologically EBF cannot be distinguished from fracture callus.5
Immediately following the traumatic incident there is haemorrhage and fibrin deposition into the injury site. The centre of the ‘wound’ will contain variable amounts of necrotic material, blood clot and fibrin.7 Unlike other contusional injuries the wound displays an aberrant mesenchymal reaction directed towards callus formation. Under the microscope, the lesions exhibit a wide range of histological features with different amounts of immature fibroblastic cells, osteoid, cartilage, and young or mature bone accompanied by fibrous connective tissue. Comparing these appearances with those of pseudomalignant myositis ossificans, histologically there is microscopic evidence of zonation in which peripheral maturation is present with the central proliferating zone usually causing the diagnostic problems. This zonation is a characteristic of EBF and differentiates it from extraskeletal osteosarcoma.7 This differentiation was recognized as long ago as 1958 when Ackerman described how the bone is formed first at the periphery of the lesion in EBF but in sarcoma calcification extends centrifugally.2,4
Using electron microscopy, Povysil and Matejovsky demonstrated cells showing the morphological features of myofibroblasts and monocytic cells of the macrophage type. They considered that these previously unreported features together with the zonal pattern of the lesions indicated their reparative nature.25 EBF represents a polyclonal hyperplastic proliferative, regenerative process. This has been demonstrated by Leithner et al.24 The osteoinductor releasing cells are blood-borne monocytoid cells which enter the tissues by diapedesis and become histiocytes, macrophages, matrixclasts and osteoclasts; their precursors are derived from bone marrow at sites remote from the area of bone induction. The cell populations responding to the osteoinductor released by this mechanism develop into osteoblasts and osteocytes, and are the progeny of perivascular mesenchymal cells.26
Presentation, investigation and treatment options
Presentation
EBF occurs particularly in young and vigorous male adults participating in sports such as football.27 This male preponderance is consistent with the fact that males are more susceptible to injury in sports than are females.7 Geschickter and Maseritz reported on 25 patients with EBF. Fifteen gave a history of injury and 21 were males.28 However, Mirra stated that up to 50% of patients may not give a history of trauma when the patient understands the type of trauma necessary to induce the onset of EBF. With a detailed history and careful estimation of the age of the lesion, however, a history of trauma can be elicited in most patients.7
The presenting symptoms may develop over a number of days or weeks and can consist of one or more of the triad of local pain, a hard palpable mass in the muscle and a flexion contracture of the elbow.29 These symptoms have been noted to develop up to a year after the injury.30,31 Unexplained increase in pain, relative increase in spasticity or guarding of muscle should alert the examiner to the possibility of EBF regardless of the aetiology.18 However, the condition can be asymptomatic and may be diagnosed either incidentally2 or because of progressive loss of motion of the elbow.6
The clinical examination provides important diagnostic information. Usually, the earliest physical findings are those of a doughy mass present within a few hours of the injury.28 Increased joint stiffness, a limited range of motion, warmth, swelling and erythema are the principal clinical signs.5 The most common, and often the earliest, clinical sign is limited range of motion.18 The next most common sign is localized swelling.30 Because these clinical signs are not exclusive to EBF and are common in inflammatory conditions they cannot be regarded as pathognomic of EBF. The early inflammatory stage may mimic cellulitis, thrombophlebitis, osteomyelitis or a tumorous process.32
Sites
The most common sites for the development of ectopic bone around the elbow are medially and laterally where the bone is adjacent to or surrounding the collateral ligaments.33 Posterolateral is the most frequent site to ankylose and, consequently, the most common site about the elbow to require surgical resection.18 Lateral EBF in the vicinity of the radial collateral ligament occurs more commonly after traumatic EBF.33 Anterior ectopic bone is usually formed beneath the brachialis muscle and posteriorly it is usually deep to the triceps.18 When the ectopic bone surrounds the ulnar nerve, an entrapment neuropathy may ensue.34,35
Investigations
Laboratory investigations
The laboratory investigation of choice is the assessment of serum alkaline phosphatase (AP).18 Elevated levels occur after 3 weeks,18 and 4 weeks after injury AP levels may reach 3.5 times the normal value, with a peak concentration around the twelfth week. If the EBF is small, AP levels may remain unchanged.31 When the levels are elevated, this is a good parameter to assess, but only in the absence of fractures.5 Contrary to these findings in adults, AP levels are not elevated in children.19 Prostaglandin E2 (PGE2) excretion in 24-hour urine is felt to be a reliable bone marker not only for the early detection but also for determining the efficacy of treatment. A sudden increase in 24-hour urinary PGE2 excretion would be an indication to perform bone scintigraphy.6 The erythrocyte sedimentation rate (ESR) can also be elevated.36 Calcium and phosphorus levels are often elevated or may remain normal, making these unreliable markers.37
Three-phase bone scintigraphy
Three-phase bone scintigraphy is usually positive for EBF after 2–4 weeks18 and is the most sensitive imaging modality for the early detection of EBF. Repeated bone scans can be used to determine the optimal timing for surgical resection (see below), and to monitor postoperative recurrence.5
Ultrasonography
Ultrasonography detects EBF sooner than does conventional radiography.38 Thomas and Amstutz described the zone phenomenon that is evident on ultrasound scans and is specific for EBF.39 Ultrasound scanning is regarded by a number of authors as being the best investigation not only for the early detection, but also for the follow-up of EBF5,40 as well as for distinguishing the condition from extraosseous sarcomas.40
Radiography
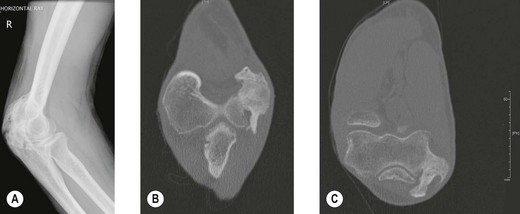
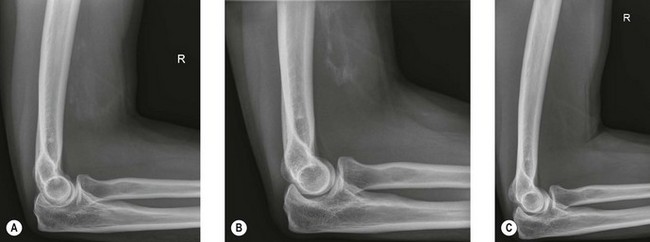
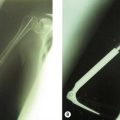
Radiography (Fig. 50.1A) and computed tomography (CT) (Fig. 50.1B,C) have low specificity in the early stages of EBF. As part of the surgical planning process, CT in combination with magnetic resonance imaging (MRI) are valuable to assess the spatial relationships with other anatomical structures such as blood vessels and peripheral nerves. Angiography is rarely used for the diagnosis of EBF, but may aid in delineating important vessels in cases of massive EBF.5 New bone is demonstrable on radiographs from 4 to 6 weeks after the injury (Fig. 50.2A). Geschichter and Maseritz showed that the first evidence of ossification was a small, dense shadow in the soft tissue some distance from the bone,28 although Gruca claims that bone can be seen as early as 3–4 weeks, reaching a maximum size from 10 weeks to 6 months41 (Fig. 50.2B). This has been corroborated by Chantraine and Minaire.42 Mirra recommended that to elucidate the early calcifications in EBF radiologically the kilovoltage must be kept within the range used for weak soft tissue densities.7
Magnetic resonance imaging
EBF typically presents as a soft tissue mass and MRI is commonly used to evaluate such swellings. de Smet et al scanned seven patients with EBF to determine if typical patterns were present.43 They concluded that typical MRI appearances of EBF do exist. A low signal intensity rim was a common finding. However, the patterns are not unique to EBF and resemble those that have been reported in other lesions. They warned that it is important to be aware of the spectrum of MRI findings of EBF when considering the differential diagnosis of a soft tissue mass.43 Kransdorf et al found that on T2-weighted spin-echo images, the lesions were relatively well defined and heterogeneous with intermediate to high signal intensity.44 When compared with the histology of the lesions, the latter corresponded to a central proliferating core of fibroblasts and myofibroblasts with a myxoid stroma resembling nodular fasciitis, rimmed by osteoblasts with bone production. Oedema surrounded lesions less than a few months old. T1-weighted images of early lesions were normal or showed evidence of a mass by displacement of fat planes. Mature lesions tended to be well defined with heterogeneous signal intensity, similar to that of fat, representing areas of fat situated between bone trabeculae within the lesion. Kransdorf et al noted that the varying appearance of EBF related to the histological changes that occur as the disorder progresses.44
The use of gadolinium is useful in distinguishing between EBF and malignant lesions. Cvitanic et al found that unenhanced sequences could be helpful in excluding malignancy, particularly when viewed serially.45 While the use of gadolinium can allow primary sarcoma to be excluded, it is not useful in the exclusion of early abscess formation or necrotic metastasis45 because the MRI features mimic those of an inflammatory mass or neoplasm. MRI is non-specific in the diagnosis of the early stages of EBF.46
Clinical Pearl 50.4
Biopsy
The diagnosis of EBF in the early stages is difficult. A needle aspirate of the lesion will determine if the mass is solid or contains pus. If it is solid and a biopsy is contemplated then this should be delayed because an early lesion of EBF is difficult to distinguish from a sarcoma. Close monitoring should be implemented with AP measurements, PGE2 excretion in 24-hour urine, bone scans, X-rays and CT scans (see above). Once a wedge or excisional biopsy has been performed of an ‘unripened’ mass it is very important to give as much clinical information to the pathologist as possible – history of trauma, behaviour of the lesion, rate of growth and the results of laboratory investigations. The lesion may recur within 2 weeks and the patient should be made aware of this. The growth of this reparative lesion should, however, cease after 7 weeks and is rarely larger that half the size of the original.7 Mirra recommends total excision of the remaining mass and the recurrence within 4 weeks to ensure the benign nature by the appearance of maturation and zonal patterns.7
Treatment options
EBF is difficult to treat once the process has been initiated, therefore it is important to begin prophylactic treatment as early as possible in susceptible patients.6 Some patients with minimal ectopic bone may require no specific treatment whereas others may require the full gamut of treatment including medication, physiotherapy, radiotherapy and surgery.
Natural history
The natural history of EBF when recorded radiologically is similar and predictable regardless of the aetiology. Only minor variations are seen between the different aetiologies and within similar patient populations.18 Most of the studies of the natural history of the condition are of EBF following total hip arthroplasty or in traumatic brain injury patients. From these studies some inferences can be made regarding ectopic bone formation about the elbow. The growth of the mass is usually complete by 7–8 weeks7 and the majority of ossification occurs by 3 months after the initial injury with radiological evolution for up to 6 months. In some patients with spinal cord injury, increased scintigraphic activity within ectopic bone may persist for some years.54,55 Occasional total regression has also been reported by Leithner et al24 and others56 (Fig. 50.2C).
Non-steroidal antiinflammatory drugs
Although there is some evidence that non-steroidal antiinflammatory drug (NSAID) use for EBF prophylaxis around the elbow is effective, there have been no studies that show that it leads to a better outcome. The use of pharmacological agents in EBF prophylaxis of the elbow is somewhat anecdotal.6 Much of the work on the pharmacological suppression of EBF has been done in patients with spinal cord injuries and after total hip joint replacement arthroplasty. As a result of this work, indometacin has been shown to have a beneficial effect. Banovac et al conducted a randomized, prospective, double-blinded, placebo-controlled clinical trial on its effect after spinal cord injury (n = 33).57 Bone scans were performed for the early detection of bone formation. Early stages of EBF were demonstrated in 25% of patients in the NSAID group and in 65% of the placebo group (p < 0.001). Bone formation was significantly more delayed in the NSAID group (p < 0.5) and the inflammatory signs (swelling, redness and fever) were also significantly less. Radiographically visible, late bone formation occurred in 12.5% of the NSAID group and in 41% of the placebo group (p < 0.001). After administration of indomethacin only solitary joint involvement was found with significantly less bone formation.57 The recommended dose is either 25 mg three times a day or 75 mg twice daily. Both appear to be equally effective.58,59 Garland recommended continuing the indometacin for 3 months.18 Several trials of cyclooxygenase-2 inhibitors have shown promising results in preventing EBF about the hip60 but no comparable trials have reported their use in EBF of the elbow.
Bisphosphonates
Bisphosphonates block the mineralization of osteoid but not the formation of osteoid itself.6 Treatment with biphosphonates appears to be effective as long as it is continued. It only retards osteoid calcification because ossification continues once the biphosphonates have been stopped. Although this has been referred to as a rebound effect it is actually not a true rebound effect; rather it is merely a physiological process that progresses. Biphosphonates are no longer used in the prevention of EBF.39
Etidronate
Most of the work on the efficacy of disodium etidronate in suppressing the formation of heterotopic bone has been done in patients with spinal cord injuries, although some studies have looked at its use as prophylaxis in preventing recurrence of EBF after surgical resection. Banovac et al studied 40 patients 2–5 weeks following spinal cord injuries, in all of whom there were clinical and scintigraphic signs of early EBF.57 Each patient received etidronate, 300 mg/day intravenously for 3 days, followed by 20 mg/kg/day orally for 6 months. The authors concluded that rebound ossification after early and prolonged administration of etidronate is rare; new EBF foci show a milder course without severe functional impairment and total joint ankylosis does not occur. However, it is difficult to establish whether a true rebound of the initial early scintigraphic diagnosis occurred here, or rather de novo formation of heterotopic bone.61
Disodium etidronate was used in another clinical trial including four patients to study its effectiveness in preventing the recurrence of mineralization after surgical removal of ectopic bone. These patients, who had sustained injuries of their spinal cords followed by ankylosis of the hips, had seven surgical wedge resections as part of a double-blinded study. X-rays showed that in the resections done without the drug there was recurrence within 3 weeks, whereas with the drug there was no sign of recurrence as long as the drug continued to be administered. After discontinuation of drug therapy, recurrence was variable but less than expected. The authors suggested that disodium etidronate is the first therapeutic agent that may delay or partially prevent postoperative recurrence of EBF.62
Despite these encouraging results, Haran et al reviewed the literature and concluded that there really was not enough evidence to support the continued use of this agent to treat acute EBF.63 These authors carried out a Cochrane review of the use of disodium etidronate in the prevention of bone formation in soft tissues. They found only two randomized trials by Ono et al and Stover et al that satisfied their inclusion criteria of comparing disodium etidronate versus placebo from which 92 participants with spinal cord injury had radiographically proven EBF. The two trials examined in this review had significant heterogeneity in their treatment effects when the outcome of improvement in radiographic EBF grade was used. The Ono study suggested that 12 weeks of disodium etidronate given soon after the onset of radiographically diagnosed EBF significantly improves the likelihood of either preventing the progression of, or improving the EBF grade at the completion of treatment. The Stover study did not support these conclusions, although the estimated treatment effect and confidence intervals for the prevention of progression were consistent with the Ono study. Longer-term radiographical, clinical or side effect outcomes are unavailable. Data were not pooled due to this heterogeneity and the inadequate duration of follow-up. The reviewers concluded that given the absence of long-term radiographical outcomes in these studies, there is insufficient evidence to recommend the use of disodium etidronate or other pharmacological agents for the treatment of acute EBF. It has been previously suggested that disodium etidronate acts by delaying, rather than preventing, the mineralization of EBF, and that in many cases mineralization may occur after treatment cessation, thereby negating the benefit of disodium etidronate on eventual EBF grade. They recommended that further long-term studies are undertaken to assess all pharmacological treatments for acute EBF.63
Radiotherapy
The use of radiotherapy to prevent the differentiation of mesenchymal stem cells into osteoblasts thus blocking the EBF process has some support.64 Its use has been confined mainly to the prevention of recurrence of EBF after surgical excision. Thurston and Spry reported on three cases of posttraumatic EBF of the forearm that had resulted in radio-ulnar synostosis.65 The management involved surgical excision of the ectopic bone followed by radiotherapy. Irradiation was commenced on the first postoperative day and was continued daily. The first patient received 20 Gy midline in 10 fractions and the second and third patients 10 Gy in five fractions. No side effects were observed. All three patients regained a good, functional range of forearm rotation with no evidence of recurrence of the EBF after 2 years. The authors recommended this method of treatment as an alternative to other adjunctive therapies such as perioperative pharmacological suppression.65 Similar results were reported by Poggi et al in three patients with EBF of the elbow treated with radiotherapy, delivered in a single fraction of 7–8 Gy within 48 hours of the surgery. After a median follow-up of 10.5 months, all three patients demonstrated a significant increase in range of motion without any evidence of recurrence.66
McAuliffe and Wolfson reviewed the results in eight patients after surgical excision of ectopic bone at the elbow performed 3–10 months (average, 7 months) after the initial injury, followed by radiotherapy.67 Each patient received a total dose of radiation of 1000 cGy, in five fractions starting on the first postoperative day and at least three of the remaining four treatments were administered on consecutive days. At an average of 46 months (25–72 months), there was no substantial recurrence of ossification either visible radiographically or that limited motion. No complications attributable to the radiotherapy were noted.67 In a larger study, Sautter-Bihl et al irradiated 46 joints in 36 patients with an early stage of EBF;68 11 patients had radiologically evident ossifications that were resected before the radiotherapy. The mean duration of follow-up was 23.6 months (4–98 months). Three patients were lost to follow-up. A dose of 10 Gy in fractions of 2–2.5 Gy was administered, except in three patients who received a total dose of 12–20 Gy. Of the 32 primarily irradiated joints, 16 showed no additional radiological abnormalities. Three showed progressive bone formation, although in these the timing of treatment had not been optimal, i.e. as soon as possible after the onset of symptoms. No significant differences were seen in the postoperative group (11 joints) and no differences were found between single-dose and fractionated radiation therapy. The authors admitted that the design of this study was hampered by some flaws and they recommended that further investigation using stricter population criteria was needed.68
One concern about the use of radiotherapy is the risk of impairment of wound healing and dehiscence, although a number of small studies on the use of radiotherapy for elbow EBF did not report any particular wound problems.64 Another concern is the induction of malignancy.64 Several studies, however, have shown that at doses of less than 3000 cGy administered in less than 3 weeks, no sarcomas developed in soft tissue or bone.69
Manipulation
Forceful manipulation of the rabbit thigh over a period has been shown to induce EBF.70 It is claimed by some authors that range of motion exercises increase the inflammatory response and potentiate the EBF process. Conversely, other authors have stated that range of motion exercises maintain joint mobility without promoting EBF.71 In the study reported by Garland et al, eight elbows that were manipulated under general anaesthesia gained an average of 47° without exacerbation of the EBF process.72
Surgical techniques and rehabilitation
Indications
According to Hastings and Graham there is no indication for surgery in the early stages of EBF and they recommend that the patient should be managed primarily by observation, serial radiographs and appropriate physiotherapy regimens.73 When EBF is noted within the first 6 weeks, they recommend the use of antiinflammatory agents. If the patient has a history of having developed motion-limiting ectopic bone in the past, then they recommend that consideration should be given to a single dose of radiotherapy. When EBF is seen to develop in the period between 6 and 12 weeks after the injury, they recommend that physiotherapy is used with the purpose of maintaining a full range of motion and an antiinflammatory agent continued or started.
The presence of ectopic bone around the elbow joint is not, in itself, an indication for surgical resection. However, when the ectopic bone is causing pain or altered function either in the joint or in the adjacent soft tissue structures, then surgery is indicated. The common problem is either that of ankylosis of the joint or progressive loss of movement. A less common problem is that of an entrapment neuropathy of the ulnar nerve.35,74 Vascular compression and lymphoedema attributable to EBF have also been reported in the lower limbs.75
Timing
The optimal timing for surgery is still controversial, although the tendency is to attach more importance to the functional and neurological recovery, rather than to the maturity of the bone. One should realize that surgical resection is often associated with complications and a high risk of recurrence.18 Secondary prevention by means of radiotherapy and/or administration of NSAID is essential. The focus of research into EBF has mainly centred on its occurrence after total hip arthroplasty. More and more attention is now being paid to the neurogenic form, which has the same preventive and therapeutic possibilities.5
The traditional time for surgery is generally accepted as being when the AP has returned to normal, the new bone appears radiologically mature and the appearance on the nuclear bone scan is similar to that of normal bone. Garland has stated that in adults this is generally 1 year after injury18 and this view is supported by others,76 even in children.19 Procrastination for longer that 2 years allows secondary changes such as bone weakening as a result of osteopenia and intra-articular ankylosis.18 It has, however, now been shown that these traditional indicators are not reliable and there is no guarantee that the ectopic bone will not recur even if these indicators are normal.77 While some authors have suggested that surgical excision can be performed as early as 3 months after the injury,59 in reality, the risk of recurrence after early excision compared with those of late excision has not been fully determined.6 If the ‘unripened’ mass has been biopsied then Mirra recommends the total extirpation of the mass within 4 weeks (see above). Schajowicz advocated waiting until the maturation of the ectopic bone, as a certain percentage may regress spontaneously.55
Technical considerations
The surgical resection of ectopic bone from around the elbow is technically challenging. The goal is to remove all bone and to release any soft tissue contractures to restore movement.78 In planning surgery on the ankylosed elbow a number of risk factors must be borne in mind and the surgeon must also be prepared for any one of a number of reconstructive procedures. Muscular and capsular contractures can be masked by the bony ankylosis, only to be unmasked by the resection of the ectopic bone. Elbows with EBF secondary to severe burns are often ankylosed in extension and frequently have more skin problems than those resulting from direct trauma. Occasionally, a free microvascular muscle transfer will be required in these difficult cases.79
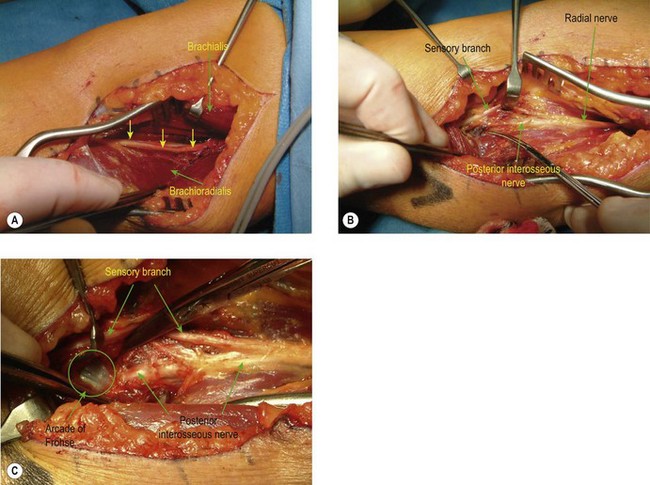
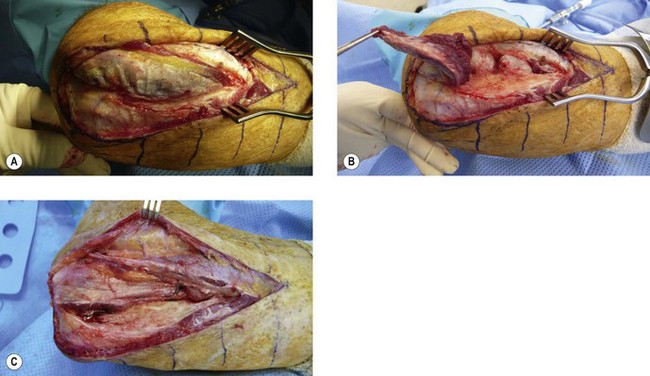
Three standard surgical approaches to the elbow are used. An anterolateral approach is preferred for anterior bony bridges as it allows the radial nerve to be exposed and protected along with the median nerve and brachial artery, if necessary. Through an anterolateral incision the radial nerve is identified between the brachialis and brachioradialis muscles (Fig. 50.3A) and is protected, as is the lateral antebrachial nerve. The ectopic bone is palpated within the brachialis muscle and is exposed through a muscle-splitting approach.75 More extensive bony deposition may extend into the tendon of biceps brachii and/or the anterior capsule. Dissecting more distally necessitates exposure and protection of the posterior interosseous nerve (Fig. 50.3B) by division of the arcade of Frohse (supinator muscle – Fig. 50.3C). Resection of an anterior bony bridge usually has to be combined with a capsulotomy. This resection of ectopic bone from the anterior aspect of the elbow is frequently complicated by soft tissue contractures that have to be addressed by soft tissue releases and lengthening of the contracted biceps tendon with fractional lengthening of brachialis muscle. These elbows also require careful neurolysis and protection of vascular structures and the meticulous attention to haemostasis, postoperative drainage and the removal of bone dust is sine qua non. Arthroplasty may have to be considered when injury has led to joint derangement or deterioration. If there is ectopic bone in the proximal portion of the interosseous membrane this can be excised by extending the incision distally. The common site for EBF in the interosseous membrane is adjacent to the bicipital tuberosity (Fig. 50.4A). Alternatively, the anconeus approach to the posterior aspect of the proximal radius and interosseous membrane can be utilized (Fig. 50.4B,C) (see below).
Posteriorly, limitation of motion is caused by a contracted and scarred triceps, capsular contracture or bony impingement and synostosis. The treatment of these elbows is through a standard posterolateral (Kocher) approach. Bridging bone, usually passing from the posterior aspect of the lateral humeral condyle to the posterolateral aspect of the olecranon process, has to be excised, the tip of the olecranon may also need to be removed and the olecranon fossa cleared of bone and scar. When the ankylosis is more extensive it may be necessary to reflect the triceps insertion subperiosteally by dividing Sharpey’s fibres and reflect it completely to the medial side. Attempts should be made to preserve the fat pad of the olecranon fossa, which can act as an effective interposition material. Garland has described the forceful manipulation of the elbow, at this stage, into flexion to stretch the contracted triceps and over a bolster into extension to stretch the flexor muscles.18 Failure of this to restore motion necessitates a capsular release and triceps tenolysis. The posterolateral incision can be extended distally and with the separation of anconeus (Fig. 50.4B,C) and the extensor carpi ulnaris muscles from the lateral aspect of the ulna, access can be gained to the proximal portion of the interosseous membrane to remove any ectopic bone that is blocking pronation and supination. When this is not possible because the synostosis is too extensive or the joint surfaces have been damaged, Kamineni et al have described the creation of a pseudarthrosis in the shaft of the proximal radius by resecting a 1 cm thick section.80 They claim that this provides a safe and reliable method of improving forearm rotation in patients with EBF of the elbow who have radio-ulnar synostosis. They mentioned that a single technical factor that seems to influence positively the result is the application of bone wax at the resection site.80
Rehabilitation
There is almost universal support for the use of some form of prophylaxis against recurrent EBF after surgical resection of ectopic bone.5 Garland recommended radiotherapy starting on the day of the surgery (see above).18 As an alternative he recommended the use of indomethacin for 3 months.54 The use of indomethacin for elbow EBF prophylaxis is by no means clinically proven but it is justified given the excellent results reported for prophylaxis after total hip joint replacement.
Physiotherapy to encourage range of motion usually begins when the patient is comfortable and pain is under control. Patients with poor neurological control will require more intensive physiotherapy than those who are able to exercise their own elbows. Supervised physiotherapy should continue until the joint has stabilized; this may take up to 6 months. If the range of motion diminishes significantly from that achieved intraoperatively then manipulation under anaesthesia can be beneficial.72
Outcomes including literature review
Aggressive treatment of posttraumatic EBF has led to improved long-term outcomes with up to 80–90% of patients having a good functional result following surgical excision of their ectopic bone.78 Patients with more severe injuries tend to have less satisfactory results.81
There are numerous studies giving the results of late excision of ectopic bone from around the elbow with reasonably predictable outcome. More recently some groups have become more aggressive and are resecting the ectopic bone much earlier. Denormandie et al reported on the results of 20 patients who were operated upon for EBF around the elbow following intra-cranial trauma, without prophylactic diphosphonates or radiotherapy.80 The average delay between the accident and the surgery was 34 months (5 months to 9 years). Associated procedures included lengthening of the brachialis (four cases), lengthening of the triceps tendon (four cases) and an anterior capsulotomy in three cases. Twenty-four elbows were reviewed with an average follow-up period of 18 months (6 months to 4 years). In 58% of the cases, there was a gain in mobility of greater than 70% while in the remaining cases, the improvement was between 40% and 70%.82
In advocating earlier release of the elbow, Viola and Hanel expressed a belief that delayed treatment beyond the time of fracture healing is unnecessary to obtain results comparable with those of previous studies.59 Similarly, they proposed that radiotherapy is also not necessary after excision of heterotopic ossification. Their cohort of 14 patients (15 elbows) was prospectively managed with early excision of posttraumatic heterotopic ossification, immediate postoperative mobilization, and a 5-day course of indometacin. The average time from injury to release was 23 weeks. The mean preoperative arc of flexion/extension was 43°; that of pronation/supination was 79°. After 2 years, the corresponding values were 120° and 152°. Cubital tunnel syndrome, present in five patients, resolved after surgery. There were no recurrent contractures or loss of motion.59 Moritomo et al reported on a similar review of wide excision of ectopic bone performed on nine consecutive patients with a stiff elbows.83 The average duration between initial injury and operation was 7.7 months. The thick, elastic mass on the posterior oblique ligament of the medial collateral ligament, which included mature and immature heterotopic ossification, was resected widely along with the posterior oblique ligament, the posteromedial joint capsule, and the surrounding scar tissue. After an average follow-up of 52 months, the total arc of motion improved from a preoperative mean of 37.3° to a postoperative mean of 112.8°. No patient complained of instability or pain on movement.83
In another review of ‘early’ surgical intervention, this time in burns patients, Tsionos et al reviewed 35 elbows in 28 patients in whom the surgery was undertaken as soon as the patient’s general and local condition allowed.84 The mean preoperative range of movement was 22° of flexion–extension and 94° of pronation–supination. The mean time between the burn and operation was 12 months with the median being 9.5. The mean follow-up period was 21 months. At the last review, the mean range of movement was 123° of flexion–extension and 160° of pronation–supination. Clinical evidence of recurrence was seen in four patients, occurring within the first 2 months after operation. Nevertheless, three of these elbows gained 60° or more in flexion–extension and in pronation–supination. Based on this experience, the authors recommended early surgical treatment of heterotopic ossification of the elbow in patients with severe burns.84 Gaur et al reported the results of a review of elbow function following excision of ectopic bone around the elbow in seven children (nine elbows) that developed as a result of severe burns. Excision of the ectopic bone was undertaken if the patient had limitation of function and if movement was restricted to a total arc of motion of <50°. The procedure was performed at an average of 17.3 months following the burn. At an average of 56 months after surgery, all nine elbows had an improved arc of motion (an average increase of 57°) with no recurrences. All of the children were able to reach their faces and their perineums following the procedure.85
Conclusions
1 Rosenberg A. Myositis Ossificans and Fibroosseous Pseudotumors of Digits. Lyon: International Agency for Research on Cancer; 2002.
2 Person D, Pattekar M. Myositis Ossificans: Multimedia. http://emedicine.medscape.com/article/1007104-overview, 2006. Available at accessed 2010, 19th July
3 Cushner F, Morwessel R. Myositis ossificans traumatica. Orthop Rev. 1992;21(11):1319-1326.
4 Ackerman L. Extraosseous localized, non-neoplastic bone and cartilage formation (so-called myositis ossificans). Clinical and pathological confusion with malignant neoplasms. J Bone Joint Surg (Am). 1958;40:279-298.
5 Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37(3):129-136.
6 Martin B, Johansen J, Edwards S. Complications related to simple dislocations of the elbow. Hand Clin. 2008;24(1):9-25.
7 Mirra J, editor. Myositis ossificans. Philadelphia: Lea & Febinger, 1989.
8 Chalmers J, Gray D, Rush J. Observation on the induction of bone in soft tissues. J Bone Joint Surg (Br). 1975;57(1):36-45.
9 Kozawa O, Tokuda H, Miwa M, et al. Cross-talk regulation between cyclic AMP production and phosphoinositide hydrolysis induced by prostaglandin E2 in osteoblast-like cells. Exp Cell Res. 1992;198(1):130-134.
10 Tonna E, Cronkite E. Autoradiographic studies of cell proliferation in the periosteum of intact and fractured femora of mice utilizing DNA labeling with H3-thymidine. Proc Soc Exp Biol Med. 1961;107:719-721.
11 Bosch P, Musgrave D, Ghivizzani S, et al. The efficiency of muscle-derived cell-mediated bone formation. Cell Transplant. 2000;9(4):463-470.
12 Kaplan F, Glaser D, Hebela N, et al. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12(2):116-125.
13 Thompson H, Garcia A. Myositis ossificans: aftermath of elbow injuries. Clin Orthop. 1967;50:129-134.
14 Ilahi O, Strausser D, Gabel G. Post-traumatic heterotopic ossification about the elbow. Orthopedics. 1998;21(3):265-268.
15 Hierton C. Regional blood flow in experimental myositis ossificans. A microsphere study in conscious rabbits. Acta Orthop Scand. 1983;54(1):58-63.
16 Drouet A, Marcel S, Guilloton L, et al. Myosite ossifiante circonscrite et sequelles d’ischemie musculaire. Deux observations. Rev Med Int. 1998;19(10):734-739.
17 Heinrich S, Zembo M, MacEwen G. Pseudomalignant myositis ossificans. Orthopedics. 1989;12(4):599-602.
18 Garland D. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop. 1991;263:13-29.
19 Kluger G, Kochs A, Holthausen H. Heterotopic ossification in childhood and adolescence. J Child Neurol. 2000;15(6):406-413.
20 Karapinar H, Yagdi S. A case of myositis ossificans as a complication of tetanus treated by surgical excision. Acta Orthop Belg. 2003;69(3):285-288.
21 Tompkins G, Lachiewicz P. Myositis ossificans after tetanus: treatment aided by quantitative technetium Tc 99m pyrophosphate radionuclide imaging. J South Orthop Assoc. 1995;4(3):239-243.
22 Cohen M. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
23 Estrada-Villasenor E, Cedillo E, Martinez G. Scrape cytology of myositis ossificans: report of a case and analysis of the cytologic findings described previously. Diagn Cytopathol. 2008;36(1):50-53.
24 Leithner A, Weinhaeusel A, Zeitlhofer P, et al. Evidence of a polyclonal nature of myositis ossificans. Virchows Archiv. 2005;446(4):438-441.
25 Povysil C, Matejovsky Z. Ultrastructural evidence of myofibroblasts in pseudomalignant myositis ossificans. Virchows Arch A Pathol Anat Histol. 1979;381(2):189-203.
26 Buring K. On the origin of cells in heterotopic bone formation. Clin Orthop. 1975;110:293-301.
27 Coley V. Myositis ossificans traumatica. A report of three cases illustrating the difficulties of diagnosis from sarcoma. Ann Surg. 1913;57:305-337.
28 Geschickter C, Maseritz I. Myositis ossificans. J Bone Joint Surg (Am). 1938;26(3):661-674.
29 Huss C, Puhl J. Myositis ossificans of the upper arm. Am J Sports Med. 1980;8(6):419-424.
30 Hardy A, Dickson J. Pathological ossification in traumatic paraplegia. J Bone Joint Surg (Br). 1963;45:76.
31 Orzel J, Rudd T. Heterotopic bone formation: clinical, laboratory, and imaging correlation. J Nucl Med. 1985;26(2):125-132.
32 Ragone D, Kellerman W, Bonner F. Heterotopic ossification masquerading as deep venous thrombosis in head-injured adult: complications of anticoagulation. Arch Phys Med Rehabil. 1986;67(5):339-341.
33 Garland D, O’Hollaren R. Fractures and dislocations about the elbow in the head-injured adult. Clin Orthop. 1982;168:38-41.
34 Keenan M, Kauffman D, Garland D, et al. Late ulnar neuropathy in the brain-injured adult. J Hand Surg (Am). 1988;13(1):120-124.
35 Gallucci G, Gallucci J, de Carli P, et al. Entrapment of the ulnar nerve in heterotopic ossification of the elbow: a case report. J Shoulder Elbow Surg. 2003;12(6):637-640.
36 Beiner J, Jokl P. Muscle contusion injury and myositis ossificans traumatica. Clin Orthop. 2002;403(Suppl):S110-S119.
37 Evans E. Heterotopic bone formation in thermal burns. Clin Orthop. 1991;263:94-101.
38 Pistarini C, Carlevati S, Contardi A. Diagnosi ecografica delle paraosteoartropatie neurogene in pazienti mielolesi. G Ital Med Lav. 1993;15(5–6):1159-1163.
39 Thomas B, Amstutz H. Results of the administration of diphosphonate for the prevention of heterotopic ossification after total hip arthroplasty. J Bone Joint Surg (Am). 1985;67(3):400-403.
40 Okayama A, Futani H, Kyo F, et al. Usefulness of ultrasonography for early recurrent myositis ossificans. J Orthop Sci. 2003;8(2):239-242.
41 Chantraine A, Minaire P. Para osteo arthropathies. A new theory and mode of treatment. Scand J Rehabil. 1981;13:31-37.
42 Gruca A. Myositis ossificans circumscripta. Ann Surg. 1925;LXXXII:883.
43 de Smet A, Norris M, Fisher D. Magnetic resonance imaging of myositis ossificans: analysis of seven cases. Skeletal Radiol. 1992;21(8):503-507.
44 Kransdorf M, Meis J, Jelinek J. Myositis ossificans: MR appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1991;157(6):1243-1248.
45 Cvitanic O, Sedlak J. Acute myositis ossificans. Skeletal Radiol. 1995;24(2):139-141.
46 Shirkhoda A, Armin A, Bis K, et al. MR imaging of myositis ossificans: variable patterns at different stages. J Magn Reson Imaging. 1995;5(3):287-292.
47 Torigoe T, Yazawa Y, Takagi T, et al. Extraskeletal osteosarcoma in Japan: multiinstitutional study of 20 patients from the Japanese Musculoskeletal Oncology Group. J Orthop Sci. 2007;12(5):424-429.
48 Coleman R. Fibro-osseous pseudotumour of the digit–amputation for a benign but aggressive lesion. J Hand Surg (Br). 2005;30(5):504-506.
49 Yeon H, Kaplan F, Shore E, et al. Focal fibronodular heterotopic ossification. A case report. J Bone Joint Surg (Am). 2007;89(6):1329-1336.
50 Alaani A, Hogg R, Warfield A, et al. Air bag injury as a cause of inflammatory myofibroblastic pseudotumour of the subglottic larynx progressing to myositis ossificans. Acta Oto-Laryngologica. 2005;125(6):674-677.
51 Allen A, Wetzel L, Borek D. Malignant myositis ossificans. A case report. Tumori. 1992;78(1):55-58.
52 Deller A, Heuer B, Wiedeck H. Is myositis ossificans following ARDS a complication of prone-dependency or is it osteoarthropathia hypertrophicans (Bamberger-Marie syndrome)? Intensive Care Med. 1998;24(12):1345-1346.
53 Ogilvie-Harris D, Fornasier V. Pseudomalignant myositis ossificans: heterotopic new-bone formation without a history of trauma. J Bone Joint Surg (Am). 1980;62(8):1274-1283.
54 Garland D. Clinical observations on fractures and heterotopic ossification in the spinal cord and traumatic brain injured populations. Clin Orthop. 1988;233:86-101.
55 Schajowicz F. Tumors and Tumorlike Lesions of Bone and Joints, 1st edn. New York: Springer-Verlag; 1981.
56 Bottu Y, Vannoyen G. Un cas d’ossification réversible des tissus mous chez une petite patiente paraplégique. Acta Paediatr Belg. 1963;17:223-230.
57 Banovac K, Williams J, Patrick L, et al. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001;39(7):370-374.
58 Naraghi F, DeCoster T, Moneim M, et al. Heterotopic ossification. Orthopedics. 1996;19(2):145-151.
59 Viola R, Hanel D. Early ‘simple’ release of posttraumatic elbow contracture associated with heterotopic ossification. J Hand Surg (Am). 1999;24(2):370-380.
60 Grohs J, Schmidt M, Wanivenhaus A. Selective COX-2 inhibitor versus indomethacin for the prevention of heterotopic ossification after hip replacement: a double-blind randomized trial of 100 patients with 1-year follow-up. Acta Orthop. 2007;78(1):95-98.
61 Banovac K. The effect of etidronate on late development of heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2000;23(1):40-44.
62 Stover S, Niemann K, Miller J. Disodium etidronate in the prevention of postoperative recurrence of heterotopic ossification in spinal-cord injury patients. J Bone Joint Surg (Am). 1976;58(5):683-688.
63 Haran M, Bhuta T, Lee B. Pharmacological interventions for treating acute heterotopic ossification. Cochrane Database Syst Rev 2004;(4):CD003321.
64 Ayers D, Pellegrini V, Evarts C. Prevention of heterotopic ossification in high-risk patients by radiation therapy. Clin Orthop. 1991;263:87-93.
65 Thurston A, Spry N. Post-traumatic radio-ulnar synostosis treated by surgical excision and adjunctive radiotherapy. Aust N Z J Surg. 1993;63:976-980.
66 Poggi M, Thomas B, Johnstone P. Excision and radiotherapy for heterotopic ossification of the elbow. Orthopedics. 1999;22(11):1059-1061.
67 McAuliffe J, Wolfson A. Early excision of heterotopic ossification about the elbow followed by radiation therapy. J Bone Joint Surg (Am). 1997;79(5):749-755.
68 Sautter-Bihl M, Liebermeister E, Nanassy A. Radiotherapy as a local treatment option for heterotopic ossifications in patients with spinal cord injury. Spinal Cord. 2000;38(1):33-36.
69 Kim J, Chu F, Woodard H, et al. Radiation-induced soft-tissue and bone sarcoma. Radiology. 1978;129(2):501-508.
70 Michelsson J, Granroth G, Andersson L. Myositis ossificans following forcible manipulation of the leg. A rabbit model for the study of heterotopic bone formation. J Bone Joint Surg (Am). 1980;62(5):811-815.
71 Stover S, Hataway C, Zeiger H. Heterotopic ossification in spinal cord-injured patients. Arch Phys Med Rehabil. 1975;56(5):199-204.
72 Garland D, Razza B, Waters R. Forceful joint manipulation in head-injured adults with heterotopic ossification. Clin Orthop. 1982;169:133-138.
73 Hastings H, Graham T. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand clin. 1994;10:417-437.
74 Kayalar M, Ozerkan F, Bal E, et al. Elbow arthrolysis in severely stiff elbows. Arch Orthop Trauma Surg. 2008;128(10):1055-1063.
75 Varghese G, Williams K, Desmet A, et al. Nonarticular complication of heterotopic ossification: a clinical review. Arch Phys Med Rehabil. 1991;72(12):1009-1013.
76 Abrams R, Simmons B, Brown R, et al. Treatment of posttraumatic radioulnar synostosis with excision and low-dose radiation. J Hand Surg (Am). 1993;18(4):703-707.
77 Garland D, Hanscom D, Keenan M, et al. Resection of heterotopic ossification in the adult with head trauma. J Bone Joint Surg (Am). 1985;67(8):1261-1269.
78 Akeson W, Amiel D, Abel M, et al. Effects of immobilization on joints. Clin Orthop. 1987;219:28-37.
79 Ring D, Jupiter J. Operative release of ankylosis of the elbow due to heterotopic ossification. Surgical technique. J Bone Joint Surg (Am). 2004;86(Suppl 1):2-10.
80 Kamineni S, Maritz N, Morrey B. Proximal radial resection for posttraumatic radioulnar synostosis: a new technique to improve forearm rotation. J Bone Joint Surg (Am). 2002;84(5):745-751.
81 Ring D, Jupiter J. Operative release of complete ankylosis of the elbow due to heterotopic bone in patients without severe injury of the central nervous system. J Bone Joint Surg (Am). 2003;85(5):849-857.
82 Denormandie P, Viguie G, Denys P, et al. Results of excision of heterotopic new bone around the elbow in patients with head injuries. A series of 25 cases. Chir Main. 1999;18(2):99-107.
83 Moritomo H, Tada K, Yoshida T. Early, wide excision of heterotopic ossification in the medial elbow. J Shoulder Elbow Surg. 2001;10(2):164-168.
84 Tsionos I, Leclercq C, Rochet J. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg (Br). 2004;86(3):396-403.
85 Gaur A, Sinclair M, Caruso E, Peretti G, Zaleske D. Heterotopic ossification around the elbow following burns in children: results after excision. J Bone Joint Surg (Am). 2003;85A–8:1538-1543.