Musculoskeletal Infections
Overview of Musculoskeletal Infections
Acute Pyogenic Osteomyelitis
Etiology, Pathophysiology, and Clinical Presentation: Osteomyelitis may occur from direct or hematogenous inoculation; it may be iatrogenic, related to orthopedic implants; or it may be from secondary extension from primary septic arthritis or pyomyositis. Hematogenous osteomyelitis is preponderantly a disease of children; however, infantile and even neonatal cases are not uncommon. Bacteria are the most common inflammatory agents, but growing bones may also be invaded by other pathogens, including viruses, spirochetes, and fungi.
The incidence of pediatric osteomyelitis in the United States is 1 in 5150 and has increased 2.8-fold in 20 years.1 This increased incidence is confounded, however, because of differences in access to health care and advances in imaging diagnosis. Staphylococcus aureus remains the most common causative organism of acute osteomyelitis in children. Unfortunately, community-acquired methicillin-resistant S. aureus (MRSA) strains are increasing in prevalence.2 Haemophilus influenzae osteomyelitis and septic arthritis have become less common since the availability of effective vaccination (H. influenzae type B vaccine).3
In sickle cell disease, bone complications include osteonecrosis and osteomyelitis. Osteonecrosis is approximately 50 times more frequent than osteomyelitis.4 The proposed mechanism of osteomyelitis is hematogenous, with bacteria gaining entrance to blood vessels through ischemic bowel and finding suitable culture material in foci of infarcted bone marrow. Both S. aureus and Salmonella commonly occur in sickle cell patients.2
In chronic granulomatous disease of childhood, an X-linked recessive disorder of leukocyte function, repeated infections occur in solid organs, skin, and bone. Approximately one third of patients develop osteomyelitis. Phagocytes are unable to kill catalase-positive organisms such as Staphylococcus and Aspergillus.5
Hematogenous osteomyelitis usually involves the highly vascularized metaphysis of the fastest growing bones, such as the distal femur and radius and the proximal tibia and humerus. The most common location for hematogenous osteomyelitis is about the knee (distal femur, proximal tibia).2 Pain, localized signs, fever, reduced range of motion, and reduced weightbearing are the most common initial clinical features. A history of trauma is seen in approximately 30% of cases,2 and the male-to-female ratio is approximately 1.8 : 1.
Organisms lodge most frequently in the terminal capillary sinusoids of the metaphyses.6 Rarely, they may locate initially in the epiphyses related to the terminal capillary sinusoids of the metaphyseal equivalent region immediately next to the spherical growth plate (Fig. 138-1). A small abscess forms in the marrow of the metaphysis, followed by local decalcification and destruction of the adjacent bone. When focal abscesses are generated, multiple small foci of bone destruction develop and later coalesce. Inflammatory swelling increases the intraosseous pressure because of the rigid bony walls of the marrow cavity; this can force extension of the infected exudate into several sites, as indicated in Figure 138-2. The most common route is via the haversian canals of the cortex to the subperiosteal space, where a subperiosteal abscess is formed. Simultaneously, spread also occurs farther within the medullary cavity. Rupture of the periosteal abscess is responsible for extension of infection into the adjacent soft tissues. Inflammation and rapidly increased intraosseous pressure may cause thrombosis of the vascular channels.
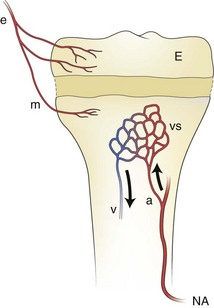
Figure 138-1 The blood supply to the metaphysis and epiphysis of a child and the arterial channels through which invading organisms enter the growing bone.
An epiphyseal artery (e) supplies the epiphysis (E) and may branch to give (minor) metaphyseal vessels (m). The major blood supply of the metaphysis comes from the nutrient artery. a, arteriole; NA, nutrient artery; v. venule; vs, venous sinuses in the metaphysis.
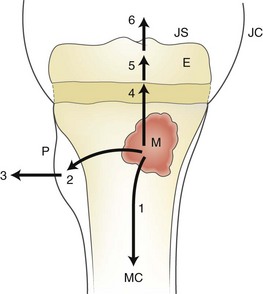
Figure 138-2 Pathways of infection after hematogeneous implantation in the metaphysis and formation of a metaphyseal focus (M) of bone infection (bone abscess).
1, spread to the medullary canal (MC); 2, formation of a subperiosteal abscess; 3, penetration of the periosteum (P) and spread to the adjacent soft tissues; 4, 5, and 6, spread across the growth plate to the epiphysis (E), and eventually to the joint space (JS). JC, joint capsule.
The most common location for direct inoculation osteomyelitis is the foot. Plantar puncture wounds secondary to walking on broken glass, metal (nail), or vegetable matter (thorn, toothpick) may result in infectious cellulitis, plantar fasciitis, and osteomyelitis, whether the foreign body is removed or retained. The calcaneus is often involved, and Pseudomonas aeruginosa is often found related to direct inoculation, usually with a history of a puncture through a shoe.5
Imaging: Imaging guidelines for the evaluation of suspected osteomyelitis include radiographs first to exclude alternative etiologies, such as a fracture or neoplasm, as an explanation for symptoms. If radiographs are normal, ultrasound (US) is recommended if the symptoms are localized to an osteoarticular region. If US is negative, scintigraphy is the next study of choice, if symptoms are nonlocalizable.7 If symptoms can be localized, targeted magnetic resonance imaging (MRI) of the affected region should be performed both for diagnostic purposes and for planning surgical treatment.8,9
Radiography: With acute osteomyelitis, the earliest change on radiographs is soft tissue swelling; osseous changes are seldom present until the second week of disease (Fig. 138-3).10 The earliest bone changes seen on conventional images are one or more small radiolucencies, usually in the metaphyseal region, where necrosis and destruction of bone has occurred (Fig. 138-4). On serial examinations, these areas of bone destruction enlarge and become confluent.
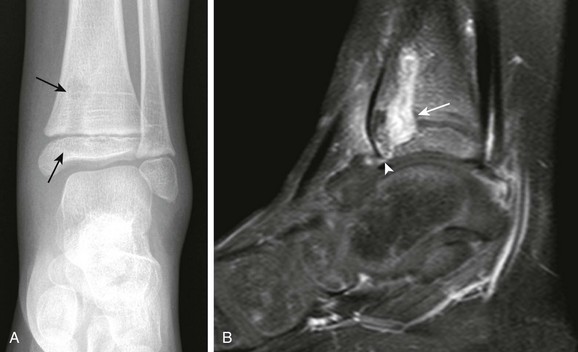
Figure 138-3 Osteomyelitis in a 6-year-old girl.
A, Frontal radiograph demonstrates a lytic lesion in the distal tibia metaphysis extending into the epiphysis (arrows). B, T1-weighted fat-saturated post-gadolinium sagittal view demonstrates a thick, rim-enhancing lesion with a small amount of nonenhancing fluid consistent with early abscess formation with epiphyseal extension (arrow) and a small cloaca (arrowhead) extending to the tibiotalar joint.
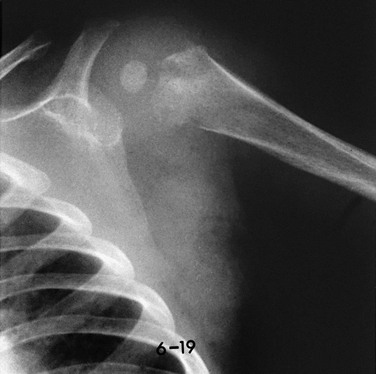
Figure 138-4 Early radiographic changes of osteomyelitis in the proximal left humeral metaphysis.
This 9-month-old boy had fever and local signs and symptoms for 12 days. Metaphyseal areas of bone destruction are visualized as irregular, ill-defined lucencies.
With continuing appropriate antibiotic therapy, periostitis is visible when the periosteum begins to produce new bone on its undersurface after the second or third week (Fig. 138-5). Osteogenic function by the periosteum suggests that infection has been at least partly locally controlled. Subsequent healing may involve remodeling of the cortical new bone and reconstitution of the underlying bone or, if damage has been extensive, it may involve an increase in the amount of periosteal reaction to form an involucrum (Fig. 138-6), a living bone sheath around the fragments of the old devitalized bone (sequestrum).
Scintigraphy: Early bone scans may demonstrate a “cold” metaphyseal lesion as a result of compression or occlusion of the metaphyseal vessels. In these cases, increased activity is observed toward the diaphysis, beyond the cold metaphyseal area, which subsequently becomes “hot” and merges with the adjacent increased activity. The multiphase bone scan is very sensitive and is usually positive 24 to 48 hours after the onset of symptoms.7 It can detect extension of metaphyseal osteomyelitis into the epiphysis through the growth plate (Fig. 138-7).
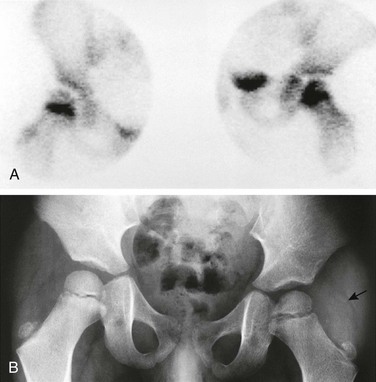
Figure 138-7 A 4-year-old boy with fever and inability to walk or move the left lower extremity for 6 days.
A, Bone scan shows increased uptake in the femoral neck and head on the left side, likely resulting from extension of an initial metaphyseal focus of infection into the epiphysis. The right hip shows normal increased uptake in the region of the growth plate. B, Radiograph of the hips 2 weeks after the beginning of symptoms shows osteopenia on the left side, ill-defined medial metaphyseal and epiphyseal bone lucencies on each side of the growth plate, and indirect evidence of left hip joint effusion. Fat line (arrow) is displaced laterally by the joint fluid. Adjacent deep soft tissues also appear swollen and edematous compared with the normal right side.
In the early detection of acute bone infection, radionuclide imaging is more sensitive than radiographs and can identify additional foci of disease not clinically apparent. Vascular phase images done within the first 5 minutes following injection, delayed images with pinhole collimators, and special attention to the affected area have proven of great value. Osteomyelitis appears as an area of increased tracer activity that reflects the hyperemia and bone turnover induced by the infectious process. In a study of 100 children with acute limb pain, the sensitivity and specificity of three-phase bone scans for acute osteomyelitis were 84% and 97%, respectively.11 Errors arise from simulation of infection by fracture or sickle cell disease, obscuration of osteomyelitis by septic arthritis, prior antibiotic treatment, and “cold” defects that result from ischemia. It is difficult to detect infection close to the growth plate, because both the growing physis and the nearby area of infection show increased activity.
Computed Tomography: Computed tomography (CT) is of limited clinical value in acute osteomyelitis.12 It is more useful in advanced or chronic disease to help determine the quality of bone stock, including determinations of cortical destruction, involucrum, and sequestra (see Fig. 138-6).
Magnetic Resonance Imaging: MRI is the optimal study to evaluate for infection and alternative etiologies for symptoms, particularly when radiographs are normal. MRI can identify early bone changes, and it delineates the anatomy and extent of marrow involvement; for this reason, it has become an important tool for imaging of suspected osseous infection. However, MRI does carry additional cost, and depending on the availability of scanner time and the need for sedation or anesthesia, delays in definitive diagnosis and treatment are possible. Imaging should aim at guiding or modifying treatment if necessary.
When precontrast MRI exams are entirely normal and show no evidence to suggest osteomyelitis, routine post-gadolinium images may not be necessary.13,14 When abnormal, MRI can reveal marrow alterations and extent of disease in bone, soft tissues, or adjacent joints (Fig. 138-8).15 Early MRI findings of osteomyelitis may have a tumefactive appearance and may be paradoxically hypointense on fluid-sensitive sequences (e-Fig. 138-9). Over time, the lesion may remain masslike and demonstrates the expected, more homogeneous hyperintense signal on fluid-sensitive sequences, indicative of its inflammatory nature (Fig. 138-10). Eventually, periostitis and adjacent soft tissue involvement may be seen in the early phase of osteomyelitis (e-Fig. 138-11). Subperiosteal abscess formation may be seen by sonography (Fig. 138-12) or MRI (Fig. 138-13), preceding radiographic bony changes. A salt-and-pepper appearance to the marrow may be seen in the late acute phase of osteomyelitis and is presumed to represent small areas of noncoalescent microabscess formation and early bone destruction (Fig. 138-14).
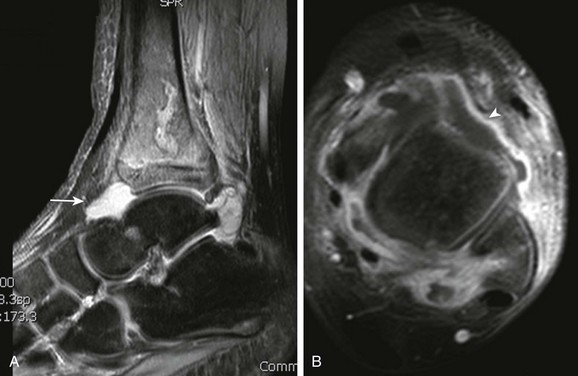
Figure 138-8 Distal tibial osteomyelitis and tibiotalar septic arthritis in an 11-year-old boy.
A, T2-weighted fat-saturated sagittal magnetic resonance image demonstrates diffuse marrow edema that includes transphyseal extension to the epiphysis and juxtacortical soft tissue edema. A large joint effusion is present (arrow) with T1-weighted fat-saturated post-gadolinium axial images (B) that demonstrate thick synovial enhancement (arrowhead).
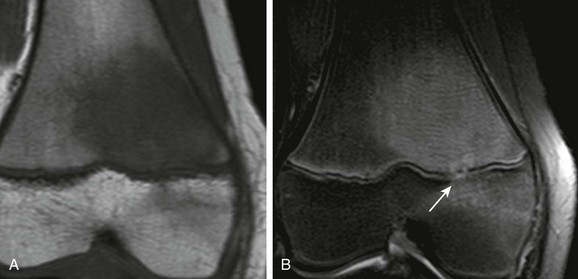
Figure 138-10 Child with biopsy-confirmed osteomyelitis of the distal femur.
A, T1-weighted coronal magnetic resonance imaging (MRI) demonstrates tumefactive marrow replacement in the distal femur. B, Short tau inversion recovery coronal MRI demonstrates diffuse homogeneous hyperintensity in the same distribution with early physeal extension of infection (arrow).
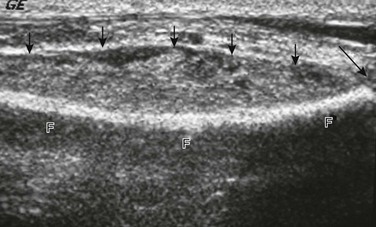
Figure 138-12 Acute osteomyelitis of the distal fibula in a 12-year-old boy.
Ultrasound shows a large subperiosteal abscess (small arrows) and distal fibular growth plate (long arrow). F, fibula.
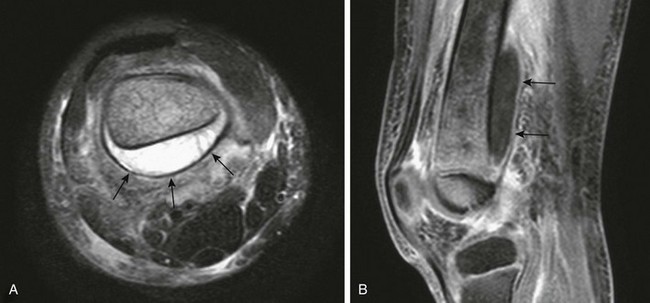
Figure 138-13 Acute osteomyelitis of the distal femur in a 5-year-old boy.
A, T2-weighted fat-saturated axial magnetic resonance imaging (MRI) shows a large subperiosteal abscess (arrows) at the posterior aspect of the femur. Increased signal is seen within the bone, and there is adjacent soft tissue edema. B, T1-weighted fat-saturated post-gadolinium sagittal MRI shows the longitudinal extent of the subperiosteal abscess with enhancing wall (arrows).
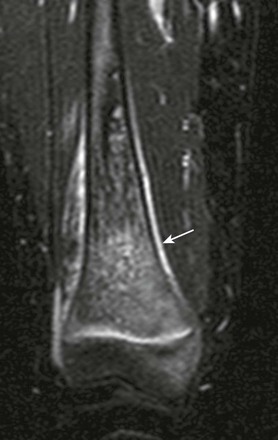
Figure 138-14 Femoral osteomyelitis in a 13-year-old girl.
Short tau inversion recovery coronal magnetic resonance image demonstrates a salt-and-pepper appearance to marrow edema and periosteal reaction (arrow).
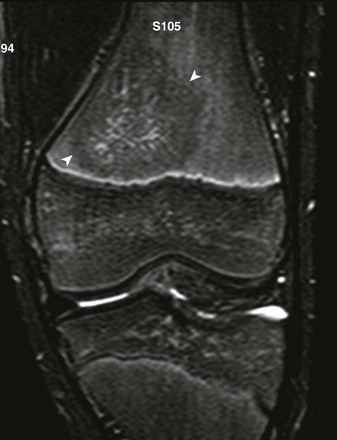
e-Figure 138-9 Biopsy-confirmed osteomyelitis in a 13-year-old boy.
short tau inversion recovery coronal sequence demonstrates round masslike hypointensity in the distal femur metadiaphysis (arrowheads).
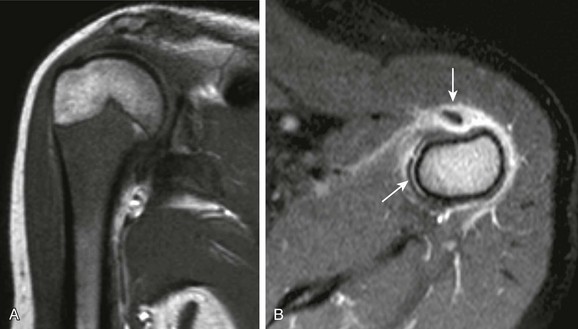
e-Figure 138-11 A 12-year-old girl with proximal humeral osteomyelitis.
A, T1-weighted coronal magnetic resonance imaging (MRI) demonstrates diffuse masslike marrow replacement in the proximal humerus. B, T1-weighted fat-saturated post-gadolinium axial MRI demonstrates periostitis and juxtacortical soft tissue edema. Note tenosynovitis of the long head of the biceps tendon (arrow).
Subacute and Chronic Osteomyelitis
Subacute or chronic osteomyelitis may develop as a result of partial host response to contain the infection. Distinguishing between subacute and chronic osteomyelitis is arbitrary.16 The initial purulent exudate is replaced by granulation tissue, and the clinical manifestations are mild and consist mainly of local pain. A Brodie abscess may then develop, typically in the metaphysis and less commonly in the epiphysis, because the growth plate is only a partial barrier against the spread of infection. A Brodie abscess is characterized radiographically by a central or eccentric round or oval radiolucency.17 The cavity may contain a small, dense sequestrum. On MRI, lesions have a characteristic layered appearance with a high-signal periphery as a result of edema (penumbra sign)18 and a double-line sign (rim sign), which on fluid-sensitive sequences is delineated as a low-signal outer rim because of sclerosis; an inner rind of intermediate signal because of granulation tissue; and a central, hyperintense region related to abscess (Fig. 138-15).19 With contrast-enhanced imaging, the inner granulation layer will show enhancement around the nonenhancing central abscess.
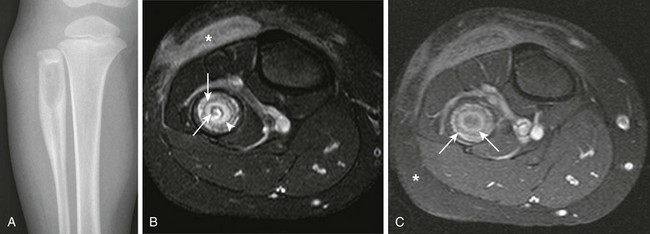
Figure 138-15 Brodie abscess in a 3-year-old boy.
A, Radiograph demonstrates a lytic lesion in the proximal fibula with laminated thick periostitis. T2-weighted fat-saturated axial (B) and T1-weighted fat-saturated post-gadolinium axial (C) magnetic resonance images demonstrate a Brodie abscess with sclerotic outer rim (asterisk) and inner granulation tissue with enhancement (arrowhead). Note the nonenhancing central abscess, which contains a small sequestrum (arrows) that is only seen on the T2-weighted fat-saturated sequence.
Adjacent soft tissue swelling and edema and periosteal new bone formation may be present. In spite of occasional growth plate involvement, the incidence of premature growth plate fusion after subacute osteomyelitis is rare.20
Cortical and trabecular bone sclerosis, cavities, involucra, and sequestra are characteristic of advanced osteomyelitis.21 The affected bone is thickened, and its outline may be wavy, with or without periosteal cloaking of new bone. An involucrum of reactive, viable bone may cloak an area of infection (Fig. 138-16). The involucrum may be perforated by a cloaca, which is a tract or communication between bone and the surrounding soft tissues (e-Fig. 138-17). If the cloaca extends to the skin surface, it is termed a sinus tract (see Fig. 138-16).22 The necrotic, devitalized bone of a sequestrum is surrounded by inflammatory granulation tissue and may be located within a bone abscess cavity. The dead bone of a sequestrum is relatively sclerotic. Sequestra may be demonstrated on radiography, CT, or MRI, but it is best seen with CT (see Figs. 138-6 and 138-16). Sequestration is now relatively rare owing to earlier diagnosis, largely because of advances in imaging, and more effective antibiotic therapies.
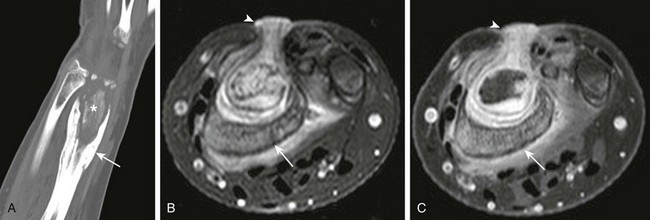
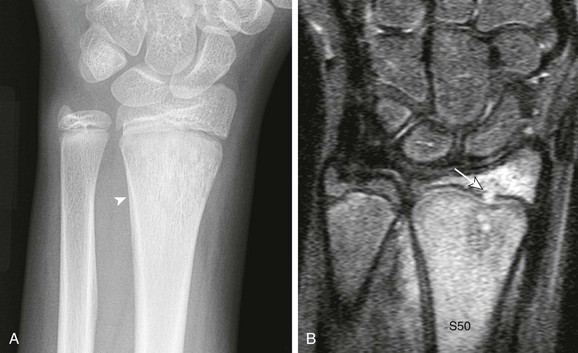
Figure 138-16 Chronic osteomyelitis of the distal radius in a 13-year-old girl.
A, Computed tomographic coronal reformat shows involucrum (arrow) surrounding sequestrum (asterisk). T2-weighted fat-saturated axial (B) and T1-weighted fat-saturated axial (C) post-gadolinium magnetic resonance image shows involucrum (arrow) that surrounds the nonenhancing sequestrum. Note granulation along the sinus tract that extends from the radial metaphysis sequestrum and abscess to the skin surface (arrowhead).
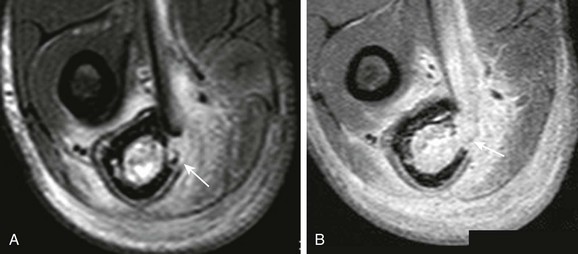
e-Figure 138-17 Chronic osteomyelitis and cloaca in a 17-year-old boy.
T2-weighted fat-saturated axial (A) and post-gadolinium T1-weighted fat-saturated axial (B) magnetic resonance image demonstrate cortical destruction with communication (arrow in A) between marrow and adjacent soft tissues (cloaca).
Radiographs will suggest the diagnosis of subacute or chronic osteomyelitis and may be used to follow up for gross changes. Although CT is of limited clinical value in acute osteomyelitis, it is very useful in imaging chronic disease to evaluate bone stock quality and to detect cortical metaphyseal tracts and channels, sequestra, and bone destruction. MRI will detect any reactivation or persistence of infection by showing focal active disease in the bone marrow and also showing juxtacortical soft tissue hyperemia and edema. Serial MRI after an established diagnosis of osteomyelitis has been made, and after medical or surgical treatment, has limited utility.23
Treatment: Although the mortality and morbidity of bone infection have decreased significantly, permanent sequelae do occur, largely as a result of delay in diagnosis or inadequate treatment with complications related to generalized bacterial sepsis.24 Complications of bone infection include pathologic fracture through regions of bone destruction,25 venous thrombosis,26 and adjacent infectious arthritis and destruction of joints (Fig. 138-18).
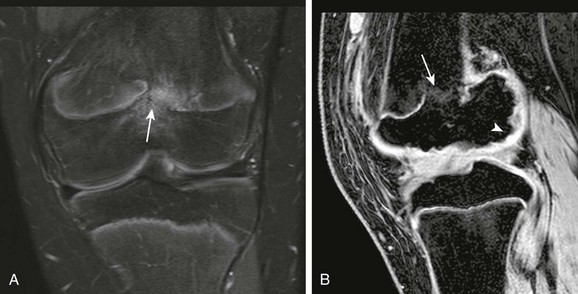
Figure 138-18 A 14-year-old boy with a remote history of distal femoral osteomyelitis and septic arthritis.
Physeal bar (arrows) and femoral condylar epiphyseal irregularity (arrowhead) are seen on T2-weighted fat-saturated coronal (A) and gradient recalled echo sagittal (B) magnetic resonance image.
Identification of a bacterial pathogen for underlying osteomyelitis ranges from 9% to 22% by blood culture and 40% to 50% by bone or joint aspiration.24 MRI is useful for confirming or excluding the diagnosis of osteomyelitis, identifying fluid for culture, and preoperative planning for incision and drainage of potential intraosseous and extraosseous abscesses.8
To decrease the chance of complications, empiric antibiotic therapy is often initiated even before bacterial culture is isolated.27 Treatment includes parenteral antibiotics followed by a long course of oral antibiotics, provided the child is not immunocompromised.28
Surgical drainage is recommended if the child has not responded to antibiotics after 48 to 72 hours, or if a substantial abscess is identified by MRI.24 If adjacent septic arthritis is present, the joint will need to be débrided as well. Aseptic reactive effusions may occur, so not all adjacent joint effusions near an area of osteomyelitis should be categorically diagnosed as secondary septic arthritis. Sequestrae are foci for continued infection, and their detection and localization are crucial in view of surgical excision.24
Neonatal Osteomyelitis
Etiology, Pathophysiology, and Clinical Presentation: In the neonatal period, the incidence of epiphyseal and joint involvement is higher, and multifocal disease may be seen.29 This is because of the persistence of metaphyseal vessels that cross the physis and extend into the intraarticularly located epiphysis. Also, osteomyelitis is often indolent in onset or is masked by other medical conditions. Risk factors include prematurity and vascular catheter-related sepsis.30 The most common causative organism is S. aureus. Other isolated organisms include Escherichia coli, group B streptococci, Gram-negative rods, and Candida albicans.29 The hip is frequently involved,31 and neonatal calcaneal osteomyelitis may develop after heel pad puncture for blood drawing.32
Imaging: Bone scan is very helpful for early diagnosis, particularly if symptoms and clinical findings are nonlocalizable.7 The bone scan will be positive several days before radiographic findings become manifest (~ 2 weeks). The initial radiographic findings may be soft tissue swelling with bone rarefaction and destruction seen later. Because of the high incidence of coexisting septic arthritis related to neonatal osteomyelitis, US of the adjacent joint may be performed to identify effusion. MRI may better delineate early epiphyseal cartilage involvement (Fig. 138-19).33 Complications related to neonatal osteomyelitis include premature physeal fusion, epiphyseal osteonecrosis, joint dislocation, and joint arthrodesis.29
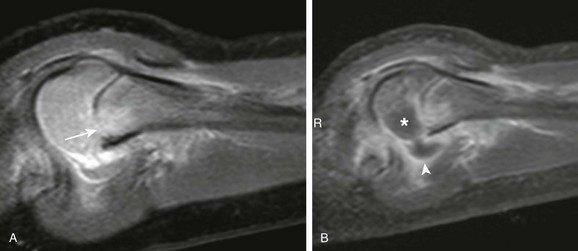
Figure 138-19 Acute proximal humeral osteomyelitis and septic arthritis in a 3-year-old boy.
A, Short tau inversion recovery sagittal magnetic resonance imaging (MRI) demonstrates proximal metaphyseal edema with physeal involvement (arrow). B, T1-weighted post-gadolinium sagittal MRI demonstrates glenohumeral effusion with synovial enhancement (arrowhead) and nonenhancing humeral epiphyseal cartilage, consistent with chondral involvement (asterisk).
Epiphyseal Osteomyelitis
Etiology, Pathophysiology, and Clinical Presentation: In tubular bones, the early changes of pyogenic hematogenous osteomyelitis are usually localized to the metaphysis, where capillary vascularization is very rich, and blood flow is slow on the venous side of the sinusoidal loops. Infection may spread from the metaphysis through the growth plate to the epiphysis (see Fig. 138-2).12 Epiphyseal osteomyelitis and septic arthritis occur more commonly in infants younger than 15 months of age, because metaphyseal vessels penetrate the growth plate and enter the epiphysis.34
Imaging: Radiography usually demonstrates a focus of epiphyseal bone destruction (Fig. 138-20, A). In subacute and chronic cases, a small round or oval epiphyseal abscess cavity with well-defined borders is seen. CT is useful for imaging of the bone cavity and detection of a sequestrum. MRI reveals the epiphyseal focus of infection as low signal on T1-weighted images and high signal on T2-weighted images (Fig. 138-20, B). Imaging after gadolinium injection may show rim enhancement outlining the abscess cavity of the epiphyseal ossification center. Browne et al showed epiphyseal cartilage involvement was seen in 52% of cases of S. aureus infections in children under 18 months of age.33 Enhancement defects in the epiphyseal cartilage are commonly seen in the setting of osteomyelitis, but they do not routinely reflect true epiphyseal cartilage abscesses (see Fig. 138-19); only one fifth to one third represent true epiphyseal cartilage abscesses.34 The importance of identifying epiphyseal cartilage involvement is both to determine underlying epiphyseal abscess formation and to provide prognostic information related to growth disturbance of the spherical growth plate of the epiphysis.
Treatment: Epiphyseal osteomyelitis may lead to growth disturbance of the epiphyseal ossification center and physis.33 Early osteoarthritis may also occur with epiphyseal and articular cartilage destruction. Because the epiphysis is an intraarticular structure, coexisting septic arthritis may often be present. Surgically, these children will require exploration and débridement of both the epiphyseal bone and joint space, when those are involved, based on preoperative MRI. Care should be made to differentiate epiphyseal cartilage enhancement defects from true epiphyseal abscesses.34
Fungal Osteomyelitis
Fungal infections of bone are becoming more common as the immunosuppressed population increases. Aspergillus, Candida, Histoplasma, Blastomyces, and other pathogens have been implicated. The radiographic changes are similar to those of chronic pyogenic osteomyelitis or tuberculosis (TB): destruction of bone and periosteal reaction with areas of cortical thickening and trabecular sclerosis. Fungal osteomyelitis may be multicencentric, masslike, and may superficially mimic osteonecrosis.31,35,36
Tuberculous Osteomyelitis
Etiology, Pathophysiology, and Clinical Presentation: Although osseous TB is a relatively rare condition today, cases are still encountered that are not diagnosed until an extensive, and expensive, workup has been completed. Hematogenous metastases of tubercle bacilli to the skeleton may take place early during the active phase of the primary complex in the thorax or later from postprimary tuberculous foci. Musculoskeletal involvement of TB is seen in approximately 10% to 15% of all TB cases.37 After implantation in the bone, an immediate active inflammatory reaction may develop, or the bacilli may be dormant for years until activated by local factors, such as trauma to the bone or joint. The synovial surface may be infected before the bones are involved; the infection may then spread from the joint into the contiguous epiphysis and metaphysis. In a large South African experience, the distribution of childhood skeletal TB is 60% to 70% vertebral, 20% to 25% large joints, and 10% to 15% tubular and flat bones.38
Imaging: TB produces a chronic inflammatory reaction in the bones that is similar in its macroscopic aspects to chronic pyogenic osteomyelitis. Local necrosis of the intraosseous tissues develops at the site of implantation and is then followed by regional decalcification and bone destruction. Spread of infection takes place through the same pathways as those described in the pathogenesis of pyogenic osteomyelitis. During infancy and early childhood, when the epiphyseal cartilages are relatively thick, direct transfer of the infection from the joint to the bone, or infection across joints, is uncommon. The articular cartilages are preserved longer in tuberculous osteomyelitis and arthritis than in pyogenic arthritis because of the lack of a destructive proteolytic enzyme in tuberculous exudates.39 Sinus formation and cold abscesses are common; involucrum formation and sequestration are rare.
The radiographic findings also are similar to those of chronic pyogenic osteomyelitis, so that TB should be added regularly to the differential diagnosis of focal bone disease. Unlike pyogenic osteomyelitis, radiographs are usually abnormal at the initial clinical presentation of tuberculous osteomyelitis.37
Certain imaging features can suggest the diagnosis of TB. In the metaphyses and epiphyses, destruction of bone is more prominent than production of bone (Fig. 138-21).39 The joint space is characteristically preserved in the early phases of tuberculous arthritis. The epiphysis is a site of predilection in primary skeletal TB. In the diaphyses of tubular bones, long segments may exhibit destructive and productive changes, whereas metaphyseal regions are unaffected (e-Fig. 138-22). Sometimes, sharply defined rarefactions are present that gave rise to the term cystic TB of bone. In the short bones of the hands and feet, tuberculous lesions may cause bone expansion, so-called spina ventosa (Fig. 138-23). Skeletal TB may affect multiple sites, and a bone scan is recommended to detect the presence of quiescent lesions. MRI is useful in certain cases and clearly demonstrates the full extent of the bone marrow, subperiosteal, and soft tissue involvement as well as any extension into the adjacent joint.
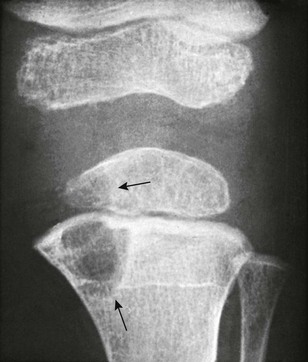
Figure 138-21 Tuberculous metaphysitis and epiphysitis of the tibia and arthritis of the knee in a 3-year-old boy.
Large areas of destruction are present in the medial aspects of the metaphysis and the epiphyseal ossification center (arrows).
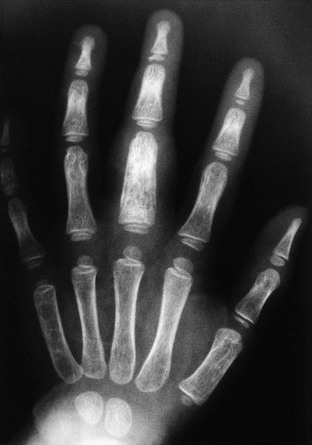
Figure 138-23 Tuberculous dactylitis (spina ventosa) in the proximal phalanx of the third finger in a 2-year-old girl.
The whole diaphysis is involved, but the epiphysis is spared. Expansion of the rest of the phalanx shows mixed areas of bone destruction and sclerosis.
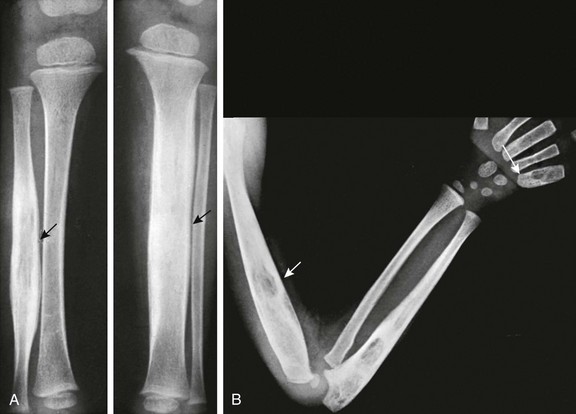
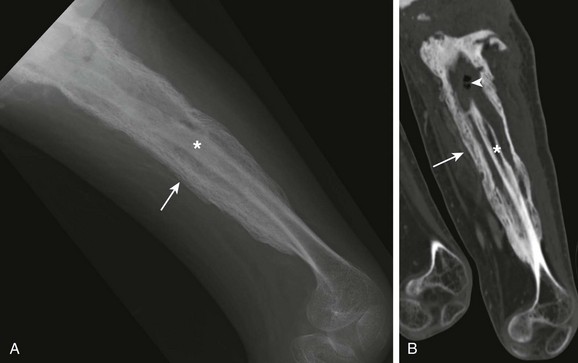
e-Figure 138-22 Multiple sites of tuberculous diaphysitis with cold abscesses on the dorsal surfaces of the hands in an 18-month-old boy.
A, Lower extremities. The left tibia and right fibula are enlarged; the medullary canals are expanded, and the overlying cortex is thickened (arrows). B, Left upper extremity. The distal half of the humerus is enlarged into a sausage-shaped contour, and the cortex is thickened; the medullary canal is expanded and exhibits cystic rarefaction (short arrow). The proximal half of the ulna is enlarged and irregularly cystic; its cortex is thickened. The fifth left metacarpal is expanded and irregularly lytic and osteoporotic (long arrow). All of these lesions healed slowly but completely. On images made 2 years later, the skeleton appeared to be normal.
Congenital Syphilis
Etiology, Pathophysiology, and Clinical Presentation: Congenital syphilis (Treponema pallidum) causes hepatosplenomegaly, lymphadenopathy, skin rash, and anemia.40 Bones are often involved, but this may not become clinically or radiologically manifest in the first weeks of life. Bone pain in one or more extremities may be severe and may result in lack of movement of those extremities, a condition termed Parrot pseudoparalysis. Syphilis of bone can be suggested on the basis of radiologic signs, when it has not been considered on clinical grounds. The diagnosis, however, still rests on appropriate serologic tests. Two main forms are observed, infantile and juvenile, and their radiographic features are different.
Imaging: The unique imaging characteristic of infantile syphilis is multiple bone involvement with almost selective localization in the metaphyses. Broad bands of metaphyseal radiolucency were considered evidence of “metaphysitis” but are now known to be nonspecific responses to the stress of disseminated infection.41 Syphilitic granulation tissue can, however, occur in these regions (e-Fig. 138-24). Radiographically, the two causes cannot be distinguished, but when associated with metaphyseal serration, the so-called sawtooth metaphysis (Fig. 138-25), the diagnosis of congenital syphilis is practically assured. Similar diagnostic “specificity” is provided by the Wimberger sign after the newborn period, when destructive foci are found in the metaphyseal regions of tubular bones, particularly the medial tibial metaphyses at the knee (Fig. 138-26). Metaphyseal destructive lytic lesions may lead to pathologic fractures, and child abuse is in the differential diagnosis.42 Although metaphyseal lesions are characteristic of congenital syphilis, they are not pathognomonic and may be seen with ordinary osteomyelitis, hyperparathyroidism, skeletal infantile myofibromatosis, and metastases. Diaphyseal involvement, especially with periosteal new bone formation, also tends to occur after the first month and may be associated with some destructive foci and even scattered focal cortical destruction and expansion of the medullary cavity (e-Fig. 138-27). The epiphyses are generally spared. Healing of the bone lesions in the infantile form of the disease occurs with or without therapy, usually without deformity.
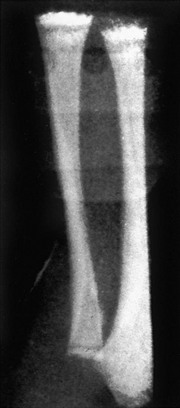
Figure 138-25 Newborn infant with congenital syphilis and sawtooth metaphysis.
This patient had a rash and mucocutaneous lesions characteristic of the disease.
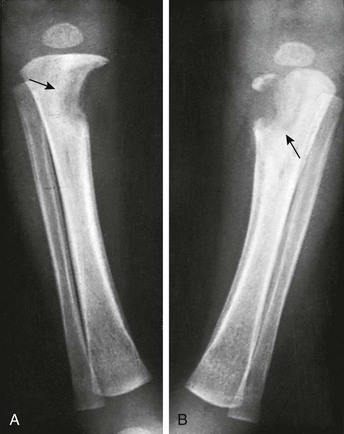
Figure 138-26 Bilateral, symmetric, destructive syphilitic metaphysitis of the proximal ends of the tibias (Wimberger sign) in a 2-month-old infant.
Large areas of destruction of the spongiosa and overlying cortex are seen at the medial aspects of the tibias (arrows). In the left tibia, the medial segment of the epiphyseal plate is partially destroyed. Note also diffuse periosteal thickening of the diaphyses.
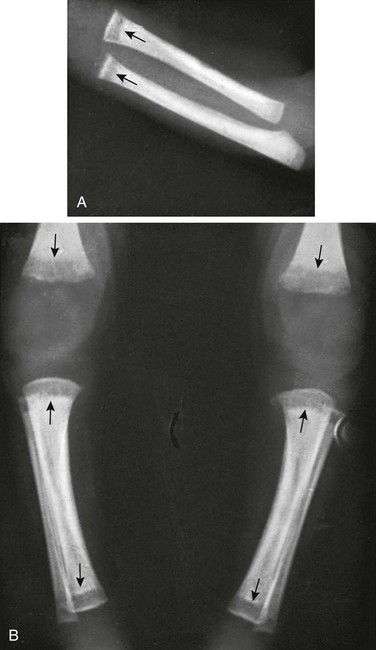
e-Figure 138-24 Syphilitic metaphysitis in a 1-month-old former premature infant.
Radiographs show deep segments of diminished density (arrows). The spongiosa in these segments has been replaced by radiolucent syphilitic granulation tissue. A, Upper limb. B, Lower limbs.
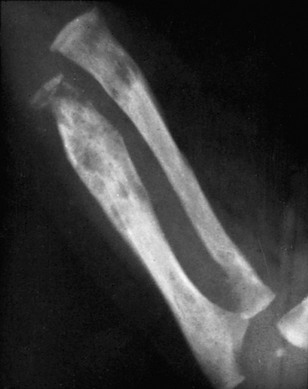
e-Figure 138-27 Expansion and destructive metaphyseal and diaphyseal changes in a 3-month-old syphilitic infant.
Juvenile syphilis is observed radiographically in childhood and is manifested by diffuse or localized subperiosteal thickening of the cortex (Fig. 138-28). Associated focal destructive lesions, resembling cystic TB, occasionally are present. Thickening of the anterior cortex of the tibia is responsible for the “saber shin” deformity of congenital syphilis that appears in late childhood.43
Septic Arthritis
Etiology, Pathophysiology, and Clinical Presentation: The annual per capita incidence of septic arthritis in a study based in the United States is approximately 1 in 93,500.1 S. aureus is the most commonly isolated organism, the majority of which are MRSA. The knee is most commonly affected (41.4%) followed by the hip (22.6%) and ankle (13.6%).
Bacterial entry into the joint can occur by two mechanisms: direct inoculation from a penetrating injury and hematogenous inoculation. The vascular synovium may be hematogenously inoculated.12 Alternatively, inoculation may occur secondarily by way of osteomyelitis. This may occur with higher frequency in infants when transphyseal vessels extend from the metaphysis to the intraarticular epiphysis.44
Septic arthritis causes rapid cartilage loss and joint destruction and therefore is a medical and surgical emergency. Diagnosis is based on clinical grounds and laboratory data. A history of fever, non-weightbearing, an erythrocyte sedimentation rate above 40 mm/hour, and a white blood cell count greater than 12,000 cells/mm3 are four consistent variables that occur frequently in the setting of septic arthritis as extensively studied in the hip.45 When present in isolation, each of these individual variables has low accuracy for the diagnosis of septic arthritis, but when three or more of these variables are present at the same time, the chance of a positive diagnosis of septic arthritis of the hip is greater than 93%.45
Imaging: Radiography is the first line of imaging in the setting of suspected septic arthritis. Radiographs are used to rule out an obvious alternative cause for symptoms, such as an underlying fracture. The initial findings may be nonspecific soft tissue swelling about a joint. In certain joints—such as the elbow, knee, and ankle—a joint effusion may be seen and is best delineated on a lateral radiograph.
The role of US is to identify the presence or absence of an underlying joint effusion or to determine whether pathology is intraarticular or extraarticular, such as adjacent tenosynovitis.46,47 When a joint effusion is present, no sonographic features are evident that predict the presence or absence of septic arthritis.48–50 Capsular distension with anechoic or hypoechoic fluid is usually seen. Debris and septations within the joint, as well as hyperemic flow in the synovium, can be seen on power Doppler; but this can also be seen in JIA, transient synovitis,50 and posttraumatic arthritis (Fig. 138-29).
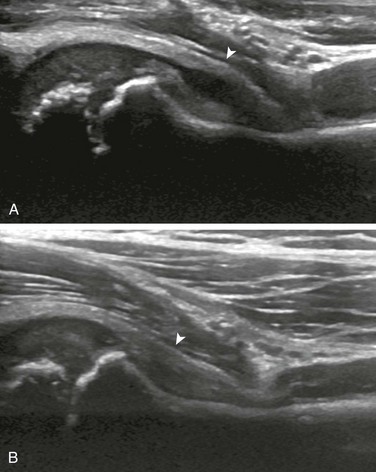
Figure 138-29 Transient synovitis of the hip in a 1-year-old girl.
A, Longitudinal imaging of the symptomatic right hip shows capsular distension (arrowhead) because of a large effusion. B, Imaging of the asymptomatic left hip shows a normal amount of hip fluid; note the capsule (arrowhead).
The MRI findings of isolated septic arthritis are also nonspecific.51,52 Synovial thickening, enhancement, effusions, and juxtasynovial soft tissue edema may be seen, but this is indistinguishable from transient synovitis and JIA. The role of MRI is not to diagnose septic arthritis but to identify the presence or absence of coexisting osteomyelitis (see Fig. 138-8), epiphyseal cartilage involvement (e-Fig. 138-30), and juxtasynovial muscle inflammation (Fig. 138-31) or abscesses, because this will affect medical and surgical management.
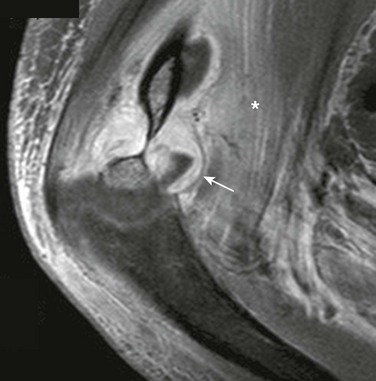
Figure 138-31 Elbow septic arthritis and distal humeral osteomyelitis in an 11-year-old boy.
T1-weighted fat-saturated post-gadolinium sagittal magnetic resonance image demonstrates thick synovial enhancement (arrow) and juxtasynovial myositis (asterisk). Marrow edema is also seen in the distal humerus.
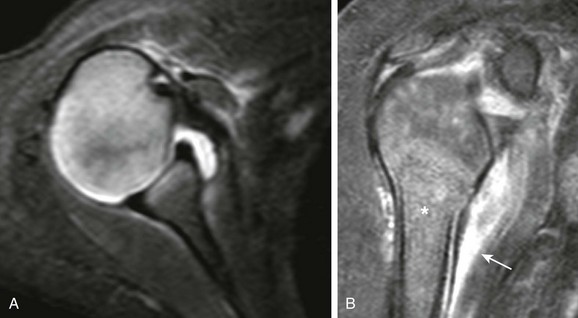
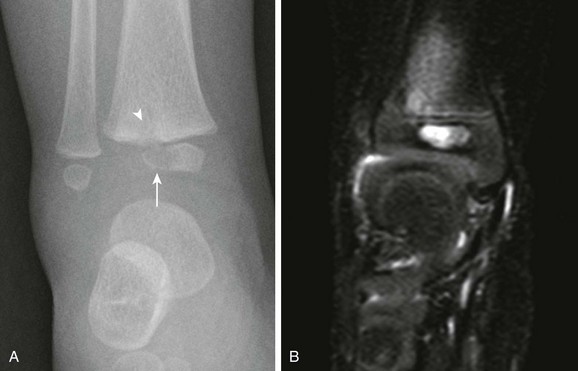
e-Figure 138-30 Glenohumeral septic arthritis related to humeral osteomyelitis in a 4-month-old boy.
A, T2-weighted fat-saturated axial magnetic resonance imaging (MRI) demonstrates a nonspecific glenohumeral joint effusion. B, T1-weighted fat-saturated post-gadolinium coronal MRI shows abnormal patchy enhancement of the proximal humeral epiphyseal cartilage, abnormal heterogeneous marrow enhancement (asterisk), and periostitis with juxtacortical edema (arrow).
Treatment: US may be used to both identify the presence or absence of a joint effusion and to provide needle guidance for diagnostic joint aspiration.47 Emergent arthrotomy and washout is recommended to prevent complications of septic arthritis. If septic arthritis is a complicating feature of osteomyelitis, the underlying bone should also be débrided. Complications of septic arthritis include sepsis, chondrolysis, and osteonecrosis.53 Antibiotic treatment is similar to the treatment of osteomyelitis at all ages: a total of 4 to 6 weeks of therapy.54
Pyomyositis
Etiology, Pathophysiology, and Clinical Presentation: Pyomyositis is a localized primary infection of muscle characterized by progression to abscess formation that can mimic tumor, trauma (hematoma), osteomyelitis, septic arthritis, cellulitis, or thrombophlebitis. This disorder is endemic in Africa, South America, southeastern Asia, and the South Pacific; it is increasingly reported in temperate climates, including the northern United States. Pyomyositis occurs in otherwise healthy children and in those predisposed to infection by debilitating diseases, including human immunodeficiency virus infection. S. aureus is the most commonly identified organism; it is isolated in 45% of cases in a U.S.-based study, followed by group A β-hemolytic streptococci (GABHS; 10%).1 GABHS infections may occur in the setting of Varicella infection,55 and multifocal pyomyositis may be seen as a complication of bacterial endocarditis. Secondary pyomyositis may also occur as the result of extension from adjacent osteomyelitis, particularly in the pelvis. Damaged muscle with some degree of underlying immune suppression and a source of bacteremia are the key predisposing elements.
Clinically, the gluteal muscles, thighs, and calves are more commonly affected than the upper extremities, trunk, and chest wall.56 Approximately 25% of patients have a history of antecedent trauma. Usually, only a single muscle is involved. Early in the course of illness, patients experience low-grade fevers, general malaise, and dull, cramping pain.57 The involved area lacks the cutaneous erythema usually evident with cellulitis, and it has a “woody” nonfluctuant feel. Fever usually occurs as infection advances. Infections of the pelvic musculature may clinically mimic osteomyelitis or septic arthritis by producing hip or groin pain. Infection of the piriformis muscle may irritate the adjacent sciatic nerve and produce pain that extends into the ipsilateral lower extremity.58 Pyomyositis is usually accompanied by elevations in white blood cell count, erythrocyte sedimentation rate, and C-reactive protein level.
Imaging: Early in the development of pyomyositis, the affected muscle is enlarged and edematous without a frank abscess. Without treatment, the process progresses to form an intramuscular abscess. US may be helpful in localizing relatively superficial sites of pyomyositis (Fig. 138-32).59 Infections at deeper sites and infections that have not yet formed an intramuscular abscess are less readily detected with US. Although CT is not the imaging modality of choice, most intramuscular abscesses are readily seen at all locations. CT findings indicative of abscess include enlargement of the involved muscle, central low-attenuation fluid collection, and a peripheral rim that enhances with contrast (e-Fig. 138-33).60
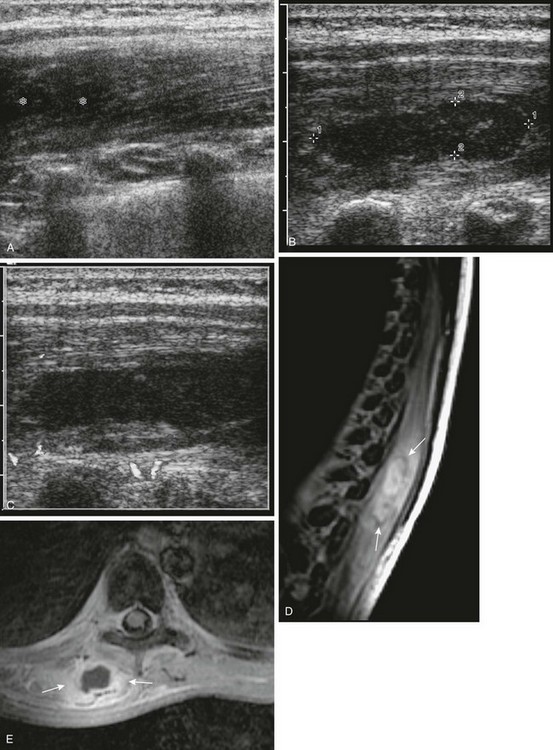
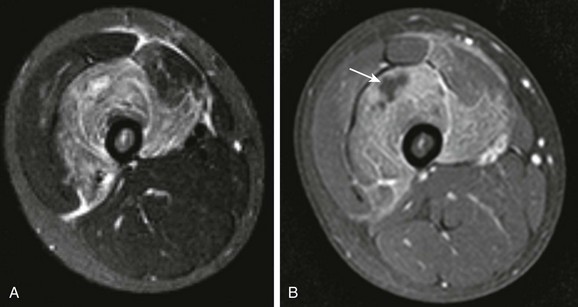
Figure 138-32 Pyomyositis.
A 10-year-old girl with aplastic anemia came to medical attention with fever and back pain. During renal sonography (A), hypoechoic enlargement of the right paraspinal muscles (asterisk) was seen that 2 days later had progressed to localized phlegmon/abscess (cursors), with no Doppler signal seen centrally (B and C). D, Sagittal T2-weighted magnetic resonance imaging shows right paraspinal muscle edema with a central higher signal intensity collection surrounded by a lower signal intensity rim (arrows). E, On axial fat-suppressed post-gadolinium T1-weighted images, the collection (arrows) is characterized by an irregular rim of enhancement and lack of contrast enhancement centrally. Note low signal intensity marrow, likely the result of iron deposition from multiple transfusions.
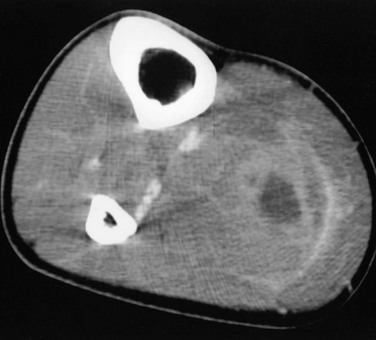
e-Figure 138-33 Pyomyositis.
An abscess in the leg of a 16-year-old boy with leukemia. A contrast-enhanced computed tomographic scan image shows a central low-attenuation collection with an ill-defined enhancing peripheral rim.
MRI is the preferred modality for imaging of suspected pyomyositis in children, and it is particularly valuable in cases of pelvic involvement.61 On MRI, the involved muscle appears enlarged and edematous with increased signal on T2-weighted and inversion recovery sequences. On T1-weighted images, the signal intensity of some abscesses is homogeneously greater than that of the involved muscle. On T2-weighted images, the abscess is seen as high signal with a hypointense rim and less well-defined hyperintensity of the adjacent muscle (Fig. 138-34). Post-gadolinium T1-weighted images best reveal the abscess, and the abscess wall densely enhances (see Fig. 138-32). Changes may result from associated cellulitis and include skin thickening, stranding of subcutaneous fat, and swelling of fascial planes; these findings are best seen on fat-saturated T2-weighted and inversion recovery images. A sympathetic effusion may be present if infection is near a joint. Often, the chief clinical differential diagnosis is osteomyelitis. In pyomyositis, underlying bones usually appear normal, and the process is displaced or eccentric to underlying bone, whereas in osteomyelitis, signal abnormality is seen within the medullary space, and the process is centered on involved bone.
Necrotizing Fasciitis/Cellulitis
Etiology, Pathophysiology, and Clinical Presentation: Necrotizing fasciitis is a rapidly progressive, sometimes fatal soft tissue infection that characteristically involves the deep fascia of the extremities and trunk.57 The disease tends to affect the elderly and those with impaired immunity, but it can occur in immune-competent patients of any age. Underlying skin infection related to lesions such as furuncles, insect bites, and even minor trauma or surgery has been associated. Necrotizing fasciitis is most commonly a polymicrobial infection with both aerobic and anaerobic organisms. In children with chicken pox, secondary infection of vesicular skin lesions with S. aureus or GABHS has been linked to development of cellulitis and, less frequently, necrotizing fasciitis.63
Imaging: Imaging can facilitate the diagnosis. Radiographs are insensitive to changes of necrotizing fasciitis until they are advanced, at which time gas can be seen in the soft tissues. Characteristic CT findings include soft tissue air, deep fascial fluid collections, and fascial enhancement, although these findings are not consistently seen (e-Fig. 138-35). Ultrasonography may depict deep soft tissue changes and fluid collections and offers the benefits of portability along with the ability to guide diagnostic aspiration.
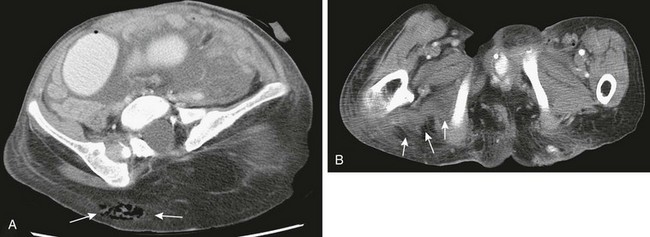
e-Figure 138-35 Necrotizing fasciitis.
This female patient with spinal dysraphism and cloacal exstrophy developed sepsis after bladder augmentation. Contrast-enhanced computed tomography (A and B) shows gas in the soft tissues of the right buttock (A, arrows) and diffuse deep fascial thickening with low attenuation (B, arrows).
Pathologic changes and extent of necrotizing fasciitis and cellulitis are detected most sensitively on MRI with fat-suppressed T2-weighted, short tau inversion recovery sequences, and post-gadolinium fat-suppressed T1-weighted imaging (Fig. 138-36). Cellulitis is characterized by high T2 signal thickening and gadolinium enhancement with or without fluid collection in the subcutaneous tissue and superficial fascia (e-Fig. 138-37). Necrotizing fasciitis should be considered when these changes extend to the deep fascia. Patients with necrotizing fasciitis may have thick (> 3 mm) fascial signal intensity or low signal intensity in the deep fascia on T2-weighted images, along with focal or diffuse nonenhancing portions of deep fascia and involvement of three or more compartments in one extremity.64 Soft tissue gas appears as areas of signal absence with susceptibility artifact. To help guide therapy, it is important to distinguish by MRI whether necrotizing fasciitis is related to underlying osteomyelitis and/or septic arthritis versus infection confined to the muscles and fascia.
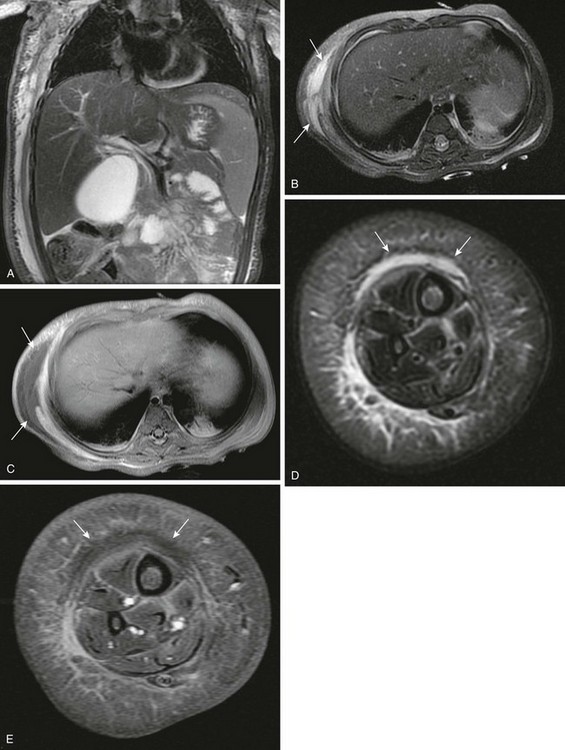
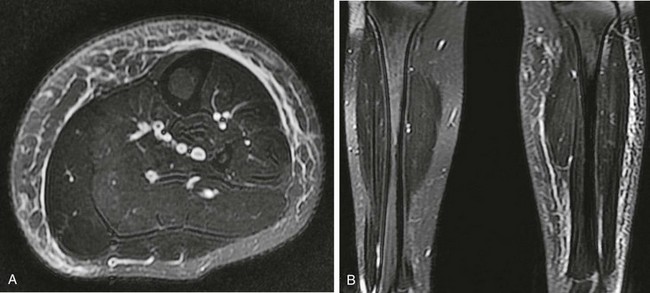
Figure 138-36 Necrotizing fasciitis.
A 1-year-old girl with a 2 week history of progressive fever, leukocytosis, and swelling of the right side of the chest wall and right calf. Coronal (A) and axial (B) T2-weighted magnetic resonance images show asymmetric high signal in the muscle and superficial and deep fascial layers of the right chest and abdominal wall (arrows). Axial fat-suppressed T1-weighted sequence (C) after intravenous gadolinium administration shows enhancement of these areas with more superficial nonenhancing fluid lacking rim enhancement (arrows). Similar changes (arrows) are seen in the right lower leg on axial T2-weighted (D) and post-contrast fat-suppressed T1-weighted images (E). Biopsy of both sites showed acute inflammation with coagulative necrosis. Cultures were negative, but dramatic clinical improvement was noted with intravenous antibiotic therapy.
Jaramillo, D. Infection: musculoskeletal. Pediatr Radiol. 2011;41(Suppl 1):S127–S134.
Kocher, MS, Zurakowski, D, Kasser, JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg. 1999;81-A(12):1662–1670.
Tureck, MB, Taljanovic, MS, Stubbs, AY, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010;39(10):957–971.
References
1. Gafar, OA, Copley, LAB, Hollmig, ST, et al. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008;28(7):777–785.
2. Dartnell, J, Ramachandran, M, Katchburian, M. Hematogeneous acute and subacute paediatric osteomyelitis. J Bone Joint Surg Br. 2012;94-B:584–595.
3. Howard, AW, Viskontas, D, Sabbagh, C. Reduction in osteomyelitis and septic arthritis related to Haemophilus influenza type B vaccination. J Pediatr Orthop. 1999;19(6):705–709.
4. Lonergan, GJ, Cline, DB, Abbondanzo, SL. Sickle cell anemia. Radiographics. 2001;21(4):971–994.
5. Puffinbarger, WR, Gruel, CR, Herndon, WA, et al. Osteomyelitis of the calcaneus in children. J Pediatr Orthop. 1996;16(2):224–230.
6. Blickman, JG, van Die, CE, de Rooy, JWJ. Current imaging concepts in pediatric osteomyelitis. Eur Radiol. 2004:14–L55.
7. DiPoce, J, Jbara, ME, Brenner, AI. Pediatric osteomyelitis: a scintigraphic case based review. Radiographics. 2012;32:865–878.
8. Kan, JH, Hilmes, MA, Martus, JE, et al. Value of MRI after recent diagnostic or surgical intervention in children with suspected osteomyelitis. AJR. 2008;191(5):1595–1600.
9. Metwalli, ZA, Kan, JH, Munjal, KA, et al. MR imaging of suspected lower extremity musculoskeletal infection in the pediatric patient: how useful is bilateral imaging? AJR. 2012. [in press].
10. Saigal, G, Azouz, EM, Abdenour, G. Imaging of osteomyelitis with special reference to children. Semin Musculoskelet Radiol. 2004;8(3):255–265.
11. Park, HM, Rothschild, PA, Kernek, CB. Scintigraphic evaluation of extremity pain in children: its efficacy and pitfalls. AJR. 1985;145(5):1079–1084.
12. Jaramillo, D. Infection: musculoskeletal. Pediatr Radiol. 2011;41(Suppl 1):S127–S134.
13. Kan, JH, Young, RS, Yu, C, et al. Clinical impact of gadolinium in the MRI diagnosis of musculoskeletal infections in children. Pediatr Radiol. 2010;40(7):1197–1205.
14. Averill, LW, Hernandez, A, Gonzalez, L, et al. Diagnosis of osteomyelitis in children: utility of fat-suppressed contrast enhanced MRI. AJR. 2009;192:1232–1238.
15. Connolly, SA, Connolly, LP, Drubach, LA, et al. MRI for detection of abscess in acute osteomyelitis of the pelvis in children. AJR. 2007;189:867–872.
16. Bohndorf, K, Infection of the appendicular skeleton. Eur Radiol, 2004;14:E53–E63.
17. McWilliams, CA. Central bone abscess: Brodie’s abscess; chronic suppurative osteomyelitis. Ann Surg. 1921;74(5):568–578.
18. Grey, AC, Davies, AM, Mangham, DC, et al. The “penumbra sign” on T1-weighted MR imaging in subacute osteomyelitis: frequency, cause, and significance. Clin Radiol. 1998;53(8):587–592.
19. Erdman, WA, Tamburro, F, Jayson, HT, et al. Osteomyelitis: characteristics and pitfalls of diagnosis with MRI. Radiology. 1991;180:533–539.
20. Rasool, MN. Primary subacute haematogenous osteomyelitis in children. J Bone Joint Surg Br. 2001;83-B:93–98.
21. Jones, HW, Harrison, JW, Bates, J, et al. Radiologic classification of chronic hematogenous osteomyelitis in children. J Pediatr Orthop. 2009;29(7):822–827.
22. Pineda, C, Espinosa, R, Pena, A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrsonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg. 2009;23:80–89.
23. Courtney, PM, Flynn, JM, Jaramillo, D, et al. Clinical indications for repeat MRI in children with acute hematogenous osteomyelitis. J Pediatr Orthop. 2010;30(8):883–887.
24. Fause, SN, Clark, J, Pallett, A, et al. Managing bone and joint infection in children. Arch Dis Child. 2012;97:545–553.
25. Taylor, MN, Chaudhuri, R, Davis, J, et al. Childhood osteomyelitis presenting as a pathological fracture. Clin Radiol. 2008;63:348–351.
26. Mantadakis, E, Plessa, E, Vouloumanou, EK, et al. Deep venous thrombosis in children with musculoskeletal infections: the clinical evidence. Int J Infect Dis. 2012;16(4):e236–e243.
27. Lew, DP, Waldvogel, FA. Osteomyelitis. Lancet. 2004;364:369–379.
28. Hatzenbuehler, J, Pulling, TJ. Diagnosis and management of osteomyelitis. American Family Physician. 2011;84(9):1027–1033.
29. Offiah, AC. Acute osteomyelitis, septic arthritis, and discitis: differences between neonates and older children. European Journal of Radiology. 2006;60:221–232.
30. Kothari, NA, Pelchovitz, DJ, Meyer, JS. Imaging of musculoskeletal infections. Radiol Clin North Am. 2001;39(4):653–671.
31. Winterstein, AR, Bohndorf, K, Vollert, K, et al. Invasive aspergillosis osteomyelitis in children-a case report and review of the literature. Skeletal Radiol. 2010;39:827–831.
32. Canale, ST, Manugian, AH. Neonatal osteomyelitis of the os calcis: a complication of repeated heel punctures. Clin Orthop Relat Res. 1981;156:178–182.
33. Browne, LP, Guillerman, RP, Orth, RC, et al. Community-acquired staphylococcal musculoskeletal infection in infants and young children: necessity of contrast-enhanced MRI for the diagnosis of growth cartilage involvement. AJR. 2012;198:194–199.
34. Johnson, DP, Hernanz-Schulman, M, Martus, JE, et al. Significance of epiphyseal enhancement defects in pediatric osteomyelitis identified by MRI with surgical correlation. Pediatr Radiol. 2011;41(3):355–361.
35. Gamaletsou, MN, Kontoyiannis, DP, Sipsas, NV, et al. Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970-2011). Clin Infect Dis. 2012;55(10):1338–1351.
36. Moore, RM, Green, NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097–1101.
37. Shikhare, SN, Singh, DR, Shimpi, TR, et al. Tuberculous osteomyelitis and spondylodiscitis. Semin in Musculoskeletal Radiology. 2011;15(5):446–458.
38. Cremin, BJ. Tuberculosis: the resurgence of our most lethal infectious disease: a review. Pediatr Radiol. 1995;25:620–625.
39. Teo, HEL, Peh, WCG. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34:853–860.
40. Woods, CR. Congenital syphilis-persisting pestilence. Pediatr Infect Dis J. 2009;28(6):536–537.
41. Coblentz, DR, Cimini, R, Mikity, VG, et al. Roentgenographic diagnosis of congenital syphilis in the newborn. JAMA. 1970;212(6):1061–1064.
42. Fiser, RH, Kaplan, J, Holder, JC. Congenital syphilis mimicking the battered child syndrome. How does one tell them apart? Clin Pediatr. 1972;11(5):305–307.
43. Fiumara, NJ, Manifestations of late congenital syphilis: an analysis of 271 patients. Arch Derm, 1970;102:78–83.
44. Daldrup-Link, HE, Steinbach, L. MR imaging of pediatric arthritis. Magn Reson Imaging Clin N Am. 2009;17:451–467.
45. Kocher, MS, Zurakowski, D, Kasser, JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg. 1999;81-A(12):1662–1670.
46. Lim-Dunham, JE, Ben-Ami, TE, Yousefzadeh, DK. Septic arthritis of the elbow in children: the role of sonography. Pediatr Radiol. 1995;25(7):556–559.
47. Zawin, JK, Hoffer, FA, Rand, FF, et al. Joint effusion in children with an irritable hip: US diagnosis and aspiration. Radiology. 1993;187(2):459–463.
48. Zamzam, MM. The role of ultrasound in differentiating septic arthritis from transient synovitis of the hip in children. J Pediatr Orthop B. 2006;15(6):418–422.
49. Chau, CL, Griffith, JF. Musculoskeletal infections: ultrasound appearances. Clin Radiol. 2005;60(2):149–159.
50. Strouse, PJ, DiPietro, MA, Adler, RS. Pediatric hip effusions: evaluation with power Doppler sonography. Radiology. 1998;206(3):731–735.
51. Lee, SK, Suh, KJ, Kim, YW, et al. Septic arthritis versus transient synovitis at MR imaging: Preliminary assessment with signal intensity alterations in bone marrow. Radiology. 1999;211:459–465.
52. Graif, M, Schweitzer, ME, Deely, D, et al. The septic versus nonseptic inflamed joint: MRI characteristics. Skeletal Radiol. 1999;28:616–620.
53. Nunn, TR, Cheung, WY, Rollinson, PD. A prospective study of pyogenic sepsis of the hip in childhood. J Bone Joint Surg. 2007;89-B:100–106.
54. Faust, SN, Clark, J, Pallett, A, et al. Managing bone and joint infection in children. Arch Dis Child. 2012;97:545–553.
55. Cowan, MR, Primm, PA, Scott, SM, et al. Serious group A beta-hemolytic streptococcal infections complicating varicella. Ann Emerg Med. 1994;23(4):818–822.
56. Chiedozi, LC. Pyomyositis: Review of 205 cases in 112 patients. Am J Surg. 1979;137(2):255–259.
57. Frank, G, Mahoney, HM, Eppes, SC. Musculoskeletal infections in children. Pediatr Clin N Am. 2005;52:1083–1106.
58. Burton, DJ, Enion, D, Shaw, DL. Pyomyositis of the piriformis muscle in a juvenile. Ann R Coll Surg Engl. 2005;87(1):W9–W12.
59. Tureck, MB, Taljanovic, MS, Stubbs, AY, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010;39(10):957–971.
60. Gordon, BA, Martinez, S, Collins, AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology. 1995;197(1):279–286.
61. Karmazyn, B, Loder, RT, Kleiman, MB, et al. The role of pelvic magnetic resonance in evaluating nonhip sources of infection in children with acute nontraumatic hip pain. J Pediatr Orthop. 2007;27(2):158–164.
62. Mitsionis, GI, Manoudis, GN, Lykissas, MG, et al. Pyomyositis in children: early diagnosis and treatment. J Pediatr Surg. 2009;44(11):2173–2178.
63. Shirley, R, Mackey, S, Meagher, P. Necrotising fasciitis: a sequelae of varicella zoster infection. J Plast Reconstr Aesthet Surg. 2011;64(1):123–127.
64. Kim, KT, Kim, YJ, Won Lee, J, et al. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology. 2011;259(3):816–824.
65. Endorf, FW, Garrison, MM, Klein, MB, et al. Characteristics, therapies, and outcome of children with necrotizing soft tissue infections. Pediatr Infect Dis J. 2012;31(3):221–223.